Abstract
Setting
Tertiary hospital in Gaborone, Botswana.
Objective
To examine whether exposure to wood smoke worsens outcomes of childhood pneumonia.
Design
Prospective cohort study of children 1-23 months of age meeting clinical criteria for pneumonia. Household use of wood as a cooking fuel was assessed during a face-to-face questionnaire with caregivers. We estimated crude and adjusted risk ratios (RR) and 95% confidence intervals (CI) for treatment failure at 48 hours by household use of wood as a cooking fuel. We assessed for effect modification by age (1-5 vs. 6-23 months) and malnutrition (none vs. moderate vs. severe).
Results
Median age of the 284 enrolled children was 5.9 months and 17% had moderate or severe malnutrition. Ninety-nine (35%) children failed treatment at 48 hours and 17 (6%) died. In multivariable analyses, household use of wood as a cooking fuel increased the risk of treatment failure at 48 hours (RR: 1.44, 95% CI: 1.09-1.92, P=0.01). This association differed by child nutritional status (P=0.02), with a detrimental effect observed only among children with no or moderate malnutrition.
Conclusions
Exposure to wood smoke worsens outcomes from childhood pneumonia. Efforts to prevent exposure to smoke from unprocessed fuels may improve pneumonia outcomes among children.
Introduction
Pneumonia remains the leading killer of children beyond the neonatal period, but substantial progress was made in the past decade in reducing pneumonia-related mortality. Global deaths from pneumonia among children decreased 35% from 1.8 million in 2000 to 1.1 million in 2012.1 Most of this decline occurred in low- and middle-income countries (LMICs), with Haemophilus influenzae type B (HIB) and pneumococcal conjugate vaccines contributing to the health gains experienced by many countries.1 However, pneumonia still disproportionately impacts the world's poorest children, with more than 99% of deaths occurring in LMICs.2
While the global proportion of households using unprocessed solid fuels for cooking or heating declined between 1980 and 2010, the number of African households using these fuels nearly doubled.3, 4 Burning of solid fuels, particularly unprocessed biomass fuels such as wood or animal dung, emits particulate matter, carbon monoxide, and a number of other hazardous substances.5-8 When these fuels are burned indoors or in close proximity to the living space, exposure to high concentrations of these pollutants can occur.5, 8 The World Health Organization (WHO) estimates that 3.9% of all deaths in LMICs are attributable to air pollution from the burning of solid fuels.4 Infants and young children may receive the highest exposures as they spend more time within the home and are often carried by their mothers during meal preparation.4, 9, 10 A number of studies support an association between exposure to smoke from unprocessed solid fuels and the incidence of childhood pneumonia.11-13 In a recent meta-analysis that included 24 such studies, exposure to smoke from unprocessed solid fuels increased the risk of pneumonia by 80%.11 However, it is not known if exposure to smoke from solid fuels also affects outcomes among children with established pneumonia.
Within the context of a hospital-based, prospective cohort study of pneumonia in Botswana, we examined whether household use of wood as a cooking fuel was associated with worse outcomes among children less than two years of age. As a secondary objective, we assessed for effect modification of this exposure-outcome relationship by age and malnutrition.
Study Population and Methods
Setting
The study was conducted from April 2012 – April 2014 at a tertiary hospital in Gaborone, Botswana. HIB vaccine was introduced in Botswana's immunization schedule in 2010, while 13-valent pneumococcal conjugate vaccine was included in July 2012. Wood is used for cooking in 46% of households in Botswana; use of other unprocessed solid fuels such as animal dung or coal is uncommon.14
Study population
Children 1 to 23 months of age with pneumonia, defined by the WHO as cough or difficulty in breathing with lower chest wall indrawing,15 were eligible for inclusion, provided that a legal guardian provided written informed consent. The presence of one or more danger signs (central cyanosis, convulsions, inability to drink, or abnormal sleepiness) further classified children as having severe pneumonia.15 We excluded children with a chronic medical condition predisposing to pneumonia, hospitalization in the prior 14 days, diagnosis of asthma, wheezing with resolution of lower chest wall indrawing after ≤2 bronchodilator treatments, or prior study enrollment. All subjects were recruited within six hours of the triage time in the Emergency Department.
Data collection
Sociodemographic and clinical information were collected at enrollment from a physical examination, review of infant and maternal medical records, and a detailed face-to-face interview with the child's caregiver(s). Exposure to smoke from solid fuels was assessed by asking caregivers if the child's household uses wood as a fuel for cooking. Moderate malnutrition was defined as weight-for-length between -3 and -2 standard deviations on WHO growth curves or, for children ≥6 months of age, mid-upper arm circumference (MUAC) between 115mm and 125mm.16 Severe malnutrition was defined as weight-for-length <-3 standard deviations on WHO growth curves, MUAC <115mm (for children ≥6 months), or bilateral edema of nutritional origin.16 Proximity to health care services was categorized as travel of <1 or ≥1 hour prior to first contact with the health care system on the enrollment date.
Outcomes assessment
The primary outcome, treatment failure, was assessed at 48 hours and defined as persistent lower chest wall indrawing, development of new WHO danger signs, oxygen saturation <80% (on room air), requirement for continuous positive airway pressure (CPAP) or mechanical ventilation, or death. This definition was adapted for our setting from criteria used in a study of childhood pneumonia sponsored by the WHO.17 Treatment failure assessments were performed by a study physician or nurse blinded to enrollment data, including household use of wood as a cooking fuel. Children discharged before 48 hours were considered treatment responders although caregivers were contacted by telephone to confirm treatment response.
Statistical analysis
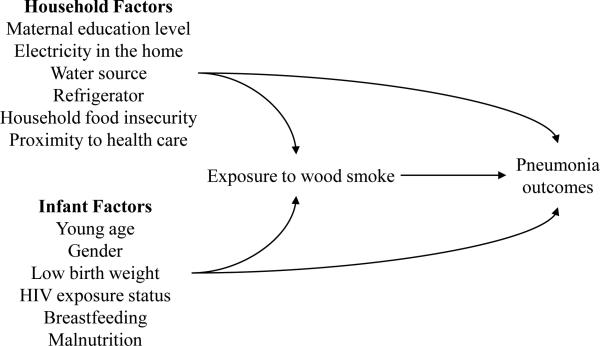
Baseline characteristics of the exposure groups were described using frequencies and percentages for categorical variables and median and interquartile ranges for continuous variables. We used Cox proportional hazards to directly estimate crude and adjusted risk ratios (RR) for treatment failure at 48 hours. Potential confounding variables were identified based on a literature review and subject matter knowledge of the proposed causal pathway between exposure to wood smoke and treatment outcomes (Figure 1). We considered the following covariates: age (1-5 months, 6-23 months), gender (male, female), low birth weight (yes, no), HIV exposure status (HIV-infected, HIV-exposed uninfected, HIV-unexposed), malnutrition (none, moderate, severe), current breastfeeding (yes, no), maternal education level (none or primary, secondary, tertiary), household electricity (yes, no), municipal water or private water source (yes, no), refrigerator in home (yes, no), proximity to health care services (<1 or ≥1 hour), and two questions from the Household Food Insecurity Access Scale assessing for insufficient food intake and its physical consequences.18
Figure 1.
The hypothesized relationship between exposure to wood smoke and pneumonia outcomes.
To examine the association between exposure to wood smoke and treatment failure, we used a “change-in-estimate” approach to empirically select confounding variables for inclusion in a “reduced” multivariable model. We began by estimating the RR for household use of wood as a cooking fuel in a “full” multivariable model containing all of the hypothesized confounding variables. Thereafter, each variable was removed from the full model and the RR for use of wood as a cooking fuel was again estimated. The covariate for which removal caused the smallest change in the RR of the exposure was dropped, provided that the change in RR was less than 10%. Those variables whose removal from the model changed the RR for exposure by more than 10% were retained in the reduced multivariable model. To assess whether the association differed by age and nutritional status, a χ2 test was used to assess the homogeneity of the stratum-specific RR's for age and malnutrition, comparing each to the overall RR obtained from the reduced multivariable model. For all models containing nutritional status, inverse probability weighting was used to adjust for children with missing anthropometric data (n=15). All statistical analyses were conducted using SAS software version 9.3 (SAS Institute, Cary, NC).
Results
Patient Characteristics
Median age of the 284 enrolled children was 5.9 months, and 55% were male. Ninety-seven (34%) children presented with severe pneumonia. Two hundred twenty-five (79%) children received antibiotic therapy during the first 48 hours, with the most common regimen being ampicillin and gentamicin.
Table 1 presents baseline characteristics of the study population by household use of wood as a cooking fuel. Children whose caregivers reported use of wood as a cooking fuel had significantly lower maternal education levels, were less likely to have electricity, municipal water or a private water source, or a refrigerator, and more likely to have household food insecurity in the prior four weeks. There were no significant differences in WHO disease severity, respiratory rate, or hypoxia at enrollment by household use of wood as a cooking fuel.
Table 1.
Baseline characteristics overall and according to household use of wood as a cooking fuel among N=284 children 1 to 23 months of age presenting with pneumonia to a tertiary hospital in Gaborone, Botswana, April 2012 to April 2014.a
| Household use of wood as a cooking fuel | ||||
|---|---|---|---|---|
| Characteristic (n with data) | Overall (N = 284) | Yes (N = 94) | No (N = 190) | Pb |
| Demographics | ||||
| Age (n=284) | 0.08 | |||
| 1 to 5 months | 145 (51.1) | 55 (58.5) | 90 (47.4) | |
| 6 to 23 months | 139 (48.9) | 39 (41.5) | 100 (52.6) | |
| Male gender (n=284) | 157 (55.3) | 53 (56.4) | 104 (54.7) | 0.79 |
| Birth weight <2500 grams (n=284) | 62 (21.8) | 25 (26.6) | 37 (19.5) | 0.17 |
| HIV exposure status (n=282) | 0.56 | |||
| HIV-unexposed | 177 (62.8) | 55 (58.5) | 122 (64.9) | |
| HIV-exposed, uninfected | 82 (29.1) | 31 (33.0) | 51 (27.1) | |
| HIV-infected | 23 (8.1) | 8 (8.5) | 15 (8.0) | |
| Nutrition and infant feeding practices | ||||
| Malnutrition (n=269) | 0.89 | |||
| None | 222 (82.5) | 74 (84.1) | 148 (81.8) | |
| Moderatec | 24 (8.9) | 7 (8.0) | 17 (9.4) | |
| Severed | 23 (8.6) | 7 (8.0) | 16 (8.8) | |
| Current breastfeeding (n=284) | 116 (40.9) | 43 (45.7) | 73 (38.4) | 0.24 |
| Socioeconomic factors | ||||
| Maternal education level (n=284) | 0.009 | |||
| None or primary | 33 (11.6) | 17 (18.1) | 16 (8.4) | |
| Secondary | 192 (67.6) | 65 (69.2) | 127 (66.8) | |
| Tertiary | 59 (20.8) | 12 (12.8) | 47 (24.7) | |
| Electricity in home (n=284) | 183 (64.4) | 45 (47.9) | 138 (72.6) | <0.0001 |
| Municipal water or private water source (n=284) | 244 (85.9) | 75 (79.8) | 169 (89.0) | 0.04 |
| Refrigerator in home (n=284) | 171 (60.2) | 43 (45.7) | 128 (67.4) | 0.0005 |
| Household food insecurity in the prior 4 weeks | ||||
| Smaller meal than needed because of a lack of resources (n=284) | 39 (13.7) | 20 (21.3) | 19 (10.0) | 0.009 |
| Fewer meals in a day because of a lack of resources (n=284) | 40 (14.1) | 19 (20.2) | 21 (11.1) | 0.04 |
| Current illness factors | ||||
| WHO severe diseasee (n=284) | 97 (34.2) | 35 (37.2) | 62 (32.6) | 0.44 |
| Respiratory rate (breaths per minute), median (IQR) (n=284) | 62 (56-74) | 64 (56-74) | 62 (54-74) | 0.27 |
| Oxygen saturation <90%, room air (n=283) | 107 (37.8) | 42 (44.7) | 65 (34.4) | 0.09 |
| Travel of more than 1 hour to clinic or hospital (n=283) | 25 (8.8) | 12 (12.8) | 13 (6.9) | 0.10 |
IQR, interquartile range; WHO, World Health Organization; μL, microliter; dL, deciliter
Data are N (column %) unless otherwise stated; values may not sum to 100% due to rounding
Wald χ2 P-values
Moderate malnutrition defined as weight-for-length between -3 and -2 standard deviations on World Health Organization (WHO) growth curves or, for children ≥6 months of age, mid-upper arm circumference (MUAC) between 115mm and 125mm
Severe malnutrition defined as weight-for-length <-3 standard deviations on WHO growth curves, MUAC <115mm (for children ≥6 months), or bilateral edema of nutritional origin
Pneumonia accompanied by WHO danger signs (central cyanosis, convulsions, inability to drink, or abnormal sleepiness)
Outcomes
Ninety-nine (35%) children failed treatment at 48 hours, and 17 (6%) children died. One hundred and seventy-three (61%) children required supplemental oxygen, CPAP, or mechanical ventilation during the hospitalization. Among the 267 children surviving to hospital discharge, median [interquartile range (IQR)] length of stay was 3.9 days (IQR: 2.7, 13.1 days). Sixty-three (22%) children were discharged before the 48-hour treatment failure assessment; the caregivers of 59 (94%) of these children were contacted by phone, and only one child discharged early was reported to have required further medical care after discharge.
Table 2 shows the bivariable and full and reduced multivariable models for treatment failure at 48 hours. In the reduced multivariable model adjusting only for age, household use of wood as a cooking fuel was associated with an increased risk of treatment failure at 48 hours (RR: 1.44; 95% CI: 1.09-1.92; P=0.01). A similar although less precise association was observed in the full multivariable model. Table 3 shows the results of analyses assessing for effect modification by age and nutritional status. The effect of household use of wood smoke tended to be stronger among children <6 months, although this did not reach statistical significance (P=0.07). There was evidence for effect modification by nutritional status (P=0.02), with a detrimental effect from exposure to wood smoke observed only among children with no or moderate malnutrition.
Table 2.
Bivariable and multivariable-adjusted effect of household use of wood as a cooking fuel on treatment failure at 48 hours among N=284 children 1 to 23 months of age presenting with pneumonia to a tertiary hospital in Gaborone, Botswana, April 2012 to April 2014.
| Bivariable Models | Full Multivariable Model | Reduced Multivariable Modela | ||||
|---|---|---|---|---|---|---|
| RR (95% CI) | P b | RR (95% CI) | P b | RR (95% CI) | P b | |
| Exposure of interest | ||||||
| Use of wood as a cooking fuel | 1.62 (1.19, 2.21) | 0.002 | 1.48 (1.08, 2.03) | 0.01 | 1.44 (1.09, 1.92) | 0.01 |
| Demographics | ||||||
| Age | ||||||
| 1 to <6 months | 3.36 (2.22, 5.07) | <0.0001 | 3.48 (2.27, 5.35) | <0.0001 | 3.23 (2.14, 4.88) | <0.0001 |
| 6 to <24 months | 1 (ref) | 1 (ref) | 1 (ref) | |||
| Male gender | 1.10 (0.80, 1.52) | 0.57 | 1.02 (0.77, 1.34) | 0.91 | ||
| Birth weight <2,500 grams | 1.71 (1.25, 2.34) | 0.001 | 1.27 (0.95, 1.69) | 0.11 | ||
| HIV exposure statusc | ||||||
| HIV-infected | 2.34 (1.55, 3.53) | <0.0001 | 1.63 (1.08, 2.46) | 0.02 | ||
| HIV-exposed, uninfected | 1.78 (1.27, 2.51) | 0.001 | 1.32 (0.85, 2.07) | 0.22 | ||
| HIV-unexposed | 1 (ref) | 1 (ref) | ||||
| Nutrition and infant feeding practices | ||||||
| Malnutritiond | ||||||
| None | 1 (ref) | 1 (ref) | ||||
| Moderatee | 1.71 (1.14, 2.57) | 0.01 | 1.55 (0.99, 2.43) | 0.06 | ||
| Severef | 1.35 (0.81, 2.22) | 0.25 | 1.66 (0.93, 2.95) | 0.08 | ||
| Currently breastfeeding | 0.90 (0.65, 1.25) | 0.54 | 0.90 (0.61, 1.33) | 0.61 | ||
| Socioeconomic factors | ||||||
| Maternal education level | ||||||
| None or primary | 2.30 (1.32, 4.00) | 0.003 | 1.44 (0.83, 2.48) | 0.19 | ||
| Secondary | 1.47 (0.90, 2.42) | 0.13 | 1.31 (0.82, 2.07) | 0.26 | ||
| Tertiary | 1 (ref) | 1 (ref) | ||||
| Electricity in home | 0.69 (0.50, 0.94) | 0.02 | 1.15 (0.63, 2.12) | 0.65 | ||
| Municipal water or private water source | 0.54 (0.39, 0.75) | 0.0002 | 0.72 (0.49, 1.06) | 0.09 | ||
| Refrigerator in home | 0.67 (0.49, 0.92) | 0.01 | 0.95 (0.54, 1.68) | 0.86 | ||
| Household food insecurity in the prior 4 weeks | ||||||
| Smaller meal than needed because of a lack of resources | 1.12 (0.73, 1.73) | 0.60 | 1.06 (0.53, 2.13) | 0.87 | ||
| Fewer meals in a day because of a lack of resources | 1.01 (0.64, 1.59) | 0.98 | 0.66 (0.31, 1.39) | 0.28 | ||
| Travel of more than 1 hour to clinic or hospitalg | 1.04 (0.60, 1.81) | 0.88 | 0.72 (0.48, 1.09) | 0.12 | ||
RR, risk ratio; CI, confidence interval
Reduced multivariable model was constructed using a change-in-estimate approach. At each stage, the covariate for which removal caused the smallest change in the RR of the exposure was dropped. Only those variables whose removal from the model changed the RR for exposure by more than 10% were retained in the reduced multivariable model.
Wald χ2 P-values
HIV exposure status could not be established for N=2 children
Data on nutritional status were missing from N=15 children
Moderate malnutrition defined as weight-for-length between -3 and -2 standard deviations on World Health Organization (WHO) growth curves or, for children ≥6 months of age, mid-upper arm circumference (MUAC) between 115mm and 125mm
Severe malnutrition defined as weight-for-length <-3 standard deviations on WHO growth curves, MUAC <115mm (for children ≥6 months), or bilateral edema of nutritional origin
Data on duration of travel to clinic or hospital were missing from N=1 child
Table 3.
Multivariable-adjusted effect of household use of wood as a cooking fuel on treatment failure at 48 hours according to age and nutritional status.
| Household Use of Wood as a Cooking Fuel | |||
|---|---|---|---|
| RRa | (95% CI) | Pb | |
| Age at enrollment | 0.07 | ||
| 1 to 5 months | 1.51 | (1.13, 2.03) | |
| 6 to 23 months | 1.20 | (0.53, 2.71) | |
| Malnutrition | 0.02 | ||
| None | 1.52 | (1.08, 2.15) | |
| Moderatec | 1.87 | (1.02, 3.45) | |
| Severed | 0.30 | (0.05, 1.96) | |
RR, risk ratio; CI, confidence interval
Risk ratios estimated from Cox proportional hazards models adjusted for age
Wald χ2 P for test of homogeneity of the stratum-specific risk ratios
Moderate malnutrition defined as weight-for-length between -3 and -2 standard deviations on World Health Organization (WHO) growth curves or, for children ≥6 months of age, mid-upper arm circumference (MUAC) between 115mm and 125mm
Severe malnutrition defined as weight-for-length <-3 standard deviations on WHO growth curves, MUAC <115mm (for children ≥6 months), or bilateral edema of nutritional origin
Discussion
Household use of wood as a cooking fuel was associated with a nearly 50% increase in the risk of treatment failure at 48 hours among children under two years of age with pneumonia.
There are a number of potential mechanisms by which exposure to wood smoke might worsen pneumonia outcomes. The air pollutants generated by the burning of solid fuels can cause lung injury through direct damage to lipids and proteins, as well as indirectly through activation of stress signaling pathways in lung epithelial cells.6, 19 Alveolar macrophages phagocytose particulate matter and respond by producing pro-inflammatory cytokines including interleukin-1β, interleukin-6, and tumor necrosis factor alpha, while release of anti-inflammatory cytokines may be suppressed.20-22 This recruits activated neutrophils and T lymphocytes to the airways resulting in acute tissue damage and a more exuberant inflammatory response to pathogens.6, 19, 20 Finally, exposure to air pollutants reduces pulmonary clearance of bacteria by alveolar macrophages in animal models.23, 24
Several prior studies examined the effect of exposure to smoke from solid fuels on outcomes of childhood pneumonia in sub-Saharan Africa.9, 25-27 In a pooled analysis of data from 16 African countries, solid fuel use more than doubled mortality from acute lower respiratory tract infection (ALRI) in children.25 Similarly, exposure to cooking smoke was more common among Gambian children dying from pneumonia than among healthy controls,9 while sleeping in the room used for cooking was associated with ALRI mortality among children in Tanzania.26 However, these studies used verbal autopsies to ascertain exposure, introducing the potential for recall bias, and the case-control design prevented determination of whether the observed effect resulted from an increased incidence or worse outcomes from pneumonia. In a cohort study of 103 Nigerian children hospitalized with ALRI, nearly two-thirds (63%) of the children who died were potentially exposed to wood smoke, despite only 16% of the cohort reporting this exposure.27 These studies did not account for several potential confounders. In particular, malnutrition, HIV infection, and formula feeding are established risk factors for poor pneumonia outcomes and may be more prevalent among African children exposed to smoke from solid fuels.28-31 In our cohort, HIV infection and moderate or severe malnutrition tended to increase the risk of treatment failure in multivariable analyses. However, these variables were not significant confounders because they were not associated with use of wood as a cooking fuel in the study population (Table 1).
The effect of exposure to wood smoke on treatment outcomes tended to be more pronounced among children less than six months of age. Although this did not reach statistical significance, this finding is consistent with prior studies suggesting that the impact of exposure to smoke from solid fuels differs by age in children.11, 32 Gurley et al. found that high levels of indoor particulate matter increased the incidence of ALRI in children aged 0–11 months, but not in older children.32 Similarly, pooled odds ratios for studies of children <24 months or <36 months of age were slightly higher than the pooled odds ratio for older children in a meta-analysis of the effect of indoor air pollution on pneumonia incidence.11 Whether the effect modification observed in our study is the result of greater exposure or an enhanced susceptibility to air pollutants in young infants is unclear.
Although malnutrition is a risk factor for severe ALRI and treatment failure,31 few studies investigating exposure to smoke from solid fuels collected nutritional data. In our cohort, household use of wood as a cooking fuel increased the risk of treatment failure among children with no or moderate malnutrition, but a similar effect was not observed among children with severe malnutrition. While we can only speculate as to why wood smoke exposure did not affect the outcomes of severely malnourished children, protein-energy malnutrition does impair cell-mediated immune function and could blunt the tissue inflammatory response to air pollutants.33
Our results identify children who may be particularly susceptible to smoke from biomass fuels while highlighting the urgent need for interventions to prevent exposure in LMICs. Clean cookstoves have the potential to substantially reduce the pollutants generated from the burning of solid fuels. The Randomized Exposure Study of Pollution Indoors and Respiratory Effects (RESPIRE) trial conducted in Guatemala compared households receiving a clean woodstove with a chimney to control households that continued to cook with open fires.34 Use of a chimney stove was associated with a 50% reduction in child carbon monoxide levels and significantly reduced the incidence of WHO-defined pneumonia among children in the household.34 The Global Alliance for Clean Cookstoves is currently mobilizing support to distribute 100 million clean and efficient cookstoves to households that are currently using solid fuels. This initiative has the potential to substantially reduce the burden of ALRI among children in LMICs.
Our study has several limitations. First, it was conducted at a single hospital in Botswana, and the results may not be generalizable to other countries. In addition, we only assessed use of wood as a cooking fuel, leading to potential exposure misclassification of children in households that used other solid fuels. However, data suggest that use of other solid fuels is uncommon in Botswana,14 and such misclassification would tend to bias our results toward the null. Additionally, child exposure was assessed crudely based on household use of wood as a cooking fuel, which may have resulted in random exposure error and an underestimate of the effect size. Ventilation, cooking location, and a number of behavioral factors also affect personal exposure levels to smoke within households using biomass fuels.10, 35 Finally, the case fatality rate was relatively low in our population and we did not have sufficient power to evaluate whether wood smoke exposure also increased mortality among children with pneumonia.
Conclusions
This study is one of few to assess the effect of exposure to biomass smoke on outcomes from childhood pneumonia. Exposure to smoke from biomass fuels is one of the most important modifiable risk factors for poor health outcomes in LMICs. Our results indicate that efforts to prevent or minimize exposure to air pollutants have the potential to improve pneumonia outcomes among children.
Acknowledgements
This research was supported by an Early Career Award from the Thrasher Research Fund (to MSK), by the Children's Hospital of Philadelphia (to APS and KAF) and Pincus Family Foundation, and through core services and support from the Penn Center for AIDS Research (CFAR), a National Institutes of Health (NIH)-funded program (P30-AI045008). CKC received financial support from the NIH through the Duke Center for AIDS Research (P30-AI064518). APS received financial support from the NIH through the Penn Center for AIDS Research (P30-AI045008). Funding for this project was also made possible in part by a CIPHER grant from the International AIDS Society (to MSK), supported by ViiV Healthcare. The views expressed in this publication do not necessarily reflect the official policies of the International AIDS Society or ViiV Healthcare.
Footnotes
Conflicts of interest
None declared.
References
- 1.United Nations Children's Fund (UNICEF) Progress report 2013. UNICEF; New York: 2013. Committing to child survival: a promise renewed. [Google Scholar]
- 2.United Nations Children's Fund (UNICEF) Pneumonia and diarrhea: tackling the deadliest diseases for the world's poorest children. UNICEF; New York: 2013. [Google Scholar]
- 3.Bonjour S, Adair-Rohani H, Wolf J, Bruce NG, Mehta S, Pruss-Ustun A, et al. Solid fuel use for household cooking: country and regional estimates for 1980-2010. Environmental health perspectives. 2013;121(7):784–90. doi: 10.1289/ehp.1205987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO) Global health risks: mortality and burden of disease attributable to selected major risks. WHO; Geneva: 2009. [Google Scholar]
- 5.Smith KR, Samet JM, Romieu I, Bruce N. Indoor air pollution in developing countries and acute lower respiratory infections in children. Thorax. 2000;55(6):518–32. doi: 10.1136/thorax.55.6.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunekreef B, Holgate ST. Air pollution and health. Lancet. 2002;360(9341):1233–42. doi: 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein JA, Alexis N, Barnes C, Bernstein IL, Bernstein JA, Nel A, et al. Health effects of air pollution. The Journal of allergy and clinical immunology. 2004;114(5):1116–23. doi: 10.1016/j.jaci.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 8.Smith KR, Uma R, Kishore VVN, Lata K, Joshi V, Zhang J, et al. Prepared for: United States Environmental Protection Agency / Office of Research and Development / Office of Air and Radiation. Prepared by: National Risk Management Research Laboratory; Washington, DC: 2000. Greenhouse gases from small-scale combustion devices in developing countries. Phase IIa: household stoves in India. [Google Scholar]
- 9.de Francisco A, Morris J, Hall AJ, Armstrong Schellenberg JR, Greenwood BM. Risk factors for mortality from acute lower respiratory tract infections in young Gambian children. International journal of epidemiology. 1993;22(6):1174–82. doi: 10.1093/ije/22.6.1174. [DOI] [PubMed] [Google Scholar]
- 10.Northcross A, Chowdhury Z, McCracken J, Canuz E, Smith KR. Estimating personal PM2.5 exposures using CO measurements in Guatemalan households cooking with wood fuel. Journal of environmental monitoring : JEM. 2010;12(4):873–8. doi: 10.1039/b916068j. [DOI] [PubMed] [Google Scholar]
- 11.Dherani M, Pope D, Mascarenhas M, Smith KR, Weber M, Bruce N. Indoor air pollution from unprocessed solid fuel use and pneumonia risk in children aged under five years: a systematic review and meta-analysis. Bulletin of the World Health Organization. 2008;86(5):390–8C. doi: 10.2471/BLT.07.044529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bates MN, Chandyo RK, Valentiner-Branth P, Pokhrel AK, Mathisen M, Basnet S, et al. Acute lower respiratory infection in childhood and household fuel use in Bhaktapur, Nepal. Environmental health perspectives. 2013;121(5):637–42. doi: 10.1289/ehp.1205491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramesh Bhat Y, Manjunath N, Sanjay D, Dhanya Y. Association of indoor air pollution with acute lower respiratory tract infections in children under 5 years of age. Paediatrics and international child health. 2012;32(3):132–5. doi: 10.1179/2046905512Y.0000000027. [DOI] [PubMed] [Google Scholar]
- 14.Ministry of Environment, Wildlife and Tourism, Finance and Development Planning . Reflecting on the challenges of attaining a green economy for Botswana: energy policy brief. Gaborone, Botswana: 2013. [Google Scholar]
- 15.World Health Organization (WHO) Pocket book of hospital Care for children: guidelines for the management of common childhood illnesses. WHO; Geneva: 2013. [PubMed] [Google Scholar]
- 16.World Health Organization (WHO), United Nations Children's Fund (UNICEF) WHO child growth standards and the identification of severe acute malnutrition in infants and children. WHO; Geneva: 2009. [PubMed] [Google Scholar]
- 17.Addo-Yobo E, Chisaka N, Hassan M, Hibberd P, Lozano JM, Jeena P, et al. Oral amoxicillin versus injectable penicillin for severe pneumonia in children aged 3 to 59 months: a randomised multicentre equivalency study. Lancet. 2004;364(9440):1141–8. doi: 10.1016/S0140-6736(04)17100-6. [DOI] [PubMed] [Google Scholar]
- 18.Swindale A, Bilinsky P. Development of a universally applicable household food insecurity measurement tool: process, current status, and outstanding issues. The Journal of nutrition. 2006;136(5):1449s–52s. doi: 10.1093/jn/136.5.1449S. [DOI] [PubMed] [Google Scholar]
- 19.Bayram H, Sapsford RJ, Abdelaziz MM, Khair OA. Effect of ozone and nitrogen dioxide on the release of proinflammatory mediators from bronchial epithelial cells of nonatopic nonasthmatic subjects and atopic asthmatic patients in vitro. The Journal of allergy and clinical immunology. 2001;107(2):287–94. doi: 10.1067/mai.2001.111141. [DOI] [PubMed] [Google Scholar]
- 20.van Eeden SF, Tan WC, Suwa T, Mukae H, Terashima T, Fujii T, et al. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)). American journal of respiratory and critical care medicine. 2001;164(5):826–30. doi: 10.1164/ajrccm.164.5.2010160. [DOI] [PubMed] [Google Scholar]
- 21.Hiraiwa K, van Eeden SF. Contribution of lung macrophages to the inflammatory responses induced by exposure to air pollutants. Mediators of inflammation. 2013;2013:619523. doi: 10.1155/2013/619523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin XJ, Ma JY, Antonini JM, Castranova V, Ma JK. Roles of reactive oxygen species and heme oxygenase-1 in modulation of alveolar macrophage-mediated pulmonary immune responses to Listeria monocytogenes by diesel exhaust particles. Toxicological sciences : an official journal of the Society of Toxicology. 2004;82(1):143–53. doi: 10.1093/toxsci/kfh255. [DOI] [PubMed] [Google Scholar]
- 23.Castranova V, Ma JY, Yang HM, Antonini JM, Butterworth L, Barger MW, et al. Effect of exposure to diesel exhaust particles on the susceptibility of the lung to infection. Environmental health perspectives. 2001;109(Suppl 4):609–12. doi: 10.1289/ehp.01109s4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drummond JG, Curtis SE, Simon J. Effects of atmospheric ammonia on pulmonary bacterial clearance in the young pig. American journal of veterinary research. 1978;39(2):211–2. [PubMed] [Google Scholar]
- 25.Rehfuess EA, Tzala L, Best N, Briggs DJ, Joffe M. Solid fuel use and cooking practices as a major risk factor for ALRI mortality among African children. Journal of epidemiology and community health. 2009;63(11):887–92. doi: 10.1136/jech.2008.082685. [DOI] [PubMed] [Google Scholar]
- 26.Mtango FD, Neuvians D, Broome CV, Hightower AW, Pio A. Risk factors for deaths in children under 5 years old in Bagamoyo district, Tanzania. Tropical medicine and parasitology : official organ of Deutsche Tropenmedizinische Gesellschaft and of Deutsche Gesellschaft fur Technische Zusammenarbeit (GTZ) 1992;43(4):229–33. [PubMed] [Google Scholar]
- 27.Johnson AW, Aderele WI. The association of household pollutants and socio-economic risk factors with the short-term outcome of acute lower respiratory infections in hospitalized pre-school Nigerian children. Annals of tropical paediatrics. 1992;12(4):421–32. doi: 10.1080/02724936.1992.11747609. [DOI] [PubMed] [Google Scholar]
- 28.Jackson S, Mathews KH, Pulanic D, Falconer R, Rudan I, Campbell H, et al. Risk factors for severe acute lower respiratory infections in children: a systematic review and meta-analysis. Croatian medical journal. 2013;54(2):110–21. doi: 10.3325/cmj.2013.54.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNally LM, Jeena PM, Gajee K, Thula SA, Sturm AW, Cassol S, et al. Effect of age, polymicrobial disease, and maternal HIV status on treatment response and cause of severe pneumonia in South African children: a prospective descriptive study. The Lancet. 2007;369(9571):1440–51. doi: 10.1016/S0140-6736(07)60670-9. [DOI] [PubMed] [Google Scholar]
- 30.Asbjornsdottir KH, Slyker JA, Weiss NS, Mbori-Ngacha D, Maleche-Obimbo E, Wamalwa D, et al. Breastfeeding is associated with decreased pneumonia incidence among HIV-exposed, uninfected Kenyan infants. AIDS (London, England) 2013;27(17):2809–15. doi: 10.1097/01.aids.0000432540.59786.6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webb C, Ngama M, Ngatia A, Shebbe M, Morpeth S, Mwarumba S, et al. Treatment failure among Kenyan children with severe pneumonia--a cohort study. The Pediatric infectious disease journal. 2012;31(9):e152–7. doi: 10.1097/INF.0b013e3182638012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gurley ES, Homaira N, Salje H, Ram PK, Haque R, Petri W, et al. Indoor exposure to particulate matter and the incidence of acute lower respiratory infections among children: a birth cohort study in urban Bangladesh. Indoor air. 2013;23(5):379–86. doi: 10.1111/ina.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keusch GT. Immune function in the malnourished host. Pediatric annals. 1982;11(12):1004–14. doi: 10.3928/0090-4481-19821201-08. [DOI] [PubMed] [Google Scholar]
- 34.Smith KR, McCracken JP, Weber MW, Hubbard A, Jenny A, Thompson LM, et al. Effect of reduction in household air pollution on childhood pneumonia in Guatemala (RESPIRE): a randomised controlled trial. Lancet. 2011;378(9804):1717–26. doi: 10.1016/S0140-6736(11)60921-5. [DOI] [PubMed] [Google Scholar]
- 35.Akunne AF, Louis VR, Sanon M, Sauerborn R. Biomass solid fuel and acute respiratory infections: the ventilation factor. International journal of hygiene and environmental health. 2006;209(5):445–50. doi: 10.1016/j.ijheh.2006.04.009. [DOI] [PubMed] [Google Scholar]