Abstract
The purpose of this meta-analysis was to establish the time for achievement of maximal blood pressure (BP) efficacy of a sodium reduction (SR) intervention and the relation between the amount of SR and the BP response in individuals with hypertension and normal BP. Relevant studies were retrieved from a pool of 167 randomized controlled trials (RCTs) published in the period 1973–2010 and integrated in meta-analyses. Fifteen relevant RCTs were included in the maximal efficacy analysis. After initiation of sodium reduction (range: 55–118 mmol/d), there were no significant differences in systolic blood pressure (SBP) or diastolic blood pressure (DBP) between measurements at weeks 1 and 2 (∆SBP: −0.18 mmHg/∆DBP: 0.12 mmHg), weeks 1 and 4 (∆SBP: −0.50 mmHg/∆DBP: 0.35 mmHg), weeks 2 and 4 (∆SBP: −0.20 mmHg/∆DBP: −0.10 mmHg), weeks 2 and 6 (∆SBP: −0.50 mmHg/∆DBP: −0.42 mmHg), and weeks 4 and 6 (∆SBP: 0.39 mmHg/∆DBP: −0.22 mmHg). Eight relevant RCTs were included in the dose-response analysis, which showed that within the established usual range of sodium intake [<248 mmol/d (5700 mg/d)], there was no relation between the amount of SR (range: 136–188 mmol) and BP outcome in normotensive populations [∆SBP: 0.99 mm Hg (95% CI: 2.12, 4.10), P = 0.53; ∆DBP: −0.49 mm Hg (95% CI: −4.0, 3.03), P = 0.79]. In contrast, prehypertensive and hypertensive populations showed a significant dose-response relation (range of sodium reduction: 77–140 mmol/d) [∆SBP: 6.87 mmHg (95% CI: 5.61, 8.12, P < 0.00001); ∆DBP: 3.61 mmHg (95% CI: 2.83, 4.39, P < 0.00001)]. Consequently, the importance of kinetic and dynamic properties of sodium reduction, as well as baseline BP, should probably be considered when establishing a policy of sodium reduction.
Keywords: dietary guidelines, meta-analysis, sodium, blood pressure, pharmacokinetics, pharmacodynamics, salt
Introduction
The need to define more precisely the efficacy of sodium reduction (SR)9 for the general population has been brought into focus by conflicting Institute of Medicine (IOM) reports (1–3). One has defined a recommended upper sodium intake amount of 2300 mg/d (100 mmol/d) (1). In contrast, the most recent IOM report concluded that “Science was insufficient and inadequate to establish whether reducing sodium intake below 2300 mg/d either decreases or increases cardiovascular disease risk in the general population” (2). Furthermore, the 2300 mg/d recommendation is in conflict with the IOM’s own rules on how to define adequate intake for a nutrient, which is “the approximate intake found in apparently healthy populations” (3). This is much higher than 2300 mg/d (4). The justification for the 2300 mg/d recommendation is based on the controversial association between sodium intake and blood pressure (BP) (5–8) and on modeling studies (9, 10). These use selected cross-sectional sodium intake data and BP data to create a sodium-dose–BP-response relation and successively apply this dose-response relation to BP mortality data. Disregarding side effects, these analyses predict millions of saved lives (9, 10). However, these artificial data are in contrast to the outcome of real data from population studies, which indicate that both low and excessive sodium intake are associated with increased mortality, thus demonstrating a J-shape relation between sodium intake and outcome (11). The disagreement between the modeling studies and the observational studies could be because of incorrect premises for the modeling studies caused by a lack of knowledge of kinetic and dynamic consequences of a changed sodium intake. The purpose of the present supplementary analysis of a previous meta-analysis of randomized trials (8) was to clarify the significance of duration of SR and amount of SR by estimating 1) the requisite time for establishing maximal BP efficacy in response to SR, and 2) the dose-response relation between the reduction in sodium intake and the BP effect in normotensive and hypertensive subjects.
Methods
Relevant studies were retrieved from a pool of 167 randomized controlled trials (RCTs) published in the period 1973–2010 and identified in a Cochrane review in 2011 (8). Search methods for identification of these 167 studies are previously described in detail (8). The general eligibility criteria of the Cochrane review are described previously (8).
Inclusion criteria for estimation of maximal efficacy
Criteria for estimation of maximal efficacy were studies with at least 2 measurements of BP effects between week 1 and week 4 after initiation of SR intervention.
Inclusion criteria for estimation of dose-response relation
Criteria for estimation of dose-response relations were studies randomly allocating participants to at least 3 different amounts of SR.
Data extraction
Most data were retrieved as explained previously (8). Two authors independently retrieved additional data (NG and THG). The following data were recorded: 1) mean baseline systolic blood pressure (SBP) and diastolic blood pressure (DBP) (millimeters of mercury); 2) mean effect measurements (change in SBP and DBP in millimeters of mercury) obtained weekly until termination of study; 3) effect measurements (change in SBP and DBP in millimeters of mercury) for different doses of SR; 4) Cochrane risk of bias sources (12).
Because not all studies provided SE values for all intermediate measurements, we derived the SE values from SDs, CIs or figure graphs when necessary (12).
Risk of bias
The Cochrane Collaboration’s tool for assessing risk of bias (12) was used to assess the risk of bias in the individual randomized trials. Funnel plots were used to estimate publication bias across studies.
Data synthesis
Review Manager (12, 13) was used to synthesize data and heterogeneity was estimated by I2 (I2 is the proportion of the total variability explained by heterogeneity and is expressed as a percentage) (12).
Time of maximal BP efficacy.
Studies were integrated irrespective of baseline BP. The effect of SR on SBP and DBP (difference between BP at low sodium intake and higher sodium intake) was calculated for each week. In the comparison of week 1 vs. week 2, only those studies that had measurements at both week 1 and week 2 were included. The 2 summary estimates for weeks 1 and 2 were then compared. The same procedure was used for all week comparisons. For each week investigated, data were categorized as generic inverse variance data and the mean difference for the week compared with baseline was calculated by means of the inverse variance method. The summary estimates (mean difference in SBP and DBP) for each week were then categorized as continuous data and compared by means of the inverse variance method (12, 13). Normotensive participants (BP <140/90) and hypertensive participants (BP ≥140/90) were combined in the analyses.
Dose-response relation.
The mean usual sodium intake in 197 population samples from 45 countries was 159 mmol/d (3657 mg/d) and varied in the range of 90–248 mmol/d (2070–5700 mg/d) (4). We therefore categorized the sodium doses as follows: 1) low (<90 mmol/d (2070 mg/d)) 2) low usual [90–159 mmol/d (2070–3657 mg/d)], 3) high usual [159–248 mmol/d (3657–5700 mg/d)], and 4) high [>248 mmol/d (5700 mg/d)]. However, if a study had 2 groups within one category, we allowed the one closest to one of the cutoff values to be moved to the neighboring category. BPs at different sodium doses were recorded and depicted as a function of sodium intake. The effects of higher sodium intake categories (low usual sodium, high usual sodium, and high sodium) were compared with the low sodium category. Normotensive and hypertensive studies were integrated separately.
Normotensive participants (BP <140/90) and hypertensive participants (BP ≥140/90) were compared separately in Review Manager (13). Because there is general agreement about the effect of SR on BP in hypertensive participants (5, 6) but not in normotensive participants (5, 6), we classified mixed groups of participants with normal BP and hypertension as hypertensive, even if the mean BP was below 140/90, in order to be able to evaluate a clean normotensive population. The significance level was defined to be 5%.
Supplementary analyses
Sensitivity analyses were intended to investigate the importance of significant risk of bias factors and to explore sources of heterogeneity.
Results
Description of studies
Inclusion and exclusion criteria.
Ten studies included healthy normotensive participants, 10 studies included hypertensive participants, and 3 studies included both. Eighteen studies specifically mentioned that participants with diseases were excluded. One study included regular drinkers (3 drinks/d) who were otherwise healthy. Five studies did not mention exclusion criteria.
Time to maximal efficacy.
The 15 studies (14–28) of the 167 SR RCTs that were included in the “time to maximal efficacy” analysis are shown in Table 1. Seven studies included hypertensive participants, 7 included normotensive participants, and 1 (28) included both. Three studies included treated hypertensive participants (17, 20, 28). Eight studies used crossover design and 7 used a parallel design. Analysis of the individual studies showed that there were multiple BP measurements at weeks 1, 2, 4, and 6. Because of the few studies with measurements at weeks 3, 5, 7, 8, and beyond, separate analyses of these measurements compared with weeks 1, 2, 4, and 6 were not performed. In 2 studies that measured BP at weeks 1, 2, and 3 (26, 27), the week 3 measurement was included as a week 4 measurement. In one study that measured BP at weeks 2 and 5 (22), the week 5 measurement was included as a week 4 measurement. In another study that measured BP at weeks 2, 4, 8, 12, and 14 (28), the week 8 measurement was included as a week 6 measurement.
TABLE 1.
Characteristics of studies included in maximal efficacy analysis1
| Reference | Study design | SR method | Washout period | Order effect | Participants, n | Data source from reference | Exclusion of sick participants | Mean age, y | BMI, kg/m2 or weight, kg | BP | Baseline SBP, mm Hg | Baseline DBP, mm Hg | Week of 24-hr urine sodium measurement |
| MacGregor et al. (14) | CO | T | No | No | 19 | Table 1 | Yes | 49 | 80 kg | HT | 156 | 98 | 2, 4 |
| Chalmers et al. (15) | P | T | — | — | 100 | Figure 2 | Yes | 53 | N/A | HT | 155 | 96 | 2, 4, 6 |
| ANMMRC 89 (16) | P | T | — | — | 103 | Figure 2 | Yes | 58 | N/A | HT | 154 | 95 | 2, 4, 6 |
| Parker et al. (17) | P | T | — | — | 31 | Figure 4 | Yes | 52 | 29 kg/m2 | THT | 138 | 85 | 1, 2, 3, 4 |
| Mascioli et al. (18) | CO | T | Yes | No | 48 | Figures 3 and 4 | Yes | 52 | 27.6 kg/m2 | NT | 131 | 84 | 4 |
| Cobiac et al. (19) | P | T | — | — | 52 | Figure 2 | Yes | 66 | 25 kg/m2 | NT | 132 | 77 | 2, 4 |
| Sciarrone et al. (20) | P | T | — | — | 91 | Figure 3 | Yes | 54 | 25 kg/m2 | THT | 136 | 83 | 5 times3 |
| Nestel et al. (21) | P | T | — | — | 36 | Tables 3 and 4 | Yes | 66 | 25 kg/m2 | NT | 125 | 73 | 2, 4, 6 |
| Fotherby et al. (22) | CO | T | No | N/I | 17 | Tables 3 and 4 | N/I | 73 | 26 kg/m2 | HT | 184 | 100 | 5 |
| Ruppert et al. (23) | CO | T | No | N/I | 25 | Table 3 | Yes | 46 | 70 kg | NT | 112 | 73 | 1, 4 |
| Schorr et al. (24) | CO | SW | Yes | N/I | 16 | Figure 1 | Yes | 64 | 26.1 kg/m2 | NT | 140 | 84 | 1, 4 |
| Gates et al. (25) | CO | T | No | No | 12 | Figure 4 | Yes | 64 | 25.1 kg/m2 | HT | 148 | 84 | 1, 2, 3, 4 |
| Palacios et al. (26) | CO | D | Yes | N/I | 40 | Table 3 | Yes | 13 | 22 kg/m2 | NT | 113 | 57 | 8 times3 |
| Forrester et al. (27)2 | CO | D + T | Yes | N/I | 114 | Figure 4 | Yes | 44 | 25.8 kg/m2 | NT | 119 | 75 | 3 |
| Nowson et al. (28) | P | D | — | — | 111 | Figure 2 | Yes | 59 | 29.6 kg/m2 | THT + NT | 127 | 81 | 4, 8 |
Abbreviations: ANHMRC, Australian National Health and Medical Research Council BP, blood pressure; CO, crossover; D, diet; DBP, diastolic blood pressure; HT, hypertension; N/A, not applicable; N/I, no information; NT, normotension; P, parallel; SBP, systolic blood pressure; SR, sodium reduction; SW, salt water; T, low-sodium diet followed by random assignment to salt or placebo tablets; THT, treated hypertension.
Included only blacks.
The precise time of the measurement was not specified.
Dose-response analysis.
The 8 studies (9 dose-response relations) (7, 29–35) of the 167 studies included in the dose-response relation analysis are shown in Table 2. Four studies included hypertensive participants who were all untreated. All studies but one used a crossover design.
TABLE 2.
Characteristics of studies included in dose-response analysis1
| Reference | Study design | SR method | Washout period | Order effect | Participants, n | Data source from reference | Exclusion of sick participants | Mean age, y | BP | Baseline SBP, mm Hg | Baseline DBP, mm Hg | Week of 24-h urine sodium measurement |
| Sachs et al. (7) | CO | D | No | N/I | 192 | Figure 1 | Yes | 49 | HT | 135 | 86 | 4 |
| Fuchs et al. (29) | CO | T | Yes | N/I | 17 | Table1 | Yes | 21 | NT | 117 | 68 | 1 |
| MacGregor et al. (30) | CO | T | No | No | 20 | Table | Yes | 57 | HT | 163 | 100 | |
| Bruun et al. (31) | CO | T | Yes | N/I | 10 | Table1 | N/I | 46 | NT | 110 | 65 | 1 |
| Bruun et al. (31) | CO | T | Yes | N/I | 12 | Table 2 | N/I | 47 | HT | 148 | 98 | 1 |
| Gow et al. (32) | CO | D | Yes | N/I | 9 | Page 636 | N/I | — | NT | 120 | 68 | 1 |
| Heer et al. (33) | P | D | No | N/I | 32 | Page F592 | Yes | 25 | NT | 109 | 67 | 1 |
| Burnier et al. (34) | CO | D | No | N/I | 15 | Table 2 | Yes | 23 | NT | 126 | 75 | 1 |
| Johnson et al. (35) | CO | T | Yes | N/I | 40 | Tables1 and 2 | N/I | 69 | HT | 159 | 84 | 2 |
BP, blood pressure; CO, crossover; D, diet; DBP, diastolic blood pressure; HT, hypertension; N/I, no information; NT, normotension; P, parallel; SBP, systolic blood pressure; SR, sodium reduction; T, low-sodium diet followed by random assignment to salt or placebo tablets.
Descriptive data are shown in Tables 1 and 2 and the risk of bias sources are shown in Supplemental Table 1. None of the studies described the random assignment procedure. A main source of bias was lack of observer blinding (detection bias) in 5 of the maximal efficacy studies and 5 of the dose-response studies. The differences in 24-h urinary sodium excretions between the usual and low-sodium diets during the investigation period of the individual studies included in the maximal efficacy analysis are shown in Supplemental Table 2.
Outcomes
All single-week analyses included in the final comparison of different weeks are shown in Supplemental Figures 1–8 and the dose-response analyses are shown in Supplemental Figures 9–12. Sample size, SE, and weight of the individual studies in each of the analyses appear in Supplemental Figures 1–12. In all but 5 of the analyses, there was no significance for heterogeneity and we therefore used the fixed effect model. We also calculated the effect sizes with the use of the random effect model for all analyses in which I2 was bigger than 0. The results of the random effect models are also shown in Supplemental Figures 1–8, where applicable.
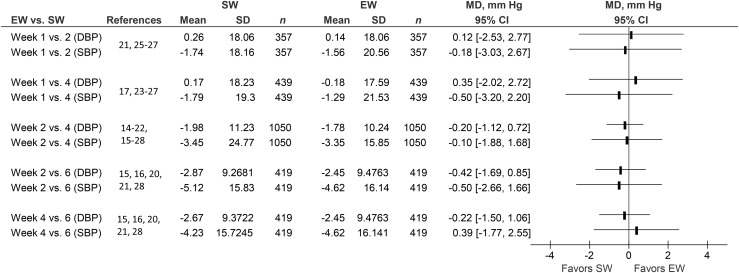
Comparisons of the effects of SR on SBP and DBP measured at week 1 vs. week 2 (number of studies = 4) and week 4 (number of studies = 6), week 2 vs. week 4 (number of studies = 13) and week 6 (number of studies = 5), and week 4 vs. week 6 (number of studies = 5) are shown in Figure 1. Weeks 1 and 6 were not compared, because there was no paired measurement for these time points.
FIGURE 1.
Paired comparisons of BP responses obtained at successive weeks (1, 2, 4, and 6) after initiation of sodium reduction in otherwise healthy normotensive and hypertensive individuals. (Individual study data are shown in Supplemental Figures 1–8). BP, blood pressure; DBP, diastolic blood pressure; EW, early week; MD, mean difference; SBP, systolic blood pressure; SW, successive week.
The numbers of participants with paired measurements are shown in Supplemental Table 3. All but 3 studies (18, 22, 27) measured 24-h urinary sodium at each BP measurement and these measurements were stable (Table 1, Supplemental Table 2). None of the comparisons were statistically significant, with P values varying between 0.52 and 0.92.
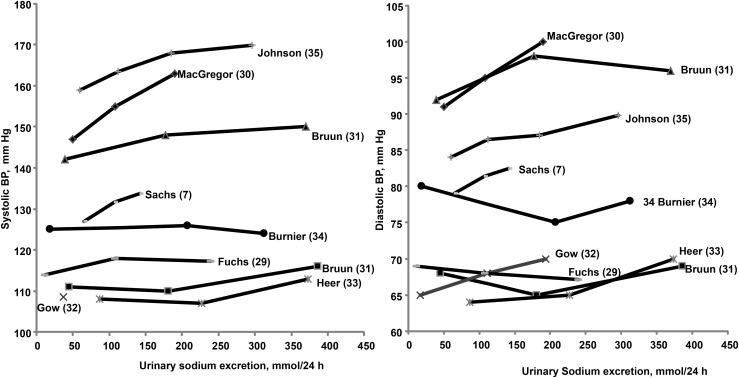
The individual study data used in the dose-response analysis are shown in Supplemental Table 4. Nine dose-response relations extracted from 8 studies are shown in Figure 2. Baseline BP varied significantly between the studies, with SBP ranging from 110 mm Hg to 160 mm Hg and DBP ranging from 65 mm Hg to 95 mm Hg. Figure 2 indicates that in studies in which participants’ baseline BP was below 130/80 mm Hg, there was no dose-response relation until the intervention exceeded the upper limit for usual sodium intake (248 mmol/d). In the 3 studies (31, 33, 34) in which the upper limit of the intervention was greater than the upper limit of usual human sodium intake, an increase in BP was observed. In 4 studies (7, 30, 31, 35) with a BP above 130/80 mm Hg (4 upper studies in Figure 2), a dose-response relation is suggested across the entire range of sodium intake. Three of these studies (30, 31, 35) include only hypertensive participants, whereas the fourth included a mixture of borderline hypertensive and hypertensive participants with a mean baseline BP of 135/86 mm Hg (7).
FIGURE 2.
Individual study diastolic and systolic BP response to increasing changes in sodium urinary excretion (as a measure of sodium intake) in otherwise healthy normotensive and hypertensive individuals. (Individual study data are shown in Supplemental Figures 9–12 and Supplemental Table 4.) The study by Gow et al. (32) presented data only on diastolic BP. BP, blood pressure.
Normal BP.
There was only one study that measured SBP at the low usual sodium dose (90–159 mmol/d) (Figure 2) (29). Therefore, no statistical calculations were performed for the low usual dose.
Compared with the low sodium dose, there was no significant increase in SBP at the high usual sodium dose [159–248 mmol/d (3657–5700 mg/d)] [∆SBP: 0.99 mmhg; (95% CI: 2.12, 4.10, P = 0.53)], or at the high sodium dose [>248 mmol/d (5700 mg/d)] [∆SBP: 3.06 mmHg (95% CI: −1.47, 7.60), P = 0.19] (29, 31, 33, 34) (Supplemental Figure 9).
Compared with the low sodium dose, there was no significant increase in DBP when sodium intake was increased at the low usual sodium intake [∆DBP −0.49 mmHg (95% CI: −4.00, 3.03), P = 0.79], or the high usual sodium dose [∆DBP −1.67 mmHg (95% CI: −4.17, 0.82), P = 0.19]. There was no change at the high sodium dose [∆DBP: 0.38 mmHg (95% CI: −4.03, 4.79), P = 0.87] (29, 31–34) (Supplemental Figure 10).
Hypertension.
As mentioned, we included 1 study of borderline hypertensive and hypertensive participants (7) in the hypertensive group. Compared with the low sodium dose, there was a significant increase in SBP at the low usual sodium dose [∆SBP: 4.65 mmHg (95% CI: 3.39, 5.91), P < 0.00001], at the high usual sodium dose [∆SBP: 6.87 mmHg (95% CI: 5.61, 8.12), P < 0.00001], and at the high dose [∆SBP: 10.03 mmHg (95% CI: 4.12, 15.95), P = 0.0009] (7, 30, 31, 35) (Supplemental Figure 11).
Compared with the low sodium dose, there was a significant increase in DBP at the low usual sodium dose [∆DBP: 2.44 mmHg (95% CI: 1.57, 3.31), P = 0.00001], at the high usual sodium dose [∆DBP: 3.61 mmHg (95% CI: 2.83, 4.39), P = 0.00001], and at the high dose [∆DBP: 5.55 mmHg (95% CI: 1.53, 9.56), P = 0.007] (7, 30-31, 35) (Supplemental Figure 12).
Supplementary analyses
Funnel plots (Supplemental Figures 13 and 14) for SBP and DBP were not obviously asymmetric.
Because lack of blinding was the most obvious source of bias, we performed separate maximal efficacy analyses of 10 studies with blinded blood pressure measurements. Eight of the studies were double blind. These analyses (Supplemental Figure 15) were in accordance with the main analysis (Figure 1). Exclusion of the week 1 and week 3 results (26, 27) from the week 1 vs. wk 4 analysis (Supplemental Figure 4) did not change the result.
Exploration of heterogeneity
Five analyses showed significant heterogeneity [Supplemental Figure 5 (weeks 2 and 4), Supplemental Figure 6 (week 2), and Supplemental Figure 7 (weeks 2 and 4)]. In all 5 analyses, heterogeneity disappeared when separate analyses of participants with untreated hypertension and participants with a baseline BP below 140/90 (normotensive and treated hypertensive participants) were performed.
Discussion
Our review of 167 RCTs of the effect of SR on BP showed that the vast majority only measured the effect on BP 1 time during the observation period, and with only 1 amount or 1 target level of SR. However, the longitudinal and randomized nature of the limited amount of data from the 23 studies addressing the objectives of this study is more reliable than the cross-sectional meta-regression data obtained in studies biased in favor of study populations with high BP (9, 10). The present study provides evidence to indicate that the maximal efficacy of SR on BP may be evident and stable after 1 wk. The statistically strongest comparison between SBP and DBP involving 2192 BP measurements in 853 participants measured at weeks 2 and 4 showed no difference between the 2 values (Figure 1, Supplemental Table 3). The CI for this comparison is reasonably narrow, but many of the other CIs were broader; for instance, the one comparing week 1 with week 4 (Figure 1). This indicates that the existing data are not sufficient to firmly establish that maximal efficacy is achieved at 1 wk. However, the identical patterns found for all other pairwise comparisons during the first 6 wk, including comparisons of weeks 1 and 2 and weeks 1 and 4 (Figure 1, Supplemental Figures 1–8), indicate that at least there are no scientific data to support the assumption that more than 1 wk is required to achieve maximal efficacy. In addition, the results could not be explained by variation in dose or adherence, because the 24-h excretions were relatively stable at all measurements (Supplemental Table 2). Furthermore, there were no borderline significant effects to indicate that a true difference may have been overlooked (P values between 0.52 and 0.92) and separate analyses of blinded studies (Supplemental Figure 15) were in accordance with the main analysis. Finally 18 of 23 studies specifically excluded sick individuals (apart from hypertension), and in the remaining 5 there is no indication that such individuals were included. It is therefore reasonable to assume that the included studies were relatively homogeneous. The paucity of studies does complicate an interpretation of the funnel plots (Supplemental Figures 13 and 14), but at a minimum they did not reveal obvious bias. If a publication bias does exist, it should favor a significant result, but because our analyses were nonsignificant, the potential elimination of publication bias would strengthen, rather than weaken, our findings. Therefore, the finding that SR has its full effect after 1 wk is a reasonable conclusion with no scientific evidence supporting the notion that the achievement of maximal efficacy requires more than 1 wk.
To consider the dose-response relation, we categorized sodium intake in relation to usual sodium intake (4) for which there is general agreement between critics (4) and advocates (10) of SR. Our analysis indicates that there is no relation between sodium dose and BP in subjects whose BP is <130/80. However, there was a significant dose-response relation in 4 studies that included hypertensive individuals or both borderline and hypertensive individuals with a mean baseline BP >130/80, consistent with the assumption that the effect of SR on BP is proportional to baseline BP. This was further emphasized by our analysis to explore the reason for heterogeneity in 5 of the intermediate analyses [Supplemental Figure 5 (weeks 2 and 4), Supplemental Figure 6 (week 2), and Supplemental Figure 7 (weeks 2 and 4)], which showed that heterogeneity disappeared when studies of hypertensive and normotensive participants were analyzed separately. In general, baseline BP was high in the included studies. In the maximal efficacy analysis, 12 of 15 studies included participants with an SBP that was higher than the mean of the population (119 mm Hg) (36), and 14 of 15 included participants with a DBP that was higher than the mean of the population (71 mm Hg) (36). The fact that the majority of RCTs were carried out in subjects with a BP higher than those of the general population likely explains why effects of SR on SBP up to 5.12 mm Hg (Supplemental Figure 8) were identified in the present analyses (Supplemental Figures 1–8), but should not be taken as representative of the BP response to SR that would be expected in subjects with a BP corresponding to the mean of the general population, i.e., normotensive subjects. The reason for the larger effect size in hypertensive patients is probably general sensitivity to BP-lowering interventions, rather than specific sodium sensitivity.
Recently, it was found that total body sodium fluctuates in an infradian rhythm that is independent of sodium intake and BP (37). This complicates the understanding of the assumed association between body sodium content and BP and is in contrast to the frequent association between the physiologic steady state of a drug and the time of maximal efficacy of the drug. This, however, does not prevent the possibility of defining the time of maximal efficacy or a dose-response relation. A potential limitation of our analysis is that only 8 of 16 crossover studies used washout periods between treatment interventions. Because our analysis documented that maximal efficacy is reached in 1 wk and all studies lasted for at least 1 wk, it is unlikely that this limitation influenced our results. This is confirmed by the fact that 3 of the studies, which did not use washout periods, found no order effect.
Inaccurate assumptions concerning the significance of the duration of an intervention, i.e., the time of maximal efficacy and the dose-response relation, can lead to biases in meta-analyses (6, 38) and modeling projections of the effect of SR on BP to effects on mortality (9, 10). Advocates of SR have used the putative difference between the 2 Cochrane Reviews (8, 38) as justification to accept the findings of the meta-analysis that excluded RCTs with an SR duration <4 wk (38). A detailed analysis of the mean baseline BP of included RCTs indicates that, at entry, the study populations’ BP was ∼130/80 (38), a baseline BP significantly higher than the mean BP in the all-inclusive, larger meta-analysis (8) and substantially higher than the mean BP of the general population (36). Consequently, baseline BP, rather than duration of RCTs, seems to be the determinant of the apparent difference in effect size between these 2 frequently quoted meta-analyses.
In addition He and MacGregor showed a significant sodium-dose–BP-response relation, which was based on a cross-sectional meta-regression analysis (6). However, 9 of 12 studies in the “normotensive” analysis included borderline hypertensive participants, the dose-response data were not linearly distributed, and the statistical significance of the dose-response relation depended on 1 study of overweight, borderline hypertensive participants (7). In contrast, a later update of the large meta-analysis, which included 167 studies (8), confirmed the findings from the first version (5). In addition, both large meta-analyses (5, 8) showed significant adverse effects from SR on hormones and lipids. In spite of this, the 2 modeling studies (9, 10) did not include adverse effects in the statistical model. The first (9) is based on a dose-response analysis of the borderline hypertensive meta-analysis (6) and the borderline hypertensive Dietary Approaches to Stop Hypertension study (7). In the second analysis (10), the authors borrowed their literature search from the Cochrane Review of 167 studies (8) and performed a cross-sectional sodium dose–blood pressure response meta-regression analysis on 107 of the 167 studies without revealing precisely which studies or how the data were extracted. Furthermore, individual study data were not shown. The authors found a dose-response relation of 3.7 mm Hg/100 mmol in 50-y-old participants (10) based on selected studies drawn from the large Cochrane Review (8). The regular meta-analysis had shown this effect to be ∼1.2 mm Hg in all studies of normotensive participants (8) and ∼1.4 mm Hg in studies of normotensive participants with a mean age between 40 and 60 y (39). On the basis of the modeling analysis of indirectly associated sodium intake data and mortality data, which were not documented, the authors projected millions of saved lives in stark contrast to an observational study in the same journal issue that showed that sodium intake simultaneously was inversely associated with mortality (40) and directly associated with BP (41) by means of genuine scientific data directly linking sodium intake with mortality.
Other variables influenced by SR, such as renin, aldosterone, noradrenalin, adrenalin, cholesterol, and TGs, have also been shown to change within 1 wk after the introduction of SR (5, 8). Despite the claim that it takes time for BP to adapt to SR (6), it has been maintained that hormones and lipids only change for a short time after SR and then regress to original concentrations (6). Thus, the question is whether the duration of the physiologic effects of SR differs among variables. Concerning renin and aldosterone, there is scientific evidence that these hormones are permanently increased in individuals on a low sodium diet (42, 43). In the case of catecholamines and lipids, there is evidence that these variables increase in short-term studies up to 2 wk (5, 8), but little evidence that these changes are permanent. Conversely, there is also no evidence to support the claim that these variables revert to normal.
The present findings, like those from the 2013 IOM Report (2), several population studies (40, 44–48), and a meta-analysis of population studies (11), raise valid concerns about whether there is any benefit from SR in individuals whose BP is below 130/80, i.e., the normotensive general population. Recently, advocates of SR, in rejecting the findings of the 2013 IOM Report and the 25 reports of health outcomes (11), argued that the body of evidence linking sodium to elevated BP was a “robust” basis for the recommendations of a population-wide policy of SR (49). This postulate is in contrast to the population studies linking SR to increased all-cause mortality (11, 40, 44, 45, 47, 48). The remarkably limited data addressing the kinetic and dynamic properties of SR indicate that the assumption of a robust link between sodium and BP is questionable, in particular for normotensive subjects across the usual range of sodium intake.
In conclusion, the present data may be considered to be insufficient to definitively answer the question on the time of maximal efficacy of SR and the dose-response relation. The acknowledgment of such a conclusion by policymakers instead of the present acceptance of flawed dose-response meta-regression analyses would be appropriate progress. Although available data are limited, our analysis indicates that the effect of SR is evident primarily in persons with borderline BP elevation and hypertension. Specifically, the effect of SR on BP appears to reach maximal efficacy at 1 wk and remain stable over subsequent time intervals. At present, it is reasonable to assert that there is no statistically significant evidence to show that the time of maximal efficacy requires more than 1 wk to be established. In normotensive individuals, the effect of SR is not evident within the range of sodium intake consumed by 95% of the population. An effect of SR in normotensive participants was only evident when sodium intake was reduced from an intake >248 mmol/d (5600 mg/d), an amount consumed by <2% of individuals worldwide (50). On the population level, it is important that the baseline BP distribution of studies to be included in meta-analyses corresponds to the distribution of BP in the population and that the chosen amount of SR is relevant, i.e., within the level of 95% of usual intake. Finally, the fact that SR has been so widely touted without knowledge of these simple and fundamental kinetic and dynamic properties merits thoughtful consideration vis-a-vis the appropriateness of the current population-wide recommendation of sodium restriction. This conclusion echoes that of the editorial (51) accompanying recent reports that presented divergent conclusions with respect to the impact of SR on cardiovascular disease events and mortality (10, 40, 41).
Acknowledgments
We thank Molly Reusser for her technical editing of the manuscript. NG designed the analysis, extracted the data, performed the statistical analyses, and prepared the first draft of the manuscript. TH-G extracted the data, performed the statistical analyses, and revised the manuscript. GJ extracted the data and revised the manuscript. DAM contributed to the manuscript and revised the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: BP, blood pressure; DBP, diastolic blood pressure; IOM, Institute of Medicine; RCT, randomized controlled trial; SBP, systolic blood pressure; SR, sodium reduction.
References
- 1.Institute of Medicine. Dietary reference intakes: water, potassium, sodium, chloride, and sulfate. Washington (DC): National Academies Press, 2004.
- 2.Institute of Medicine. Sodium intake in populations: assessment of evidence. Washington (DC): National Academies Press, 2013. [DOI] [PMC free article] [PubMed]
- 3.Institute of Medicine. Dietary reference intakes: Essential Guide to Nutrient Requirements. Washington (DC): National Academies Press; 2006.
- 4.McCarron DA, Kazaks AG, Geerling JC, Stern JS, Graudal NA. Normal range of human dietary sodium intake: a perspective based on 24-hour urinary sodium excretion worldwide. Am J Hypertens 2013;26:1218–23. [DOI] [PubMed] [Google Scholar]
- 5.Graudal NA, Galløe AM, Garred P. Effects of sodium restriction on blood pressure, renin, aldosterone, catecholamines, cholesterols, and triglyceride: a meta-analysis. JAMA 1998;279:1383–91. [DOI] [PubMed] [Google Scholar]
- 6.He FJ, MacGregor G. Effect of modest salt reduction on blood pressure: a meta-analysis of randomized trials. Implications for public health. J Hum Hypertens 2002;16:761–70. [DOI] [PubMed] [Google Scholar]
- 7.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller, 3rd ER, Simons-Morton DG, et al.; DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. N Engl J Med 2001;344:3–10. [DOI] [PubMed]
- 8.Graudal NA, Hubeck-Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev 2011;11:CD004022. [DOI] [PubMed] [Google Scholar]
- 9.Bibbins-Domingo K, Chertow GM, Coxson PG, Moran A, Lightwood JM, Pletcher MJ, Goldman L. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med 2010;362:590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mozaffarian D, Fahimi S, Singh GM, Micha R, Khatibzadeh S, Engell RE, Lim S, Danaei G, Ezzati M, Powles J. Global sodium consumption and death from cardiovascular causes. N Engl J Med 2014;371:624–34. [DOI] [PubMed] [Google Scholar]
- 11.Graudal N, Jürgens G, Baslund B, Alderman MH. Compared with usual sodium intake, low and excessive sodium diets are associated with increased mortality. A meta-analysis. Am J Hypertens 2014;27:1129–37. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JPT, Green S (editors) [Internet]. Cochrane handbook for systematic reviews of interventions, Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011. [cited 2014 Feb 6]. Available from: http://handbook.cochrane.org.
- 13. Review Manager (RevMan) [Computer program]. Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2011.
- 14.MacGregor GA, Markandu ND, Best FE, Elder DM, Cam JM, Sagnella GA, Squires M. Double-blind randomised crossover trial of moderate sodium restriction in essential hypertension. Lancet 1982;1:351–5. [DOI] [PubMed] [Google Scholar]
- 15.Chalmers J, Morgan T, Doyle A, Dickson B, Hopper J, Mathews J, Matthews G, Moulds R, Myers J, Nowson C, et al. Australian National Health and Medical Research Council dietary salt study in mild hypertension. J Hypertens Suppl 1986;4: Supp:S629–37. [PubMed] [Google Scholar]
- 16.Australian National Health And Medical Research Council Dietary Salt Study Management Committee Fall in blood pressure with modest reduction in dietary salt intake in mild hypertension. Lancet 1989;1:399–402. [PubMed] [Google Scholar]
- 17.Parker M, Puddey IB, Beilin LJ, Vandongen R. Two-way factorial study of alcohol and salt restriction in treated hypertensive men. Hypertension 1990;16:398–406. [DOI] [PubMed] [Google Scholar]
- 18.Mascioli S, Grimm R, Jr, Launer C, Svendsen K, Flack J, Gonzalez N, Elmer P, Neaton J. Sodium chloride raises blood pressure in normotensive subjects. The study of sodium and blood pressure. Hypertension 1991;17: Suppl:I21–6. [DOI] [PubMed] [Google Scholar]
- 19.Cobiac L, Nestel PJ, Wing LMH, Howe PRC. A low sodium diet supplemented with fish oil lowers blood pressure in the elderly. J Hypertens 1992;10:87–92. [DOI] [PubMed] [Google Scholar]
- 20.Sciarrone SEG, Beilin LJ, Rouse IL, Rogers PB. A factorial study of salt restriction and a low-fat/high-fibre diet in hypertensive subjects. J Hypertens 1992;10:287–98. [DOI] [PubMed] [Google Scholar]
- 21.Nestel PJ, Clifton PM, Noakes M, McArthur R, Howe PR. Enhanced blood pressure response to dietary salt in elderly women, especially those with small waist:hip ratio. J Hypertens 1993;11:1387–94. [DOI] [PubMed] [Google Scholar]
- 22.Fotherby MD, Potter JF. Effects of moderate sodium restriction on clinic and twenty-four-hour ambulatory blood pressure in elderly hypertensive subjects. J Hypertens 1993;11:657–63. [DOI] [PubMed] [Google Scholar]
- 23.Ruppert M, Overlack A, Kolloch R, Kraft K, Göbel B, Stumpe KO. Neurohormonal and metabolic effects of severe and moderate salt restriction in non-obese normotensive adults. J Hypertens 1993;11:743–9. [DOI] [PubMed] [Google Scholar]
- 24.Schorr U, Distler A, Sharma AM. Effect of sodium chloride- and sodium bicarbonate-rich mineral water on blood pressure and metabolic parameters in elderly normotensive individuals: a randomized double-blind crossover trial. J Hypertens 1996;14:131–5. [PubMed] [Google Scholar]
- 25.Gates PE, Tanaka H, Hiatt WR, Seals DR. Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension 2004;44:35–41. [DOI] [PubMed] [Google Scholar]
- 26.Palacios C, Wigertz K, Martin BR, Jackman L, Pratt JH, Peacock M, McCabe G, Weaver CM. Sodium retention in black and white female adolescents in response to salt intake. J Clin Endocrinol Metab 2004;89:1858–63. [DOI] [PubMed] [Google Scholar]
- 27.Forrester T, Adeyemo A, Soarres-Wynter S, Sargent L, Bennett F, Wilks R, Luke A, Prewitt E, Kramer H, Cooper RS. A randomized trial on sodium reduction in two developing countries. J Hum Hypertens 2005;19:55–60. [DOI] [PubMed] [Google Scholar]
- 28.Nowson CA, Wattanapenpaiboon N, Pachett A. Low-sodium Dietary Approaches to Stop Hypertension-type diet including lean red meat lowers blood pressure in postmenopausal women. Nutr Res 2009;29:8–18. [DOI] [PubMed] [Google Scholar]
- 29.Fuchs FD, Wannmacher CM, Wannmacher L, Guimarães FS, Rosito GA, Gastaldo G, Hoeffel CP, Wagner EM. Effect of sodium intake on blood pressure, serum levels and renal excretion of sodium and potassium in normotensives with and without familial predisposition to hypertension. Braz J Med Biol Res 1987;20:25–34. [PubMed] [Google Scholar]
- 30.MacGregor GA, Markandu ND, Sagnella GA, Singer DRJ, Cappucio FP. Double-blind study of three sodium intakes and long-term effects of sodium restriction in essential hypertension. Lancet 1989;2:1244–7. [DOI] [PubMed] [Google Scholar]
- 31.Bruun NE, Skøtt P, Nielsen MD, Rasmussen S, Schütten HJ, Leth A, Pedersen EB, Giese J. Normal renal tubular response to changes of sodium intake in hypertensive man. J Hypertens 1990;8:219–27. [PubMed] [Google Scholar]
- 32.Gow IF, Dockrell M, Edwards CRW, Elder A, Grieve J, Kane G, Padfield PL, Waugh CJ, Williams BC. The sensitivity of human blood platelets to the aggregation agent ADP during different dietary sodium intakes in healthy men. Eur J Clin Pharmacol 1992;43:635–8. [DOI] [PubMed] [Google Scholar]
- 33.Heer M, Baisch F, Kropp J, Gerzer R, Drummer C. High dietary sodium chloride consumption may not induce body fluid retention in humans. Am J Physiol Renal Physiol 2000;278:F585–95. [DOI] [PubMed] [Google Scholar]
- 34.Burnier M, Monod M, Chiolero A, Maillard M, Nussberger J, Brunner HR. Renal sodium handling in acute and chronic salt loading/depletion protocols: the confounding influence of acute water loading. J Hypertens 2000;18:1657–64. [DOI] [PubMed] [Google Scholar]
- 35.Johnson AG, Nguyen TV, Davis D. Blood pressure is linked to salt intake and modulated by the angiotensinogen gene in normotensive and hypertensive elderly subjects. J Hypertens 2001;19:1053–60. [DOI] [PubMed] [Google Scholar]
- 36.Wright JD, Hughes JP, Ostchega Y, Yoon SS, Nwankwo T. Mean systolic and diastolic blood pressure in adults aged 18 and over in the United States, 2001–2008. Natl Health Stat Report 2011;35:1–22. [PubMed] [Google Scholar]
- 37.Rakova N, Jüttner K, Dahlmann A, Schröder A, Linz P, Kopp C, Rauh M, Goller U, Beck L, Agureev A, et al. Long-term space flight simulation reveals infradian rhythmicity in human Na(+) balance. Cell Metab 2013;17:125–31. [DOI] [PubMed] [Google Scholar]
- 38.He FJ, MacGregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev 2004;CD004937. [DOI] [PubMed] [Google Scholar]
- 39.Graudal N. Sodium and cardiovascular disease. N Engl J Med 2014;371:2136–7. [DOI] [PubMed] [Google Scholar]
- 40.O'Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, Yan H, Lee SF, Mony P, Devanath A, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med 2014;371:612–23. [DOI] [PubMed] [Google Scholar]
- 41.Mente A, O'Donnell MJ, Rangarajan S, McQueen MJ, Poirier P, Wielgosz A, et al. Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med 2014;371:601–11. [DOI] [PubMed] [Google Scholar]
- 42.Brunner HR, Laragh JH, Baer L, Newton MA, Goodwin FT, Krakoff LR, Bard RH, Buhler FR. Essential hypertension: renin and aldosterone, heart attack and stroke. N Engl J Med 1972;286:441–9. [DOI] [PubMed] [Google Scholar]
- 43.Oliver WJ, Cohen EL, Neel JV. Blood pressure, sodium intake, and sodium related hormones in the Yanomamo Indians, a “no-salt” culture. Circulation 1975;52:146–51. [DOI] [PubMed] [Google Scholar]
- 44.Ekinci EI, Clarke S, Thomas MC, Moran JL, Cheong K, MacIsaac RJ, Jerums G. Dietary salt intake and mortality in patients with type 2 diabetes. Diabetes Care 2011;34:703–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas MC, Moran J, Forsblom C, Harjutsalo V, Thorn L, Ahola A, Wadén J, Tolonen N, Saraheimo M, Gordin D, et al. FinnDiane Study Group. The association between dietary sodium intake, ESRD, and all-cause mortality in patients with type 1 diabetes. Diabetes Care 2011;34:861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stolarz-Skrzypek K, Kuznetsova T, Thijs L, Tikhonoff V, Seidlerova ÅLJ, Richart T, Jin Y, Olszanecka A, Malyutina S, Casiglia E, et al. ; European Project on Genes in Hypertension (EPOGH) InvestigatorsFatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA 2011;305:1777–85. [DOI] [PubMed] [Google Scholar]
- 47.O’Donnell MJ, Yusuf S, Mente A, Gao P, Mann JF, Teo K, McQueen M, Sleight P, Sharma AM, Dans A, et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA 2011;306:2229–38. [DOI] [PubMed] [Google Scholar]
- 48.Pfister R, Michels G, Sharp SJ, Luben R, Wareham NJ, Khaw KT. Estimated urinary sodium excretion and risk of heart failure in men and women in the EPIC-Norfolk study. Eur J Heart Fail 2014;16:394–402. [DOI] [PubMed] [Google Scholar]
- 49.Cobb LK, Anderson CA, Elliott P, Hu FB, Liu K, Neaton JD, Whelton PK, Woodward M, Appel LJ; on behalf of the American Heart Association Council on Lifestyle and Metabolic Health. Methodological issues in cohort studies that relate sodium intake to cardiovascular disease outcomes: A science advisory from the American Heart Association. Circulation 2014;129:1173–86. [DOI] [PubMed] [Google Scholar]
- 50.Powles J, Fahimi S, Micha R, Khatibzadeh S, Shi P, Ezzati M, Engell RE, Lim SS, Danaei G, Mozaffarian D. Global Burden of Diseases Nutrition and Chronic Diseases Expert Group Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide.(NutriCoDE). BMJ Open 2013;3:e003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oparil S. Low sodium intake–cardiovascular health benefit or risk? N Engl J Med 2014;371:677–9. [DOI] [PubMed] [Google Scholar]