Abstract
Eight cultivars of Brassica oleracea var. capitata and two types of explant (hypocotyl and cotyledon) were tested for their potential to regenerate under in vitro conditions. Hypocotyl and cotyledon explants from 10-d-old seedlings were subcultured onto different callus induction media based on Murashige and Skoog (MS) basal medium supplemented with 1% sucrose and different concentrations and combinations of plant growth regulators. Hypocotyl explants were found to be more suitable for callus induction and organogenesis than cotyledon explants for all cultivars tested. In terms of regeneration, the cv. ‘Amager’ was significantly more responsive than the other cultivars tested and produced the highest number of shoots/buds per explant. Moreover, among five types of media tested, MS + 8.88 μM 6-benzyloaminopurine (BAP) + 0.53 μM α-naphthylacetic acid (NAA) was most effective for shoot regeneration. Rooting was achieved within 10–15 d on all the rooting media, but MS medium containing 5.37 μM NAA produced the maximum number of strong and healthy roots. Plantlets (95%) were subsequently established in the greenhouse, and no phenotypic variations were observed among regenerated plants. This plant regeneration protocol could be suitable for a wide range of cabbage cultivars.
Keywords: In vitro regeneration, Brassica oleracea var. capitata, Hypocotyl, Cotyledon
Introduction
The popular crop plant cabbage belongs to the species Brassica oleracea (Capitata Group) of the family Brassicaceae (or Cruciferae). China is the leading producer of cabbage worldwide, followed by India and the Russian Federation, whereas Poland takes the eight place (FAOSTAT 2012). This crop is an excellent source of calcium, vitamins C, K, and A, and folic acid; it also contains significant amounts of glutamine and amino acids that have anti-inflammatory properties. Cabbage can be also included in diet programs, as it contains crude fiber and is a low-calorie food. In contrast to seed brassicas, cabbage is mainly used for pickle and fodder. The quality of cabbage suffers due to its susceptibility to a number of pests and diseases. Generally, this crop is damaged by cabbage butterfly worm or fungal diseases caused by Botrytis cinerea, Alternaria brassicola, Plasmodiophora brassicae, or Pythium spp. To improve the yield of B. oleracea, considerable research has been conducted to optimize tissue culture and transformation protocols. Brassica spp. are generally considered to be recalcitrant in tissue culture. However, there are several reports regarding cabbage, canola, and broccoli transformation (Metz et al.1995; Jin et al.2000; Li et al.2005; Sretenović-Rajičić et al.2006; Sretenović-Rajičić et al.2007; Bhala and Singh 2008). Also, in vitro regeneration from different explants via organogenesis has been achieved (Bajaj and Nietsch 1975; Sparrow et al.2004; Munshi et al.2007; Pavlović et al.2010). So far, with respect to cabbage, shoot regeneration has been achieved from various tissues and organs, including hypocotyls, cotyledons, roots, leaves, peduncle segments, callus and cell cultures, thin cell layers, protoplasts, and immature zygotic embryos (reviewed by Cardoza and Stewart 2004; Kiełkowska and Adamus 2012; Pavlović et al.2013; Ravanfar et al.2014). A range of studies have noted substantial variation even if the same species or cultivar were investigated. Therefore, we deemed it necessary to investigate the shoot-regeneration ability in B. oleracea var. capitata that could be a prospective material for further transformation and breeding. All cultivars tested in this study are among those mostly cultivated in Poland. According to our best knowledge, there have been no reports concerning in vitro regeneration of these cultivars using hypocotyl- or cotyledon-derived explants. Furthermore, only scanty data is available for regeneration of B. oleracea var. capitata. While, regeneration of hypocotyl-derived explants has been reported, in the case of cotyledon explants, the success was limited (Jin et al.2000; reviewed by Cardoza and Stewart 2004; reviewed by Ravanfar et al.2014). Furthermore, since it is known that genotype specificity strongly influences plant regeneration, the development of a reliable and reproducible regeneration protocol for different genotypes or cultivars is indispensable. Therefore, it is beneficial to study the regeneration potential of the wider range of cabbage cultivars. Here, we present a protocol for in vitro regeneration of eight cabbage cultivars via indirect organogenesis using two types of explants, namely hypocotyls and cotyledons.
Materials and Methods
Eight commercial cultivars of B. oleracea var. capitata, i.e., ‘Zora’, ‘Brunświcka’, ‘Ula’, ‘Ditmarska’, ‘Kamienna głowa’, ‘Amager’, ‘Sława of Enkhuizen’, and ‘Replika’, were used in this study. The seeds of B. oleracea var. capitata cultivars were provided by PNOS PlantiCo Ltd (Zielonki, Poland) and KHNO POLAN Ltd. (Kraków, Poland) Mature seeds were first rinsed in 50% (v/v) ethanol for 5 min, and then rinsed in distilled sterile water (32,009× 3 min). Next, they were surface-sterilized in 50% (v/v) commercial bleach for 5 min and subsequently rinsed in distilled sterile water (3 × 3 min). The sterilized seeds were germinated in Petri dishes (6 seeds per dish) on basic Murashige and Skoog (MS) medium containing 1% (w/v) sucrose and 0.8% agar (w/v), pH 5.8 (Murashige and Skoog 1962), without plant growth regulators.
Cotyledon and hypocotyl explants were aseptically excised from 10-d-old seedlings and cultured on five types of MS solid shoot-regenerating media supplemented with 1% sucrose and with different concentrations and combinations of plant growth regulators (i.e., MS alone; MS + 4.44 μM 6-benzyloaminopurine (BAP); MS + 2.22 μM BAP + 0.53 μM α-naphthylacetic acid (NAA); MS + 4.44 μM BAP + 0.53 μM NAA; MS + 8.88 μM BAP + 0.53 μM NAA). All cultures were maintained in a growth room under an 8-h dark/16-h light photoperiod (~3000 lx) at 23 ± 2°C. Explants were subcultured at regular intervals of 4 wk. Data on callus and the bud/shoot induction, multiplication from explants as well as rooting were collected after 4 wk of culture, while growth characteristics and time required for the process initiation were observed every week. Each of eight cultivars was cultured on five different media to assess the regeneration ability of the tested material, each experiment was repeated three times, and the total number of explants was 180 (60 explants per repeat).
After 4 wk of culture on shoot-regenerating medium, single-regenerated shoots (1.0–1.5 cm in length) were transferred to rooting media consisting of MS salts, 1% sucrose and 0.7% agar (pH 5.8), supplemented with either 0, 2.68, 5.37, or 8.05 μM NAA.
The percentage of explants with bud regeneration ([the number of explants with adventitious buds/total number of explants] × 100) and the average number of buds per explant (the number of explants forming adventitious buds) were calculated for the explants that had been cultured for 4 wk (Table 2).
Table 2.
The effect of media and explant type on shoot regeneration in eight cv. of B. oleracea var. capitata.
| Medium | Cultivar of B. oleracea var. capitata (frequency of explants with shoots (%)/mean no. of shoots/buds per explants) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amager | Kamienna głowa | Ula | Ditmarska | Sława z Enkhuizen GOF | Replika | Zora | Brunświcka | |||||||||
| MS | 16.6a | 1.1a ± 0.18 | 22.2a | 1.6a ± 0.45 | 16.6a | 0.72a ± 0.33 | 1.11a | 0.50a ± 0.12 | 16.6a | 0.66a ± 0.16 | 22.2a | 1.61a ± 0.49 | 5.5a | 0.05a ± 0.06 | 22.2a | 1.05a ± 0.33 |
| MS + 4.4 μM BAP | 88.8bc | 7.50a ± 0.38 | 94.4bc | 6.8a ± 0.34 | 33.3a | 1.88a ± 0.40 | 27.7a | 0.83a ± 0.17 | 33.3a | 3.66a ± 0.62 | 33.2a | 2.16a ± 0.53 | 16.6a | 0.83a ± 0.19 | 44.4a | 4.16a ± 0.44 |
| MS + 2.2 μM BAP + 0.5 μM NAA | 77.2b | 6.66a ± 0.58 | 55.5abd | 4.0a ± 0.55 | 44.4a | 2.33a ± 0.33 | 38.8a | 0.94a ± 0.15 | 44.4a | 4.66a ± 0.57 | 38.8a | 2.11a ± 0.54 | 38.8a | 1.83a ± 0.20 | 38.8a | 1.27a ± 0.21 |
| MS + 4.4 μM BAP + 0.5 μM NAA | 44.4abd | 3.72a ± 0.58 | 44.4a | 3.8a ± 0.60 | 27.7a | 1.44a ± 0.28 | 33.3a | 0.55a ± 0.15 | 83.3a | 6.55a ± 0.51 | 55.5a | 3.5a ± 0.47 | 27.7a | 1.22a ± 0.21 | 27.7a | 1.22a ± 0.21 |
| MS + 8.8 μM BAP + 0.5 μM NAA | 55.5ab | 5.20a ± 0.47 | 61.1ac | 7.1a ± 0.46 | 38.8a | 2.11a ± 0.21 | 27.7a | 1.16a ± 0.20 | 66.6a | 5.11a ± 0.65 | 66.6a | 4.0a ± 0.61 | 22.2a | 1.05a ± 0.24 | 22.2a | 1.22a ± 0.15 |
All values are means and standard error (±SE) and those followed by a different letter within a column (for each variety separately) are significantly different at P < 0.05 according to Tukey’s test
Experimental design and statistical analysis.
The data were subjected to one-way analysis of variance (ANOVA), and the differences among means were compared by Tukey’s test (P < .05).
Results and Discussion
The seeds of each B. oleracea cultivar were germinated on plant growth regulator-free MS medium. After 10 d, the percentage of germination was highest in ‘Replika’ (94.4%) followed by ‘Zora’, ‘Brunświcka’, ‘Kamienna głowa’, ‘Amager’, ‘Ula’, ‘Sława of Enkhuizen’, and ‘Ditmarska’ (90.7, 90.5, 68.5, 65.9, 61, 52.7, and 58%, respectively). Ten days after germination, seedlings were about 4–5 cm long and were harvested for use as explants.
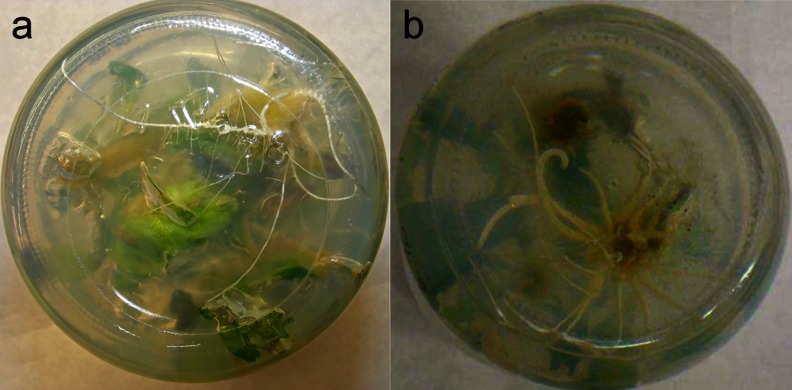
The use of hypocotyls and/or cotyledons as explants for in vitro plant regeneration has received significant attention. Moreover, as many studies indicate, these explants possess a high capacity for shoot organogenesis, somatic embryogenesis, and protoplast culture (Cardoza and Stewart 2004). Here, we tested the ability of two types of cabbage explants: hypocotyls and cotyledons, to regenerate entire plants via indirect organogenesis. Hypocotyl and cotyledon explants excised from aseptically grown 10-d-old cabbage seedlings were cultured on MS and MS containing BAP alone or in combination with NAA to promote shoot initiation and multiplication (Fig. 1a, d–h). In general, callus was readily obtained, with hypocotyls producing much more callus than cotyledons (Table 1). Swelling of explants was observed within 1 wk (Fig. 1b, c). Callus was initiated within 10–14 d, and adventitious bud formation occurred soon after (Fig. 1d–g). In general, the hypocotyl-derived callus produced a higher bud regeneration frequency than the cotyledon-derived callus. The bud regeneration frequency varied among cultivars, explant type, and medium composition (Table 2). The formation of calli and shoots was observed on cut edges and/or in the central region of explant tissues (Fig. 1c). Moderate callus induction was observed both in hypocotyl and cotyledon cultures. Calli raised from hypocotyl explants were green or pale yellow and friable (Fig. 1c, g), while those from cotyledons were compact and slow growing (data not shown).
Figure 1.
Plant regeneration form hypocotyls and cotyledons Brassica oleracea var. capitata cv. Amager. a, d–g Shoot proliferation. b Swollen hypocotyl after 10-d culture. c Differentiating callus tissue after 14-d culture on MS + 4.44 μM BAP + 0.537 μM NAA. h Regenerated plantlets after 42 d.
Table 1.
Effect of BA and NAA on callus induction from different explants of Brassica oleracea var. capitata.
| Cultivar/genotype | Growth regulator (μM) | Callus induction (%) | ||
|---|---|---|---|---|
| BAP | NAA | Hypocotyls | Cotyledons | |
| Amager | 0 | 0 | 55.5a | 5.5a |
| 4.44 | 0 | 94.4b | 11.1a | |
| 2.22 | 0.53 | 100.0b | 0.0a | |
| 4.44 | 0.53 | 100.0b | 0.0a | |
| 8.88 | 0.53 | 83.39ab | 0.0a | |
| Kamienna głowa | 0 | 0 | 50.0a | 0.0a |
| 4.44 | 0 | 55.5a | 22.2b | |
| 2.22 | 0.53 | 66.6ab | 0.0a | |
| 4.44 | 0.53 | 100.0b | 0.0a | |
| 8.88 | 0.53 | 77.7ab | 0.0a | |
| Ula | 0 | 0 | 38.8a | 0.0a |
| 4.44 | 0 | 100.0b | 16.6a | |
| 2.22 | 0.53 | 100.0b | 0.0a | |
| 4.44 | 0.53 | 100.0b | 0.0a | |
| 8.88 | 0.53 | 88.8b | 5.5a | |
| Ditmarska | 0 | 0 | 44.4a | 5.5a |
| 4.44 | 0 | 66.6ac | 11.1a | |
| 2.22 | 0.53 | 94.4bc | 0.0a | |
| 4.44 | 0.53 | 100.0b | 0.0a | |
| 8.88 | 0.53 | 100.0b | 0.0a | |
| Sława of Enkhuizen | 0 | 0 | 38.8a | 5.5a |
| 4.44 | 0 | 77.7b | 0.0ab | |
| 2.22 | 0.53 | 94.4b | 0.0ab | |
| 4.44 | 0.53 | 100.0b | 16.6a | |
| 8.88 | 0.53 | 100.0b | 27.7ac | |
| Replika | 0 | 0 | 61.1a | 0.0a |
| 4.44 | 0 | 72.7ac | 11.1a | |
| 2.22 | 0.53 | 88.8ac | 16.6a | |
| 4.44 | 0.53 | 100.0bc | 16.6a | |
| 8.83 | 0.53 | 100.0bc | 22.2a | |
| Zora | 0 | 0 | 44.4a | 0.0a |
| 4.44 | 0 | 100.0b | 16.6ab | |
| 2.22 | 0.53 | 83.3b | 50.0b | |
| 4.44 | 0.53 | 100.0b | 44.4b | |
| 8.88 | 0.53 | 100.0b | 44.4b | |
| Brunświcka | 0 | 0 | 66.6a | 5.5a |
| 4.44 | 0 | 16.6bc | 11.1a | |
| 2.22 | 0.53 | 55.5ac | 33.3a | |
| 4.44 | 0.53 | 27.7acd | 38.8a | |
| 8.88 | 0.53 | 88.3ae | 16.6a | |
All values are means and those followed by a different letter within a column (for each variety separately) are significantly different at P < 0.05 according to Tukey’s test
The results of callus induction varied with respect to treatments and cultivars. For all B. oleracea cultivars, use of hypocotyl explants resulted in a higher percentage of callus formation, ranging from 16.5 to 100% on different media (Table 1). Moreover, among five tested media, MS + 8.88 μM BAP + 0.53 μM NAA gave the best results on average, with 100% of explants producing callus for cv. ‘Zora’, ‘Replika’, and ‘Sława of Enkhuizen’, while the minimum rate of callus formation on this medium was 77.7% for ‘Kamienna głowa’ (Table 1). Across all media, the results showed that ‘Zora’ closely followed by ‘Replika’ produced the most callus. For ‘Sława of Enkhuizen’, ‘Amager’, ‘Ula’, and ‘Ditmarska’, the results were much lower, while ‘Kamienna głowa’ and ‘Brunświcka’ hypocotyl explants revealed the lowest callus response (Table 1). The lowest responses were noted for MS medium without plant growth regulators.
On the other hand, callus induction for cotyledon explants was low overall (between 0 and 50%), and callus was only generated in five cultivars, i.e., ‘Ula’, ‘Sława of Enkhuizen’, ‘Replika’, ‘Zora’, and ‘Brunświcka’, but not in ‘Amager’, ‘Kamienna głowa’, or ‘Ditmarska’. Only the ‘Brunświcka’ cultivar produced callus from cotyledon explants on all tested media. Similar results were obtained for ‘Zora’, but excluded MS only medium. However, as we later discovered, the amount of callus produced from either explant did not positively correlate with the shoot-regeneration response (Table 2).
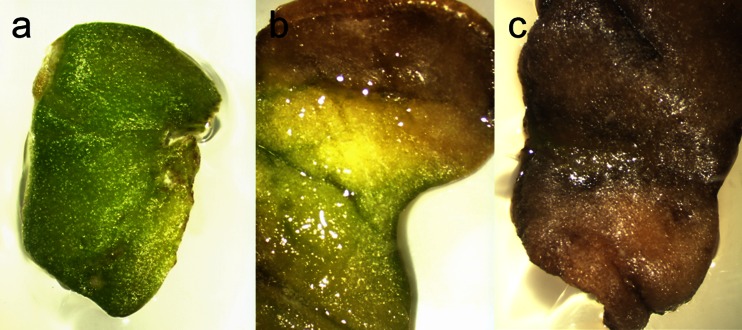
In in vitro cultures of different plant species are vulnerable to chlorosis as well as necrosis when explants remain in the medium for long periods of time. This might result from leaking of phenolic compounds from explants to the medium followed by their oxidation producing toxic compounds, and Brassica species including cabbage are rich sources of phenolic compounds. Accumulation of ethylene in the culture plates as a result of low gas exchange can be another explanation. During our experiments over time of 4 wk, in all tested cultivars, cotyledon explants showed progressive chlorosis (they turned yellow), and they finally became brown and died (cv. ‘Ula’ 82% of explants, ‘Ditmarska’ 62%, ‘Amager’ 23.3%, ‘Kamienna głowa’ 99%, ‘Sława of Enkhuizen’ 100%, ‘Replika’ 100%, ‘Zora’ 99%, and ‘Brunświcka’ 95.5%; Fig. 2). Qin et al. (2007) observed similar effects during in vitro culture of broccoli. Addition of small amounts of silver nitrate (AgNO3) to the medium could be a possible solution to this problem. This compound is known to inhibit ethylene and has been shown to increase shoot proliferation in B. oleracea var. italica (Qin et al.2007), Brassica rapa (Cogbill et al.2010), and Brassica napus (Maheshwari et al.2011).
Figure 2.
Progressive chlorosis of cotyledon explants (a, b) and necrosis (c) on MS medium containing MS + 2.22 μM BAP + 0.537 μM NAA. After culture for 14 d (a), 28 d (b), and 40 d (c).
The present study showed that hypocotyl explants derived from all the tested B. oleracea var. capitata cultivars were also efficient in in vitro shoot regeneration. Each of the five media induced different regeneration responses (Table 2). Among eight varieties used, ‘Amager’ was most responsive in terms of percent of explants with shoot regeneration (88.8%) as well as the highest number of shoots/buds per explant (7.5). Although ‘Zora’ produced the most calli in the same culture period (data not shown), its capacity for shoot regeneration was the lowest. Thus, our results indicated that the genotype played an important role in callus induction and shoot formation. Similar studies of Zhang and Bhalla (2004) and Cardoza and Stewart (2004) showed that genotype effect was one of the most important factors of in vitro regeneration of B. oleracea. While the hypocotyl explants revealed high regeneration responses, for the cotyledon explants, it was generally very poor for all cultivars; ‘Ditmarska’, ‘Sława’, and ‘Replika’ did not show any response, whereas ‘Kamiena głowa’, ‘Ula’, ‘Zora’, and ‘Brunświcka’ showed negligible responses to all the media tested. Only ‘Amager’ showed a slightly higher regeneration response in the tested media, except MS + 2.22 μM BAP + 0.53 μM NAA (data not shown). As many independent studies showed, seedling hypocotyls are preferred for regeneration and transformation of Brassica spp. (Li et al.2005; Sretenović-Rajičić et al.2006; Munshi et al.2007; Rafat et al.2010).
BAP either alone or in combination with auxin has been previously shown to be optimal for shoot regeneration and multiplication in different Brassica species (Metz et al.1995; Jin et al.2000; Munshi et al.2007; Sretenović-Rajičić et al.2007; Maheshwari et al.2011). The presence of BAP in the medium significantly increased the number of shoots produced per explant in rapid cycling B. oleracea in vitro (Cheng et al.2001). Furthermore, the addition of NAA was shown to significantly enhance shoot regeneration (Guo et al.2005). Therefore, in this study, we used the media containing BAP alone or in combination with NAA. The maximum number of shoots/buds per explant (7.5) was obtained with cv. ‘Amager’ from the hypocotyl explants cultured on MS + 4.44 μM BAP. It is worth noting that relatively good responses were obtained using the same medium for ‘Kamienna głowa’ and ‘Brunświcka’ cultivars (6.8 and 4.16 buds/explant, respectively). However, on MS medium without supplementation, significantly lower values were obtained (1.1, 1.6, and 1.05 buds/explant, respectively, for ‘Amager’, ‘Kamienna głowa’, and ‘Brunświcka’). A similar trend was observed in the others cultivars (Table 2). However cv. ‘Ula’, ‘Ditmarska’, ‘Sława of Einkhuizen’, ‘Replika’, and ‘Zora’ required both BAP (2.22 to 8.88 μM) and addition of a small amount of NAA (0.53 μM) to obtain increased numbers of shoots/buds per explants. Therefore, on the basis the results, we conclude that the presence of BAP in the medium was required to enhance the mean number of shoots/buds per explant. Moreover, in some cases, this could be improved further upon auxin supplementation. The Brassica cultivars exhibited enhanced frequency of shoot regeneration (from hypocotyl explants) on the media containing both phytohormones. The results obtained for ‘Ula’, ‘Sława of Enkhuizen’, ‘Replika’, and ‘Brunświcka’ were similar (frequency of explants with shoots ranged from 16.6 to 83.3%), whereas two cultivars ‘Ditmarska’ and ‘Zora’ showed poor shoot regeneration response (Table 2). On the other hand, ‘Amager’ and ‘Kamiena głowa’ revealed higher results on the media with BAP alone (88.8 and 94.4%, respectively).
In most Brassica species, regeneration depends on the age of explants (Bhala and Singh 2008; Cogbill et al.2010), with younger explants resulting in better responses (Ovesna et al.1993). Explants from 3- or 4-d-old seedlings gave optimal regeneration rates in different Brassica spp.; however, these explants are too small and consequently difficult to manipulate (reviewed by Cardoza and Stewart 2004; Sparrow et al.2004; Zhang and Bhalla 2004; Bhala and Singh 2008). Thus, most researchers used explants excised from 5- to 7- or even 10-d-old seedlings (Munshi et al.2007; Pavlović et al.2010; Rafat et al.2010). Therefore, it can be concluded that age of explants is important for their ability to regenerate. During the present study, hypocotyls from 10-d-old seedlings showed better response toward indirect organogenesis. We also noted that the subtypes of hypocotyl explants (upper, middle, and lower sections of hypocotyls) did not show any differences in shoot regeneration (data not shown). This result was in agreement with a study using B. napus (Zhang and Bhalla 2004).
Successful rooting of in vitro-derived shoots is an integral part of each regeneration protocol. To achieve this, we used MS medium alone or MS medium supplemented with different concentrations of NAA (2.68, 5.37, or 8.05 μM). Our results showed that Brassica napus var. capitata had a high rooting ability, but there were slight differences among the tested cultivars (Table 3). Overall, we observed that the addition of auxin significantly improved rooting in all tested cultivars. Similar correlations were reported by Munshi et al. (2007) for cabbage and Ravanfar et al. (2009) for broccoli (cv. ‘Green Marvel’). Our best results were obtained using 5.37 μM NAA: 100% rooting was obtained for ‘Amager’, ‘Kamienna głowa’, ‘Sława of Enkhuizen’, and ‘Brunświcka’; 93.3% for ‘Ula’ and ‘Replika’; and 86.6% for ‘Ditmarska’ and ‘Zora’. MS medium containing 5.37 μM NAA produced the maximum number of strong and healthy roots (Fig. 3). It should be noted that while addition of NAA increased the number of roots produced per shoot, the average root length decreased (Fig. 3b; Table 3). For example the maximum root length (6.7 cm) was attained on MS medium without NAA for ‘Ula’ while for the same cultivar on MS + NAA (2.68, 5.37, or 8.05 μM), the average root length was reduced to 5.8, 4.5, and 4.0 cm, respectively. This phenomenon was consistent in all tested cultivars. Pavlović et al. (2010) noticed a similar correlation in their studies on red cabbage (cv. ‘Rubin’), broccoli (cv. ‘Korvet’), savoy cabbage (cv. ‘Vertus’), and cauliflower (cv. ‘Rasa’). In our study, coinciding with the increasing NAA concentration, the root morphology became shorter and more stumpy when growing inside the medium or grew as a fluffy mass on the medium surface. MS medium without NAA on the other hand, resulted in fewer roots raised above the surface and were longer and thinner (Fig. 3a). These results are in agreement with the observations of Ravanfar et al. (2009) on broccoli. Moreover, higher concentrations of NAA (5.2–8.0 μM) in the medium triggered callus formation at the base of the shoot, similar to the findings of Munshi et al. (2007).
Table 3.
Effect of different concentration of NAA in MS medium on root formation of in vitro grown shoots.
| Cultivar | Concentration (mg/L) | % of shoots rooted | Average no. of roots (±SE) | Average length of roots (cm) (±SE) | Days to rooting |
|---|---|---|---|---|---|
| Amager | MS | 73.3a | 4.5 ± 0.82 | 6.5 ± 0.26 | 10–12 |
| Kamienna głowa | MS | 66.6a | 3.5 ± 0.80 | 5.5 ± 0.48 | 10–12 |
| Ula | MS | 73.3a | 3.8 ± 0.68 | 6.7 ± 0.37 | 10–12 |
| Ditmarska | MS | 66.6a | 3.5 ± 0.54 | 6.5 ± 0.61 | 10–12 |
| Sława of Enkhuizen | MS | 80.0a | 4.1 ± 0.50 | 6.2 ± 0.73 | 10–12 |
| Replika | MS | 73.3a | 3.8 ± 0.66 | 5.8 ± 0.38 | 10–12 |
| Zora | MS | 66.6a | 3.5 ± 0.46 | 6.0 ± 0.26 | 10–12 |
| Brunświcka | MS | 73.3a | 4.5 ± 0.40 | 5.5 ± 0.22 | 10–12 |
| Amager | MS + 2.68 μM NAA | 86.6a | 5.8 ± 0.60 | 5.7 ± 0.46 | 13–15 |
| Kamienna głowa | MS + 2.68 μM NAA | 86.6a | 4.1 ± 0.61 | 5.1 ± 0.45 | 13–15 |
| Ula | MS + 2.68 μM NAA | 80.0a | 4.0 ± 0.61 | 5.6 ± 0.44 | 13–15 |
| Ditmarska | MS + 2.68 μM NAA | 73.3a | 4.1 ± 0.62 | 5.8 ± 0.61 | 13–15 |
| Sława of Enkhuizen | MS + 2.68 μM NAA | 93.3a | 5.7 ± 0.58 | 5.7 ± 0.44 | 13–15 |
| Replika | MS + 2.68 μM NAA | 86.6a | 4.1 ± 0.24 | 5.2 ± 0.31 | 13–15 |
| Zora | MS + 2.68 μM NAA | 73.3a | 4.0 ± 0.55 | 5.8 ± 0.48 | 13–15 |
| Brunświcka | MS + 2.68 μM NAA | 86.6a | 5.7 ± 0.64 | 5.1 ± 0.48 | 13–15 |
| Amager | MS + 5.37 μM NAA | 100a | 8.8 ± 0.54 | 4.5 ± 0.61 | 13–15 |
| Kamienna głowa | MS + 5.37 μM NAA | 100a | 6.8 ± 0.59 | 4.3 ± 0.22 | 13–15 |
| Ula | MS + 5.37 μM NAA | 93.3a | 6.3 ± 0.60 | 4.5 ± 0.39 | 13–15 |
| Ditmarska | MS + 5.37 μM NAA | 86.6a | 5.0 ± 0.82 | 4.7 ± 0.61 | 13–15 |
| Sława of Enkhuizen | MS + 5.37 μM NAA | 100a | 8.7 ± 0.41 | 4.5 ± 0.46 | 13–15 |
| Replika | MS + 5.37 μM NAA | 93.3a | 5.7 ± 0.64 | 4.6 ± 0.45 | 13–15 |
| Zora | MS + 5.37 μM NAA | 86.6a | 5.5 ± 0.62 | 4.3 ± 0.30 | 13–15 |
| Brunświcka | MS + 5.37 μM NAA | 100a | 7.1 ± 0.64 | 4.4 ± 0.48 | 13–15 |
| Amager | MS + 8.05 μM NAA | 93.3a | 7.2 ± 0.76 | 3.9 ± 0.47 | 13–15 |
| Kamienna głowa | MS + 8.05 μM NAA | 73.3a | 6.2 ± 0.79 | 4.0 ± 0.39 | 13–15 |
| Ula | MS + 8.05 μM NAA | 86.6a | 5.7 ± 0.89 | 4.0 ± 0.44 | 13–15 |
| Ditmarska | MS + 8.05 μM NAA | 80.0a | 4.5 ± 0.83 | 3.8 ± 0.52 | 13–15 |
| Sława of Enkhuizen | MS + 8.05 μM NAA | 86.6a | 7.0 ± 0.61 | 3.8 ± 0.39 | 13–15 |
| Replika | MS + 8.05 μM NAA | 86.6a | 4.8 ± 0.97 | 3.5 ± 0.57 | 13–15 |
| Zora | MS + 8.05 μM NAA | 80.0a | 4.2 ± 0.78 | 3.3 ± 0.50 | 13–15 |
| Brunświcka | MS + 8.05 μM NAA | 93.3a | 6.7 ± 0.83 | 3.7 ± 0.61 | 13–15 |
All values are means and standard error (±SE) and those followed by a different letter within a column (for each variety separately) are significantly different at P < 0.05 according to Tukey’s test
Figure 3.
Rooting on in vitro regenerated shoots in a MS and b MS supplemented with 5.37 μM NAA.
Rooting was achieved within 10–15 d for all the tested media. In the present study, supplementation of auxin did not accelerate the rooting process; however, it increased the quantity and quality of roots as compared to MS medium without auxin. Rooted plantlets were successfully acclimatized in a potting medium containing soil and perlite (3:1) and grew naturally in a greenhouse. Survival rate of the regenerated plants all of eight cultivars tested was 95%. No apparent phenotypic variations were observed among the regenerated plants.
Conclusions
The results showed that in B. oleracea var. capitata, a higher frequency of shoot regeneration was achieved form hypocotyl as compared to cotyledon explants. Nevertheless, considerable variation in shoot regeneration from hypocotyl explants were observed among the eight cultivars tested. The best results were obtained for ‘Amager’ followed by high levels of response observed in ‘Kamienna głowa’, ‘Sława of Enkhuizen’, ‘Replika’, ‘Brunświcka’, and ‘Ula’, whereas ‘Ditmarska’ and ‘Zora’ showed poor regeneration responses. ‘Amager’ was most responsive, showing the highest rate of shoot regeneration (88.8% of explants) as well as the highest number of shoot buds per explant (7.5).
In general, the regenerated plants from all tested cultivars showed high rooting ability. NAA, at all three concentrations (2.68, 5.37, and 8.05 μM), significantly improved the percentage of explants forming roots and mean number of roots produced per explant in all tested cultivars, as compared to MS treatment without plant growth regulators. The best rooting results were achieved on MS + 5.37 μM NAA (for ‘Amager’, ‘Kamienna głowa’, ‘Sława of Enkhuizen’, and ‘Brunświcka’ cultivars). The regenerated plants (all tested cultivars) survived and grew in a greenhouse. From the above findings, it can be concluded that the regeneration protocol developed in this study is simple, reproducible, and applicable to a wide variety of B. oleracea var. capitata cultivars.
References
- Bajaj YPS, Nietsch P. In vitro propagation of red cabbage (Brassica oleracea L. var. capitata) J Exp Bot. 1975;95:883–890. doi: 10.1093/jxb/26.6.883. [DOI] [Google Scholar]
- Bhala PL, Singh MB. Agrobacterium mediated transformation of Brassica napus and Brassica oleracea. Nat Protoc. 2008;3:181–189. doi: 10.1038/nprot.2007.527. [DOI] [PubMed] [Google Scholar]
- Cardoza V, Stewart NC. Brassica biotechnology: progress in cellular and molecular biology. In Vitro Cell Dev Biol Plant. 2004;40:542–551. doi: 10.1079/IVP2004568. [DOI] [Google Scholar]
- Cheng PK, Lakshmanan P, Swarup S. High-frequency direct shoot regeneration and continuous production of rapid-cycling Brassica oleracea in vitro. In Vitro Cell Dev Biol Plant. 2001;37:592–598. doi: 10.1007/s11627-001-0104-0. [DOI] [Google Scholar]
- Cogbill S, Faulcon T, Jones G, McDaniel M, Harmon G, Blacmon R, Young M. Adventitious shoot regeneration from cotyledonary explants of rapid-cycling fast plants of Brassica rapa L. Plant Cell Tiss Organ Cult. 2010;101:127–133. doi: 10.1007/s11240-010-9669-9. [DOI] [Google Scholar]
- FAOSTAT. Food and Agricultural Organization of United Nations: Economic and Social Department: The Statistical Division. Food and Agriculture Organization. http://faostat3.fao.org/faostat-gateway/go/to/home/E (2012)
- Guo DP, Zhu ZJ, Hu XX, Zheng SJ. Effect of cytokinins on shoot regeneration from cotyledon and leaf segment of team mustard (Brassica juncea var. tsatsai) Plant Cell Tiss Organ Cult. 2005;83:123–127. doi: 10.1007/s11240-005-3799-5. [DOI] [Google Scholar]
- Jin RG, Liu YB, Tabashnik B, Borthakur D. Development of transgenic cabbage (Brassica oleracea var. capitata) for insect resistance by Agrobacterium tumefaciens-mediated transformation. In Vitro Cell Dev Biol Plant. 2000;36:231–237. doi: 10.1007/s11627-000-0043-1. [DOI] [Google Scholar]
- Kiełkowska A, Adamus A. An alginate-layer technique for culture of Brassica oleracea L. protoplasts. In Vitro Cell Dev Biol Plant. 2012;48:265–273. doi: 10.1007/s11627-012-9431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Peng RH, Fan HQ, Xiong AS, Yao QH, Cheng ZM, Li Y. Vitreoscilla hemoglobin overexpression increases submergence tolerance in cabbage. Plant Cell Rep. 2005;23:710–715. doi: 10.1007/s00299-004-0872-1. [DOI] [PubMed] [Google Scholar]
- Maheshwari P, Selvaraj G, Kovalchuk I (2011) Optimization of Brassica napus (canola) explant regeneration for genetic transformation. Nat Biotechnol 29:144–155. http://www.ncbi.nlm.nih.gov/pubmed/21722759. doi:10.1016/j.nbt.2011.06.014 [DOI] [PubMed]
- Metz TD, Dixit R, Earle ED. Agrobacterium tumefaciens-mediated transformation of broccoli (Brassica oleracea var. italica) and cabbage (B. oleracea var. capitata) Plant Cell Rep. 1995;15:287–292. doi: 10.1007/BF00193738. [DOI] [PubMed] [Google Scholar]
- Munshi MK, Roy PK, Kabir MH, Ahmed G (2007) In vitro regeneration of cabbage (Brassica oleracea L. var. capitata) through hypocotyl and cotyledon culture. Plant Tissue Cult Biotechnol 2:131–136. http://www.banglajol.info/index.php/PTCB/article/view/3233. doi:10.3329/ptcb.v17i2.3233
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Ovesna J, Ptacek L, Opatrny Z. Factors influencing the regeneration capacity of oilseed rape and cauliflower in transformation experiments. Biol Plant. 1993;35:107–112. doi: 10.1007/BF02921131. [DOI] [Google Scholar]
- Pavlović S, Vinterhalter B, Mitić N, Adžić S, Pavlović N, Zdravković M, Vinterhalter D. In vitro shoot regeneration from seedling explants in Brassica vegetables: red cabbage, broccoli, savoy cabbage and cauliflower. Arch Biol Sci. 2010;62:337–345. doi: 10.2298/ABS1002337P. [DOI] [Google Scholar]
- Pavlović S, Vinterhalter B, Zdravković-Korać S, Vinterhalter D, Zdravkowić J, Cvikić D, Mitić N. Recurrent somatic embryogenesis and plant regeneration from immature zygotic embryos of cabbage (Brassica oleracea var. capitata) and cauliflower (Brassica oleracea var. botrytis) Plant Cell Tiss Organ Cult. 2013;113:397–406. doi: 10.1007/s11240-012-0279-6. [DOI] [Google Scholar]
- Qin Y, Li HL, Guo YD. High frequency embryogenesis, regeneration of broccoli (Brassica oleracea var. italic) and analysis of genetic stability by RAPD. Sci Hortic. 2007;3:203–208. doi: 10.1016/j.scienta.2006.10.022. [DOI] [Google Scholar]
- Rafat A, Aziz MA, Rashid AA, Abdullach SNA, Kamaladini H, Sirchi MHT, Javadi MB. Optimization of Agrobacterium tumefaciens-mediated transformation and shoot regeneration after co-cultivation of cabbage (Brassica oleracea subsp. capitata) cv. KY Cross with AtHSP101 gene. Sci Hortic. 2010;124:1–8. doi: 10.1016/j.scienta.2009.11.015. [DOI] [Google Scholar]
- Ravanfar SA, Aziz MA, KAdir MA, Rashid AA, Sirchi MHT (2009) Plant regeneration of Brassica oleracea subsp. italica (broccoli) cv Green Marvel as affected by plant growth regulators. Afr J Biotechnol 11:2523–2528. http://www.ajol.info/index.php/ajb/article/view/60750
- Ravanfar SA, Aziz MA, Rashid AA, Salim S (2014) In vitro adventitious shoot regeneration from cotyledon explants of Brassica oleracea subsp. italica and Brassica oleracea subsp. capitata using TDZ and NAA. Pak J Bot 46:329–335. http://www.pakbs.org/pjbot/PDFs/46%281%29/41.pdf
- Sparrow PAC, Townsend TM, Morgan CL, Dale PJ, Arthur AE, Irwin JA. Genetic analysis of in vitro shoot regeneration from cotyledonary petioles of Brassica oleracea. Theor Appl Genet. 2004;108:1249–1255. doi: 10.1007/s00122-003-1473-z. [DOI] [PubMed] [Google Scholar]
- Sretenović-Rajičić T, Ninković S, Miljuš-Dukić J, Vinterhalter B, Vinterhalter D. Agrobacterium rhizogenes-mediated transformation of Brassica oleracea var. sabauda and B. oleracea var. capitata. Biol Plant. 2006;50:525–530. doi: 10.1007/s10535-006-0083-4. [DOI] [Google Scholar]
- Sretenović-Rajičić T, Ninković S, Uzelać B, Vinterhalter B, Vinterhalter D. Effects of plant genotype and bacterial strain on Agrobacterium tumefaciens-mediated transformation of Brassica oleracea L. var. capitata. Russ J Plant Physiol. 2007;54:653–658. doi: 10.1134/S1021443707050135. [DOI] [Google Scholar]
- Zhang Y, Bhalla PL. In vitro shoot regeneration from commercial cultivars of Australian canola (Brassica napus L.) Aust J Agric Res. 2004;55:753–756. doi: 10.1071/AR03209. [DOI] [Google Scholar]