Abstract
Nutrition is an important modifiable risk factor that plays a role in the strategy to prevent or delay the onset of dementia. Research on nutritional effects has until now mainly focused on the role of individual nutrients and bioactive components. However, the evidence for combined effects, such as multinutrient approaches, or a healthy dietary pattern, such as the Mediterranean diet, is growing. These approaches incorporate the complexity of the diet and possible interaction and synergy between nutrients. Over the past few years, dietary patterns have increasingly been investigated to better understand the link between diet, cognitive decline, and dementia. In this systematic review we provide an overview of the literature on human studies up to May 2014 that examined the role of dietary patterns (derived both a priori as well as a posteriori) in relation to cognitive decline or dementia. The results suggest that better adherence to a Mediterranean diet is associated with less cognitive decline, dementia, or Alzheimer disease, as shown by 4 of 6 cross-sectional studies, 6 of 12 longitudinal studies, 1 trial, and 3 meta-analyses. Other healthy dietary patterns, derived both a priori (e.g., Healthy Diet Indicator, Healthy Eating Index, and Program National Nutrition Santé guideline score) and a posteriori (e.g., factor analysis, cluster analysis, and reduced rank regression), were shown to be associated with reduced cognitive decline and/or a reduced risk of dementia as shown by all 6 cross-sectional studies and 6 of 8 longitudinal studies. More conclusive evidence is needed to reach more targeted and detailed guidelines to prevent or postpone cognitive decline.
Keywords: Mediterranean diet, healthy diet, dietary pattern, cognitive decline, dementia
Introduction
It has been estimated that, worldwide, 44 million people lived with dementia in 2013. With the aging of the population and with an estimated 7.7 million new cases per year this number doubles every 20 y and will reach 135 million patients with dementia by 2050 (1). The impact of dementia worldwide and the public health importance has been described by the WHO and Alzheimer’s Disease International (2). These organizations propose to make dementia a global health priority, which underlines the importance of finding strategies to prevent dementia. Because there is currently still no effective treatment to modify the course of dementia, prevention is an urgent priority, both to reduce incidence and to slow down progression. Important risk factors need to be further identified and, in particular, the factors that can be modified, such as lifestyle factors. In this systematic review we focus on the risk factor nutrition, for which promising indications exist that it can contribute in reducing the risk of developing dementia. Over the past years the attention has shifted from the role of single nutrients or foods to the role of dietary patterns, such as the promising association of the Mediterranean diet with cognitive decline and dementia. A dietary pattern approach better reflects the complexity of the diet and our daily eating behavior (3–6). Multiple reviews and meta-analyses have been written in the past years that summarize the evidence of a substantial number of studies investigating the influence of a Mediterranean diet on cognitive decline and dementia (7–13). In addition to the Mediterranean diet, there are several other knowledge-based (a priori) dietary patterns, such as the Healthy Diet Indicator (HDI)4 and the Healthy Eating Index (HEI)–2005, and empirically (a posteriori) derived dietary patterns (e.g., by using factor analysis or principal components analysis) that could be associated with cognitive decline and dementia. So far, there has only been one review summarizing the literature on different dietary patterns and cognitive aging until 2011 (14). Therefore, the reviews describing studies on the Mediterranean diet and cognitive decline can be updated with several new studies that have been published since that review.
The aim of this systematic review is to summarize and evaluate available evidence from studies investigating dietary patterns, both a priori and a posteriori, in relation to cognitive decline and dementia in older adults and elderly persons. Although underlying biological mechanisms will be touched on briefly, this review is not intended to provide an extended description of mechanisms underlying the association between dietary patterns and cognitive performance. Instead, our specific goals are as follows: 1) to summarize studies on the Mediterranean diet, 2) to summarize studies of other dietary patterns, and 3) to critically evaluate and summarize all evidence emerging from these studies on associations between dietary patterns and cognitive performance and/or dementia.
Methods
Search strategy.
The role of a healthy diet (Mediterranean diet, dietary patterns) in the development of cognitive decline and dementia has been the subject of several recent systematic reviews that were the starting point of the current review. Medline databases and the Cochrane database were searched up to May 2014 for additional, recently published studies. The search strategies used text words and relevant indexing [medical subject heading (MeSH) terms] to capture studies investigating the association between healthy diet (Mediterranean diet, dietary patterns) with cognitive decline and dementia. When no systematic reviews were found, narrative reviews were used and both checked and updated by using a combination of MeSH and text-based terms: “Diet, Mediterranean” (MeSH terms) or “Mediterranean diet” or (“Mediterranean” and “Diet”) or “Dietary pattern” and “memory” (MeSH terms) or “memory” (all fields) or “cognition” (MeSH terms) or “cognition” (all fields) or cognitive (all fields) or “alzheimer disease” (MeSH terms) or “alzheimer” (all fields) and “disease” (all fields) or “alzheimer disease” (all fields) or “alzheimer” (all fields) or “dementia” (MeSH terms) or “dementia” (all fields) and “humans” (MeSH terms). We included only studies performed in older adults and elderly persons and for which full articles were published.
Study selection process.
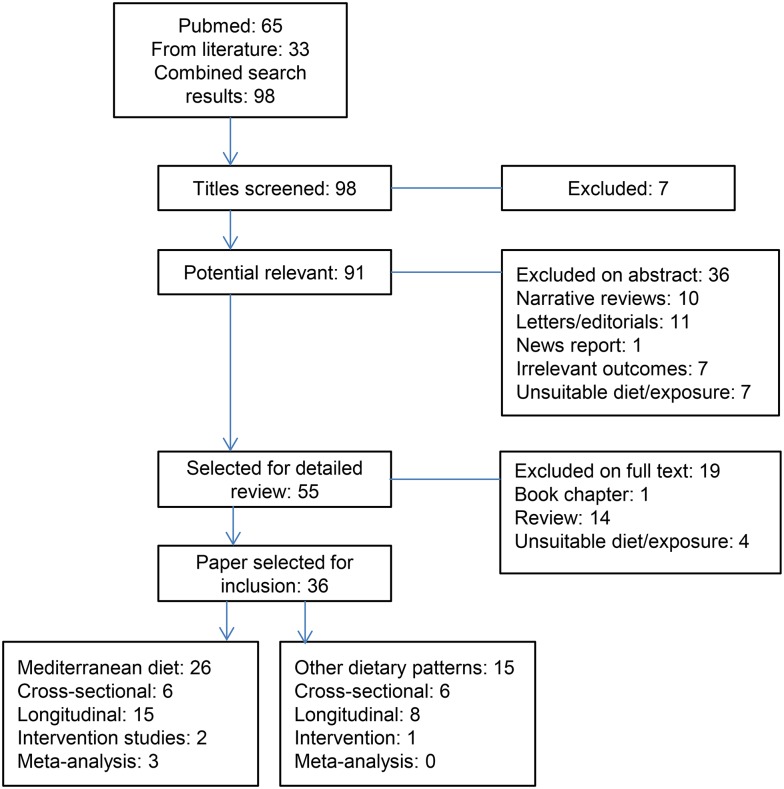
Our search strategy resulted in 65 studies, reviews, and meta-analyses on diet and cognition and an additional search via reference lists of reviews and meta-analyses resulted in 33 more studies. This resulted in a total of 98 studies, of which 36 were selected based on full abstracts and texts. In total, we found 26 studies on the Mediterranean diet and cognitive function or dementia and 15 on other dietary patterns in relation to cognitive function (Figure 1). Five studies performed analyses on both the Mediterranean diet and other patterns (15–19). Study selection, data extraction, and quality assessment were performed independently by 2 reviewers (OVDR and AAMB).
FIGURE 1.
Flow chart of selection process resulting in 36 studies included in the review, 5 of which performed analyses on both the Mediterranean diet and other dietary patterns.
Results
Specification and potential underlying mechanisms
In Table 1, characteristics of frequently reported a priori (20–25) and a posteriori (3, 7, 26–28) dietary patterns are shown. The majority of a priori dietary patterns consist of adequacy components, such as fruits, vegetables, cereals, fatty fish, and dairy, and limitation components, such as total fat, SFAs, cholesterol, and sodium. In an a posteriori data-driven approach, data are reduced into dietary patterns either based on differences in intakes between subjects or on intercorrelations between dietary items (29). Usually this results in dietary patterns consisting of combinations of high or low loadings of similar components as defined by the a priori methods. It has been proposed that these components in combination could affect several biological mechanisms, which may explain how certain healthy dietary patterns can exert their effects on cognitive health and decline. Prevailing mechanisms that are believed to play a role in the pathogenesis of age-related diseases, including cognitive impairment and Alzheimer disease (AD), are oxidative stress, inflammation, and vascular risk factors, which are mechanisms that are ideal targets for nutritional intervention with dietary patterns such as the Mediterranean diet that are abundant in antioxidants and MUFAs and have a balanced ratio of essential n–6 and n–3 FAs. Based on the multiple plausible biological mechanisms, there is a strong theoretical basis that the intake and status of these nutrients may affect the known mechanisms for neurodegeneration.
TABLE 1.
Characterization of most investigated a priori– and a posteriori–defined dietary patterns in relation to neurodegeneration1
| Dietary pattern (ref) | Characterization |
| A priori (hypothesis driven approach) | |
| MeDi (25) | Based on traditional eating habits in Crete, south Italy, and other Mediterranean countries |
| • High in fruits, vegetables, cereals, and legumes | |
| • Low in saturated fats; olive oil main fat source | |
| • Moderate in fish | |
| • Low to moderate in dairy products | |
| • Low in red meat and meat products | |
| • Moderate in alcohol (wine) | |
| HDI (21) | Based on WHO recommendations for the prevention of chronic diseases |
| • SFAs: ≤10en% | |
| • PUFAs: 3–7en% | |
| • Protein: 10–15en% | |
| • Complex carbohydrates: 50–70en% | |
| • Dietary fiber: 27–40 g/d | |
| • Fruits and vegetables: >400 g/d | |
| • Pulses, nuts, and seeds: >30 g/d | |
| • Oligo-/mono- and disaccharides: ≤10en% | |
| • Cholesterol: ≤300 mg/d | |
| HEI (23) | Based on the food patterns found in MyPyramid and is a sum of 10 individual components |
| • Adequacy of total whole fruits, vegetables, dark-green and orange vegetables and legumes, total grains, whole grains, milk, meat and beans, oil | |
| • Low intakes of saturated fat, sodium, and calories from solid fats, alcoholic beverages, and added sugars | |
| RFS (22) | Based on the Dietary Guidelines for Americans and calculated as the sum of 23 items that are consumed at least once a week |
| 1) Apples or pears; 2) oranges; 3) cantaloupe; 4) orange or grapefruit juice; 5) grapefruit; 6) other fruit juices; 7) dried beans; 8) tomatoes; 9) broccoli; 10) spinach; 11) mustard, turnip, or collard greens; 12) carrots or mixed vegetables with carrots; 13) green salad; 14) sweet potatoes, yams; 15) other potatoes; 16) baked or stewed chicken or turkey; 17) baked or broiled fish; 18) dark breads, such as whole wheat, rye, or pumpernickel; 19) cornbread, tortillas, and grits; 20) high-fiber cereals, such as bran, granola, or shredded wheat; 21) cooked cereals; 22) 2%-fat milk and beverages with 2%-fat milk; 23) 1%-fat or skim milk | |
| DASH trial (24) | Based on intakes of nutrients hypothesized to alter blood pressure |
| • Rich in fruits and vegetables | |
| • Rich in low-fat dairy food | |
| • Reduced amounts of saturated fat, total fat, and cholesterol | |
| French National Nutrition and Health Program (PNNS-GS) (20) | Based on French National Nutrition and Health Program recommendations to improve the health status of the general population |
| • Fruits and vegetables at least 5 servings/d | |
| • Bread, cereals, potatoes, and legumes at each meal | |
| • Choose whole-grain food and whole-grain bread more often | |
| • Milk and dairy products 3 servings/d | |
| • Meat and poultry, seafood, eggs 1–2 servings/d | |
| • Seafood at least twice per week | |
| • Limit consumption of added fats | |
| • Favor fats of vegetable origin | |
| • Drink water as desired | |
| • Limit sweetened beverages | |
| • Limit salt consumption | |
| • At least 30 min of brisk walking or equivalent per day of physical activity | |
| A posteriori (exploratory approach) | |
| Cluster analysis (3, 7, 27) | Classification technique, which aggregates subjects with similar defined variables such as dietary pattern and the energy contribution of each food group |
| PCA (7, 15) | Common approach of factor analysis to define dietary patterns, aggregates highly correlated food items to identify underlying dietary patterns |
| RRR (7, 26, 28) | Mix of an exploratory and hypothesis-driven approach, involves the elements of an a priori approach to derive dietary patterns |
DASH, Dietary Approaches to Stop Hypertension; en%, percentage of energy intake; HDI, Healthy Diet Indicator; HEI, Healthy Eating Index; MeDi, Mediterranean diet; PCA, principal component analysis; PNNS-GS, Program National Nutrition Santé guideline score; ref, reference; RFS, Recommended Food Score; RRR, reduced rank regression.
Mediterranean diet
The role of the Mediterranean diet on cognitive decline and dementia risk was only recently systematically reviewed by Lourida et al. (8). This review included literature published until January 2012. In addition, Alzheimer’s Disease International published a report on the available evidence on this subject in the beginning of 2014 (30). However, because this area of research is developing rapidly, the results of these 2 reviews can already be updated by adding at least 10 new studies on the Mediterranean diet and cognition and dementia. We found a total of 6 cross-sectional studies, 15 prospective studies, and 1 intervention trial. The characteristics of these studies are summarized in Table 2.
TABLE 2.
Characteristics of included studies on Mediterranean diet, cognitive decline, and dementia1
| Author, year (ref), country, study name | Population (sample size, mean age) | Follow-up, y | Exposure/ intervention measure | Outcome measure | Effect measure |
| Cross-sectional studies | |||||
| Samieri et al., 2013 (16), USA, NHS | n = 10,670 | — | FFQ, A-MeDi score | Cognitive decline (TICS), mental health (SF-36) | No significant association between the A-MeDi and mental health or cognitive impairment (ORQ5vsQ1: 1.12; 95% CI: 1.01, 1.20; P-trend < 0.001; and OR: 0.97; 95% CI: 0.95, 1.00; P-trend = 0.020, respectively) |
| 59 y | |||||
| Chan et al., 2013 (15), Hong Kong | n = 3670 | — | FFQ, MeDi score | Cognitive function (CSI-D) | No significant association between the MeDi and cognitive function in either men or women (ORT3vsT1: 0.89; 95% CI: 0.56, 1.41; P-trend = 0.882; and OR: 1.02; 95% CI: 0.75, 1.41; P-trend = 0.952, respectively). |
| 71.8 y | |||||
| Ye et al., 2013 (19), Puerto Rico, BPRHS | n = 1269 | — | FFQ, MeDi score | Cognitive function and cognitive impairment (MMSE) | A significant association between a higher MeDi score, higher MMSE scores (P-trend = 0.012) and a lower risk of cognitive impairment (OR: 0.80; 95% CI: 0.80, 0.94; P < 0.001) |
| 57.3 y | |||||
| Katsiardanis et al., 2013 (33), Greece, Velestino Study | n = 557 | 157-item FFQ, MeDi score | Cognitive impairment (MMSE) | Significant lower risk of cognitive impairment in men per 1-unit increase in adherence to the MeDi (OR: 0.88; 95% CI: 0.80, 0.98; P = 0.02) but a higher risk in women (OR: 1.11; 95% CI: 1.00, 1.22; P = 0.04) | |
| >65 y | |||||
| Gardener et al., 2012 (31), Australia, AIBL study | n = 970 | — | 74-item CCV FFQ, MeDi score | MCI (MMSE), AD (DSM-IV, NINCDS-ADRDA) | Each unit increase in MeDi score was significantly associated with a reduced risk of MCI or AD (OR: 0.87; 95% CI: 0.75, 1.00; P < 0.05, and OR: 0.81; 95% CI: 0.71, 0.92; P < 0.01, respectively) |
| >60 y | |||||
| Scarmeas et al., 2006 (32), USA, WHICAP | n = 1984 | Nested case control | 61-item FFQ, MeDi score | Prevalent AD (NINCDS-ADRDA) | Better adherence to the MeDi was significantly associated with lower risk of AD (OR: 0.76; 95% CI: 0.67, 0.87; P < 0.01; ORT3vsT1: 0.32; 95% CI: 0.17, 0.59; P-trend < 0.001) |
| 76.3 y | |||||
| Longitudinal studies | |||||
| Wengreen et al., 2013, (18), USA, CCMS | n = 3831 | 11 | 142-item FFQ, MeDi score | Cognitive impairment (3MS) | Better adherence to MeDiet was significantly associated with higher 3MS scores (MeDietQ5vsQ1: 0.94 ± 0.29; P-trend = 0.0022) |
| 74.1 y | |||||
| Tsivgoulis et al., 2013 (37), USA, REGARDS study | n = 17,478 | 4 | FFQ, MeDi score | Incident cognitive impairment (SIS) | Higher adherence to MeDiet was significantly associated with a lower likelihood of ICI (OR: 0.87; 95% CI: 0.76, 1.00), especially in nondiabetic participants (OR: 0.81; 95% CI: 0.70, 0.94; P = 0.0066), but not in diabetic participants (OR: 1.27; 95% CI: 0.95, 1.71; P = 0.1063) |
| 64.6 y | |||||
| Samieri et al., 2013 (35), USA, NHS | n = 16,058 | 6 | 116-item FFQ, A-MeDi score | Cognitive status and cognitive decline (TICS), verbal memory, global cognition | Highest adherence to MeDi was significantly associated with cognitive status at older ages [adjusted mean differences in z scores Q5vsQ1 (95% CI): 0.06 (0.01, 0.11), P-trend = 0.004 for TICS; 0.05 (0.01, 0.08), P-trend = 0.002 for global score; and 0.06 (0.03, 0.10), P-trend < 0.001 for verbal memory score], but not with cognitive decline [0.004 (−0.011, 0.019), P-trend 0.31; −0.001 (−0.010, 0.007), P-trend = 0.84; −0.001 (−0.011, 0.010), P-trend = 0.70] |
| 74.3 y | |||||
| Samieri et al., 2013 (39), USA, Women’s Health Study | n = 6174 | 2 | 131-item FFQ, A-MeDi score | Cognitive decline (TICS), global cognition, verbal memory | No significant associations between higher A-MeDi scores and mean differences in averaged measures of global cognition and verbal memory (Q5vsQ1: 0.02; 95% CI: −0.03, 0.06; P-trend = 0.63; and 0.03; 95% CI: −0.02, 0.07. P-trend = 0.44, respectively), nor over time (P for quintile medians × time interaction = 0.26 for global score and 0.40 for score and cognitive decline) |
| 72 y | |||||
| Kesse-Guyot et al., 2013 (41), France, SU.VI.MAX | n = 3083 | 13 | Repeated 24-h records, MeDi score, and MSDPS | Cognitive function; episodic and lexical-semantic memory, mental flexibility | No significant association between higher adherence to MeDi and MSDPS and cognitive scores, except for a lower phonemic fluency (−1.00; 95% CI: −1.85, −0.15; P = 0.048) with decreasing MSDPS and lower backward digit with decreasing MDS (−0.64; 95% CI: −1.60, 0.32; P = 0.03) |
| 65.4 y | |||||
| Vercambre et al., 2012 (40), USA, WACS | n = 2504 | 5.4 | 116-item FFQ, MeDi score | Cognitive decline (TICS), verbal memory, category fluency score | No significant association between higher adherence to MeDi and adjusted mean differences in annual rates of cognitive decline [T3vsT1 (95% CI): 0.00 (−0.02, 0.01), P = 0.88, for global cognition; −0.03 (−0.11, 0.05), P = 0.53, for TICS; 0.00 (−0.02, 0.02), P = 0.97, for verbal memory; and −0.03 (−0.14, 0.08), P =0.64 for category fluency] |
| 72.3 y | |||||
| Cherbuin and Anstey, 2012 (42), Australia, PATH | n = 1528 | 4 | 215-item FFQ, MeDi score | MCI, cognitive decline, any MCD (ICC, CDR) | No significant association between the MeDiet and transition from normal aging to MCI, CDR 0.5, and any MCD [OR (95% CI): 1.41 (0.95, 2.10); 1.18 (0.88, 1.57); and 1.20 (0.98, 1.47), respectively] |
| 62.5 y | |||||
| Tangney et al., 2011 (17), USA, CHAP | n = 3790 | 7.6 | 139-item FFQ, MeDi score | Global cognitive function (MMSE, EBMT, SDMT) | Higher MeDi scores were significantly associated with better global cognitive scores at baseline (β = 0.0070; SEE = 0.0022, P = 0.0013) and with slower rates of decline over time (β = 0.0014; SEE = 0.0004, P = 0.0004) |
| 75.4 y | |||||
| Roberts et al., 2010 (38), USA, MCSA | n = 1233 | 2.2 | 128-item FFQ, MeDi score | MCI (CDR) | A high MeDi score was not statistically associated with risk of incident MCI or dementia (HRT3vsT1: 0.75; 95% CI: 0.46, 1.21; P = 0.24) |
| 79.6 y | |||||
| Gu et al., 2010 (45), USA, WHICAP | n = 1219 | 3.8 | 61-item SFFQ, MeDi score | AD (NINCDS-ADRDA) | Better adherence to MeDi was borderline significantly associated with lower risk for AD in fully adjusted model (HR: 0.87; 95% CI: 0.78, 0.97; P = 0.01; and HRT3vsT1: 0.68; 95% CI: 0.42, 1.08; P-trend = 0.06) |
| 76.7 y | |||||
| Scarmeas et al., 2009 (36), USA, WHICAP | n = 1393 | 4.5 | FFQ, MeDi score | MCI (DSM-III-R), AD (NINCDS-ADRDA) | Better adherence to MeDi was significantly associated with a lower risk of MCI (HR: 0.85; 95% CI: 0.72, 1.00; P-trend = 0.05; HRT3vsT1: 0.72; 95% CI: 0.52, 1.00; P = 0.05) and a lower risk of developing AD after MCI (HR: 0.71; 95% CI: 0.53, 0.95; P-trend = 0.02; HRT3vsT1: 0.52; 95% CI: 0.30, 0.91; P = 0.02) |
| 76.9 y | |||||
| Scarmeas et al., 2009 (46), USA, WHICAP | n = 1880 | 5.4 | 61-item FFQ, MeDi score | AD (NINCDS-ADRDA) | Better MeDi adherence was significantly associated with lower AD risk (HRT3vsT1: 0.60; 95% CI: 0.42, 0.97; P-trend = 0.008) |
| 77.2 y | |||||
| Feart et al., 2009 (34), France, 3C study | n = 1410 | FFQ and 24-HR, MeDi score | Cognitive performance, dementia risk, and AD risk (MMSE, DSM-III-R) | A 1-point increase in the MeDi was significantly associated with fewer MMSE errors (β = −0.006, P = 0.04) and was borderline significant across categories of MeDi (βT3vsT1 = −0.02, P = 0.06); there was no significant association with dementia risk and AD risk (HR: 1.06; 95% CI: 0.92, 1.21; P = 0.43; HRT3vsT1: 1.12; 95% CI: 0.60, 2.10; P = 0.72; HR: 1.00; 95% CI: 0.85, 1.19; P = 0.96; and HRT3vsT1: 0.86; 95% CI: 0.39, 1.88; P = 0.71, respectively) | |
| 75.9 y | |||||
| Psaltopoulou et al., 2008 (43), Greece, EPIC | n = 732 | 8 | 150-food FFQ, MeDi score | Cognitive decline, MMSE | No significant association per 1-unit increase in MeDi and MSSE (β = 0.05; 95% CI: −0.09, 0.19; P = 0.485) |
| >60 y | |||||
| Scarmeas et al., 2006 (44), USA, WHICAP | n = 2258 | 4 | 61-item SFFQ, MeDi score | AD (NINCDS-ADRDA) | Better adherence to MeDi was associated with lower risk of AD (HR: 0.91; 95% CI: 0.83, 0.98; P = 0.015; HRT3vsT1: 0.60; 95% CI: 0.42, 0.87; P-trend = 0.007) |
| 77.2 y | |||||
| Randomized controlled trials | |||||
| Martinez-Lapiscina et al. 2013 (47, 48), Spain, PREDIMED-Navarra | n = 522 (47) | 6.5 | 137-item FFQ; MedDiet intervention | Cognitive performance (MMSE, CDT) | Participants in the MeDi + olive oil and the MeDi + nuts group showed better cognitive performance compared with the control group for MMSE and CDT [adjusted differences (95% CI): 0.62 (0.18, 1.05, P = 0.005, and 0.57 (0.11, 1.03), P = 0.015 for MMSE; 0.51 (0.20, 0.82), P = 0.001, and 0.33 (0.003, 0.67), P = 0.048 for CDT] |
| 74.6 y | 3 arms: | ||||
| n = 268 (48) | MeDi + olive oil | Cognitive performance, MCI | MeDi with olive oil was related to better cognitive performance (for 5 of 16 tests) and lower MCI (OR: 0.34; 95% CI: 0.12, 0.97) compared with control group | ||
| 74.1 y | MeDi + nuts | MeDi with nuts was not related to better cognitive performance or MCI (OR: 0.56; 95% CI: 0.22, 1.43) | |||
| Low-fat diet |
AD, Alzheimer disease; AIBL, Australian Imaging, Biomarkers, and Lifestyle Study of Ageing cohort; A-MeDi, alternate Mediterranean diet; BPRHS, Boston Puerto Rican Health Study; CCMS, Cache County Memory Study; CDR, Clinical Dementia Rating; CDT, Clock Drawing Test; CHAP, Chicago Health and Aging Project; CSI-D, Community Screening Instrument for Dementia; DSM, Diagnostic and Statistical Manual of Mental Disorders; EBMT, East Boston Memory Test; EPIC, European Prospective Investigation into Cancer and Nutrition; ICC, International Consensus Criteria; MCD, mild cognitive disorder; MCI, mild cognitive impairment; MCSA, Mayo Clinic Study of Aging; MeDi, Mediterranean diet; MMSE, Mini-Mental State Examination; MSDPS, Mediterranean Style Dietary Pattern Score; NHS, Nurses’ Health Study; NINCDS-ADRDA, National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer Disease and Related Disorders Association; PATH, Personality and Total Health Through Life Project; PREDIMED, PREvencion con DIeta MEDiterranea; Q, quintile; ref, reference; REGARDS, Reasons for Geographic and Racial Differences in Stroke; SDMT, Symbol Digit Modalities Test; SF-36, Medical Outcomes Short-Form 36 Health Survey; SIS, Six-item Screener; SU.VI.MAX, Supplementation en VItamines et Mineraux Anti-oXydants; T, tertile; TICS, Telephone Interview for Cognitive Status; WACS, Women’s Antioxidant Cardiovascular Study; WHICAP, Washington Heights-Inwood Columbia Aging Project; 3C, Three-City; 3MS, Modified Mini-Mental State Examination; 24-HR, 24-h dietary recall.
Observational evidence.
Four of the 6 cross-sectional studies showed an inverse association of the Mediterranean diet with cognitive functioning (16, 19, 31) or AD (31, 32) in American, Puerto Rican, and Australian older adults and elderly persons. One cross-sectional study in Greek elderly individuals observed a protective association with a 1-unit increase in Mediterranean diet score in men (OR: 0.88; 95% CI: 0.80, 0.98) but, in contrast, a suggestion of an increased risk of cognitive impairment in women (OR: 1.11; 95% CI: 1.00, 1.22) (33). A study in Hong Kong did not find associations in either men or women [ORs (95% CIs) for tertile 3 vs. tertile 1: 0.89 (0.56, 1.41) vs. 1.02 (0.75, 1.41)] (15).
Of the 12 prospective studies on adherence to the Mediterranean diet and better cognitive performance, 6 observed a beneficial association after 3 to 7.6 y of follow-up (17, 18, 34–37). Of these studies, 5 were performed in the United States (17, 18, 35–37) and 1 in France (34). All 5 studies performed in the United States showed marginal, but significant, associations. The French study showed that a 1-point increase in the Mediterranean diet was associated with fewer errors on the Mini-Mental State Examination (MMSE) but was only marginally significant across categories of the Mediterranean diet (βT3vsT1: −0.02, P = 0.06). In the other 6 studies, of which 3 were performed in the United States (38–40) and the others in France (41), Australia (42), and Greece (43), the association was beneficial but not significant after 2–3 y of follow-up (38–43).
With respect to dementia and AD, 4 of 6 studies showed a reduced risk of AD with better adherence to the Mediterranean diet after 3–5.4 y of follow-up in US populations [HR: 0.91; 95% CI: 0.83, 0.98 (44); HR: 0.87; 95% CI: 0.78, 0.97 (45); HR: 0.60; 95% CI: 0.42, 0.97 (46)] and a lower risk of developing AD after mild cognitive impairment (MCI; HR: 0.71; 95% CI: 0.53, 0.95) (36). Two studies, performed in a France (34) and in a US population (38), did not find associations.
Trial evidence.
The only trial until now that investigated the effect of a Mediterranean-type diet either rich in olive oil or rich in nuts was performed in 522 participants with a high cardiovascular risk profile. After 6.5 y of intervention, participants in both types of the Mediterranean diet had a higher cognitive performance than did the control group [adjusted differences for MMSE (95% CI): +0.62 (+0.18, +1.05), P = 0.005; and +0.57 (+0.11, +1.03), P = 0.015] (47). In a smaller subgroup (n = 285) of this same trial, only the Mediterranean diet with olive oil showed better results for 5 of 16 specific cognitive tests and MCI (OR: 0.34; 95% CI: 0.12, 0.97) (48).
Meta-analyses.
An updated systematic review and meta-analysis from 2010 pooled data of prospective cohort studies on adherence to the Mediterranean diet and risk of AD, Parkinson disease, cognitive decline, dementia, and MCI showed an inverse association between a 2-point increase of adherence to the Mediterranean diet and neurodegenerative diseases (RR: 0.87; 95% CI: 0.81, 0.94; I2 = 0%; P = 0.73) (Table 3) (12).
TABLE 3.
Characteristics of included reviews and meta-analyses on Mediterranean diet, cognitive decline, and dementia1
| Author, year (ref) | Studies included | Update until | Exposure | Outcomes measure | Effect |
| Singh et al., 2014 (11) | Five prospective cohort studies with at least 1 y follow-up | November 2012 | MeDi score | From normal to MCI, from normal to AD, cognitive impairment | Better adherence to MeDi was associated with a lower risk of MCI (HRT3vsT1: 0.73; 95% CI: 0.56, 0.96; I2 = 0%; HRT2vsT1; 0.82; 95% CI: 0.64, 1.05; I2 = 0%; HR: 0.98; 95% CI: 0.84, 1.08; I2 = 33%), AD (HR: 0.92; 95% CI: 0.85, 0.99; HRT2vsT1: 0.87; 95% CI: 0.66, 1.14; HRT3vsT1: 0.64; 95% CI: 0.46, 0.89), and cognitive impairment (HR: 0.92; 95% CI: 0.88; 0.97; HRT2vsT1: 0.80; 95% CI: 0.67, 0.95; HRT3vsT1: 0.67; 95% CI: 0.55, 0.81; I2 = 0%) |
| Psaltopoulou et al., 2013 (9) | 9 studies; case-control, longitudinal, cross-sectional | 31 October 2012 | MeDi score | Cognitive impairment, depression | Both a moderate and a high adherence to MeDi were associated with a reduced risk of cognitive impairment and depression (RR: 0.79; 95% CI: 0.67, 0.94; I2 = 28.3%; P = 0.193, vs. RR: 0.60; 95% CI: 0.43, 0.83; I2 = 76.4%; P = 0.000; and RR 0.77; 95% CI: 0.62, 0.95; I2 = 54.4%, vs. RR: 0.68; 95% CI: 0.54, 0.86; I2 = 53.4%, respectively). No effect measure modification by sex was observed |
| Sofi et al., 2010 (12) | Five prospective cohort studies | June 2010 | MeDi score | Neurodegenerative diseases (cognitive decline, risk of dementia, MCI, AD, Parkinson disease) | Per 2-point increase of adherence to the MeDi score, the risk of incidence of neurodegenerative diseases decreased (RR: 0.87; 95% CI: 0.81, 0.94) |
MCI, mild cognitive impairment; MeDi, Mediterranean diet; ref, reference.
Another meta-analysis (9) pooled data of 2 case-control studies, 5 longitudinal studies, and 5 cross-sectional studies on moderate and high adherence to the Mediterranean diet and risk of cognitive impairment, which resulted in inverse associations [RRs (95% CIs): 0.79 (0.67, 0.94), I2 = 28.3%, and 0.60 (0.43, 0.83), I2 = 76.4%; P = 0.000].
A more recent systematic review and meta-analysis by Singh et al. (11) analyzed data of prospective cohort studies with at least 1 y of follow-up on the Mediterranean diet and cognitive outcomes (MCI or AD). On the basis of 2 longitudinal studies performed in the United States, there was an association with high adherence to the Mediterranean diet (HRT3vsT1: 0.73; 95% CI: 0.56, 0.96; I2 = 0%), but there was no association with moderate adherence compared with poor adherence [HRT2vsT2: 0.82; 95% CI: 0.64, 1.05; I2 = 0%], nor per 1-unit increase in the Mediterranean diet score (HR: 0.95; 95% CI: 0.84, 1.08; I2 = 33%). Pooled analyses of 2 other longitudinal studies showed an inverse association between adherence to the Mediterranean diet (both continuous and categorical) and risk of AD among cognitively normal individuals [HR (95% CI): 0.92 (0.85, 0.99), I2 = 0%; and HRT3vsT1: 0.64 (0.46, 0.89), I2 = 0%]. When combining all of the data, this resulted in an inverse association between a better adherence to the Mediterranean diet and cognitive impairment: HR (95% CI): 0.92 (0.88, 0.97), I2 = 0%; HRT2vsT1: 0.80 (0.67, 0.95), I2 = 0%; HRT3vsT1: 0.67 (0.55, 0.81), I2 = 0%.
Other dietary patterns
Fewer studies have been performed on dietary patterns other than the Mediterranean diet. So far, there has been only 1 review summarizing the literature of studies on different dietary patterns and cognitive aging until 2011 and this review included a total of 13 studies (14). We found a total of 16 studies of which there were 8 cross-sectional studies, 7 prospective studies, and 1 trial investigating a priori–derived dietary patterns and a posteriori–derived dietary patterns in relation to cognitive decline (Table 4).
TABLE 4.
Characteristics of included studies on dietary patterns other than the Mediterranean diet, cognitive decline, and dementia1
| Author, year (ref), country, study name | Population (sample size, mean age) | Follow-up | Exposure/ intervention measure | Outcome measure | Effect |
| Cross-sectional studies | |||||
| Ye et al., 2013 (19), Puerto Rico, BPRHS | n = 1269 | — | FFQ, HEI-2005 | Cognitive function and cognitive impairment (MMSE) | A higher HEI-2005 score was significantly associated with a higher MMSE score (P-trend = 0.011) and lower risk of cognitive impairment (OR10points: 0.86; 95% CI: 0.74, 0.99; P = 0.033) |
| 57.3 y | |||||
| Chan et al., 2013 (15), Hong Kong | n = 1926 | FFQ, factor analysis, 3 patterns: “vegetables-fruits,” “snacks-drinks-milk products,” “meat-fish” | Cognitive function (CSI-D) | A higher “vegetables-fruits” and “snacks-drinks-milk products” pattern score was significantly associated with a reduced risk of cognitive impairment in women (ORQ4vsQ1: 0.73; 95% CI: 0.54, 1.00; P-trend = 0.018; and ORQ4vsQ1: 0.65; 95% CI: 0.47, 0.90; P-trend = 0.003, respectively) but not in men | |
| 71.8 y | |||||
| Samieri et al., 2013 (16), USA, NHS | n = 10,670 | — | FFQ, AHEI-2010 | Cognitive decline (TICS), mental health (SF-36) | The AHEI-2010 was significantly associated with greater likelihood of no major limitations in mental health (ORQ5vsQ1: 1.31; 95% CI: 1.05, 1.22; P-trend < 0.001) and marginally associated with no cognitive impairment (ORQ5vsQ1: 0.99; 95% CI: 0.97, 1.01; P-trend = 0.09) |
| 59 y | |||||
| Samieri et al., 2008 (51), France, 3C | n = 1724 | — | FFQ, cluster analysis, 5 patterns: “small eaters,” “biscuits and snacking,” “healthy,” “charcuterie, meat, and alcohol,” “pasta eaters” | Cognitive function (MMSE) | Better adherence to the “healthy” dietary pattern was significantly associated with fewer errors on the MMSE (β = −0.11; 95% CI: −0.22, −0.004, in men; and β = −0.13; 95% CI: −0.22, −0.04, in women) |
| 76.0 y | |||||
| Corrêa Leite et al., 2001 (49), Pavia, Italy | n = 1651 | — | 180-item FFQ, HDI | Cognitive deficit (NPT) | Better adherence to the HDI was associated with a lower prevalence of cognitive deficit (OR: 0.85; 95% CI: 0.77, 0.93) |
| 76.6 y | |||||
| Huijbregts et al., 1998 (50), Seven Countries Study | n = 1049 | — | Cross-check dietary history, HDI | Cognitive impairment (MMSE) | Better adherence to the HDI had a protective effect on cognitive impairment, although not consistent over all cohorts (Zutphen OR: 0.81; 95% CI: 0.63, 1.04; Italian OR: 0.75; 95% CI: 0.58, 0.97) |
| 76.1 y | |||||
| Longitudinal studies | |||||
| Wengreen et al., 2013 (18), USA, CCMS | n = 3831 | 11 y | 142-item FFQ, DASH score | Cognitive impairment, 3MS | Better adherence to DASH diet was significantly associated with higher 3MS scores (DASHQ5vsQ1: 0.97 ± 0.29 points; P-trend = 0.0001) |
| 74.1 y | |||||
| Kesse-Guyot et al., 2011 (54), France, SU.VI.MAX | n = 2135 | 12 y | Repeated 24-HR, PNNS-GS scores | Verbal memory, executive functioning | Better adherence to nutritional recommendations was significantly associated with the verbal memory factor (β = 0.41; 95% CI: 0.17, 0.64), whereas no association was shown with the executive functioning factor |
| 65.5 y | |||||
| Ozawa et al., 2013 (56), Japan, Hisayama Study | n = 1006 | 15 y | 70-item FFQ, RRR, 7 patterns; only “Japanese” presented | Dementia risk, AD, vascular dementia (HDS, HDSR, MMSE) | Better adherence to the “Japanese” pattern was associated with a reduced risk of dementia (all-cause dementia HR: 0.66; 95% CI: 0.46, 0.95; AD HR: 0.65; 95% CI: 0.40, 1.06; and VaD HR: 0.45; 95% CI: 0.22, 0.91) |
| 68.5 y | |||||
| Kesse-Guyot et al., 2012 (55), France, SU.VI.MAX | n = 3054 | 13 y | Repeated 24-HR, factor analysis, 2 patterns: “healthy,” “traditional” | Global cognitive function, verbal memory, executive functioning | Significantly higher cognitive function scores were found with better adherence to the “healthy” pattern vs. “traditional” pattern (adjusted means ± SDs: 50.1 ± 0.7 vs. 48.9 ± 0.7, P-trend = 0.001 for global cognitive function; 49.7 ± 0.4 vs. 48.7 ± 0.4, P-trend 0.01 for verbal memory) |
| 65.4 y | |||||
| Shatenstein et al., 2012 (52), Canada, NuAge | n = 1488 | 3 y | 78-item FFQ, C-HEI | Cognitive decline (3MS) | There was no significant association between global diet quality (total C-HEI/100) and cognitive decline after 3-y follow-up (β = 0.00008, P = 0.852) |
| 74.2 y | |||||
| Tangney et al., 2011 (17), USA, CHAP | n = 3790 | 7.6 y | 139-item FFQ, HEI-2005 | Cognitive function (MMSE) | HEI-2005 was neither associated with better global cognitive score at baseline nor with changes in global cognitive score at follow-up (β = −0.0011 ± 0.001, P = 0.236, and β = 0.00002 ± 0.0002, P = 0.214, respectively) |
| 75.4 y | |||||
| Gu et al., 2010 (26), USA, WHICAP | n = 2148 | 3.9 y | FFQ, RRR, 7 patterns: DP1–DP7 | AD (DSM) | Better adherence to the “healthy” pattern was significantly associated with a lower AD risk (HRT3vsT1: 0.62; 95% CI: 0.43, 0.89) |
| 77.2 y | |||||
| Wengreen et al., 2009 (53), USA, Cache County | n = 3634 | 11 y | 142-item FFQ, RFS vs. non-RFS | Cognitive decline, 3MS | Better adherence to RFS at baseline was associated with less decline in 3MS scores after 11 y of follow-up (RFST3vsT1: 1.79 points, P = 0.0013) |
| 74.7 y | |||||
| Randomized controlled trials | |||||
| Smith et al., 2010 (57), USA, ENCORE | n = 124 | 4 mo | DASH diet 3-arms: | Cognitive functioning | DASH diet alone resulted in better psychomotor speed (Cohen’s d = 0.440; P = 0.036) compared with control group in subjects with high blood pressure |
| 52.3y | DASH alone | ||||
| DASH weight Management | |||||
| Usual care |
AD, Alzheimer disease; AHEI-2010, Alternative Healthy Eating Index–2010; BPRHS, Boston Puerto Rican Health Study; CCMS, Cache County Memory Study; CHAP, Chicago Health and Aging Project; C-HEI, Canadian Healthy Eating Index; CSI-D, Community Screening Instrument for Dementia; DASH, Dietary Approaches to Stop Hypertension; DSM, Diagnostic and Statistical Manual of Mental Disorders; ENCORE, Exercise and Nutrition Interventions for Cardiovascular Health Study; HDI, Healthy Diet Indicator; HDS, Hasegawa Dementia Scale; HDSR, Hasegawa Dementia Scale–Revised; HEI, Healthy Eating Index, MMSE, Mini-Mental State Examination; NHS, Nurses’ Health Study; NPT, neuropsychological test; NuAge, Longitudinal Study on Nutrition and Successful Aging; PNNS-GS, Program National Nutrition Santé guideline score; Q, quartile/quintile; ref, reference; RFS, Recommended Food Score; RRR, reduced rank regression; SF-36, Medical Outcomes Short-Form 36 Health Survey; SU.VI.MAX, Supplementation en VItamines et Mineraux Anti-oXydants; T, tertile; TICS, Telephone Interview for Cognitive Status; VaD, vascular dementia; WHICAP, Washington Heights-Inwood Columbia Aging Project; 3C, Three-City; 3MS, Modified Mini-Mental State Examination; 24-HR, 24-h dietary recall.
Observational evidence.
Of the 6 cross-sectional studies, 4 used a priori knowledge to study dietary patterns. Better adherence to the HDI was associated with a lower prevalence of cognitive deficit [OR: 0.85; 95% CI 0.77, 0.93 (49)] and a reduced risk of cognitive impairment (OR: 0.75; 95% CI: 0.58, 0.97) in an Italian cohort but not in a Dutch cohort (OR: 0.81; 95% CI: 0.63, 1.04) (50). Higher adherence to the HEI-2005 was associated with a lower risk of cognitive impairment in a Puerto Rican population (OR: 0.86; 95% CI: 0.74, 0.99) (19), but this was not found for higher adherence to the 2010 alternative HEI in a US population (OR for quintile 5 vs. quintile 1: 0.99; 95% CI: 0.97, 1.01) (16). Two studies used an empirical approach such as principal components analysis (15) or cluster analysis (51). One study observed fewer errors on the MMSE with a better adherence to a healthy dietary pattern in both men and women [β (95% CI): −0.11 (−0.22, −0.0004) and −0.13 (−0.22, −0.04), respectively] (51). The other study found a beneficial association only in Chinese women with a higher “vegetables-fruits” and “snacks-drinks-milk products” pattern score and cognitive function but not in men (15).
Of the 8 prospective studies, 5 used a priori–defined dietary scores (17, 18, 52–54) and 3 studies used data-driven approaches (26, 55, 56). Results from studies using a priori diet scores showed mixed results, with higher cognitive function scores for the Dietary Approaches to Stop Hypertension (DASH) diet [Modified MMSE (3MS) score for DASH quintile 5 vs. quintile 1: 0.97 ± 0.29] (18) and less cognitive decline for the Recommended Food Score after 11 y of follow-up (3MS score for Recommended Food Score quartile 4 vs. quartile 1: 1.79) (53), whereas there was no association between the HEI-2005 (β = 0.00002, P = 0.214) (17) and the Canadian Healthy Eating Index (β = 0.00008, P = 0.852) (52) and cognitive decline after 7.6 and 3 y of follow-up, respectively. A better adherence to the French guidelines [Program National Nutrition Santé guideline score (PNNS-GS)] was associated with better cognitive function as measured by many specific cognitive function tests (54). The other 3 of the 7 studies used data-driven approaches showing consistent beneficial associations between dietary patterns and risk of dementia (56), cognitive function (55), and AD (26, 56). Two studies used reduced rank regression, for which 1 study observed a typical “Japanese” pattern and the other study reported on a “healthy” pattern. After 15 y of follow-up, the Japanese pattern was associated with a reduced risk of dementia (HR: 0.66; 95% CI: 0.46. 0.95), AD (HR: 0.65; 95% CI: 0.40, 1.06), and vascular dementia (HR: 0.45; 95% CI: 0.22, 0.91) (56). The healthy pattern was strongly associated with a lower risk of AD (HRT3vsT1: 0.62; 95% CI: 0.43, 0.89) after 3.9 y of follow-up (26). The other study used factor analysis and reported higher cognitive function scores for the healthy pattern than for a “traditional” pattern (50.1 ± 0.7 vs. 48.9 ± 0.7, P-trend = 0.001) after 13 y of follow-up (55).
Trial evidence.
The single trial that was performed observed better scores on 1 of 9 cognitive function tests (psychomotor speed; Cohen’s d = 0.440, P = 0.036) after a 4-mo intervention with the DASH diet compared with a usual-diet control group in 124 overweight adults with high blood pressure (57).
Discussion
We reviewed the current evidence from observational studies and intervention trials investigating healthy dietary patterns in relation to cognitive decline and dementia. Overall, the results of all types of dietary pattern approaches suggest that better adherence to a healthy dietary pattern is associated with less cognitive decline and/or a lower risk of dementia. However, most studies were observational and evidence from intervention trials is limited to 2 trials, one of which investigated the effect of the Mediterranean diet (47, 48) and one the effect of the DASH diet (57). There were several different methodologic factors between the studies. This heterogeneity hinders comparison between studies; therefore, the most important points are discussed below.
Both a priori and a posteriori approaches to define dietary patterns (3, 7, 58) were used in studies included in this review and each method has its strengths and limitations. A limitation of a priori indexes is that they are based on current scientific knowledge on what a healthy diet comprises. Evolutions in knowledge should be considered each time the index is applied, which also changes the index over time (59). In addition, few cues about how to weight food groups or guidelines have been proposed. A limitation of both a priori and a posteriori approaches is that complex correlations of food matrixes are not taken into account, neither are all components specifically related to cognitive outcomes. Indexes described in this review are mainly based on improving overall health status (20–23, 25) or blood pressure (60) rather than improving cognitive health specifically. A limitation of a posteriori methods is the limited comparability and reproducibility in other study samples, because dietary patterns are based on food behavior in specific study samples (61). Another limitation of a posteriori methods is that good skills in multidimensional statistical methods are required to select the best components of which the choice is subjective (62).
Another subjective choice, which could be confusing, is the naming of the dietary patterns. Therefore, for studies performed in different populations, the food consumption characterizing the pattern should be described clearly. Populations studied were almost all Western populations, mostly from the United States and the Mediterranean countries in Europe, and a few studies were performed in Australia, China, or Japan. According to Solfrizzi and Panza (63), the components of the Mediterranean diet in Western countries could be different from the traditional Mediterranean diet, in particular for the high intakes of olive oil and regular consumption of wine with meals. In general, a healthy dietary pattern comprises a diet high in fruits, vegetables, other plant-derived products, and fish and lower intakes of meat, saturated fats, and added refined sugar. We found no clear differences in associations between healthy dietary patterns and cognitive decline and dementia across countries. In addition, Singh et al. (11) did not find any heterogeneity in their analysis; however, this could be due to the fact that they included 3 US studies and 1 French study.
Dietary intake can be assessed with different methods, such as food records, 24-h recalls, or FFQs, of which the latter have been mostly used in the studies included in the current review. Different intake methods not only limit comparisons between studies but also affect the number of variables to be used for dietary pattern analyses. This could affect both the number of derived dietary patterns as well as the dietary patterns itself (62). It has been shown in studies using principal components analyses that this could lead to attenuated disease odds (62, 64).
The outcome measures that were included in our review ranged from cognitive performance in cross-sectional studies to cognitive decline and risk of AD or dementia in longitudinal studies. There are many differences in the way cognitive outcomes were measured and reported. This makes comparison between studies more difficult and limits comparison of studies in a meta-analysis, which would provide a more quantitative understanding of the relation between dietary patterns and cognitive impairment. The length of follow-up time of longitudinal studies ranged from 2 to 15 y. To capture changes in cognitive functioning, several years of follow-up are needed, but how long exactly is sufficient and what the best period to start follow-up are not clear. It is currently suggested to already start at middle age.
Some of the inconsistency in findings may be explained by the general considerations that should be taken into account when interpreting results of observational studies, such as residual confounding, possible overadjustment, and the fact that different covariates were included across the studies. Another important issue with observational studies is that they do not allow causal inference. This can be overcome with well-designed intervention studies. However, the only trial on the effect of the Mediterranean diet and cognitive decline did not measure cognition at baseline but only at follow-up, which limits the possibility to establish a cause-effect relationship.
Another point to take into account when interpreting results could be effect modification by sex as suggested by findings from Chan et al. (15), who found that higher intakes of “vegetables-fruits” and “snacks-drinks-milk” patterns were associated with reduced risk of cognitive impairment in women, but no association was observed in men. In contrast, a Greek study reported an increased risk of cognitive impairment with better adherence to the Mediterranean diet in women, but a reduced risk in men (33). Because eating behavior may differ between men and women it should be taken into account that dietary patterns could have been derived for men and women separately. Unfortunately, none of the included studies examined dietary patterns by sex. These possible sex-specific differences merit further investigation and clarification.
Conclusions and Recommendations
The results suggest that better adherence to a Mediterranean diet is associated with less cognitive decline, dementia, or AD as shown by 4 of 6 cross-sectional studies, 6 of 12 longitudinal studies, 1 trial, and 3 meta-analyses. Other healthy dietary patterns, derived both a priori (e.g., HDI, HEI, and PNNS-GS) and a posteriori (e.g., factor analysis, cluster analysis, and reduced rank regression), were shown to be associated with reduced cognitive decline and/or a reduced risk of dementia as shown by all 7 cross-sectional studies and 5 of 7 longitudinal studies.
Investigating whole-diet approaches instead of individual nutrients is an attractive strategy, because combined effects may yield larger results since effects of individual nutrients may be small. Furthermore, a whole-diet approach is more comparable to dietary intake in daily life. A dietary index specifically aimed at improving cognitive performance would be desirable. To further advance this field of research, more intervention trials of sufficient sample size investigating what type of dietary pattern is favorable with respect to prevention of cognitive decline are recommended. In this respect, findings of the ongoing NU-AGE dietary intervention study, in which the effect of a 1-y healthful diet on cognitive performance is investigated, are to be awaited (65). Furthermore, more observational studies starting in middle-aged adults and with a sufficient duration of at least 10–15 y of follow-up are warranted. Those studies should, if possible, take into account the methodologic issues as pointed out above and should aim for more homogeneity in, for example, cognitive outcomes and composition of dietary patterns to facilitate comparison between studies. In addition, the suggestion of differences in associations between men and women needs further investigation. If effects of certain dietary approaches are proven, it will be a challenging task to change people’s dietary habits, but it is important to take up the challenge now in order to provide (pre-) dementia patients some perspective of treatment or delay of the disease process and to reach clear recommendations in the future for a cost-effective, safe, and sustainable solution. This is of special importance because there are currently no curative treatments for this disease.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AD, Alzheimer disease; DASH, Dietary Approaches to Stop Hypertension; HDI, Healthy Diet Indicator; HEI, Healthy Eating Index; MCI, mild cognitive impairment; MeSH, medical subject heading; MMSE, Mini-Mental State Examination.
References
- 1.Alzheimer's Disease International. Policy brief for heads of government: the global impact of dementia 2013–2050. London: Alzheimer's Disease International; 2013.
- 2.WHO; Alzheimer’s Disease International. Dementia: a public health priority. London: WHO, Alzheimer's Disease International; 2012.
- 3.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol 2002;13:3–9. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs DR, Jr, Steffen LM. Nutrients, foods, and dietary patterns as exposures in research: a framework for food synergy. Am J Clin Nutr 2003;78(3, Suppl):508S–13S. [DOI] [PubMed] [Google Scholar]
- 5.Jacques PF, Tucker KL. Are dietary patterns useful for understanding the role of diet in chronic disease? Am J Clin Nutr 2001;73:1–2. [DOI] [PubMed] [Google Scholar]
- 6.Kant AK. Indexes of overall diet quality: a review. J Am Diet Assoc 1996;96:785–91. [DOI] [PubMed] [Google Scholar]
- 7.Allès B, Samieri C, Feart C, Jutand MA, Laurin D, Barberger-Gateau P. Dietary patterns: a novel approach to examine the link between nutrition and cognitive function in older individuals. Nutr Res Rev 2012;25:207–22. [DOI] [PubMed] [Google Scholar]
- 8.Lourida I, Soni M, Thompson-Coon J, Purandare N, Lang IA, Ukoumunne OC, Llewellyn DJ. Mediterranean diet, cognitive function, and dementia: a systematic review. Epidemiology 2013;24:479–89. [DOI] [PubMed] [Google Scholar]
- 9.Psaltopoulou T, Sergentanis TN, Panagiotakos DB, Sergentanis IN, Kosti R, Scarmeas N. Mediterranean diet, stroke, cognitive impairment, and depression: A meta-analysis. Ann Neurol 2013;74:580–91. [DOI] [PubMed] [Google Scholar]
- 10.Roman B, Carta L, Martinez-Gonzalez MA, Serra-Majem L. Effectiveness of the Mediterranean diet in the elderly. Clin Interv Aging 2008;3:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh B, Parsaik AK, Mielke MM, Erwin PJ, Knopman DS, Petersen RC, Roberts RO. Association of Mediterranean diet with mild cognitive impairment and Alzheimer's disease: a systematic review and meta-analysis. J Alzheimers Dis 2014;39:271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr 2010;92:1189–96. [DOI] [PubMed] [Google Scholar]
- 13.Sofi F, Macchi C, Abbate R, Gensini GF, Casini A. Effectiveness of the Mediterranean diet: can it help delay or prevent Alzheimer's disease? J Alzheimers Dis 2010;20:795–801. [DOI] [PubMed] [Google Scholar]
- 14.Gu Y, Scarmeas N. Dietary patterns in Alzheimer's disease and cognitive aging. Curr Alzheimer Res 2011;8:510–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan R, Chan D, Woo J. A cross sectional study to examine the association between dietary patterns and cognitive impairment in older Chinese people in Hong Kong. J Nutr Health Aging 2013;17:757–65. [DOI] [PubMed] [Google Scholar]
- 16.Samieri C, Sun Q, Townsend MK, Chiuve SE, Okereke OI, Willett WC, Stampfer M, Grodstein F. The association between dietary patterns at midlife and health in aging: an observational study. Ann Intern Med 2013;159:584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tangney CC, Kwasny MJ, Li H, Wilson RS, Evans DA, Morris MC. Adherence to a Mediterranean-type dietary pattern and cognitive decline in a community population. Am J Clin Nutr 2011;93:601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wengreen H, Munger RG, Cutler A, Quach A, Bowles A, Corcoran C, Tschanz JT, Norton MC, Welsh-Bohmer KA. Prospective study of Dietary Approaches to Stop Hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County Study on Memory, Health and Aging. Am J Clin Nutr 2013;98:1263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye X, Scott T, Gao X, Maras JE, Bakun PJ, Tucker KL. Mediterranean diet, Healthy Eating Index 2005, and cognitive function in middle-aged and older Puerto Rican adults. J Acad Nutr Diet 2013;113(2):276–81, e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Estaquio C, Kesse-Guyot E, Deschamps V, Bertrais S, Dauchet L, Galan P, Hercberg S, Castetbon K. Adherence to the French Programme National Nutrition Sante Guideline Score is associated with better nutrient intake and nutritional status. J Am Diet Assoc 2009;109:1031–41. [DOI] [PubMed] [Google Scholar]
- 21.Huijbregts P, Feskens E, Rasanen L, Fidanza F, Nissinen A, Menotti A, Kromhout D. Dietary pattern and 20 year mortality in elderly men in Finland, Italy, and The Netherlands: longitudinal cohort study. BMJ 1997;315:13–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kant AK, Schatzkin A, Graubard BI, Schairer C. A prospective study of diet quality and mortality in women. JAMA 2000;283:2109–15. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy ET, Ohls J, Carlson S, Fleming K. The Healthy Eating Index: design and applications. J Am Diet Assoc 1995;95:1103–8. [DOI] [PubMed] [Google Scholar]
- 24.Sacks FM, Obarzanek E, Windhauser MM, Svetkey LP, Vollmer WM, McCullough M, Karanja N, Lin PH, Steele P, Proschan MA, et al. Rationale and design of the Dietary Approaches to Stop Hypertension trial (DASH): a multicenter controlled-feeding study of dietary patterns to lower blood pressure. Ann Epidemiol 1995;5:108–18. [DOI] [PubMed] [Google Scholar]
- 25.Trichopoulou A, Kouris-Blazos A, Wahlqvist ML, Gnardellis C, Lagiou P, Polychronopoulos E, Vassilakou T, Lipworth L, Trichopoulos D. Diet and overall survival in elderly people. BMJ 1995;311:1457–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu Y, Nieves JW, Stern Y, Luchsinger JA, Scarmeas N. Food combination and Alzheimer disease risk: a protective diet. Arch Neurol 2010;67:699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reedy J, Wirfalt E, Flood A, Mitrou PN, Krebs-Smith SM, Kipnis V, Midthune D, Leitzmann M, Hollenbeck A, Schatzkin A, et al. Comparing 3 dietary pattern methods—cluster analysis, factor analysis, and index analysis—with colorectal cancer risk: the NIH-AARP Diet and Health Study. Am J Epidemiol 2010;171:479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffmann K, Schulze MB, Schienkiewitz A, Nothlings U, Boeing H. Application of a new statistical method to derive dietary patterns in nutritional epidemiology. Am J Epidemiol 2004;159:935–44. [DOI] [PubMed] [Google Scholar]
- 29.Newby PK, Tucker KL. Empirically derived eating patterns using factor or cluster analysis: a review. Nutr Rev 2004;62:177–203. [DOI] [PubMed] [Google Scholar]
- 30.Alzheimer's Disease International. Nutrition and dementia: a review of available research. London: Alzheimer's Disease International; 2014. [Google Scholar]
- 31.Gardener S, Gu Y, Rainey-Smith SR, Keogh JB, Clifton PM, Mathieson SL, Taddei K, Mondal A, Ward VK, Scarmeas N, et al. Adherence to a Mediterranean diet and Alzheimer's disease risk in an Australian population. Transl Psychiatry 2012;2:e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scarmeas N, Stern Y, Mayeux R, Luchsinger JA. Mediterranean diet, Alzheimer disease, and vascular mediation. Arch Neurol 2006;63:1709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katsiardanis K, Diamantaras AA, Dessypris N, Michelakos T, Anastasiou A, Katsiardani KP, Kanavidis P, Papadopoulos FC, Stefanadis C, Panagiotakos DB, et al. Cognitive impairment and dietary habits among elders: the Velestino Study. J Med Food 2013;16:343–50. [DOI] [PubMed] [Google Scholar]
- 34.Féart C, Samieri C, Rondeau V, Amieva H, Portet F, Dartigues JF, Scarmeas N, Barberger-Gateau P. Adherence to a mediterranean diet, cognitive decline, and risk of dementia. JAMA 2009;302:638–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samieri C, Okereke OI, Devore EE. Grodstein F. Long-term adherence to the Mediterranean diet is associated with overall cognitive status, but not cognitive decline, in women. J Nutr 2013;143:493–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol 2009;66:216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsivgoulis G, Judd S, Letter AJ, Alexandrov AV, Howard G, Nahab F, Unverzagt FW, Moy C, Howard VJ, Kissela B, et al. Adherence to a Mediterranean diet and risk of incident cognitive impairment. Neurology 2013;80:1684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts RO, Geda YE, Cerhan JR, Knopman DS, Cha RH, Christianson TJ, Pankratz VS, Ivnik RJ, Boeve BF, O'Connor HM, et al. Vegetables, unsaturated fats, moderate alcohol intake, and mild cognitive impairment. Dement Geriatr Cogn Disord 2010;29:413–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samieri C, Grodstein F, Rosner BA, Kang JH, Cook NR, Manson JE, Buring JE, Willett WC, Okereke OI. Mediterranean diet and cognitive function in older age. Epidemiology 2013;24:490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vercambre MN, Grodstein F, Berr C, Kang JH. Mediterranean diet and cognitive decline in women with cardiovascular disease or risk factors. J Acad Nutr Diet 2012;112:816–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kesse-Guyot E, Andreeva VA, Lassale C, Ferry M, Jeandel C, Hercberg S, Galan P, SU.VI.MAX 2 Research Group. Mediterranean diet and cognitive function: a French study. Am J Clin Nutr 2013;97:369–76. [DOI] [PubMed] [Google Scholar]
- 42.Cherbuin N, Anstey KJ. The Mediterranean diet is not related to cognitive change in a large prospective investigation: the PATH Through Life Study. Am J Geriatr Psychiatry 2012;20:635–9. [DOI] [PubMed] [Google Scholar]
- 43.Psaltopoulou T, Kyrozis A, Stathopoulos P, Trichopoulos D, Vassilopoulos D, Trichopoulou A. Diet, physical activity and cognitive impairment among elders: the EPIC-Greece cohort (European Prospective Investigation into Cancer and Nutrition). Public Health Nutr 2008;11:1054–62. [DOI] [PubMed] [Google Scholar]
- 44.Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer's disease. Ann Neurol 2006;59:912–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gu Y, Luchsinger JA, Stern Y, Scarmeas N. Mediterranean diet, inflammatory and metabolic biomarkers, and risk of Alzheimer's disease. J Alzheimers Dis 2010;22:483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, Tang MX, Stern Y. Physical activity, diet, and risk of Alzheimer disease. JAMA 2009;302:627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martínez-Lapiscina EH, Clavero P, Toledo E, Estruch R, Salas-Salvado J, San Julian B, Sanchez-Tainta A, Ros E, Valls-Pedret C, Martinez-Gonzalez MA. Mediterranean diet improves cognition: the PREDIMED-NAVARRA randomised trial. J Neurol Neurosurg Psychiatry 2013;84:1318–25. [DOI] [PubMed] [Google Scholar]
- 48.Martínez-Lapiscina EH, Clavero P, Toledo E, San Julian B, Sanchez-Tainta A, Corella D, Lamuela-Raventos RM, Martinez JA, Martinez-Gonzalez MA. Virgin olive oil supplementation and long-term cognition: the PREDIMED-NAVARRA randomized, trial. J Nutr Health Aging 2013;17:544–52. [DOI] [PubMed] [Google Scholar]
- 49.Corrêa Leite ML, Nicolosi A, Cristina S, Hauser WA, Nappi G. Nutrition and cognitive deficit in the elderly: a population study. Eur J Clin Nutr 2001;55:1053–8. [DOI] [PubMed] [Google Scholar]
- 50.Huijbregts PP, Feskens EJ, Rasanen L, Fidanza F, Alberti-Fidanza A, Nissinen A, Giampaoli S, Kromhout D. Dietary patterns and cognitive function in elderly men in Finland, Italy and The Netherlands. Eur J Clin Nutr 1998;52:826–31. [DOI] [PubMed] [Google Scholar]
- 51.Samieri C, Jutand MA, Feart C, Capuron L, Letenneur L, Barberger-Gateau P. Dietary patterns derived by hybrid clustering method in older people: association with cognition, mood, and self-rated health. J Am Diet Assoc 2008;108:1461–71. [DOI] [PubMed] [Google Scholar]
- 52.Shatenstein B, Ferland G, Belleville S, Gray-Donald K, Kergoat MJ, Morais J, Gaudreau P, Payette H, Greenwood C. Diet quality and cognition among older adults from the NuAge study. Exp Gerontol 2012;47:353–60. [DOI] [PubMed] [Google Scholar]
- 53.Wengreen HJ, Neilson C, Munger R, Corcoran C. Diet quality is associated with better cognitive test performance among aging men and women. J Nutr 2009;139:1944–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kesse-Guyot E, Amieva H, Castetbon K, Henegar A, Ferry M, Jeandel C, Hercberg S, Galan P, Group SVMR. Adherence to nutritional recommendations and subsequent cognitive performance: findings from the prospective Supplementation with Antioxidant Vitamins and Minerals 2 (SU.VI.MAX 2) study. Am J Clin Nutr 2011;93:200–10. [DOI] [PubMed] [Google Scholar]
- 55.Kesse-Guyot E, Andreeva VA, Jeandel C, Ferry M, Hercberg S, Galan P. A healthy dietary pattern at midlife is associated with subsequent cognitive performance. J Nutr 2012;142:909–15. [DOI] [PubMed] [Google Scholar]
- 56.Ozawa M, Ninomiya T, Ohara T, Doi Y, Uchida K, Shirota T, Yonemoto K, Kitazono T, Kiyohara Y. Dietary patterns and risk of dementia in an elderly Japanese population: the Hisayama Study. Am J Clin Nutr 2013;97:1076–82. [DOI] [PubMed] [Google Scholar]
- 57.Smith PJ, Blumenthal JA, Babyak MA, Craighead L, Welsh-Bohmer KA, Browndyke JN, Strauman TA, Sherwood A. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension 2010;55:1331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schulze MB, Hoffmann K. Methodological approaches to study dietary patterns in relation to risk of coronary heart disease and stroke. Br J Nutr 2006;95:860–9. [DOI] [PubMed] [Google Scholar]
- 59.Waijers PM, Feskens EJ, Ocke MC. A critical review of predefined diet quality scores. Br J Nutr 2007;97:219–31. [DOI] [PubMed] [Google Scholar]
- 60.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997;336:1117–24. [DOI] [PubMed] [Google Scholar]
- 61.Tucker KL. Dietary patterns, approaches, and multicultural perspective. Appl Physiol Nutr Metabol 2010;35(2):211–8. [DOI] [PubMed]
- 62.Fransen HP, May AM, Stricker MD, Boer JM, Hennig C, Rosseel Y, Ocke MC, Peeters PH, Beulens JW. A posteriori dietary patterns: how many patterns to retain? J Nutr 2014;144:1274–82. [DOI] [PubMed] [Google Scholar]
- 63.Solfrizzi V, Panza F. Mediterranean diet and cognitive decline—a lesson from the whole-diet approach: what challenges lie ahead? J Alzheimers Dis 2014;39:283–6. [DOI] [PubMed] [Google Scholar]
- 64.McCann SE, Marshall JR, Brasure JR, Graham S, Freudenheim JL. Analysis of patterns of food intake in nutritional epidemiology: food classification in principal components analysis and the subsequent impact on estimates for endometrial cancer. Public Health Nutr 2001;4:989–97. [DOI] [PubMed] [Google Scholar]
- 65.Berendsen A, Santoro A, Pini E, Cevenini E, Ostan R, Pietruszka B, Rolf K, Cano N, Caille A, Lyon-Belgy N, et al. Reprint of: A parallel randomized trial on the effect of a healthful diet on inflammageing and its consequences in European elderly people: design of the NU-AGE dietary intervention study. Mech Ageing Dev 2014;136–137:14–21. [DOI] [PubMed] [Google Scholar]