Abstract
Circulating inflammatory factors and endothelial dysfunction have been proposed to contribute to the pathophysiology of hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome. To date, the occurrence of neurological complications in these women has been reported, but few studies have examined whether impairment in blood–brain barrier (BBB) permeability or cerebrovascular reactivity is present in women having HELLP syndrome. We hypothesized that plasma from women with HELLP syndrome causes increased BBB permeability and cerebrovascular dysfunction. Posterior cerebral arteries from female nonpregnant rats were perfused with 20% serum from women with normal pregnancies (n = 5) or women with HELLP syndrome (n = 5), and BBB permeability and vascular reactivity were compared. Plasma from women with HELLP syndrome increased BBB permeability while not changing myogenic tone and reactivity to pressure. Addition of the nitric oxide (NO) synthase inhibitor Nω-nitro-l-arginine methyl ester caused constriction of arteries that was not different with the different plasmas nor was dilation to the NO donor sodium nitroprusside different between the 2 groups. However, dilation to the small- and intermediate-conductance, calcium-activated potassium channel activator NS309 was decreased in vessels exposed to HELLP plasma. Thus, increased BBB permeability in response to HELLP plasma was associated with selective endothelial dysfunction.
Keywords: HELLP syndrome, blood–brain barrier permeability, myogenic response
Introduction
Hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome is a severe form of preeclampsia, that affects 10% to 20% of women with preeclampsia and 0.5% to 0.9% of women in the United States.1,2 Similar to preeclampsia, the pathophysiology of HELLP syndrome remains unclear, but placental ischemia is thought to be among one of the initiating events.2 Among the more serious maternal morbidities associated with HELLP syndrome are neurological complications such as eclampsia, brain edema, headaches, visual disturbances, cerebellar infarctions, and cognitive disturbances.3–5
Impairments or disruption in the blood–brain barrier (BBB) can enhance the passage of damaging circulating proteins and water into the brain parenchyma, thereby possibly contributing to the neurological complications that can occur in women with HELLP syndrome.6–8 Previous studies by our group and others have shown that circulating factors in women with preeclampsia and eclampsia are associated with increased permeability of the BBB.9,10 In addition, we recently reported that women with HELLP syndrome have increased circulating inflammatory markers and antiangiogenic factors such as tumor necrosis factor-α and soluble fms-like tyrosine kinase 1 receptor,11 which have been postulated to increase vessel reactivity through endothelial dysfunction. The goal of the current study was to determine whether plasma from women with HELLP syndrome increases BBB permeability by measuring hydraulic conductivity (Lp) of blood vessels exposed to plasma.10 We also set out to evaluate the effect of circulating factors in the plasma of women with HELLP syndrome on cerebral artery reactivity and endothelium-dependent vasodilator responses as these may contribute to the development of neurological complications.
Methods
Patients and Plasma Samples
To determine whether plasma from patients with HELLP syndrome increased BBB permeability, blood samples were collected from a small number of patients enrolled in an institutional review board-approved study at the University of Mississippi Medical Center. Plasma was pooled from 2 groups: a control group of normotensive pregnant women (n = 6) with uncomplicated pregnancies and a group of women with HELLP syndrome. The HELLP group (n = 6) was composed of women diagnosed with HELLP syndrome using the American College of Obstetrics and Gynecologists criteria of platelet count ≤100 000, total serum lactate dehydrogenase ≥600 IU/L, and aspartate aminotransferase ≥70 IU/L.12 Blood samples were collected from patients into vacutainer tubes containing EDTA. Blood was centrifuged at 3200 rpm, the plasma was removed, and samples were frozen at −80°C until pooling and aliquoting. One patient sample from each group was not used due to discoloration when compared to the other plasma samples. These 2 samples were also collected over the weekend and remained in the labor and delivery research refrigerator over the weekend instead of being centrifuged and the plasma immediately froze. Samples were pooled by taking equal volumes of plasma from 5 patients with HELLP syndrome and 5 normotensive pregnant women, gently mixing, and aliquoting into an appropriate volume for each experiment. Aliquots were stored at −80°C until experimentation.
Animals
All animal procedures were approved by the University of Vermont Institutional Animal Care and Use Committee and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Twelve female virgin nonpregnant Sprague Dawley rats (12-14 weeks; 250-300 g) were used for all experiments. The animals were purchased from Charles River (Saint-Constant, Quebec, Canada) and housed until experimentation at the University of Vermont Animal Care Facility, an Association for Assessment and Accreditation of Laboratory Animal Care-accredited facility. Animals had access to food and water ad libitum and maintained a 12-hour light–dark cycle.
Blood–Brain Barrier Permeability Studies
To determine the effects of plasma from normal pregnant women and women with HELLP syndrome on BBB permeability, Lp was measured in isolated posterior cerebral arteries (PCAs) from female nonpregnant rats, as described previously.9,10,13,14 Briefly, a third-order branch of the PCA was carefully dissected out of the brain and the proximal end mounted on a glass microcannula in an arteriograph chamber. Posterior cerebral arteries (n = 6/group) were perfused intraluminally with 20% (v/v) plasma pooled from normal pregnant women or women with HELLP syndrome in physiological saline solution (PSS) for 2 hours at 80 ± 0.1 mm Hg and 37.0°C ± 0.2°C. After the equilibration period, the pressure servo was disconnected from the pressure transducer, and the drop in pressure due to transvascular filtration across the vascular wall in response to hydrostatic pressure was measured for 40 minutes. The decrease in intravascular pressure per minute (mm Hg/min) was converted to volume flux across the vessel wall (µm3) using a conversion curve, as described previously.13,14 The Lp was then determined by normalizing flux to surface area and oncotic pressure of the serum perfusate.
Reactivity Studies
To determine the effect of plasma from normal pregnant women and women with HELLP syndrome on reactivity of PCAs, myogenic activity and tone were measured as described previously.14 Briefly, the pressure was returned to 80 mm Hg, and the pressure transducer and servo controller were reconnected so that intravascular pressure could be either set to a constant pressure or changed manually. Lumen diameter of the PCAs was measured via video microscopy during the experiment. To assess myogenic responses, intravascular pressure was increased in steps of 20 to 120 mm Hg and lumen diameter was recorded at each pressure once stable. Pressure was then returned to 80 mm Hg for the remainder of the experiment. NS309, a small- and intermediate-conductance Ca2+-activated K+ (SK/IK) channel activator, was cumulatively added to the bath (10−8 to 10−5 mol/L), and the dilation to NS309 was measured at each concentration. NS309 was washed out of the bath and a single dose (10−3 mol/L) of the nitric oxide synthase (NOS) inhibitor Nω-nitro-l-arginine methyl ester (l-NAME) was added to the bath. The constriction in response to NOS inhibition was measured after approximately 20 minutes. In the presence of l-NAME, the nitric oxide (NO) donor sodium nitroprusside (SNP) was cumulatively added to the bath (10−8 to 10−5 mol/L) and the dilation to SNP was measured at each concentration. Zero-Ca2+ PSS was added to the bath to obtain fully relaxed diameters. Passive diameters were then recorded at pressures from 120 to 5 mm Hg.
Data Calculations
Percentage of tone was calculated as the percentage of decrease in diameter from the fully relaxed diameter in zero-Ca2+ PSS by the following equation: ((Øpassive − Øactive)/Øpassive) × 100%, where Øpassive is the diameter in zero-Ca2+ PSS and Øactive is the diameter when the vessels had tone. Percentage of constriction to l-NAME was calculated with the following equation: ((Øbaseline − Ødrug)/Øbaseline) × 100%, where Øbaseline is the diameter before adding the l-NAME and Ødrug is the diameter with the presence of l-NAME. Percentage of sensitivity to NS309 and SNP was calculated with the equation: ((Ødrug − Øminimum)/(Ømaximum − Øminimum)) × 100%, where Ødrug is diameter at a specific concentration of NS309 or SNP, Øminimum is diameter at the lowest concentration of NS309 or SNP, and Ømaximum is the diameter at the highest concentration of SNP. The effective concentration that produced half maximal dilation of NS309 (EC50) was calculated from individual plots of each sensitivity curve and averaged. The EC50 for SNP was not calculated because the curves were not different between the groups.
Drugs and Solutions
Physiological saline solution was made weekly and stored without glucose. The pH was kept constant at 7.40 ± 0.05 during an experiment by aeration with 5% CO2, 10% O2, and 85% N2. The composition of PSS was (mmol/L) 119.0 NaCl, 24.0 NaHCO3, 4.7 KCl, 1.17 MgSO4, 0.026 EDTA, 5.0 CaCl2, 1.18 KH2PO4, and 5.5 dextrose. Dextrose was added prior to each experiment. Physiological saline solution without Ca2+ was also made daily but Ca2+ was left out of the solution. l-NAME, NS309, and SNP were purchased from Sigma (St Louis, Missouri), made as stock solutions each week, and diluted prior to use.
Statistical Analysis
All data are expressed as mean ± standard error of the mean. Data were analyzed using unpaired t test with Welch correction. Differences were considered statistically significant when P < .05.
Results
Patient Population and Demographics
Patients in the current study were similar regarding baseline demographic data with the expected exceptions of gestational age at delivery and blood pressure (Table 1). Women with HELLP syndrome had significantly higher systolic and diastolic pressure (P < .05) compared to normal pregnant women, and they also delivered significantly earlier in gestation (P < .05) compared to normal pregnant women (Table 1). None of the normal pregnant patients experienced any neurological complaints during pregnancy, whereas 50% of the patients with HELLP syndrome had some form of neurologic complaint (Table 2). Neurologic complaints were classified as those associated with HELLP syndrome1,2 and reported to the nursing staff and/or physician and further evaluated by the Maternal-Fetal-Medicine specialist on the floor. In all, 2 patients had neurological complaints consisting of headaches unrelieved with Tylenol (acetaminophen) and 1 patient had a single eclamptic seizure followed by a benign neuroradiologic examination, which demonstrated no abnormal cerebral pathology.
Table 1.
Patient Demographics.
| Normal Pregnant (n = 6) | HELLP Syndrome (n = 6) | P Value | |
|---|---|---|---|
| Age, y | 29.33 ± 3.4 | 24.00 ± 1.9 | .200 |
| Median (range) | 20-38 | 18-31 | |
| Race (% African American) | 67 | 100 | .455 |
| Gestational age at delivery, wk | 38.78 ± 0.3 | 31.52 ± 2.2 | .009a |
| Median (range) | 37.4-40 | 22.4-37.4 | |
| Systolic pressure, mm Hg | 117.7 ± 2.1 | 154.0 ± 8.1 | .002a |
| Range, mm Hg | 111-125 | 128-182 | |
| Diastolic pressure, mm Hg | 70.33 ± 2.6 | 93.50 ± 5.8 | .005a |
| Range, mm Hg | 59-77 | 79-112 |
Abbreviation: HELLP, hemolysis, elevated liver enzymes, and low platelet count. a P < 0.05.
Table 2.
Neurologic Complaints Among Women With HELLP Syndrome.
| Pt. # | Final Diagnosis | Neurologic Complaint |
|---|---|---|
| 1 | SI-PreE, HELLP syndrome | None |
| 2 | HELLP syndrome, Eclampsia | 1 tonic–clonic seizure |
| 3 | HELLP syndrome | None |
| 4 | Severe PreE, HELLP syndrome | Headache |
| 5 | Severe PreE, HELLP syndrome | None |
| 6 | Severe PreE, HELLP syndrome | Headache |
Abbreviations: HELLP, hemolysis, elevated liver enzymes, and low platelet count; SI-PreE, superimposed preeclampsia; PreE, preeclampsia.
Plasma From Women With HELLP Syndrome Significantly Increased BBB Permeability Compared to Plasma From Women With Normal Pregnancies
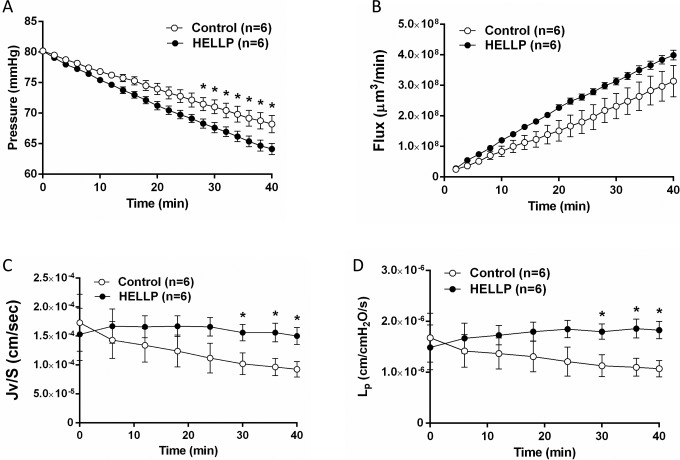
As the BBB has a central role in the development of neurological symptoms such as seizures,7 we measured BBB permeability in cerebral arteries perfused with 20% (v/v) plasma from women with normal pregnancies or women with HELLP syndrome. To assess permeability, several parameters were measured in vitro in response to a 2-hour exposure to plasma, including filtration pressure drop, volume flux, Jv/S, and Lp. As shown in Figure 1, exposure to plasma for HELLP women caused a significant decrease in intravascular pressure drop (P < .05) and an increase in Jv/S and Lp. These results demonstrate that circulating factors in plasma from women with HELLP syndrome increase BBB permeability, similar to what is seen in women with preeclampsia.
Figure 1.
Plasma from women with HELLP syndrome increases BBB permeability compared to plasma from normal pregnant women. The permeability parameters intravascular pressure drop, volume flux, Jv/S, and Lp were used to determine BBB permeability in cerebral arteries from nonpregnant female rats perfused with the different plasmas. A, Decrease in intravascular pressure in response to plasma from women with HELLP syndrome was significantly greater compared to the normal pregnant group. B, Plasma from women with HELLP syndrome tended to have an increase in flux compared to the normal pregnant group. C, Plasma from women with HELLP syndrome caused a significant increase in Jv/S compared to plasma from normal pregnant women. D, There was a significant increase in Lp in response to the HELLP plasma. *P < .05 compared to normal pregnant plasma. BBB indicates blood–brain barrier; HELLP, hemolysis, elevated liver enzymes, and low platelet count.
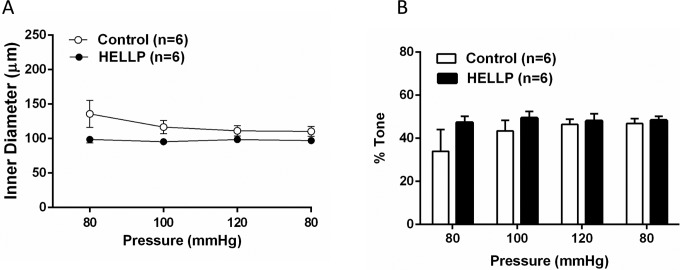
Myogenic Tone and Reactivity to Pressure Were Unchanged in Response to Plasma From Women With HELLP Syndrome
Figure 2A shows active diameter changes in response to increased intravascular pressure. The PCAs perfused with plasma from either group of patients maintained their tone in response to changes in pressure. Likewise, myogenic tone was unchanged in PCAs exposed to plasma from women with HELLP syndrome (Figure 2B).
Figure 2.
The effect of HELLP and control plasma on myogenic activity and tone. Graphs showing (A) that vessels perfused with plasma from women with normal pregnancies or HELLP syndrome had myogenic reactivity and maintained their diameters as pressure increased that was not different with the different plasmas (B) that the different plasmas did not affect the level of pressure-induced tone. HELLP indicates hemolysis, elevated liver enzymes, and low platelet count.
Reactivity to NO Was Unchanged in Response to Plasma From Women With HELLP Syndrome
As NO has been implicated in the regulation of cerebrovascular reactivity and tone,14 we set out to determine the effect of plasma from women with HELLP syndrome on NO reactivity. A single high dose of l-NAME was added to PCAs from both groups, and the constriction to NOS inhibition was measured. There was no significant difference in constriction of the PCAs exposed to plasma from women with HELLP syndrome compared to plasma from normal pregnant women (Figure 3A). In addition, there was no difference in sensitivity to SNP with the different plasmas, suggesting that smooth muscle sensitivity to NO was not different either (Figure 3B).
Figure 3.
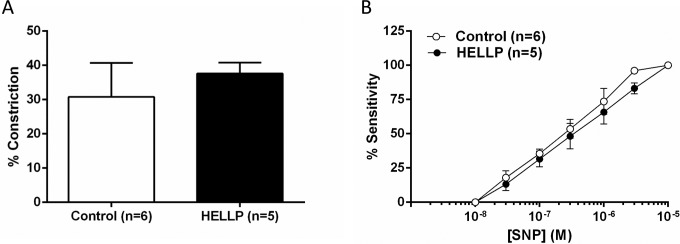
The effect of hemolysis, elevated liver enzymes, and low platelet count (HELLP) plasma on reactivity to NO. A, The constriction in response to nitric oxide synthase (NOS) inhibition with 10− 3 mol/L Nω-nitro-l-arginine methyl ester (l-NAME) was not different with the different plasmas. B, The sensitivity to the NO donor sodium nitroprusside (SNP) in posterior cerebral artery (PCA) was unchanged between the 2 groups. NO indicates nitric oxide.
Sensitivity to NS309 Was Decreased in Response to Plasma From Women With HELLP Syndrome
Figure 4A shows the effect of circulating factors in plasma from women with HELLP syndrome on dilation to NS309 in PCAs. Plasma from women with HELLP syndrome led to significantly decreased dilation of the PCAs to SK/IK channel activation. Figure 4B demonstrates a significant increase in EC50 values between the 2 groups (P < .05). As SK/IK channel activation has been shown to be critical for endothelial-derived hyperpolarization, it appears that HELLP plasma specifically affects this pathway.
Figure 4.
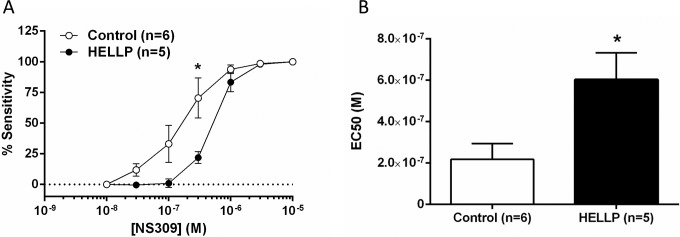
The effect of HELLP plasma on sensitivity to small- and intermediate-conductance Ca2+-activated K+ (SK/IK) channel activation with NS309. A, Plasma from women with HELLP syndrome significantly decreased sensitivity to NS309 dilation and (B) shows that the half maximal effective concentration (EC50) values for NS309 were significantly increased in arteries exposed to HELLP plasma. *P < .05 versus normal pregnant plasma. HELLP indicates hemolysis, elevated liver enzymes, and low platelet count.
Discussion
In the present pilot study, we demonstrate that, similar to plasma from preeclamptic women, plasma from women with HELLP syndrome increased BBB permeability. Additionally, we found that the increased BBB permeability with HELLP plasma was associated with decreased dilation to NS309, an activator of SK/IK channels that are expressed on cerebral endothelium and part of the endothelium-dependent hyperpolarization (EDH) pathway.15,16 In contrast, plasma from patients with HELLP syndrome did not appear to affect the response to NO or pressure-induced myogenic tone. Thus, circulating factors in patients with HELLP syndrome had specific effects on the cerebral endothelium to have increased permeability and decreased response to SK/IK channel activation that may relate to the more frequent appearance of neurologic symptoms in that group (Table 2).
Our current results suggest that acute exposure to plasma from women with HELLP syndrome increases BBB permeability; however, the mechanism by which this occurs is not clear. In our previous study on early-onset preeclamptic plasma, oxidative stress and increased circulating oxidized low-density lipoprotein was found to be responsible for the increase in BBB permeability.9 Interestingly, in the current study, we also found that HELLP plasma decreased sensitivity to activation of SK/IK channels. Although these channels are critical to the EDH pathway, they are also activated in response to increases in endothelium cell calcium through activation of transient receptor potential vanilloid type 4 channels.17 Thus, HELLP plasma may inactivate these channels either directly or indirectly through other pathways that mediate endothelial cell calcium. In any case, together with the increase in BBB permeability, these results suggest that HELLP plasma causes specific endothelial dysfunction in the cerebral circulation. It is worth noting that this dysfunction was found after an acute (2-3 hour) exposure to plasma that may significantly underestimate its effect when one considers the duration of which women are exposed to these circulating factors during pregnancy. It is possible that through using in vitro cellular BBB models,18,19 we could investigate the role of long-term plasma exposure from women with and without HELLP syndrome on endothelial function.
In contrast to BBB permeability, we found no effect of HELLP plasma on basal myogenic tone, reactivity to pressure, or response to NO. The lack of effect on cerebral artery reactivity and myogenic tone is similar to what we have previously reported, when plasma from preeclamptic women was exposed to PCAs.10 That HELLP plasma specifically decreased dilation to NS309 suggests it may diminish EDH but not NO. It is well established that NO is basally active in the cerebral circulation and inhibits tone, as is demonstrated by the ∼30% constriction in response to NOS inhibition, a role for basal EDH is less clear. Thus, it is not surprising that the EDH pathway may be affected by HELLP plasma without affecting basal tone. It is also possible that the exposure to the different plasmas was too acute to have an effect on either NO or smooth muscle contractility and that longer exposure would cause greater vascular dysfunction.
Currently, the only way to resolve preeclampsia and HELLP syndrome is to deliver the baby and placenta,1,2 which often occurs less than 36 weeks of gestation, which accounts for the earlier gestational age in this group of women compared to normal pregnant women. As women with normal pregnancies do not experience complications that require early delivery, we were not able to have gestational age-matched plasma from normal pregnant women undergoing delivery. It would be possible to compare with plasma from women who had premature delivery without HELLP syndrome, but then other factors related to prematurity would further complicate the interpretation. Thus, we chose to compare our outcome measures between plasma from pregnancies with HELLP syndrome to healthy pregnancies although they were at different gestational ages.
In summary, we show here that plasma from patients with HELLP syndrome increased BBB permeability and diminished dilation to SK/IK channel activation, without affecting myogenic tone or reactivity to NO. The relationship between SK/IK channel activation and BBB permeability has yet to be explored but could suggest a specific endothelial dysfunction in response to HELLP plasma. Due to several factors such as the severity of disease manifestation, progress of labor, and ethical considerations, physicians are often left with the choice of only reporting any neurologic disturbances that the patient may state, which oftentimes is not a true indication of a cerebral disturbance that may be occurring. The results from the current pilot study, previous studies examining BBB permeability in women with preeclampsia,10 and a recent study reporting that 11% of women with preeclampsia had a diagnosis of posterior reversible encephalopathy syndrome20 emphasize the need for a large multicenter clinical trial examining neurologic function in women with preeclampsia, HELLP syndrome, and eclampsia.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by the University of Mississippi Medical Center Intramural Research Support Program to KW; the National Institutes of Health grants NS045940 and HL095488; and the Totman Medical Research Trust to MJC.
References
- 1. Haram K, Svendsen E, Abildgaard U. The HELLP syndrome: clinical issues and management. A review. BMC Pregnancy Childbirth. 2009;9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abildgaard U, Heimdal K. Pathogenesis of the syndrome of hemolysis, elevated liver enzymes, and low platelet count (HELLP): a review. Eur J Obstet Gynecol Reprod Biol. 2013;166:117–123. [DOI] [PubMed] [Google Scholar]
- 3. Vigil-de Gracia P, Tenorio-Maranon F, Cejudo-Carranza E, Helquera-Martinez A, Garcia-Caceres E. Difference between preeclampsia, HELLP syndrome and eclampsia, maternal evaluation. Ginecol Obstet Mex. 1996;64:377–382. [PubMed] [Google Scholar]
- 4. Habli M, Eftekhari N, Wiebracht E, et al. Long-term maternal and subsequent pregnancy outcomes 5 years after hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome. Am J Obstet Gynecol. 2009;201(4):385e1–e5. [DOI] [PubMed] [Google Scholar]
- 5. Altamura C, Vasapollo B, Tibuzzi F, et al. Postpartum cerebellar infarction and haemolysis, elevated liver enzymes, low platelet (HELLP) syndrome. Neurol Sci. 2005;26 (1):40–42. [DOI] [PubMed] [Google Scholar]
- 6. Cipolla M. Cerebrovascular function in pregnancy and eclampsia. Hypertension. 2007;50 (1):14–24. [DOI] [PubMed] [Google Scholar]
- 7. Euser A, Cipolla M. Cerebral blood flow autoregulation and edema formation during pregnancy in anesthetized rats. Hypertension. 2007;49 (2):334–340. [DOI] [PubMed] [Google Scholar]
- 8. Foyouzi N, Norwitz E, Tsen L, Buhimschi C, Buhimschi I. Placental growth factor in the cerebrospinal fluid of women with preeclampsia. Int J Gynaecol Obstet. 2006;92 (1):32–37. [DOI] [PubMed] [Google Scholar]
- 9. Schreurs M, Houston E, May V, Cipolla M. The adaptation of the blood–brain barrier to vascular endothelial growth factor and placental growth factor during pregnancy. FASEB J. 2012;26 (1):355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Amburgey O, Chapman A, May V, Bernstein I, Cipolla M. Plasma from preeclamptic women increases blood–brain barrier permeability. Hypertension. 2010;56 (5):1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wallace K, Martin J, Jr, Tam Tam K, et al. Seeking the mechanisms of action for corticosteroids in HELLP syndrome: SMASH study. Am J Obstet Gynecol. 2013;208 (5):e1–e8. [DOI] [PubMed] [Google Scholar]
- 12. American College of Obstetricians and Gynecologists. Diagnosis and Management of Preeclampsia and Eclampsia. Practice Bulletin 33 Washington, DC: ACOG; 2002. [Google Scholar]
- 13. Roberts T, Chapman A, Cipolla M. PPAR-gamma agonist rosiglitazone reverses increased cerebral venous hydraulic conductivity during hypertension. Am J Physiol Heart Circ Physiol. 2009;297:H1347–H1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schreurs M, Cipolla M. Cerebrovascular dysfunction and blood–brain barrier permeability induced by oxidized LDL are prevented by apocynin and magnesium sulfate in female rats. J Cardiovasc Pharmacol. 2014;63 (1):33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hinton J, Langton P. Inhibition of EHDF by two new combinations of K+-channel inhibitors in rat isolated arteries. Br J Pharmacol. 2003;138 (6):1031–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hannah R, Dunn K, Bonev A, Nelson M. Endothelial SKCA and IKCA channels regulate brain parenchymal arteriolar diameter and cortical cerebral blood flow. J Cereb Blood Flow Metab. 2011;31 (5):1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sonkusare S, Bonev A, Ledoux J, et al. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science. 2012;336 (6081):597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Takata F, Dohgu S, Yamauchi A, et al. In vitro blood–brain barrier models using brain capillary endothelial cells isolated from neonatal and adult rats retain age-related barrier properties. PLoS One. 2013:8(1):e55166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nakagawa S, Deli MA, Kawaguchi H, et al. A new blood–brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochem Int. 2009;54 (3-4):253–263. [DOI] [PubMed] [Google Scholar]
- 20. Saraf S, Egbert NM, Mittal G, Homel P, Minkoff H, Fisher N. Predictors of posterior reversible encephalopathy syndrome in preeclampsia and eclampsia. Obstet Gynecol. 2014;123(S1):169S.24770077 [Google Scholar]