Abstract
Metabolic syndrome and its complications continue to rise in prevalence and show no signs of abating in the immediate future. Therefore, the search for effective treatments is a high priority in biomedical research. Products derived from botanicals have a time-honored history of use in the treatment of metabolic diseases including type 2 diabetes. Trigonella foenum-graecum, commonly known as fenugreek, is an annual herbaceous plant that has been a staple of traditional herbal medicine in many cultures. Although fenugreek has been studied in both clinical and basic research settings, questions remain about its efficacy and biologic mechanisms of action. Diosgenin, 4-hydroxyisoleucine, and the fiber component of the plant are the most intensively studied bioactive constituents present in fenugreek. These compounds have been demonstrated to exert beneficial effects on several physiologic markers including glucose tolerance, inflammation, insulin action, liver function, blood lipids, and cardiovascular health. Although insights into the molecular mechanisms underlying the favorable effects of fenugreek have been gained, we still do not have definitive evidence establishing its role as a therapeutic agent in metabolic disease. This review aims to summarize the currently available evidence on the physiologic effects of the 3 best-characterized bioactive compounds of fenugreek, with particular emphasis on biologic mechanisms of action relevant in the context of metabolic syndrome.
Keywords: fat cells, insulin action, botanicals, fenugreek, diosgenin, 4-hydroxyisoleucine
Introduction
Metabolic syndrome is a group of pathologies that includes obesity, glucose intolerance, dyslipidemia, and hypertension that are inextricably linked to diabetes mellitus and cardiovascular disease. Although the clinical features of metabolic syndrome have been revised since the term was first coined by Gerald Reaven in 1988 (1), its prevalence has increased to epidemic proportions and constitutes a grave threat to public health worldwide. This is especially alarming when considering the escalating incidence and prevalence of diabetes. According to estimates issued by the International Diabetes Federation, ~366 million people suffered globally from diabetes in 2011, and the number of diabetes cases is projected to increase to 552 million by 2030 (2). The United States stands as one of the most severely afflicted nations. The CDC estimated the number of diagnosed cases at 18.8 million, with roughly 7 million undiagnosed cases (3).
Despite the availability of several drugs effective for the treatment of pathologies associated with metabolic syndrome, particularly diabetes, there are disadvantages associated with long-term use of some of the relevant pharmaceuticals. Side effects and cost must be considered, especially because the incidence of diabetes continues to climb in the developing world, where access to conventional drugs is limited. Hence, the identification of natural products that are effective in ameliorating diabetes is a worthwhile endeavor.
Trigonella foenum-graecum (fenugreek) is an annual plant native to India and North Africa that has a long history of use in cooking and as a traditional herbal medicine in the treatment of a variety of conditions, including diabetes and hyperlipidemia (4, 5). Fenugreek has been studied in humans and animals and there is considerable evidence to suggest that it possesses therapeutic properties applicable to metabolic disease (6–8). Although therapeutics derived from plant sources are, in general, attractive because of their relative safety and tolerability compared with currently available pharmaceuticals, a 2011 outbreak of hemolytic-uremic syndrome in Germany, which affected 3842 people and caused 53 deaths, was caused by a strain of shiga toxin–producing Escherichia coli in which contaminated fenugreek sprouts were identified as the most likely transmission vehicle (9, 10). This incident highlights the need for careful safety measures and standardization of botanical products before they are made available in the marketplace (11, 12).
This review aims to summarize the body of literature that has evaluated the safety and efficacy of 3 of the most comprehensively studied bioactive compounds present in fenugreek: diosgenin, 4-hydroxyisoleucine (4-OH-Ile)5, and the soluble dietary fiber fraction of fenugreek seeds. A particular area of emphasis is an analysis of the effects of these bioactive chemical components on biologic mechanisms controlling peripheral insulin action, glucose homeostasis, and maintenance of normal blood lipids. This analysis provides a starting point for future research aimed at evaluating the potential of fenugreek as a potential therapeutic in the ongoing quest to develop new strategies for the treatment of metabolic syndrome and its complications.
Diosgenin
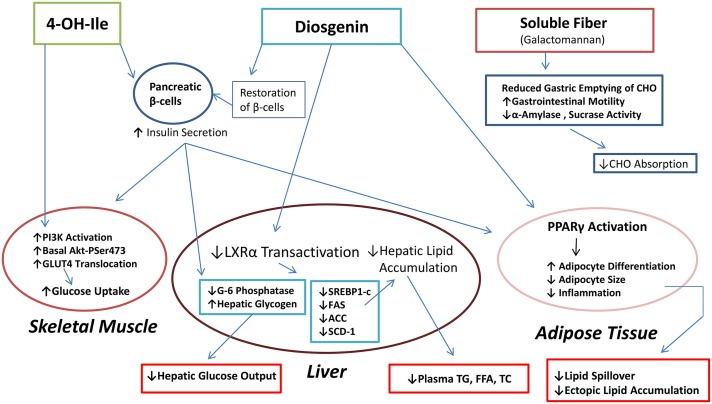
Diosgenin is a biologically active steroid sapogenin present in fenugreek. Diosgenin has been proposed to be effective against a variety of pathologies, including diabetes, hyperlipidemia, cancer, cardiovascular disease, oxidative stress, and inflammation (13–18). Studies assessing diosgenin for toxicity have shown that the compound is generally well tolerated orally in doses of up to ~500 mg/kg (19, 20). In assessing the potential of fenugreek as a therapeutic agent in combating diabetes, diosgenin has emerged as an important mediating factor in several of the biologic effects of fenugreek that contribute to the maintenance of insulin signaling and glucose homeostasis (Figure 1).
FIGURE 1.
The 3 main bioactive compounds of fenugreek and their mechanisms of action. The pancreas, skeletal muscle, liver, adipose tissue, and gut are major tissue targets of fenugreek through which serum markers such as glucose, insulin, and lipids are favorably modulated and metabolic health could thereby be improved. ACC, acetyl-CoA carboxylase; Akt-PSer473, Akt phosphorylated on Ser 473; CHO, carbohydrate; FAS, FA synthase; LXRα, liver X receptor-α; PI3K, phosphoinositide 3-kinase; SCD-1, stearoyl-CoA desaturase 1; SREBP1-c, sterol regulatory element–binding protein 1-c; TC, serum total cholesterol; 4-OH-Ile, 4-hydroxyisoleucine.
The most recent experimental evidence supporting a role for diosgenin as an antidiabetic agent has come from studies examining its effectiveness in reducing glycemia in animal models of diabetes induced pharmacologically (15, 21–23) or by a combination of drugs and diet (21, 23), although in vitro approaches have also been used (14, 24). These studies, largely performed in rodents, have assessed the effects of diosgenin on glycemic control in models of both type 1 and type 2 diabetes. These investigations have focused on various target tissues critical to the preservation of glucose homeostasis, including the pancreas, liver, and skeletal muscle. This research has yielded considerable insight into the molecular mechanisms underlying the potential of diosgenin as an antidiabetic agent. Mechanisms of action attributed to diosgenin in ameliorating experimentally induced diabetes include restoration of pancreatic β-cells (15, 21), downregulation of enzymes involved in hepatic gluconeogenesis and glucose export, upregulation of hepatic glucokinase, and increases in the amounts of hepatoprotective and antioxidant enzymes (15). Additionally, studies have begun to explore how diosgenin modulates adipose tissue function, which plays a key role in maintaining glycemic control (24). A review of the studies examining the effect of diosgenin on physiologic markers involved in metabolic syndrome is provided (Table 1).
TABLE 1.
The effect of diosgenin on markers relevant to metabolic syndrome1
| Model | Experimental outcomes | Reference |
| 3T3-L1 adipocytes, RAW 264 macrophages | ↓MCP-1, ↓TNF-α, ↓NF-κB | Hirai et al. (14) |
| Streptozotocin-induced diabetic rats | ↓BG, ↓serum LDL, ↓serum TC, ↑serum HDL, ↓Hb A1c, ↓ALT, ↓AST, ↓G6P, ↑SOD, ↑catalase, ↑GSH, ↑GK | Kalailingam et al. (15) |
| Streptozotocin-induced diabetic rats | ↓BG, ↓Hb A1c, ↑total Hb, ↑SOD, ↑catalase, ↑GSH, ↓FI | Pari et al. (16) |
| Streptozotocin-induced diabetic rats | ↓Serum TC, ↑serum HDL, ↓TBARS, ↑SOD, ↑GPx, ↑catalase | Son et al. (18) |
| Type 2 diabetic rats | ↓FFA, ↓TNF-α, ↓IL-6, ↑leptin, ↑PPARγ, ↓endoplasmic reticulum stress | Tharaheswari et al. (21) |
| Streptozotocin-induced diabetic rats | ↓BG, ↑liver glycogen | Saravanan et al. (22) |
| Type 2 diabetic rats, 3T3-L1 adipocytes | ↓BG, ↓serum TC, ↓serum TG,↓ROS, ↑SOD, ↑GSH, ↑neutral lipid accumulation | Sangeetha et al. (23) |
| Diabetic obese KK-Ay mice | ↓Adipocyte size, ↑adipogenesis, ↓macrophage infiltration, ↓adipocyte inflammation | Uemura et al. (24) |
| Diabetic obese KK-Ay mice, HepG2 cells | ↓Serum TG, ↓SREBP-1c, ↓FAS, ↓SCD-1, ↓ACC, ↓hepatic steatosis, ↓LXRα activation | Turer et al. (37) |
| NPC1L1-knockout C57BL6 mice | ↑Cholesterol excretion, ↑biliary cholesterol secretion, ↓serum TC, ↓hepatic cholesterol | Tang et al. (46) |
| Human umbilical vein endothelial cells, isolated rat aorta | ↓IKK-β, ↓NF-κB, ↓TNF-α, ↓IL-6, ↑IRS-1, ↑PI3K, ↑Akt, ↑eNOS, ↓PAI-1 | Manivannan et al. (49) |
| Chronic renal failure rats | ↑Vascular relaxation, ↑GSH, ↑eNOS, ↓serum TG, ↓ACE activity | Manivannan et al. (50) |
| Chronic renal failure rats | ↑GSH, ↑SOD, ↑catalase, ↓lipid peroxidation, ↑NO production | Salimeh et al. (51) |
| MI-induced rats | ↑GSH, ↑SOD, ↑catalase, ↓lipid peroxidation, ↓area of infarction | Badalzadeh et al. (53) |
| Hypertensive rats | ↓ROS, ↓TNF-α, ↓iNOS, ↑eNOS, ↓MAP | Ebrahimi etal. (54) |
| Ischemic rats | ↓LDH, ↓PVC, ↓VT, ↓VF | Liu et al. (48) |
| Ischemic rats | ↓TNF-α, ↓IL-6, ↓IL-1β, ↓LDH, ↑cardiac contractility | Manivannan et al. (55) |
ACC, acetyl-CoA carboxylase; ACE, angiotensin-converting enzyme; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BG, blood glucose; eNOS, endothelial NO synthase; FAS, FA synthase; FI, fasting insulin; G6P, glucose 6-phosphatase; GK, glucokinase; GSH, reduced glutathione; GPx, glutathione peroxidase; Hb A1c, glycated hemoglobin; IKK-β, inhibitor of NK-κB kinase subunit-B; iNOS, inducible NO synthase; IRS-1, insulin receptor substrate 1; LDH, lactate dehydrogenase; LXRα, liver X receptor-α; MAP, mean arterial pressure; MCP-1, monocyte chemotactic protein 1; MI, myocardial infarction; NPC1L1, Niemann-Pick C1 like 1; PAI-1, plasminogen activator inhibitor type 1; PI3K, phosphatidylinositide 3-kinase; PVC, preventricular contraction; ROS, reactive oxygen species; SCD-1, stearoyl-CoA desaturase 1; SOD, superoxide dismutase; SREBP-1c, sterol regulatory element–binding protein 1-c; TC, serum total cholesterol; total Hb, total hemoglobin; VF, ventricular fibrillation; VT, ventricular tachycardia; ↑, increase in amount or incidence; ↓, decrease in amount or incidence.
Endoplasmic reticulum (ER) stress and the unfolded protein response have been implicated in the pathogenesis of insulin resistance and diabetes, specifically in the context of β-cell dysfunction (25, 26). In a series of experiments in streptozotocin-induced type 2 diabetic rats, diosgenin attenuated pancreatic ER stress by reducing expression of the C/EBP homologous protein (CHOP) and caspases 12 and 3 (21). Diosgenin also restored normal pancreas morphology, improved serum glucose and insulin concentrations, increased amounts of antioxidant enzymes, and enhanced PPARγ expression (21). These findings were supported by a study with streptozotocin-induced diabetic rats. In this study, blood glucose (BG), glycated hemoglobin, serum LDL, total cholesterol (TC), serum TGs, alanine transaminase, glucose-6-phosphatase, serum HDL, pancreatic β-cell number, and the concentrations of several antioxidant enzymes were all restored to normal after 30 d of treatment with a daily oral dosage of 10 mg/kg of diosgenin (15). Further evidence confirming the antidiabetic potential of diosgenin was reported in a similar study with streptozotocin-induced diabetic rats, in which 45 d of oral administration of diosgenin at increasing doses (15, 30, and 60 mg/kg) resulted in a restoration of BG, carbohydrate metabolic enzymes, and glycogen content to near normal concentrations (22). These recent animal studies are in general agreement with diosgenin exerting protective effects on pancreatic function and suggest an important mechanism by which this compound could reverse several of the hallmark abnormalities characteristic of diabetes and metabolic syndrome in general.
The storage of lipids in parts of the body other than adipose tissue, known as ectopic fat accumulation, is a classic feature of metabolic syndrome intimately linked to one of its most damaging clinical consequences, cardiovascular disease (27–31). Inhibition of abnormal fat deposition is therefore a valuable component of any treatment strategy for metabolic syndrome. Of all the possible sites of ectopic lipid accumulation, the liver is probably the best studied (32, 33). Lipid synthesis in the liver is under the control of sterol regulatory element–binding proteins (SREBPs). The transcription factor SREBP-1c is vital for TG synthesis because it regulates the expression of several genes integral to FA metabolism in the liver, including acetyl-CoA carboxylase (ACC), FA synthase, and stearoyl-CoA desaturase (SCD-1) (34). Liver X receptor-α (LXRα) is an important regulator of SREBP-1c and thus plays an important role in the regulation of FA synthesis in the liver (35). A study with 4 wk of fenugreek dietary supplementation decreased plasma and hepatic TGs in KK-Ay mice subjected to a high-fat diet (HFD) (36). Gene expression analysis revealed that hepatic mRNA expression of SREBP-1c and other lipogenic genes were suppressed in the fenugreek-treated mice. Follow-up mechanistic studies in HepG2 cells demonstrated that diosgenin isolated from fenugreek accounted for fenugreek’s effects on hepatic lipid accumulation via inhibition of LXRα transactivation, which in turn decreased mRNA induction of SREBP1-c and its target genes (36). Confirmation of these findings was reported in a recent study in streptozotocin-induced diabetic rats showing that diosgenin administered for 14 d significantly reduced HFD-induced hyperglycemia and hyperlipidemia (23). Although further corroboration of these results would be advantageous, the currently available evidence supports the hypothesis that diosgenin attenuates abnormal hepatic lipid metabolism in vitro and in vivo.
The discoveries of the adipocyte-derived hormones leptin and adiponectin during the 1990s have led to recognition of the indispensable role played by adipose tissue in the maintenance of proper metabolic function (37, 38). The secretory factors produced by fully functional adipose tissue not only serve to sustain insulin sensitivity and glucose homeostasis but also regulate feeding behavior (39, 40). Conversely, when adipocyte function is compromised and inflammation occurs, it results in adverse metabolic consequences (41). Although there is a great deal of literature supporting the use of fenugreek as an antidiabetic agent, efforts to understand how bioactive compounds from this botanical could beneficially modulate adipocyte metabolism are in their infancy. However, some evidence supporting a role for diosgenin in enhancing adipocyte function has recently emerged. Diosgenin has anti-inflammatory properties in a coculture model with 3T3-L1 adipocytes and RAW macrophages. In this study diosgenin significantly decreased the production of the proinflammatory mediators TNF-α, monocyte chemoattractant protein 1 (MCP-1), and NO (14). Diosgenin can also promote adipocyte differentiation. An in vitro study of 3T3-L1 adipocytes revealed that diosgenin treatment enhanced adipogenesis and lipid accumulation while also reducing the expression of inflammatory factors (24). In addition, it was demonstrated that fenugreek ameliorated diabetes, reduced adipose tissue inflammation, decreased adipocyte size, inhibited macrophage infiltration into adipose tissue, and increased the mRNA expression of adipogenic genes in KK-Ay mice challenged with HFD (24). Although confirming the antidiabetic effect of diosgenin in streptozotocin-induced diabetic rats, a recent study has also demonstrated that diosgenin exerts its adipogenic effects at least in part by acting as a PPARγ agonist (23). These findings are of substantial interest because of the critical function played by PPARγ as the master genetic regulator of adipocyte differentiation and the chief molecular target of several insulin-sensitizing drugs (42). The thiazolidinediones, in particular, promote insulin sensitivity through their activation of PPARγ and the effectiveness of this class of drugs in the treatment of type 2 diabetes has been well established. However, adverse events associated with their use are widely reported in the literature and their use is associated with increased risk of weight gain, fluid retention, bone loss, and congestive heart failure (43, 44). In view of both the benefits and the risks associated with the use of thiazolidinediones, the development of antidiabetic agents with the insulin-sensitizing effects of classic PPARγ agonists without the undesirable side effects would provide attractive new therapeutic possibilities. Future work in this area is worthwhile and botanical products such as fenugreek containing diosgenin could have great potential in this area of investigation.
Evidence has also indicated that diosgenin could be useful in the treatment of metabolic disease by regulating cholesterol homeostasis. Aside from the studies in obese diabetic animals discussed previously, which showed that diosgenin treatment was effective in reversing hyperlipidemia, mechanistic studies indicate that diosgenin affects serum lipids by enhancing biliary cholesterol excretion (45). Diosgenin promotes fecal cholesterol excretion independently of Niemann-Pick C1-like 1 (NPC1L1), a protein present in the apical membrane of absorptive enterocytes that regulates intestinal cholesterol absorption and is targeted by ezetimibe, a cholesterol-lowering drug (45, 46). This finding is of interest in the context of botanical remedies because curcumin, a bioactive compound present in turmeric, lowers cholesterol through an NPC1L1-dependent mechanism (47). This raises the possibility that supplementation with turmeric and fenugreek could act additively in lowering cholesterol, although experimental evidence supporting this concept is still unavailable.
Diosgenin has also displayed potential as a therapeutic adjunct in the treatment of cardiovascular disease. Animal studies in the last few years have yielded evidence demonstrating that diosgenin enhances endothelial function and inhibits inflammation, insulin resistance, and oxidative stress in the vasculature induced by diabetes and palmitate treatment (16, 48). Recent studies in animal models of chronic renal failure have shown that diosgenin protects vascular function by attenuating aortic calcification, increasing the expression of endothelial NO synthase, and inhibiting osteochondrogenic differentiation in aortic vascular smooth muscle cells (49, 50). Further evidence for the cardioprotective effects of diosgenin has emerged from animal studies using several models of cardiovascular disease including pulmonary hypertension, ischemia-reperfusion injury, and isoproterenol-induced myocardial infarction (51–54). These studies support the general conclusion that diosgenin possesses anti-inflammatory, antioxidant, and vasodilatory properties. These attributes support a potential role for diosgenin in reducing cardiovascular disease risk.
The evidence thus far reported in the literature on diosgenin supports the idea that this fenugreek component exerts beneficial effects on several physiologic markers relevant to metabolic syndrome. Diosgenin exerts effects on the pancreas, liver, skeletal muscle and adipose tissue that restore insulin sensitivity, glucose homeostasis, and normal blood lipids in diabetic animals (Figure 1) (15, 21, 22, 24, 48). By restoring pancreatic β-cell function and attenuating pancreatic ER and oxidative stress diosgenin has been shown to promote insulin secretion and preserve normal glucose concentrations even in the presence of negative stimuli such as streptozotocin treatment or HFD (21). Furthermore, diosgenin can enhance insulin action in skeletal muscle and adipose tissue (21, 22, 24). This enhanced insulin action in peripheral tissues, acting in a concerted manner with augmented insulin secretion, likely accounts for the advantageous effects on glucose metabolism observed in diabetic animals treated with diosgenin. Additional mechanisms underlying the favorable metabolic effects of diosgenin are reduced hyperlipidemia mediated by increased biliary cholesterol excretion and reduced hepatic lipid accumulation via inhibition of LXRα, SREBP-1c, and other lipogenic factors in the liver (36, 45). Moreover, diosgenin positively modulates vascular function and thereby could act as a cardioprotective agent (16, 48, 49, 53–55). Taken together, these studies on diosgenin have yielded valuable insight on how this bioactive compound and fenugreek could promote metabolic health in an obesogenic environment.
4-OH-Ile
4-OH-Ile is a novel branched-chain amino acid derivative that has been shown to be present in fenugreek seeds and has been postulated to account, at least in part, for fenugreek’s antidiabetic effects. 4-OH-Ile is only present in plants and is particularly abundant in fenugreek seeds, where it comprises ~80% of the total content of free amino acids (56–58). The first studies investigating the antidiabetic properties of 4-OH-Ile determined that its effects on glycemia were attributable to its potency in stimulating insulin secretion (57). This insulinotropic effect was initially demonstrated in isolated rat and human pancreatic islet cells and in isolated perfused rat pancreas (57). Additional in vivo studies confirmed and extended these findings to animal models, where improved glucose and insulin tolerance, improved insulin secretion, and reduced hyperglycemia were observed in both diabetic and normal rats and dogs (59). A recurring finding of particular interest from these studies was the observation that 4-OH-Ile functioned as an insulin secretagogue only in the presence of elevated BG concentrations in the moderate (8.3 mM) to high (16.7 mM) range (57, 59, 60).
Although reversing defective insulin secretion is clearly beneficial in a diabetic state, enhancing insulin sensitivity in hepatic and peripheral tissues is also a valuable treatment modality. Broca and colleagues (61) conducted the first reported studies that specifically assessed the potency of 4-OH-Ile as an insulin-sensitizing agent. The results of these experiments demonstrated the efficacy of 4-OH-Ile as an insulin sensitizer in 2 rat models. Using the hyperinsulinemic clamp method, improvements in insulin sensitivity were detected in sucrose-lipid–fed rats, where peripheral glucose uptake was increased, and in Zucker fa/fa rats, where hepatic glucose output was decreased. Acute in vivo injection of 4-OH-Ile also resulted in the insulin receptor substrate 1 (IRS-1)–related activation of phosphatidylinositide 3-kinase (PI3K) in insulin-sensitive tissues (61).
Recent studies have revealed further insight into the therapeutic properties of 4-OH-Ile by probing its effects in animal models of diabetes and dyslipidemia. Narender et al. (62) broadened the scope of previous investigations by studying the effect of 4-OH-Ile on dyslipidemia. In this study, results showed that treatment of dyslipidemic hamsters with 4-OH-Ile led to decreases in plasma TGs, TC, and FFAs while simultaneously increasing the ratio of serum HDL cholesterol:TC by 39%. Haeri and colleagues (63) examined the effect of 4-OH-Ile from fenugreek in streptozotocin-induced diabetic and fructose-fed rats and demonstrated that markers of liver function and glycemic control improved after 8 wk of treatment at a dose of 50 mg/kg. Specifically, the authors found that in fructose-fed rats BG and markers of liver damage (aspartate aminotransferase and alanine aminotransferase) were restored to amounts near those observed in control animals, whereas in streptozotocin-diabetic rats serum HDL cholesterol increased. Further confirmation of the antidiabetic and antidyslipidemic effects of 4-OH-Ile were observed in the leptin receptor–deficient db/db mouse; improvements in BG, insulin, and blood lipids were reported after treatment with 4-OH-Ile (64). The beneficial effects of 4-OH-Ile appear to be also applicable to a model of type 1 diabetes, where 4 wk of 4-OH-Ile treatment decreased BG while concomitantly restoring blood lipid and uric acid concentrations to those found in normal nondiabetic controls (65).
The latest research efforts aimed at understanding the molecular mechanisms of 4-OH-Ile action have used cell culture models. In cultured rat muscle cells, glucose uptake and glucose transporter 4 (GLUT4) translocation to the plasma membrane were increased in response to a 16-h exposure to 4-OH-Ile (66). Additionally, 4-OH-Ile treatment increased the basal phosphorylation of Akt (Ser-473), while mRNA expression of total Akt, IRS-1, GLUT4, and glycogen synthase kinase-3β (GSK-3β) were unchanged. Another group extended these findings in cultured muscle cells by demonstrating that 4-OH-Ile ameliorated FA-induced insulin resistance in L6 myotubes (67). These experiments showed that 4-OH-Ile restored insulin-stimulated glucose uptake and GLUT4 recruitment to the plasma membrane in response to palmitate treatment via induction of IRS-1 phosphorylation. Interestingly, this was the first study to demonstrate that 4-OH-Ile could inhibit both the palmitate-induced production of reactive oxygen species and the associated inflammation, as was demonstrated by reduced activation of NF-κB, c-Jun N-terminal kinase isoforms 1 and 2 (JNK1/2), extracellular signal-regulated kinase isoforms 1 and 2 (ERK1/2), and p38 MAPK (67).
An understanding of the effects of 4-OH-Ile on adipocytes in the context of insulin resistance and diabetes remains a relatively undeveloped area of investigation. To date, only 1 study has examined the effects of 4-OH-Ile on adipocytes (68). This study demonstrated that treatment with 4-OH-Ile increased glucose uptake in insulin-resistant 3T3-L1 adipocytes in a dose-dependent manner. 4-OH-Ile also showed potential as an anti-inflammatory agent by reducing TNF-α mRNA expression and secretion. Given the growing recognition of the importance of the adipocyte in the maintenance of glucose homeostasis and insulin sensitivity, further studies of the effects of 4-OH-Ile on adipocyte function will be critical in development of 4-OH-Ile and other fenugreek components as potential therapeutics for metabolic syndrome (Table 2).
TABLE 2.
Effects of 4-OH-Ile and isolated soluble fiber from fenugreek on metabolic syndrome1
| Fenugreek derivative | Model | Experimental outcome | Reference |
| 4-OH-Ile | Isolated human and rat pancreas | ↑GSIS | Sauvaire et al. (57) |
| 4-OH-Ile | Normal and type 2 diabetic rats, isolated rat islets | ↑Oral glucose tolerance, ↑GSIS | Broca et al. (59) |
| 4-OH-Ile | Isolated rat islets | ↑GSIS | Broca et al. (60) |
| 4-OH-Ile | Zucker fa/fa rats, high fat + sucrose-fed rats | ↑Oral glucose tolerance, ↑IS, ↓HGP, ↑PI3K, ↓FI | Broca et al. (61) |
| 4-OH-Ile | Hamsters | ↓Serum TG, ↓TC, ↓FFA, ↑HDL:TC | Narender et al. (62) |
| 4-OH-Ile | Type 2 diabetic rats | ↓BG, ↑ serum HDL, ↓ALT, ↓AST | Haeri et al. (63) |
| 4-OH-Ile | C57BL/db/db mice | ↓BG, ↓FI, ↓serum TG, ↓serum TC, ↓serum LDL, ↑serum HDL | Singh et al. (64) |
| 4-OH-Ile | Type 1 diabetic rats | ↓BG, ↓serum TC, ↓serum LDL, ↓serum TG, ↑serum HDL | Haeri et al. (65) |
| 4-OH-Ile | Skeletal muscle (L6 myocytes) | ↑Glucose uptake, ↑pAkt, ↑PI3K, ↑GLUT4 | Jaiswal et al. (66) |
| 4-OH-Ile | Insulin-resistant skeletal muscle (L6 myocytes) | ↑pIRS-1, ↑PI3K, ↑pAkt, ↓ROS, ↓NF-κB, ↓JNK1/2, ↓p38 MAPK | Maurya et al. (67) |
| 4-OH-Ile | Insulin-resistant 3T3 L1 adipocytes | ↓TNF-α, ↑glucose uptake | Yu et al. (68) |
| Soluble fiber | Type 1 and type 2 diabetic rats | ↓Postprandial glucose, ↓sucrase activity, ↓BG, ↑liver glycogen, ↓ROS | Hannan et al. (75) |
| Soluble fiber | Lean and obese rats | ↓Intestinal glucose uptake | Srichamroen et al. (76) |
| Soluble fiber | Diabetic rats | ↓Lipase activity, ↓lipid absorption, ↓BG, ↓ALT, ↓AST, ↓uric acid | Hamden et al. (77) |
| Soluble fiber | Obese rats | ↓Body weight, ↓serum TG, ↓serum TC, ↓HMG-CoA reductase activity, ↑biliary cholesterol excretion | Ramulu et al. (78) |
| Soluble fiber | Obese rats | ↓Body weight, ↓fat pad weight, ↓BG, ↓FI, ↓ALT, ↓AST, ↓LDH, ↓TBARS, ↓serum TC, ↑GSH, ↑SOD, ↑catalase | Kumar et al. (73) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BG, blood glucose; FI, fasting insulin; GLUT4, glucose transporter 4; GSH, reduced glutathione; GSIS, glucose-stimulated insulin secretion; HGP, hepatic glucose production; HMG-CoA, β-hydroxy-β-methylglutaryl CoA; IS, insulin sensitivity; JNK, c-Jun N-terminal kinase; LDH, lactate dehydrogenase; pAkt, Akt phosphorylated on Ser473; PI3K, phosphatidylinositide 3-kinase; pIRS-1, insulin receptor substrate 1 tyrosine phosphorylation; ROS, reactive oxygen species; SOD, superoxide dismutase; TC, serum total cholesterol; 4-OH-Ile, 4-hydroxyisoleucine; ↑, increase in amount of incidence; ↓, decrease in amount or incidence.
Considering that the pathophysiology of type 2 diabetes typically progresses from insulin resistance to a failure of pancreatic β-cells to secrete sufficient insulin to control glycemia (61), the demonstrated insulinotropic properties of 4-OH-Ile suggest that it has potential as an antidiabetic pharmacologic compound. A particularly attractive feature of 4-OH-Ile is that it exhibits its secretory effect on insulin only in the presence of elevated glucose (≥8.3 mmol/l) concentrations (57). This could potentially overcome a common drawback of sulfonylureas, which carry the risk of inducing hypoglycemia (69). Although several studies have demonstrated that 4-OH-Ile stimulates insulin secretion by direct action on pancreatic islets, there is also recent evidence that 4-OH-Ile can restore insulin sensitivity in skeletal muscle with insulin resistance induced by treatment with FAs (66, 67). One recent study has produced similar findings in 3T3-L1 adipocytes, where 4-OH-Ile restored insulin sensitivity and attenuated TNF-α expression in response to palmitate-induced insulin resistance (68). The effect of 4-OH-Ile in potentiating glucose-dependent insulin secretion and its insulin-sensitizing properties, combined with its absence of acute toxicity, indicate that this novel amino acid found in fenugreek could play a part in strategies using natural products to combat metabolic syndrome.
Soluble Fiber
Dietary fiber is widely accepted as a critical component of sound nutrition and there is substantial evidence for the role of fiber in health maintenance (70, 71). Fenugreek is rich in dietary fiber, which comprises between 45% and 50% of the seeds of the plant that are commonly used in cooking (72, 73). The fiber component of fenugreek (32% insoluble and 13% soluble) (72) seeds is largely made up of galactomannans, which are polysaccharides consisting of a mannose backbone linked to galactose side groups (74). The dietary fiber fraction of fenugreek has been studied for its biologic effect, particularly in a diabetic context, and has been found to enhance glycemic control. This effect of the fiber content of fenugreek has been attributed to its ability to inhibit lipid and carbohydrate-hydrolyzing enzymes in the digestive system (75–78), which is a well-established mechanism by which fiber has been shown to inhibit lipid and glucose absorption (79) and thereby decrease postprandial hyperglycemia and hyperlipidemia (80, 81). Although synthetic disaccharidase and lipase inhibitors are effective in reducing glucose and lipid concentrations, their use often has undesirable side effects, including diarrhea and abdominal pain (82). Lipase and glucosidase inhibitors derived from botanical sources in the diet could be useful in this regard because they tend to be well tolerated and have relatively benign side effect profiles compared with many synthetics.
Data from animal studies have provided evidence that fiber isolated from fenugreek has beneficial effects on several markers pertaining to metabolic disease. The soluble dietary fiber fraction isolated from fenugreek suppressed postprandial hyperglycemia and lowered serum lipids in diabetic rats (75, 83). These effects were attributed to delayed gastric emptying of carbohydrate, inhibition of intestinal lipase and sucrase activity, and increased gut motility. Interestingly, these authors also found that the fiber fraction increased both insulin-stimulated and basal glucose uptake in 3T3-L1 adipocytes and augmented liver glycogen content (75). These data argue in favor of increased insulin action in peripheral tissues induced by fiber isolated from fenugreek. Similar findings have been reported in rats in which a comparison study of different types of fiber resulted in decreased lipidemia because of reduced hepatic production of VLDL (84).
Fiber from fenugreek has also been demonstrated to attenuate the damaging effects of an atherogenic diet in rats in which decreases in serum LDL oxidation and homocysteine were observed with concurrent increases in glutathione and α-tocopherol (85). Improvements in glucose tolerance, diminished plasma TGs, and reduced visceral fat accumulation have also been reported in rats fed a high-sucrose diet (86). Galactomannan’s inhibition of intestinal glucose uptake and antihyperlipidemic effect has been confirmed (76, 78). In addition to these effects, fenugreek galactomannan has also displayed protective effects on hepatic and renal function in a diabetic context (77). Treatment with galactomannan reduced serum concentrations of aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, urea, and creatinine in streptozotocin-induced diabetic rats (77).
The abundant dietary fiber component of fenugreek, composed mainly of the indigestible polysaccharide galactomannan, has been isolated and shown to exhibit antidiabetic properties (75–78, 86). These properties appear to be mediated by the ability of galactomannan to reduce absorption of dietary carbohydrates and lipids by inhibition of intestinal lipases and carbohydrate-hydrolyzing enzymes (75–77). Additionally, galactomannan has demonstrated antiatherogenic effects via decreased serum LDL oxidation and homocysteine (85). Galactomannan can also exert protective effects on the liver and kidney in a diabetic context (77). These findings indicate that the beneficial effects of fenugreek are, at least in part, mediated by its fiber content. Future studies aimed at assessing the relative contribution of fiber from fenugreek compared with its other bioactive constituents on metabolic health would be worthwhile. Also, research that assesses the effect of fenugreek’s fiber content on the gut microbiome could further clarify the mechanisms by which fenugreek favorably affects metabolic health.
Conclusions and Future Directions
The preponderance of the evidence summarized in the present review indicates that fenugreek has bioactive chemical components potentially useful as a part of new strategies to treat metabolic disease. Nonetheless, questions remain and more experiments will be required. This is particularly true when considering the available evidence from human studies. Data from clinical trials evaluating the effect of fenugreek on glycemia in people with diabetes indicate that fenugreek intake could reduce fasting BG and glycated hemoglobin (87). However, the results of these studies have been inconsistent and methodologic concerns exist. For example, participants have varied in terms of their diabetes status, fenugreek dosages have ranged widely from as low as <2 g/d to as much as 25 g/d, the durations of the studies vary considerably, the fenugreek preparations used in these studies have not been standardized or consistently tested for chemical composition, and the randomization methods, blinding protocols, and allocation concealment methods have not been consistently reported (87). Until results from clinical trials that overcome these methodologic issues become available, there remains insufficient evidence to support recommending the use of fenugreek as a therapeutic agent against diabetes. In addition to these concerns regarding human clinical trials, well-controlled studies designed to determine the relative mechanistic contributions to enhanced insulin sensitivity, reduced lipidemia, and improved liver and cardiovascular status conferred by whole extracts of fenugreek vs. individual bioactive components are necessary to better define how effects of this botanical are mediated.
The beneficial effects of fenugreek on metabolic status are better characterized in some tissues than in others. Adipose tissue in particular remains to be explored in greater detail. Healthy fat cells are indispensable to robust metabolic health by virtue of their endocrine function and capacity to safely store lipids. The effect of fenugreek on adipocyte function has only begun to be studied. Although there is some evidence to suggest that fenugreek could positively affect fat cell development and function (14, 23), these results await independent confirmation and more detailed characterization of the effect of fenugreek on both the fat cell directly and its interaction with other insulin-sensitive tissues via adipokine secretion. The possibility exists that fenugreek could exert global effects on metabolism that might promote overall health by enhancing adipocyte function.
The gut microbiome is a burgeoning area of research interest because of the complex role played by intestinal microbes in health and disease (88–90). In mice, evidence has demonstrated that changing the gut microbiome by fecal transplantation induces changes in metabolic phenotype and that several health conditions can be triggered by disruption of the intestinal microflora (91, 92). Furthermore, it has been shown that diet can adversely or beneficially modulate the gut microbiome and thus can have a potentially dramatic influence on health and disease (93). The soluble fiber content of fenugreek modulates gastrointestinal motility, gastric emptying, and digestive enzymes and thus presents a plausible general mechanism by which fenugreek could alter the gut microbiome. Future research directed at exploring whether and how fenugreek might favorably affect the intestinal microbial environment has the potential to reveal new insights into how natural products such as fenugreek could serve to ameliorate the burden of metabolic disease.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ACC, acetyl-CoA carboxylase; BG, blood glucose; CHOP, C/EBP homologous protein; ER, endoplasmic reticulum; ERK1/2, extracellular signal-related kinases isoforms 1 and 2; GLUT4, glucose transporter 4; GSK-3β, glycogen synthase kinase-3β; HFD, high-fat diet; IRS-1, insulin receptor substrate 1; JNK, c-Jun N-terminal kinase; LXRα, liver X receptor-α; MCP-1, monocyte chemotactic protein 1; NPC1L1, Niemann-Pick C1 like 1; PI3K, phosphatidylinositide 3-kinase; SCD-1, stearoyl-CoA desaturase 1; SREBP-1c, sterol regulatory element–binding protein 1-c; TC, total cholesterol; 4-OH-Ile, 4-hydroxyisoleucine.
References
- 1.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988;37:1595–607. [DOI] [PubMed] [Google Scholar]
- 2.Guariguata L, Whiting D, Weil C, Unwin N. The International Diabetes Federation diabetes atlas methodology for estimating global and national prevalence of diabetes in adults. Diabetes Res Clin Pract 2011;94:322–32. [DOI] [PubMed] [Google Scholar]
- 3.CDC. National diabetes fact sheet: National estimates and general information on diabetes and pre-diabetes in the United States. Atlanta (GA): US Department of Health and Human Services, CDC; 2011.
- 4.Saxena A, Vikram NK. Role of selected Indian plants in management of type 2 diabetes: a review. J Altern Complement Med 2004;10:369–78. [DOI] [PubMed] [Google Scholar]
- 5.Wang E, Wylie-Rosett J. Review of selected Chinese herbal medicines in the treatment of type 2 diabetes. Diabetes Educ 2008;34:645–54. [DOI] [PubMed] [Google Scholar]
- 6.Rizvi SI, Mishra N. Traditional Indian medicines used for the management of diabetes mellitus. J Diabetes Res 2013;3013:712092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Z, Wang J, Chan P. Treating type 2 diabetes mellitus with traditional Chinese and Indian medicinal herbs. Evid Based Complement Alternat Med 2013;2013:343594. [DOI] [PMC free article] [PubMed]
- 8.Yadav UC, Baquer NZ. Pharmacological effects of Trigonella foenum-graecum L. in health and disease. Pharm Biol 2014;52:243–54. [DOI] [PubMed] [Google Scholar]
- 9.Balabanova Y, Klar S, Delere Y, Wilking H, Faber MS, Lassen SG, Gilsdorf A, Dupke S, Nitschke M, Sayk F, et al. Serological evidence of asymptomatic infections during Escherichia coli O104:H4 outbreak in Germany in 2011. PLoS ONE 2013;8:e73052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchholz U, Bernard H, Werber D, Bohmer MM, Remschmidt C, Wilking H, Delere Y, an der Heiden M, Adlhoch C, Dreesman J, et al. German outbreak of Escherichia coli O104:H4 associated with sprouts. N Engl J Med 2011;365:1763–70. [DOI] [PubMed] [Google Scholar]
- 11.Cohen PA. American roulette–contaminated dietary supplements. N Engl J Med 2009;361:1523–5. [DOI] [PubMed] [Google Scholar]
- 12.Cohen PA. Hazards of hindsight–monitoring the safety of nutritional supplements. N Engl J Med 2014;370:1277–80. [DOI] [PubMed] [Google Scholar]
- 13.Das S, Dey KK, Dey G, Pal I, Majumder A. MaitiChoudhury S, Kundu SC, Mandal M. Antineoplastic and apoptotic potential of traditional medicines thymoquinone and diosgenin in squamous cell carcinoma. PLoS ONE 2012;7:e46641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirai S, Uemura T, Mizoguchi N, Lee JY, Taketani K, Nakano Y, Hoshino S, Tsuge N, Narukami T, Yu R, et al. Diosgenin attenuates inflammatory changes in the interaction between adipocytes and macrophages. Mol Nutr Food Res 2010;54:797–804. [DOI] [PubMed] [Google Scholar]
- 15.Kalailingam P, Kannaian B, Tamilmani E, Kaliaperumal R. Efficacy of natural diosgenin on cardiovascular risk, insulin secretion, and beta cells in streptozotocin (STZ)-induced diabetic rats. Phytomedicine 2014;21:1154–61. [DOI] [PubMed] [Google Scholar]
- 16.Pari L, Monisha P, Jalaludeen AM. Beneficial role of diosgenin on oxidative stress in aorta of streptozotocin induced diabetic rats. Eur J Pharmacol 2012;691:143–50. [DOI] [PubMed] [Google Scholar]
- 17.Yamada T, Hoshino M, Hayakawa T, Ohhara H, Yamada H, Nakazawa T, Inagaki T, Iida M, Ogasawara T, Uchida A, et al. Dietary diosgenin attenuates subacute intestinal inflammation associated with indomethacin in rats. Am J Physiol 1997;273:G355–64. [DOI] [PubMed] [Google Scholar]
- 18.Son IS, Kim JH, Sohn HY, Son KH, Kim JS, Kwon CS. Antioxidative and hypolipidemic effects of diosgenin, a steroidal saponin of yam (Dioscorea spp.), on high-cholesterol fed rats. Biosci Biotechnol Biochem 2007;71:3063–71. [DOI] [PubMed] [Google Scholar]
- 19.Lima CM, Lima AK, Melo MG, Serafini MR, Oliveira DL, de Almeida EB, Barreto RS, Nogueira PC, Moraes VR, Oliveira ER, et al. Bioassay-guided evaluation of Dioscorea villosa—an acute and subchronic toxicity, antinociceptive and anti-inflammatory approach. BMC Complement Altern Med 2013;13:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin Y, Wu X, Huang W, Gong G, Li D, He Y, Zhao Y. Acute toxicity and sub-chronic toxicity of steroidal saponins from Dioscorea zingiberensis C.H.Wright in rodents. J Ethnopharmacol 2009;126:543–50. [DOI] [PubMed] [Google Scholar]
- 21.Tharaheswari M, Jayachandra Reddy N, Kumar R, Varshney KC, Kannan M, Sudha Rani S. Trigonelline and diosgenin attenuate ER stress, oxidative stress-mediated damage in pancreas and enhance adipose tissue PPARgamma activity in type 2 diabetic rats. Mol Cell Biochem 2014;396:161–74. [DOI] [PubMed] [Google Scholar]
- 22.Saravanan G, Ponmurugan P, Deepa MA, Senthilkumar B. Modulatory effects of diosgenin on attenuating the key enzymes activities of carbohydrate metabolism and glycogen content in streptozotocin-induced diabetic rats. Can J Diabetes 2014;38:409–14. [DOI] [PubMed] [Google Scholar]
- 23.Sangeetha MK. ShriShri Mal N, Atmaja K, Sali VK, Vasanthi HR. PPAR's and diosgenin a chemico biological insight in NIDDM. Chem Biol Interact 2013;206:403–10. [DOI] [PubMed] [Google Scholar]
- 24.Uemura T, Hirai S, Mizoguchi N, Goto T, Lee J-Y, Taketani K, Nakano Y, Shono J, Hoshino S, Tsuge N, et al. Diosgenin present in fenugreek improves glucose metabolism by promoting adipocyte differentiation and inhibiting inflammation in adipose tissues. Mol Nutr Food Res 2010;54:1596–608. [DOI] [PubMed] [Google Scholar]
- 25.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004;306:457–61. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Kouri G, Wollheim CB. ER stress and SREBP-1 activation are implicated in beta-cell glucolipotoxicity. J Cell Sci 2005;118:3905–15. [DOI] [PubMed] [Google Scholar]
- 27.Lim S, Meigs JB. Ectopic fat and cardiometabolic and vascular risk. Int J Cardiol 2013;169:166–76. [DOI] [PubMed] [Google Scholar]
- 28.Morelli M, Gaggini M, Daniele G, Marraccini P, Sicari R, Gastaldelli A. Ectopic fat: the true culprit linking obesity and cardiovascular disease? Thromb Haemost 2013;110:651–60. [DOI] [PubMed] [Google Scholar]
- 29.Shimabukuro M, Kozuka C, Taira S, Yabiku K, Dagvasumberel M, Ishida M, Matsumoto S, Yagi S, Fukuda D, Yamakawa K, et al. Ectopic fat deposition and global cardiometabolic risk: new paradigm in cardiovascular medicine. J Med Invest 2013;60:1–14. [DOI] [PubMed] [Google Scholar]
- 30.Byrne CD, Targher G. Ectopic fat, insulin resistance, and nonalcoholic fatty liver disease: implications for cardiovascular disease. Arterioscler Thromb Vasc Biol 2014;34:1155–61. [DOI] [PubMed] [Google Scholar]
- 31.Lim S, Meigs JB. Links between ectopic fat and vascular disease in humans. Arterioscler Thromb Vasc Biol 2014;34:1820–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang YY, Gusdon AM, Qu S. Nonalcoholic fatty liver disease: molecular pathways and therapeutic strategies. Lipids Health Dis 2013;12:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yilmaz Y, Younossi ZM. Obesity-associated nonalcoholic fatty liver disease. Clin Liver Dis 2014;18:19–31. [DOI] [PubMed] [Google Scholar]
- 34.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest 2002;109:1125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edwards PA, Kast HR, Anisfeld AM. BAREing it all: the adoption of LXR and FXR and their roles in lipid homeostasis. J Lipid Res 2002;43:2–12. [PubMed] [Google Scholar]
- 36.Uemura T, Goto T, Kang MS, Mizoguchi N, Hirai S, Lee JY, Nakano Y, Shono J, Hoshino S, Taketani K, et al. Diosgenin, the main aglycon of fenugreek, inhibits LXR alpha activity in HepG2 cells and decreases plasma and hepatic triglycerides in obese diabetic mice. J Nutr 2011;141:17–23. [DOI] [PubMed] [Google Scholar]
- 37.Turer AT, Hill JA, Elmquist JK, Scherer PE. Adipose tissue biology and cardiomyopathy: translational implications. Circ Res 2012;111:1565–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell 2014;156:20–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia 2012;55:2319–26. [DOI] [PubMed] [Google Scholar]
- 40.Bluher M. Adipokines—removing road blocks to obesity and diabetes therapy. Mol Metab 2014;3:230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mraz M, Haluzik M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J Endocrinol 2014;222:R113–27. [DOI] [PubMed] [Google Scholar]
- 42.Spiegelman BM. Peroxisome proliferator-activated receptor gamma: a key regulator of adipogenesis and systemic insulin sensitivity. Eur J Med Res 1997;2:457–64. [PubMed] [Google Scholar]
- 43.Cariou B, Charbonnel B, Staels B. Thiazolidinediones and PPARgamma agonists: time for a reassessment. Trends Endocrinol Metab 2012;23:205–15. [DOI] [PubMed] [Google Scholar]
- 44.Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM. PPAR gamma signaling and metabolism: the good, the bad and the future. Nat Med 2013;19:557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Temel RE, Brown JM, Ma Y, Tang W, Rudel LL, Ioannou YA, Davies JP, Yu L. Diosgenin stimulation of fecal cholesterol excretion in mice is not NPC1L1 dependent. J Lipid Res 2009;50:915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang W, Jia L, Ma Y, Xie P, Haywood J, Dawson PA, Li J, Yu L. Ezetimibe restores biliary cholesterol excretion in mice expressing Niemann-Pick C1-Like 1 only in liver. Biochim Biophys Acta 2011;1811:549–55. [DOI] [PMC free article] [PubMed]
- 47.Feng D, Ohlsson L, Duan RD. Curcumin inhibits cholesterol uptake in Caco-2 cells by down-regulation of NPC1L1 expression. Lipids Health Dis 2010;9:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu K, Zhao W, Gao X, Huang F, Kou J, Liu B. Diosgenin ameliorates palmitate-induced endothelial dysfunction and insulin resistance via blocking IKKbeta and IRS-1 pathways. Atherosclerosis 2012;223:350–8. [DOI] [PubMed] [Google Scholar]
- 49.Manivannan J, Balamurugan E, Silambarasan T, Raja B. Diosgenin improves vascular function by increasing aortic eNOS expression, normalize dyslipidemia and ACE activity in chronic renal failure rats. Mol Cell Biochem 2013;384:113–20. [DOI] [PubMed] [Google Scholar]
- 50.Manivannan J, Shanthakumar J, Arunagiri P, Raja B, Balamurugan E. Diosgenin interferes coronary vasoconstriction and inhibits osteochondrogenic transdifferentiation of aortic VSMC in CRF rats. Biochimie 2014;102:183–7. [DOI] [PubMed] [Google Scholar]
- 51.Salimeh A, Mohammadi M, Rashidi B. Preconditioning with diosgenin and treadmill exercise preserves the cardiac toxicity of isoproterenol in rats. J Physiol Biochem 2013;69:255–65. [DOI] [PubMed] [Google Scholar]
- 52.Ahmed LA, Obaid AA, Zaki HF, Agha AM. Role of oxidative stress, inflammation, nitric oxide and transforming growth factor-beta in the protective effect of diosgenin in monocrotaline-induced pulmonary hypertension in rats. Eur J Pharmacol 2014;740:379–87. [DOI] [PubMed] [Google Scholar]
- 53.Badalzadeh R, Yousefi B, Majidinia M, Ebrahimi H. Anti-arrhythmic effect of diosgenin in reperfusion-induced myocardial injury in a rat model: activation of nitric oxide system and mitochondrial KATP channel. J Physiol Sci 2014;64:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ebrahimi H, Badalzadeh R, Mohammadi M, Yousefi B. Diosgenin attenuates inflammatory response induced by myocardial reperfusion injury: role of mitochondrial ATP-sensitive potassium channels. J Physiol Biochem 2014;70:425–32. [DOI] [PubMed] [Google Scholar]
- 55.Manivannan J, Barathkumar TR, Sivasubramanian J, Arunagiri P, Raja B, Balamurugan E. Diosgenin attenuates vascular calcification in chronic renal failure rats. Mol Cell Biochem 2013;378:9–18. [DOI] [PubMed] [Google Scholar]
- 56.Fowden L, Pratt HM, Smith A. 4-Hydroxyisoleucine from seed of Trigonella-Foenum-Graecum. Phytochemistry 1973;12:1707–11. [Google Scholar]
- 57.Sauvaire Y, Petit P, Broca C, Manteghetti M, Baissac Y, Fernandez-Alvarez J, Gross R, Roye M, Leconte A, Gomis R, et al. 4-Hydroxyisoleucine: a novel amino acid potentiator of insulin secretion. Diabetes 1998;47:206–10. [DOI] [PubMed] [Google Scholar]
- 58.Sauvaire Y, Girardon P, Baccou JC, Risterucci AM. Changes in growth, proteins and free amino acids of developing seed and pod of fenugreek. Phytochemistry 1984;23:479–86. [Google Scholar]
- 59.Broca C, Gross R, Petit P, Sauvaire Y, Manteghetti M, Tournier M, Masiello P, Gomis R, Ribes G. 4-Hydroxyisoleucine: experimental evidence of its insulinotropic and antidiabetic properties. Am J Physiol 1999;277:E617–23. [DOI] [PubMed] [Google Scholar]
- 60.Broca C, Manteghetti M, Gross R, Baissac Y, Jacob M, Petit P, Sauvaire Y, Ribes G. 4-Hydroxyisoleucine: effects of synthetic and natural analogues on insulin secretion. Eur J Pharmacol 2000;390:339–45. [DOI] [PubMed] [Google Scholar]
- 61.Broca C, Breil V, Cruciani-Guglielmacci C, Manteghetti M, Rouault C, Derouet M, Rizkalla S, Pau B, Petit P, Ribes G, et al. Insulinotropic agent ID-1101 (4-hydroxyisoleucine) activates insulin signaling in rat. Am J Physiol Endocrinol Metab 2004;287:E463–71. [DOI] [PubMed] [Google Scholar]
- 62.Narender T, Puri A. Shweta, Khaliq T, Saxena R, Bhatia G, Chandra R. 4-Hydroxyisoleucine an unusual amino acid as antidyslipidemic and antihyperglycemic agent. Bioorg Med Chem Lett 2006;16:293–6. [DOI] [PubMed] [Google Scholar]
- 63.Haeri MR, Izaddoost M, Ardekani MRS, Nobar MR, White KN. The effect of fenugreek 4-hydroxyisoleucine on liver function biomarkers and glucose in diabetic and fructose-fed rats. Phytother Res 2009;23:61–4. [DOI] [PubMed] [Google Scholar]
- 64.Singh AB, Tamarkar AK, Narender T, Srivastava AK. Antihyperglycaemic effect of an unusual amino acid (4-hydroxyisoleucine) in C57BL/KsJ-db/db mice. Nat Prod Res 2010;24:258–65. [DOI] [PubMed] [Google Scholar]
- 65.Haeri MR, Limaki HK, White CJ, White KN. Non-insulin dependent anti-diabetic activity of (2S, 3R, 4S) 4-hydroxyisoleucine of fenugreek (Trigonella foenum graecum) in streptozotocin-induced type I diabetic rats. Phytomedicine 2012;19:571–4. [DOI] [PubMed] [Google Scholar]
- 66.Jaiswal N, Maurya CK, Venkateswarlu K, Sukanya P, Srivastava AK, Narender T, Tamrakar AK. 4-Hydroxyisoleucine stimulates glucose uptake by increasing surface GLUT4 level in skeletal muscle cells via phosphatidylinositol-3-kinase-dependent pathway. Eur J Nutr 2012;51:893–8. [DOI] [PubMed] [Google Scholar]
- 67.Maurya CK, Singh R, Jaiswal N, Venkateswarlu K, Narender T, Tamrakar AK. 4-Hydroxyisoleucine ameliorates fatty acid-induced insulin resistance and inflammatory response in skeletal muscle cells. Mol Cell Endocrinol 2014;395:51–60. [DOI] [PubMed] [Google Scholar]
- 68.Yu H, Wu M, Lu FR, Xie J, Zheng N, Qin Y, Gao F, Du W, Jian LM. Effect of trigonella foenum-graecum 4-hydroxyisoleucine on high-glucose induced insulin resistance in 3T3–L1 adipocytes of mice. Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi jiehe zazhi [Chinese journal of integrated traditional and Western medicine] Zhongguo Zhong xi yi jie he xue hui, Zhongguo Zhong yi yan jiu yuan zhu ban 2013;33:1394–9. [PubMed]
- 69.Jennings AM, Wilson RM, Ward JD. Symptomatic hypoglycemia in NIDDM patients treated with oral hypoglycemic agents. Diabetes Care 1989;12:203–8. [DOI] [PubMed] [Google Scholar]
- 70.Overby NC, Sonestedt E, Laaksonen DE, Birgisdottir BE. Dietary fiber and the glycemic index: a background paper for the Nordic Nutrition Recommendations 2012. Food Nutr Res 2013;57. [DOI] [PMC free article] [PubMed]
- 71.Silva FM, Kramer CK, de Almeida JC, Steemburgo T, Gross JL, Azevedo MJ. Fiber intake and glycemic control in patients with type 2 diabetes mellitus: a systematic review with meta-analysis of randomized controlled trials. Nutr Rev 2013;71:790–801. [DOI] [PubMed] [Google Scholar]
- 72.Roberts KT. The potential of fenugreek (Trigonella foenum-graecum) as a functional food and nutraceutical and its effects on glycemia and lipidemia. J Med Food 2011;14:1485–9. [DOI] [PubMed] [Google Scholar]
- 73.Kumar P, Bhandari U, Jamadagni S. Fenugreek seed extract inhibit fat accumulation and ameliorates dyslipidemia in high fat diet-induced obese rats. Biomed Res Int 2014;2014:606021. [DOI] [PMC free article] [PubMed]
- 74.Doyle JP, Lyons G, Morris ER. New proposals on “hyperentanglement” of galactomannans: solution viscosity of fenugreek gum under neutral and alkaline conditions. Food Hydrocoll 2009;23:1501–10. [Google Scholar]
- 75.Hannan JMA, Ali L, Rokeya B, Khaleque J, Akhter M, Flatt PR, Abdel-Wahab YHA. Soluble dietary fibre fraction of Trigonella foenum-graecum (fenugreek) seed improves glucose homeostasis in animal models of type 1 and type 2 diabetes by delaying carbohydrate digestion and absorption, and enhancing insulin action. Br J Nutr 2007;97:514–21. [DOI] [PubMed] [Google Scholar]
- 76.Srichamroen A, Thomson AB, Field CJ, Basu TK. In vitro intestinal glucose uptake is inhibited by galactomannan from Canadian fenugreek seed (Trigonella foenum graecum L) in genetically lean and obese rats. Nutr Res 2009;29:49–54. [DOI] [PubMed] [Google Scholar]
- 77.Hamden K, Jaouadi B, Carreau S, Bejar S, Elfeki A. Inhibitory effect of fenugreek galactomannan on digestive enzymes related to diabetes, hyperlipidemia, and liver-kidney dysfunctions. Biotechnol Bioprocess Eng 2010;15:407–13. [Google Scholar]
- 78.Ramulu P, Giridharan NV, Udayasekhararao P. Hypolipidemic effect of soluble dietary fiber (galactomannan) isolated from fenugreek seeds in WNIN (GR-Ob) obese rats. J Med Plants Res 2011;5:4804–13. [Google Scholar]
- 79.Eastwood M, Kritchevsky D. Dietary fiber: how did we get where we are? Annu Rev Nutr 2005;25:1–8. [DOI] [PubMed] [Google Scholar]
- 80.Oh S-H, Park H-D, Ki C-S, Choe Y-H, Lee S-Y. Biochemical and molecular investigation of two Korean patients with glycogen storage disease type III. Clin Chem Lab Med 2008;46:1245–9. [DOI] [PubMed] [Google Scholar]
- 81.Ku S, You HJ, Ji GE. Enhancement of anti-tumorigenic polysaccharide production, adhesion, and branch formation of bifidobacterium bifidum BGN4 by phytic acid. Food Sci Biotechnol 2009;18:749–54. [Google Scholar]
- 82.Heo SJ, Hwang JY, Choi JI, Han JS, Kim HJ, Jeon YJ. Diphlorethohydroxycarmalol isolated from Ishige okamurae, a brown algae, a potent alpha-glucosidase and alpha-amylase inhibitor, alleviates postprandial hyperglycemia in diabetic mice. Eur J Pharmacol 2009;615:252–6. [DOI] [PubMed] [Google Scholar]
- 83.Hannan JM, Rokeya B, Faruque O, Nahar N, Mosihuzzaman M, Azad Khan AK, Ali L. Effect of soluble dietary fibre fraction of Trigonella foenum graecum on glycemic, insulinemic, lipidemic and platelet aggregation status of Type 2 diabetic model rats. J Ethnopharmacol 2003;88:73–7. [DOI] [PubMed] [Google Scholar]
- 84.Boban PT, Nambisan B, Sudhakaran PR. Hypolipidaemic effect of chemically different mucilages in rats: a comparative study. Br J Nutr 2006;96:1021–9. [DOI] [PubMed] [Google Scholar]
- 85.Venkatesan N, Devaraj SN, Devaraj H. A fibre cocktail of fenugreek, guar gum and wheat bran reduces oxidative modification of LDL induced by an atherogenic diet in rats. Mol Cell Biochem 2007;294:145–53. [DOI] [PubMed] [Google Scholar]
- 86.Srichamroen A, Field CJ, Thomson AB, Basu TK. The modifying effects of galactomannan from Canadian-grown fenugreek (Trigonella foenum-graecum L.) on the glycemic and lipidemic status in rats. J Clin Biochem Nutr 2008;43:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Neelakantan N, Narayanan M, de Souza RJ, van Dam RM. Effect of fenugreek (Trigonella foenum-graecum L.) intake on glycemia: a meta-analysis of clinical trials. Nutr J 2014;13:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cox AJ, West NP, Cripps AW. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol 2014;pii:S2213–8587(14)70134–2. [DOI] [PubMed]
- 89.Moreno-Indias I, Cardona F, Tinahones FJ, Queipo-Ortuño MI. Impact of the gut microbiota on the development of obesity and type 2 diabetes mellitus. Front Microbiol 2014;5:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shoaie S, Nielsen J. Elucidating the interactions between the human gut microbiota and its host through metabolic modeling. Front Genet 2014;5:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013;341:1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Walsh CJ, Guinane CM, O'Toole PW, Cotter PD. Beneficial modulation of the gut microbiota. FEBS Lett 2014;588:4120–30. [DOI] [PubMed] [Google Scholar]
- 93.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014;505:559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]