Abstract
Infant formulas have historically been developed based on providing macronutrients at intake concentrations approximately matching the composition of human milk. In most countries, targets of 1.4–1.5 g of protein/dL and 20 kcal/oz (67–68 kcal/dL) have been set as the protein and energy concentrations for formulas during the first year of life, although this may be an overestimation of these contents. Recent introduction of lower-protein and -energy formulas in full-term infants led us to systematically review the literature for its effects on growth. Following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines, our inclusion criteria were studies that enrolled healthy full-term infants and evaluated lower-protein or lower-energy formula, reported anthropometric outcomes including weight and length, and followed infants for at least 6 mo. Six studies were eligible for inclusion. These studies varied in the content of nutrients provided in the intervention and control groups, by additional dietary components in the study groups, and the timing and length of the intervention, which limit their usefulness for interpreting newly introduced lower-protein and -energy formulas in the United States. These studies suggest adequate growth during infancy and early childhood with infant formulas with concentrations of protein and energy slightly below historical standards in the United States. Further long-term research is needed to assess the impact of the use of lower-protein and/or lower-energy products, especially for nutritionally at-risk populations such as preterm infants and infants who are born small for gestational age.
Keywords: child growth, development, lactation, infant formula, obesity prevention, protein requirements
Introduction
Infant formulas have historically been developed based on providing macronutrients at intake concentrations approximately matching the composition of human milk. In most countries, targets of 1.4–1.5 g of protein/dL and 20 kcal/oz (67–68 kcal/dL) have been set as the protein and energy concentrations for formulas during the first year of life. Currently, this is the protein and energy concentrations that are used in the majority of routine cow milk protein–based infant formulas in the United States designed for feeding healthy full-term infants (1).
In the United States, specific guidelines for infant formula composition exist from 2 widely used sources. The first is the legal mandate of the Infant Formula Act (1980 and amended in 1986) (2). This act does not set an energy concentration standard but does set minimum standards for macronutrients including protein (1.2 g of protein/dL). The second source is a 1998 report on nutrient requirements of infant formulas from the Life Sciences Research Office of the American Society for Nutritional Sciences (3). This report recommends a minimum energy concentration of 63 kcal/dL (18.6 kcal/oz) for routine infant formula. This group also set a recommended minimum protein concentration of 1.7 g/100 kcal, which would be ∼1.1 g/dL for a 20-kcal/oz formula. The authors cautioned that this protein concentration was below the protein concentration of marketed products at that time; therefore, they recommended that any new formulas “at or near the minimum concentration require clinical testing to demonstrate efficacy” (3). International standards as reflected in the Codex Alimentarius mandate that formula for term infants should not contain <60 kcal/dL and 1.8–3 g of protein/100 kcal (1.08–1.8 g of protein/dL) (4).
Recently, infant formulas with less than the historically used 20-kcal/oz energy concentration have been introduced in the United States. This has led to an increased interest in understanding the effects on growth and later risks of childhood overweight and obesity of variations in protein and energy concentration in infant formulas. Reported higher rates of overweight among formula-fed children could be, in part, because of confounding and lifestyle factors, such as breastfeeding mothers being more likely to have higher education and socioeconomic levels, which may be responsible for lower rates of childhood overweight and obesity (5, 6). The potential beneficial effects of decreasing childhood obesity and overweight by providing lower-protein, lower-energy infant formulas make it potentially appealing. We systematically reviewed studies through the use of lower-protein, lower-energy formulas to understand their impact on growth and relate their findings to infant formulas marketed in the United States.
Shown in Table 1 are values for protein and energy concentration of mature human milk (7–10). Also included are the mean values for these from term infant formulas marketed in the United States providing an energy concentration of 20 kcal/oz and 1 recently marketed formula with an energy concentration of 19 kcal/oz. These values for both human milk and formulas are approximate because variations occur naturally in human milk and there are known variations introduced in the preparation and mixing of infant formulas. Values shown in Table 1 are rounded to the nearest 0.1 based on manufacturers’ statements (for protein) or nearest kilocalories per deciliter.
TABLE 1.
Human milk (mature) composition and formula comparison of commonly used formulas in the United States1
| Energy, kcal/dL | Protein, g/dL | Protein, g/100 kcal | |
| Mature human milk (7)1 | |||
| Weeks 3–4 of lactation | 66 (48–85)2 | 1.2 (0.8–1.6) | 1.82 |
| Weeks 10–12 of lactation | 68 (50–86) | 0.9 (0.6–1.2) | 1.32 |
| Routine 20-kcal/oz formula (8) | 67.6 | 1.4 | 2.1 |
| Routine 20-kcal/oz formula (9) | 67.6 | 1.5 | 2.2 |
| Routine 19-kcal/oz formula (10) | 64.3 | 1.3 | 2.0 |
Methods
Search strategy.
A systematic search of relevant published articles through July 2014 was performed with the use of inclusion criteria that were developed through the use of a Patient, Intervention, Comparators, Outcome, and Study design approach. We attempted to include all published studies of randomized and nonrandomized controlled trials that examined lower-protein, lower-energy infant formulas (intervention) in healthy full-term infants >37 completed weeks of gestation who were appropriately grown (nonsmall or large for gestational age; patient). Studies of interest also had to include a standard routine infant formula for a control comparison and evaluation of infants for ≥6 mo (comparator and study design) for growth outcomes (outcome). Studies were searched by using the terms “infant formula” or “low protein” or “low energy” or “low calorie” and “infant” or “neonate” or “newborn.”
The main outcome of interest was anthropometric measurements, specifically both weight and length (outcome). Secondary outcomes of interest were body composition, BMI, and biochemical markers for protein metabolism. Inclusion of a breastfed control group was desirable but not mandatory. This review was completed in accordance with the preferred reporting system with the use of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)6 guidelines (11).
Studies were searched through the use of the search strategy of the Cochrane Neonatal Review Group (12) without language or publication date restriction in August 2014. The following electronic databases were searched: MEDLINE (http://ovidsp.ovid.com, 1966 to August 2014) (13), PubMed (http://www.ncbi.nlm.nih.gov/pubmed) (14), Cumulative Index to Nursing and Allied Health Literature (http://www.ebscohost.com/biomedical-libraries/the-cinahl-database, 1982 to August 2014) (15), and the Cochrane Central Register of Controlled Trials (http://onlinelibrary.wiley.com/cochranelibrary/search, to August 2014) (16). Online clinical trial registries were searched [www.clinicaltrials.gov (17), http://www.controlled-trials.com (18), and the WHO International Clinical Trials Platform at www.who.int/ictrp/en (19)] to identify any ongoing or completed but unpublished studies. Conference abstracts were identified from the proceedings of Pediatric Academic Societies (American Pediatric Society, Society for Pediatric Research, and European Society for Pediatric Research, http://www.abstracts2view.com/pasall, 2002 to August 2014) (20).
Selection of studies.
The titles and the abstracts of studies identified by the search strategy were assessed for inclusion in this review by 2 review authors (KMH and MP). If this could not be done reliably by title and abstract, then the full text version was obtained for assessment. Any differences were resolved by mutual discussion. Full text versions of all eligible studies were obtained for quality assessment.
Assessment of risk of bias in included studies.
The standardized review methods of the Cochrane Neonatal Review Group were used by the 2 review authors independently to assess the methodologic quality of the included study. The included trials were assessed for the following criteria and scored as low risk, unclear risk, or high risk (Table 2):
TABLE 2.
Risk of bias1
| Reference | RSG and AC (selection bias)2 | Blinding of participants and personnel (performance bias) | Blinding of outcome assessment (detection bias) | Incomplete outcome data (attrition bias) |
| Koletzko et al. (ECOT study) (33) | Low risk of both RSG and AC: randomization in permuted blocks based on an internet platform | Low risk | Unclear risk | High risk: >40% of infants did not follow-up at 24 mo |
| Escribano et al. (ECOT study) (34) | Low risk of both RSG and AC: randomization in permuted blocks based on an internet platform | Low risk | Unclear risk | High risk: >40% of infants did not follow-up at 24 mo |
| Weber et al. (ECOT study) (35) | Low risk of both RSG and AC: randomization in permuted blocks based on an internet platform | Low risk | Unclear risk | High risk: 52% of infants did not follow-up at 6 y |
| Timby et al. (36) | Low risk of RSG: computer randomization in blocks of 8; unclear risk of AC | Low risk | Unclear risk | Medium risk: only 11% of infants lost to follow-up |
| Inostroza et al. (37) | Unclear risk of both RSG and AC | Low risk | Unclear risk | Medium risk: 16% of infants lost to follow-up at 6 mo |
| Akeson et al. (38) | Unclear risk of RSG and AC | Low risk | Unclear risk | Medium risk: <10% of infants lost to follow-up |
AC, allocation concealment; ECOT, European Childhood Obesity Trial; RSG, random sequence generation.
Each category of risk of bias was assessed as low risk if there were no concerns, unclear risk if not explicitly stated in the study report, and high risk if there were concerns of bias. For incomplete outcome data, <5% was considered low risk, 5–20% was considered medium risk, and >20% was considered high risk of bias.
Sequence generation: Was the allocation sequence adequately generated?
Allocation concealment: Was allocation adequately concealed?
Blinding of participants, personnel, and outcome assessors: Was knowledge of the allocated intervention adequately prevented during the study? At study entry? At the time of outcome assessment?
Incomplete outcome data: Were incomplete outcome data adequately addressed?
Selective outcome reporting: Are reports of the study free of suggestion of selective outcome reporting?
Other sources of bias: Was the study apparently free of other problems that could put it at a high risk of bias?
Results
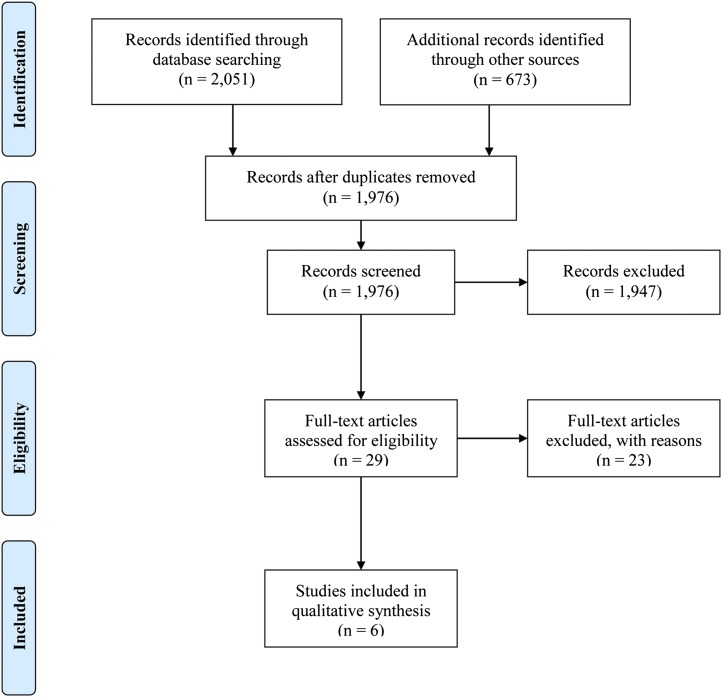
We identified 2724 records with the use of the search strategy, leaving 1976 records after duplicates were removed (PRISMA flow diagram, Figure 1). All records were screened for the inclusion criteria with the majority being excluded. Fourteen studies from clinical trials registries were excluded because not enough information was provided to determine what interventions were being performed.
FIGURE 1.
PRISMA 2009 flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
Twenty-nine full text articles were reviewed for eligibility and were identified as being controlled trials in healthy full-term infants, which might meet the remaining inclusion criteria. Of these, 3 were not studies of healthy infants (21–23), 1 did not report length outcomes (24), 5 were short-term studies of a maximum of 4–6 mo (1, 25–28), and 4 also involved use of novel or different protein sources instead of just changes in protein content (29–32) (Table 3). Relevant aspects of some of these controlled trials will be briefly discussed, but not as part of the primary group analysis.
TABLE 3.
Studies for exclusion1
| Reference | Reason for exclusion |
| Singhal et al. (21) | SGA infants |
| Clarke et al. (22) | Infants with faltering growth |
| Schulze et al. (23) | Study conducted in low birth weight infants |
| Martin et al. (24) | Did not report length measurements |
| Fomon et al. (1) | Study period was <4 mo |
| Fomon et al. (25) | Study period was <4 mo |
| Fomon et al. (26) | Study period was <2 mo |
| Huet et al. (27) | Study period was 3 mo |
| Axelsson et al. (28) | Study period was <2 mo |
| Raiha et al. 29) | Used different types and amounts of protein in each group (60:40 whey:casein vs. 70:30 modified sweet whey vs. 70:30 acid whey); study period was 4 mo |
| Turck et al. (30) | Used different types of protein in each group in addition to changing the amount of protein in each group (whey predominant vs. casein predominant) |
| Trabulsi et al. (31) | Used different types and amounts of protein in each group (α-lactalbumin vs. without α-lactalbumin) |
| Fleddermann et al. (32) | Used different types and amounts of protein in each group (α-lactalbumin and LC-PUFAs vs. standard whey and no LC-PUFAs) |
SGA, small for gestational age.
Therefore, we confirmed that there were 6 reports of randomized controlled trials with a total of 1399 healthy full-term infants who met our criteria for inclusion (33–38) (Table 4). All 6 included studies were randomized and had a low risk of selection bias. All were double-blinded controlled trials and therefore performance bias was noted to be low risk. None of the studies explicitly report blinding of outcome assessors (detection bias). There was a high loss of follow-up (attrition bias) in the studies from the ECOT (European Childhood Obesity Trial) study group (33–35). Studies by Timby et al. (36), Inostroza et al. (37), and Akeson et al. (38) had a follow-up loss of ≤16%.
TABLE 4.
Included studies1
| Reference | Participants/settings | Intervention and comparison | Outcomes | Comments |
| ECOT study 2009 (33) | Enrolled 1138 healthy full-term infants; studied for 24 mo in 5 different EU countries | Randomized double-blind controlled trial with 2 stages of formula based on age; breastfeeding group was used for comparison | The primary endpoints were length and weight at 24 mo of age, expressed as length and weight-for-length z scores based on the 2006 WHO growth standards | The EU Childhood Obesity Project is an ongoing European collaborative prospective investigation into the long-term consequences of early protein in 5 European countries (Belgium, Germany, Italy, Poland, and Spain); www.ClinicalTrials.gov registration number NCT00338689; Partially supported by the Commission of the European Community and project EarlyNutrition |
| ECOT study 2012 (34) | Enrolled 80 infants from Germany and 43 from Spain, who were selected by recruitment order from 522 eligible subjects when they were 6 mo old (between May 2003 and June 2004) | Randomized double-blind controlled trial with 2 stages of formula based on age; breastfeeding group was used for comparison | The primary endpoints were weight gain velocity and body fat mass; anthropometric measures were assessed at baseline and 6, 12, and 24 mo, and fat-free mass and fat mass were assessed by isotope dilution at 6 mo | Publication from the EU Childhood Obesity Project; www.ClinicalTrials.gov registration number NCT00338689; partially supported by the Commission of the European Community and project EarlyNutrition |
| ECOT study 2014 (35) | Enrolled 1678 infants studied from 2.5 to 6 y of age in 5 different EU countries | Randomized double-blind controlled trial with 2 stages of formula based on age; breastfeeding group was used for comparison | The primary endpoint was BMI; anthropometrics (weight, height, and head circumference) were assessed at baseline and 3, 6, 12, and 24 mo of age, and then every 6 mo until 6 y of age | Publication from the EU Childhood Obesity Project; www.ClinicalTrials.gov registration number NCT00338689; Partially supported by the Commission of the European Community and project EarlyNutrition |
| Timby et al. (36) | Enrolled 160 healthy full-term infants studied until 6 mo of age in Sweden from March 2008 to February 2012 | Randomized double-blind controlled trial of a lower-protein and lower-energy formula vs. standard protein formula; breastfeeding group was used for comparison | The primary endpoints were growth, cognitive function, and body composition; anthropometrics (weight, length, and head circumference) were measured at baseline (<2 mo) and 4, 6, and 12 mo of age; cognitive function (Bayley-III) performed at 12 mo of age and percent body fat measured at baseline and 4 mo of age | www.ClinicalTrials.gov registration number NCT00624689; supported by Sweden’s Innovation Agency (Vinnova), the Vasterbotten County Council, and Semper AB |
| Inostroza et al. (37) | Enrolled 305 healthy full-term infants of overweight mothers studied for 24 mo in Chile | Randomized double-blind controlled trial of a lower-protein formula with probiotics (Lactobacillus PR and Bifidobacterium lactis) vs. standard protein formula; breastfeeding group was used for comparison | The primary endpoint was growth; anthropometry was evaluated between 3 and 6 mo; biomarkers of protein metabolism (BUN, insulin growth factor 1, and insulinogenic amino acids) were also measured | www.ClinicalTrials.gov registration number NCT00820833; supported by Nestec SA, Vevey, Switzerland. |
| Akeson et al. (38) | Enrolled 80 healthy full-term infants who were exclusively breastfed until 3 mo of age were allocated with interventions and studied for 12 mo in Sweden (December 1989 and September 1994) | Randomized double-blind controlled trial of 2 different lower-protein formula groups, with different amounts of protein, but same sources vs. standard protein formula; breastfeeding group was used for comparison | The primary endpoint was growth; weight, length, and head circumference at birth were obtained at 3, 4, 6, 8, 10, and 12 mo of age | Supported by Ross Laboratories (Columbus, OH), Swedish Nestle AB (Bjuv, Sweden), and the Swedish Nutrition Foundation (Lund, Sweden) |
Criteria for inclusion: healthy full-term infants, lower-protein or lower-energy formula, anthropometric outcomes, and remained in study for >6 mo. BUN, blood urea nitrogen; ECOT, European Childhood Obesity Trial; EU, European Union.
Included studies.
The 6 controlled trials were characterized by subject characteristics (Table 5), timing and length of intervention (Table 6), formula characteristics (Table 7), and outcomes (Table 8) .
TABLE 5.
Subject characteristics1
| ECOT study (33–35) | Timby et al. (36) | Inostroza et al. (37) | Akeson et al. (38) | |
| Completed study, n | 934 | 213 | 181 | 71 |
| Breastfed infant comparison group | Yes | Yes | Yes | Yes |
| Lower-protein, lower-energy group:standard protein formula:breastfed, n | 313:323:298 | 73:68:72 | 54:66:61 | 49:22:Not reported |
| Definition of a breastfed infant | Exclusive breastfeeding at inclusion and intention to exclusively breastfeed ≥3 mo | Exclusive breastfeeding at inclusion and intention to exclusively breastfeed until 6 mo | No more than 1 feed/d of formula at 3 mo of age | Exclusively breastfed until 3 mo of age; if taking <125 mL of formula at 6 mo of age |
| Male:female, % | 52:48 | 50:50 | 52:48 | 53:47 |
| Location of study | Belgium, Germany, Italy, Poland, Spain | Sweden | Chile | Sweden |
| Gestational age, wk | Not specified | 37–42 | 37–42 | 38–42 |
| Age at intervention, mo | <2 | <2 | 3 | 3 |
| Birth weight for inclusion, g | Not specified, mean 3300 | 2500–4500 | 2500–4800 | ±2 SD on national growth charts |
| Excluded for SGA? <5th percentile | No | No | Yes | Yes |
| Excluded for maternal/gestational diabetes | Yes | No | Yes | Yes |
ECOT, European Childhood Obesity Trial; SGA, small for gestational age.
TABLE 6.
Timing and length of intervention1
| ECOT study (33–35) | Timby et al. (36) | Inostroza et al. (37) | Akeson et al. (38) | |
| Length of study | Inclusion at <2 mo of age until 24 mo of age | Inclusion at <2 mo of age until 12 mo of age | Inclusion at 3 mo of age until 24 mo of age | Inclusion at 3 mo of age until 12 mo of age |
| Duration of time on intervention formula | Initial formula: start at <2–5 mo of age; follow-up formula: 5–12 mo of age | Start at <2–6 mo of age | Start at 3–12 mo of age (to 6 mo exclusively, from 6 to 12 mo with complementary foods) | Start at 3–12 mo of age |
ECOT, European Childhood Obesity Trial.
TABLE 7.
Formula characteristics1
| Protein, g/dL | Protein, g/100 kcal | Energy, kcal/dL | Carbohydrate, g/dL | Lipids, g/dL | DHA, mg/dL | Bovine milk fat globule | Probiotics | |
| ECOT study (33–35): lower protein, start at 5 mo of age | 1.25 | 1.79 | 69.9 | 7.5 | 3.9 | Not reported | No | Not reported |
| ECOT study (33–35): high protein, start at 5 mo of age | 2.05 | 2.94 | 69.8 | 7.5 | 3.5 | Not reported | No | Not reported |
| ECOT study (33–35): lower protein, 5–12 mo of age | 1.6 | 2.2 | 72.7 | 7.6 | 4 | Not reported | No | Not reported |
| ECOT study (33–35): high protein, 5–12 mo of age | 3.2 | 4.4 | 72.5 | 7.6 | 3.27 | Not reported | No | Not reported |
| Timby et al. (36): lower-protein, lower-energy formula | 1.2 | 2 | 60 | 6 | 3.5 | 9 | Yes | No |
| Timby et al. (36): standard formula | 1.27 | 1.92 | 66 | 7.4 | 3.5 | 9 | No | No |
| Inostroza et al. (37): lower-protein, lower-energy formula | 1.04 | 1.66 | 62.8 | 7.16 | 3.33 | 8.34 | No | Yes |
| Inostroza et al. (37): standard formula | 1.77 | 2.7 | 65.6 | 7.2 | 3.3 | 8 | No | No |
| Akeson et al. (38): lower-protein formula 1 | 1.3 | 1.92 | 67.6 | 7.23 | 3.5 | Not reported | No | No |
| Akeson et al. (38): lower-protein formula 2 | 1.5 | 2.21 | 67.6 | 7.23 | 3.63 | Not reported | No | No |
| Akeson et al. (38): standard formula | 1.8 | 2.66 | 67.6 | 7.23 | 3.72 | Not reported | No | No |
ECOT, European Childhood Obesity Trial.
TABLE 8.
Outcome differences between lower-protein, lower-energy intervention and control groups1
| ECOT study (33–35) | Timby et al. (36) | Inostroza et al. (37) | Akeson et al. (38) | |
| Age at outcome | 6 y | 12 mo | 24 mo | 12 mo |
| Lower-protein, lower-energy group:standard protein formula:breastfed, n at outcome | 256:256:237 | 73:68:72 | 50:64:56 | 49:22:Not reported |
| Weight difference | 0.67 kg, P = 0.06 | z Score, −0.2 (NS) | 0.86 g/d (P = 0.03) | z Score not provided but reported to be NS |
| Length difference | −0.02 cm, P = 0.65 | z Score, −0.3 (NS) | Not reported | z Score not provided but reported to be NS |
| Obesity risk2 | RR, 2.43; P = 0.02 | Not reported | Not reported | Not reported |
| BMI | 0.51 kg/m2, P = 0.009 | Not reported | Difference, ∼1.0; P = 0.03 | Not reported |
| BMI z score difference | 0.33 | 0.0 | Not reported | Not reported |
NS, P > 0.05. Differences shown compare the standard protein, energy group – the lower-protein, lower-energy group (positive values indicate higher values in the standard protein, energy group). ECOT, European Childhood Obesity Trial.
Girls and boys were classified (33–35) as obese at 6 y of age if they had a BMI > 19.7 or >19.8 kg/m2, respectively.
P value not shown; 95% CI indicates significance.
ECOT study: Koletzko et al., Escribano et al., Weber et al.
Results from this study, conducted as part of the ECOT study, were first published in 2009 with data at 2 y of age [Koletzko et al. (33)], and then in 2012 with Escribano as the first author (34) providing body composition data and, finally, as a 6-y follow-up in 2014 with Weber as the first author (35). In the tables it is listed as “ECOT study” to reference the original intent and population of the studies.
Infants were enrolled from 2002 to 2004 in a variety of hospitals in Europe. The study was a controlled, double-blinded design and used a series of formulas, which varied in protein and energy (Table 7). Before the study began at 2 mo of age, infants were either breastfed or fed the parent’s choice of formula. In the first phase from ∼2 to ∼5 mo of age, their standard protein formula contained 2.05 g/dL of protein and the lower-protein formula had 1.25 g/dL of protein. Both formulas were isocaloric (69.8–69.9 kcal/dL). At 5 mo of age, the initial standard protein group was switched to a formula with 3.2 g/dL of protein and the initial lower-protein group was changed to a formula with 1.6 g/dL of protein until 12 mo of age. Again, formulas were isocaloric (72.5–72.7 kcal/dL). It is important to recognize that in the United States, routine infant formula contains 1.4–1.5 g/dL of protein and lower-protein formula contains 1.3 g/dL of protein (Table 1). Therefore, the standard protein group in this study provides protein at concentrations substantially above that of routine cow milk–based formulas marketed for healthy full-term infants in the United States. To reinforce this difference, we will refer to their standard protein formulas as high protein.
This large difference in protein content between study groups (∼65% higher in the high-protein formula than the lower-protein formula for the initial product and 100% higher in the high-protein formula than the lower-protein formula for the older infants), without a difference in energy density, led to a lower BMI of 0.71 kg/m2 (P = 0.036) in the lower-protein group than the high-protein group at 12 mo of age, but was not significantly different at 24 mo of age (33). At the 6-y follow-up, BMI was significantly higher in the high-protein group than the lower-protein group (difference of 0.51 kg/m2; 95% CI: 0.13, 0.90; P = 0.009) with an increased risk of obesity (95% CI: 1.12, 5.27; P = 0.024), defined in girls and boys as a BMI > 19.7 or 19.8 kg/m2, respectively (34).
Although not a randomized comparison, the evaluation included a breastfed reference group. The growth outcomes in the lower-protein group were similar to the breastfed group, although the rates of obesity were higher in the lower-protein group than the breastfed group, without a statistical comparison being provided between these groups. Weight at 6 y of age was similar between the breastfed and lower-protein groups. Height was similar between all groups. No significant difference was found between the infants in the lower-protein group and the breastfed reference group for BMI or obesity risk at 6 y of age.
When the relationship between feeding and body composition (as assessed by isotope dilution) at 6 mo of age was examined more closely, both fat mass index and fat-free mass index tended to be lower in the lower-protein group than the high-protein group (33). The P value and CI were not provided by the authors but were only stated not to meet significance at P > 0.05. However, the study was not powered to demonstrate a difference between groups. In addition, higher fat mass at 6 mo of age in the high-protein group predicted higher BMI at 12 and 24 mo of age, indicating the possibility that more rapid weight gain and increased fat mass deposition may be associated with protein intakes in the first months of life.
Although marketed in a range of forms in the United States, the use of follow-up infant formulas at 5–6 mo of age is not common; therefore, the feeding approach in this study is not representative of current typical practice in the United States. Because no manufacturer in the United States currently markets a routine cow milk–based infant formula for infants in the first 6 mo of life with protein intakes at or near the concentrations in the high-protein arm of this study, it is not possible to interpret these results in terms of variations in protein intake seen in formulas in the United States.
Timby et al.
This study was conducted in Sweden and published in 2014 [Timby et al. (36)]. In this controlled, double-blinded study, full-term infants were fed a formula lower in protein and energy (1.2 g of protein/dL, 60 kcal/dL) than one that was considered standard (1.27 g of protein/dL, 66 kcal/dL). The difference in energy between the products was ∼10% and the protein difference was ∼6%. These differences, especially for energy, are greater than the differences between lower and standard protein formula in the United States, but less than the differences used in the ECOT study (33–35).
Of note is that the lower-protein formula also included an additional product, a bovine milk fat globule membrane. The addition of this product meant that the 2 products could not be considered as a direct comparison of protein or energy changes. Furthermore, follow-up was only done until 12 mo of age and the study was relatively small with only ∼70 infants in each group at the end of the study.
The results showed that there were no significant differences between groups in weight or length at 12 mo. Results for growth in the lower-protein, lower-energy, and the control formula groups were similar to a breastfed control group.
The lack of difference in growth was likely because of increased formula volume intake in the lower-protein and -energy group (25). Other possible reasons may include small differences in formula composition, potential effect of the bovine milk fat globule membrane given to the lower-protein formula group, and the selection bias associated with a nonrandomized comparison.
Overall, this study provides no definitive information demonstrating any effect of lower-protein or -energy formula on infant growth outcomes, although it suggests that a small lowering such as might be proposed in the United States may lead to adequate growth rates.
Inostroza et al.
This controlled, double-blinded trial was conducted in Chile and published in 2014 [Inostroza et al. (37)]. It evaluated the effects of 2 different concentrations of protein and energy when given to infants of overweight mothers from 3 to 6 mo of age and followed infants until 24 mo of age.
Differences in energy were small (∼5%, 62.8 kcal/dL vs. 65.6 kcal/dL) but the standard protein group had ∼70% more protein than the lower-protein group (1.04 g of protein/dL vs. 1.77 g of protein/dL). In fact, this is the lowest protein content reported in any of the included studies and is remarkably lower than the protein content of formulas available in the United States.
Furthermore, the lower-protein formula also included probiotics that the standard protein group did not. Study formulas were introduced later in this study than other identified studies [3 mo of age compared with 2 mo of life in the ECOT study (33–35) and Timby et al. (36) study]. The use of an intervention beginning at 3 mo of age does not address the usual use of such products in the United States beginning earlier in life.
The study found that the lower-protein and -energy formula group had significantly less weight gain between 3 and 6 mo of age than the standard protein and energy group, but at rates similar to the breastfed reference group. In mothers who had a prepregnancy BMI > 30 kg/m2, there was a larger difference in infant weight between formula groups than the whole group and also a difference in infant BMI at 2 y of age. Because of a difference in the results between infants based on mothers’ prepregnancy BMI as an additional confounder, this area of research needs further investigation before concluding about lower-protein and -energy formulas.
This study was the only one to include biochemical markers of protein metabolism, which showed that blood urea nitrogen (BUN), a marker of protein status, was lower in the lower-protein group at 6 mo of age (P < 0.001) and no longer significantly different at 12 mo of age (P = 0.3). BUN concentrations of infants in the low-protein group were higher than those of breastfed infants. The clinical interpretation of this result is uncertain for healthy infants with normal BUN concentrations.
Overall, the use of probiotics, the large difference in protein content between study groups, and the initiation of intervention at 3 mo limit this study from providing definitive information demonstrating any effect of lower-energy or -protein formula on infant growth outcomes that might be applicable to marketed formulas for healthy term newborns in the United States.
Akeson et al.
This study was conducted in Sweden from 1989 to 1994 [Akeson et al. (38)]. It included 71 healthy full-term infants who had been exclusively breastfed until 3 mo of age at which time they were randomly assigned to either 2 different lower-protein formulas (1.3 g of protein/dL or 1.5 g of protein/dL) or the control formula (1.8 g of protein/dL). All formulas were isocaloric (67.6 kcal/dL). Infants were allowed to continue breastfeeding as desired. Breastfed infants who consumed <125 mL of formula at 6 mo of age provided the breastfeeding reference group, although the number of subjects in this group was not included in the article.
The protein content of the standard protein formula (1.8 g of protein/dL) was similar to that of the standard formula used by Inostroza et al. (1.77 g of protein/dL) but much higher than the standard formula used by Timby et al. (1.27 g of protein/dL). There was a 20–40% difference in the protein content between the 2 lower-protein formulas compared with the standard protein formula.
There was no difference in weight or length during the study period among any of the groups or between girls and boys. This early study is consistent with Timby et al. (36), demonstrating no metabolic or growth advantage to standard protein–containing formulas.
Because the standard protein formula was considerably higher than the currently marketed term formulas in the United States, this study does not directly provide evidence relative to protein content of US formulas.
Consideration of other trials.
We would like to briefly consider studies that did not meet our primary inclusion for entry into this systematic review. The first is a publication by Singhal et al. (21) evaluating the risk of obesity in small-for-gestational-age (SGA) term infants who were fed either a control (1.4 g of protein/dL, 68 kcal/dL) or nutrient-enriched formula with higher macro- and micronutrients (1.8 g of protein/dL, 72 kcal/dL). This study showed that increasing protein by 28–43% and energy by 6–12% led to a greater increase in fat mass in infants fed the nutrient-enriched formula than in infants fed the control formula at 5–8 y of age (subjects <10th percentile weight for age: 38% lower fat mass in control formula [95% CI: −67%, −10%; P = 0.009); subjects <20th percentile weight for age: 18% lower fat mass in control formula (95% CI: −36%, −0.3%; P = 0.04)]. This has been considered a concern in SGA infants related to the risk of later adiposity being associated with rapid early growth in these infants. Although this study has important possible implications, especially in the management of infants who may have suffered in utero growth restriction and provides a cautionary note to higher protein intakes in such infants, it does not address the relevant question of protein and energy concentrations of infant formulas for appropriate-for-gestational-age (AGA) infants and the effects of small differences in protein intake on growth. Further research is warranted, especially in infants who are born at the edge of definitions of SGA status or late preterm, to elucidate the risks and benefits of variations in protein and energy concentrations in formulas related to long-term outcomes.
An important group of randomized controlled trials on full-term formula-fed infants are from Fomon and his colleagues in the 1970s and 1990s (1, 25, 26). These are not included in this review because the time period of evaluation was only up to a maximum of ∼4 mo of age and thus does not provide outcomes related to growth to meet our inclusion criteria. In a study published in 1975, this group demonstrated that a formula containing 54 kcal/dL led to an increased feeding volume intake compared with one providing 100 kcal/dL (25). After a short follow-up of 41 d, weight gain was similar in the groups. This study demonstrated the ability of infants to self-adjust intake based on caloric density of an infant formula to achieve similar energy intakes. In a 1977 publication, this group compared skim milk containing 36 kcal/oz with routine 67-kcal/oz formula in infants >112 d of age (26). Gain in length over the next 8 wk was similar, but body fat was lower in the skim milk group.
In 1995 Dr. Fomon and his group (1) published a study evaluating the effects of providing a group of infants (n = 15) with formulas with 1.56 g of protein/100 kcal (1.05 g of protein/dL) decreasing to 1.25 g of protein/100 kcal (0.84 g protein/dL) over the first 4 mo of life compared with a group fed a standard protein formula with a consistent protein content (n = 13). The lower-protein group represents protein concentrations ∼20–30% below those used in current formulas in the United States (Table 1) and somewhat below the lower-protein formulas tested in recent studies (Table 4). They found similar weight but non-significantly lower gains in length in the lower-protein group. There is little specific conclusion that can be drawn from this very small study related to optimal protein in infant formulas, although the trend toward a slower length gain in the lower-protein group is concerning.
Several studies used different types of protein among formula groups in addition to changing the amount of protein. Two studies changed the whey:protein ratio (29, 30) and 2 more recent studies used α-lactalbumin with or without long-chain PUFAs (31, 32). Although these studies may offer new information they also present too many confounders to be able to distinguish the true effects of the decreased protein content of the formula.
A few studies examining preterm infants have investigated the link between body composition, growth, and other health outcomes. Roggero et al. (39) from Italy evaluated postnatal weight gain and body composition during the first 5-mo postmenstrual age in preterm infants and found that SGA infants showed the lowest mean z score of weight at term compared with AGA infants with and without extrauterine growth restriction. Consistent with previous studies, SGA preterm infants experienced more severe extrauterine growth failure than AGA counterparts. Fetal programming and the trajectory of weight and length gain postnatally may play an important role in understanding growth in these infants. Although of value in considering long-term growth and nutritional needs of some infants, these studies do not provide information relative to the nutrient requirements of most infants, especially in the United States where SGA birth is less common than in developing countries.
Euser et al. (40) found a positive association between weight gain before 32-wk postmenstrual age in very preterm infants and adult weight, height, BMI, and fat-free mass at 19 y of age, but not with other markers of body composition or fat distribution, reflecting an increased risk of obesity. Although these results are not generalizable to term infants, they do provide information about fetal growth restriction in preterm infants. Further information is needed before any conclusions can be drawn related to the risks and benefits of lower protein intake in infants born at <32 wk gestation.
A systematic review of infant size and growth as related to risk of adulthood disease showed that larger size in infancy was associated with an increased risk of insulin-dependent diabetes in both adult men and women but with reduced rates of ischemic heart disease in men only (41). Their findings suggest that there is no single optimal pattern of infant growth to achieve beneficial health outcomes in adulthood. Further research is needed related to the clinical significance of these findings associated with infant formulas for healthy full-term infants.
Discussion
Our systematic review of 6 reports of 4 randomized, controlled, double-blinded trials revealed adequate growth during infancy and early childhood over a range of protein and energy concentrations in infant formulas for healthy full-term infants. The studies varied by study design, duration of the intervention, number of participants, and inclusion of probiotics or different types of protein, in addition to different amounts of protein, which makes drawing conclusions difficult if not impossible.
A Cochrane Review published earlier this year examined standard vs. lower protein intake in formula-fed low birth weight infants (42). Most of the infants included in the review were preterm; however, birth weight rather than gestational age was used as the inclusion criteria and ranged from <800 to 2499 g. The aim of that review was to determine whether higher (≥3.0 g · kg−1 · d−1 but <4.0 g · kg−1 · d−1) vs. lower (<3.0 g · kg−1 · d−1) protein intake during the initial hospital stay of formula-fed preterm infants or low birth weight infants resulted in improved growth and neurodevelopmental outcomes without evidence of short- and long-term morbidity. Studies were excluded if infants were fed formula as a supplement to human milk. Studies in which nutrients other than protein also varied were added in a post facto analysis. The reviewers concluded that higher protein intake (3.1–3.8 g · kg−1 · d−1) led to faster weight gain in low birth weight infants than lower protein intakes (2.2–2.8 g · kg−1 · d−1). However, limited information is available regarding the impact of different protein intakes on long-term outcomes such as neurodevelopment. Insufficient evidence is available to predict growth patterns of these infants when fed lower-protein, lower-energy formulas and these data, although of interest, cannot be used to define nutrient needs of full-term infants. Preterm infants have considerably higher protein needs than term infants, and this need may persist beyond their hospital stay; thus, they should not be fed a lower-protein formula.
Risk of bias in the included studies and in the review process.
All 6 included studies were randomized and had a low risk of selection bias and performance bias was noted to be low risk. None of the studies explicitly report blinding of outcome assessors (detection bias) but we noted a high risk of attrition bias.
We attempted to decrease biases in the review process. Both authors performed the literature search with the use of an inclusive search strategy and combined their results, identifying 6 clinical trials with prespecified outcomes. We used standard methods of conducting a systematic review in accordance with PRISMA guidelines. We did not identify any other review that examined current literature on lower-protein, lower-energy formulas in full-term infants.
Conclusions
Variations of protein and/or energy in infant formulas have been evaluated in 4 trials (6 articles) in full-term infants. Our review suggests that a range of protein and energy concentrations in infant formulas is associated with adequate growth but further controlled trials are needed. The results show a small effect on growth between an intervention and a control formula in 2 studies: the ECOT study group (33–35) and Inostroza et al. (37) and no effect in the other 2 [Timby et al. (36) and Akeson et al. (38)]. All 4 studies demonstrated that growth was similar between infants in the lower-protein, lower-energy groups and breastfed infants. The 2 trials showing a significant effect on growth were both ones in which large differences (65–70%) in protein concentrations existed between the groups such that the standard protein group was well above the concentration provided in routine infant formulas in the United States and therefore does not reflect standard practice in the United States. These results show a small effect on growth. These effects may be caused by both the high concentrations of protein or energy provided in 1 group and the large group differences used in the study.
Taken together, these research studies demonstrate adequate growth during infancy and early childhood with infant formulas with concentrations of energy and protein slightly below the traditional 20 kcal/oz and 1.4–1.5 g/dL used in the United States for many decades. However, they do not provide evidence of safety or adequate growth in higher-risk populations including late preterm infants, SGA infants, or infants with a range of health problems that might affect growth.
We propose that formulas introduced into the marketplace for feeding healthy infants should be evaluated in all groups of infants that are likely to receive them. Beyond healthy full-term infants, this includes late preterm infants (34–36 wk postmenstrual age), low birth weight full-term infants (1800–2500 g), and infants who are primarily breastfed and receiving a small amount of formula supplementation. Evaluation should include outcomes at least until 1 y of age and preferably much longer.
Formulas being investigated should not have key additional components such as probiotics compared with the control group. Studies without changes in composition, except in protein or energy differences, should first be studied before 6 mo of age, especially because breast milk decreases in protein with age. If a formula composition change is made at 6 mo of age, then both study and intervention research groups should have the same change made. Furthermore, true long-term outcomes including cost:benefit analysis and risks of errors in mixing or concentrating formulas should be considered in evaluating lower-protein, lower-energy formulas. Long-term growth and risk of obesity, or prevention of obesity, should be primary outcome measures.
In conclusion, the 4 identified trials varied in the content of nutrients provided in the intervention and control groups, whether additional dietary components differed between study groups, and the timing and length of the intervention. The designs of these studies limit their interpretation or use in evaluating newly introduced lower-protein and -energy formulas in the United States. These studies suggest that lowering protein and energy from the historical standards leads to growth outcomes similar to those of breastfed infants except when very large variations in protein or energy are provided. Further long-term research is needed with caution being advised pending such research about the use of lower-protein, lower-energy products, especially for nutritionally at-risk populations such as late preterm infants.
Acknowledgments
All authors have read and approved the final manuscript.
Footnotes
Abbreviations used: AGA, appropriate for gestational age; BUN, blood urea nitrogen; ECOT, European Childhood Obesity Trial; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis; SGA, small for gestational age.
References
- 1.Fomon SJ, Ziegler EE, Nelson SE, Frantz JA. What is the safe protein-energy ratio for infant formulas? Am J Clin Nutr 1995;62:358–63. [DOI] [PubMed] [Google Scholar]
- 2.Food and Drug Administration [Internet]. Regulations and information on the manufacture and distribution of infant formula, 2002 [cited 2014 Jul 29]. Available from: www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/InfantFormula/ucm136118.htm.
- 3.Assessment of nutrient requirements for infant formulas. J Nutr 1998;128 Suppl:i-iv, 2059S–2293S. [PubMed] [Google Scholar]
- 4.Codex Alimentarius International Food Standards. Standard for infant formula and formulas for special medical purposes intended for infants [cited 2014 Sep 11]. Available from: www.codexalimentarius.org.
- 5.Walfisch A, Sermer C, Cressman A, Koren G. Breast milk and cognitive development—the role of confounders: a systematic review. BMJ Open 2013;3:e003259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owen CG, Martin RM, Whincup PH, Davey-Smith G, Gillman MW, Cook DG. The effect of breastfeeding on mean body mass index throughout life: a quantitative review of published and unpublished observational evidence. Am J Clin Nutr 2005;82:1298–307. [DOI] [PubMed] [Google Scholar]
- 7.Gidrewicz DA, Fenton TR. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr 2014;14:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enfamil Infant. [cited 2014 Jul 29]. Available from: http://www.enfamil.com/products/enfamil-infant.
- 9.Gerber Good Start Gentle. [cited 2014 Jul 29]. Available from: https://medical.gerber.com/products/formulas/gerber-good-start-gentle.
- 10.Similac Advance. [cited 2014 Jul 29]. Available from: http://abbottnutrition.com/brands/products/similac-advance.
- 11.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systemic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1–34. [DOI] [PubMed] [Google Scholar]
- 12.Cochrane Neonatal Review Group. [cited 2014 Jul 29]. Available from: http://neonatal.cochrane.org.
- 13.MEDLINE. [cited 2014 Jul 29]. Available from: http://ovidsp.ovid.com.
- 14.PubMed. [cited 2014 Jul 29]. Available from: http://www.ncbi.nlm.nih.gov/pubmed.
- 15.Cumulative Index to Nursing and Allied Health Literature (CINAHL). [cited 2014 Jul 29]. Available from: http://www.ebscohost.com/biomedical-libraries/the-cinahl-database. [PMC free article] [PubMed]
- 16.Cochrane Central Register of Controlled Trials. [cited 2014 July 29] Available from: http://onlinelibrary.wiley.com/cochranelibrary/search..
- 17.Clinical Trials. [cited 2014 Jul 29]. Available from: www.clinicaltrials.gov.
- 18.Controlled Trials. [cited 2014 Jul 29]. Available from: http://www.controlled-trials.com.
- 19.World Health Organization International Clinical Trials Platform (ICTRP). [cited 2014 Jul 29]. Available from: www.who.int/ictrp/en.
- 20.Pediatric Academic Societies(American Pediatric Society, Society for Pediatric Research and European Society for Pediatric Research) [cited 2014 Jul 29]. Available from: http://www.abstracts2view.com/pasall.
- 21.Singhal A, Kennedy K, Lanigan J, Fewtrell M, Cole TJ, Stephenson T, Elias-Jones A, Weaver LT, Ibhanesebhor S, MacDonald PD, et al. Nutrition in infancy and long-term risk of obesity: evidence from 2 randomized controlled trials. Am J Clin Nutr 2010;92:1133–44. [DOI] [PubMed] [Google Scholar]
- 22.Clarke SE, Evans S, Macdonald A, Davies P, Booth IW. Randomized comparison of a nutrient-dense formula with an energy-supplemented formula for infants with faltering growth. J Hum Nutr Diet 2007;20:329–39. [DOI] [PubMed] [Google Scholar]
- 23.Schulze KF, Stefanski M, Masterson J, Spinnazola R, Ramakrishnan R, Dell RB, Heird WC. Energy expenditure, energy balance, and composition of weight gain in low birth weight infants fed diets of different protein and energy content. J Pediatr 1987;110:753–9. [DOI] [PubMed] [Google Scholar]
- 24.Martin FP, Moco S, Montoliu I, Collino S, Da Silva L, Rezzi S, Prieto R, Kussmann M, Inostroza J, Steenhout P. Impact of breast-feeding and high- and low-protein formula on the metabolism and growth of infants from overweight and obese mothers. Pediatr Res 2014;75:535–43. [DOI] [PubMed] [Google Scholar]
- 25.Fomon SJ, Filmer LJ, Jr, Thomas LN, Anderson TA, Nelson SE. Influence of formula concentration on caloric intake and growth of normal infants. Acta Paediatr Scand 1975;64:172–81. [DOI] [PubMed] [Google Scholar]
- 26.Fomon SJ, Filer LJ, Ziegler EE, Bergmann KE, Bergmann RL. Skim milk in infant feeding. Acta Paediatr Scand 1977;66:17–30. [DOI] [PubMed] [Google Scholar]
- 27.Huet F, Lachambre E, Beck L, Van Egroo LD, Sznajder M. Evaluation of a formula with low protein content and supplemented with probiotic agents after breast milk weaning. Arch Pediatr 2006;13:1309–15. [DOI] [PubMed] [Google Scholar]
- 28.Axelsson IE, Jakobsson I, Raiha NC. Formula with reduced protein content: effects on growth and protein metabolism during weaning. Pediatr Res 1988;24:297–301. [DOI] [PubMed] [Google Scholar]
- 29.Räihä NC, Fazzolari-Nesci A, Cajozzo C, Puccio G, Monestier A, Moro G, Minoli I, Haschke-Becher E, Bachmann C, Van’t Hof M, et al. Whey predominant, whey modified infant formula with protein/energy ratio of 1.8 g/100 kcal: adequate and safe for term infants from birth to four months. J Pediatr Gastroenterol Nutr 2002;35:275–81. [DOI] [PubMed] [Google Scholar]
- 30.Turck D, Grillon C, Lachambre E, Robiliard P, Beck L, Maurin JL, Kempf C, Bernet JP, Marx J, Lebrun F, et al. Adequacy and safety of an infant formula with a protein/energy ratio of 1.8 g/100 kcal and enhanced protein efficiency for term infants during the first 4 months of life. J Pediatr Gastroenterol Nutr 2006;43:364–71. [DOI] [PubMed] [Google Scholar]
- 31.Trabulsi J, Capeding R, Lebumfacil J, Ramanujam K, Feng P, McSweeney S, Harris B, DeRusso P. Effect of an alpha-lactalbumin-enriched infant formula with lower protein on growth. Eur J Clin Nutr 2011;65:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fleddermann M, Demmelmair H, Grote V, Nikolic T, Trisic B, Koletzko B. Infant formula comparison affects energetic efficiency for growth: the BeMIM study, a randomized controlled trial. Clin Nutr 2014;33:588–95. [DOI] [PubMed] [Google Scholar]
- 33.Koletzko B, von Kries R, Closa R, Escribano J, Scaglioni S, Giovannini M, Beyer J, Demmelmair H, Gruszfeld D, Dobrzanska A, et al. ; European Childhood Obesity Trial Study Group. Lower protein in infant formula is associated with lower weight up to age 2 y: a randomized clinical trial. Am J Clin Nutr 2009;89:1836–45. [DOI] [PubMed] [Google Scholar]
- 34.Escribano J, Luque V, Ferre N, Mendez-Riera G, Koletzko B, Grote V, Demmelmair H, Bluck L, Wright A, Closa-Monasterolo R. Effect of protein intake and weight gain velocity on body fat mass at 6 months of age: the EU childhood obestiy programme. Int J Obes (Lond) 2012;36:548–53. [DOI] [PubMed] [Google Scholar]
- 35.Weber M, Grote V, Closa-Monasterolo R, Escribano J, Langhendries JP, Dain E, Giovannini M, Verduci E, Gruszfeld D, Socha P, et al. ; European Childhood Obesity Trial Study Group. Lower protein content in infant formula reduces BMI and obesity risk at school age: follow-up of a randomized trial. Am J Clin Nutr 2014;99:1041–51. [DOI] [PubMed] [Google Scholar]
- 36.Timby N, Domellöf E, Hernell O, Lönnerdal B, Domellöf M. Neurodevelopment, nutrition, and growth until 12 mo of age in infants fed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: a randomized controlled trial. Am J Clin Nutr 2014;99:860–8. [DOI] [PubMed] [Google Scholar]
- 37.Inostroza J, Haschke F, Steenhout P, Grathwohl D, Nelson SE, Ziegler EE. Low-protein formula slows weight gain in infants of overweight mothers: a randomized trial. J Pediatr Gastroenterol Nutr 2014;59:70–77. [DOI] [PMC free article] [PubMed]
- 38.Akeson PM, Axelsson IE, Raiha NC. Growth and nutrient intake in three- to twelve-month-old infants fed human milk or formulas with varying protein concentrations. J Pediatr Gastroenterol Nutr 1998;26:1–8. [DOI] [PubMed] [Google Scholar]
- 39.Roggero P, Gianni ML, Liotto N, Taroni F, Orsi A, Amato O, Morlacchi L, Piemontese P, Agosti M, Mosca F. Rapid recovery of fat mass in small for gestational age preterm infants after term. PLoS ONE 2011;6:e14489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Euser AM, Finken MJJ, Keizer-Veen MG, Hille ETM, Wit JM, Dekker FW. Associations between prenatal and infancy weight gain and BMI, fat mass, and fat distribution in young adulthood: a prospective cohort study in males and females born very preterm. Am J Clin Nutr 2005;81:480–7. [DOI] [PubMed] [Google Scholar]
- 41.Fisher D, Baird J, Payne L, Lucas P, Kleijnen J, Roberts H, Law C. Are infant size and growth related to burden of disease in adulthood? A systematic review of literature. Int J Epidemiol 2006;35:1196–210. [DOI] [PubMed] [Google Scholar]
- 42.Fenton TR, Premji SS, Al-Wassia H, Sauve RS. Higher versus lower protein intake in formula-fed low birth weight infants. Cochrane Database Syst Rev 2014;4:CD003959. [DOI] [PMC free article] [PubMed] [Google Scholar]