Abstract
BACKGROUND
Transcutaneous low-level tragus electrical stimulation (LLTS) suppresses atrial fibrillation (AF) in canines.
OBJECTIVES
We examined the antiarrhythmic and anti-inflammatory effects of LLTS in humans.
METHODS
Patients with paroxysmal AF who presented for AF ablation, were randomized to either 1 hour of LLTS (n = 20) or sham control (n = 20). Attaching a flat metal clip onto the tragus produced LLTS (20 Hz) in the right ear (50% lower than the voltage slowing the sinus rate). Under general anesthesia, AF was induced by burst atrial pacing at baseline and after 1 hour of LLTS or sham. Blood samples from the coronary sinus and the femoral vein were collected at those time points and then analyzed for inflammatory cytokines, including tumor necrosis factor (TNF)-α and C-reactive protein (CRP), using a multiplex immunoassay.
RESULTS
There were no differences in baseline characteristics between the 2 groups. Pacing-induced AF duration decreased significantly by 6.3 ± 1.9 min compared to baseline in the LLTS group, but not in the controls (p = 0.002 for comparison between groups). AF cycle length increased significantly from baseline by 28.8 ± 6.5 ms in the LLTS group, but not in controls (p = 0.0002 for comparison between groups). Systemic (femoral vein) but not coronary sinus TNF-α and CRP levels decreased significantly only in the LLTS group.
CONCLUSIONS
LLTS suppresses AF and decreases inflammatory cytokines in patients with paroxysmal AF. Our results support the emerging paradigm of neuromodulation to treat AF.
Keywords: autonomic nervous system, inflammation, neuromodulation
The most common cardiac arrhythmia, atrial fibrillation (AF) is associated with significant cardiovascular morbidity and mortality (1,2). The current therapy to maintain sinus rhythm in patients with drug-refractory AF is surgical or catheter ablation (3). Despite being more efficacious than antiarrhythmic drugs, the long-term outcome of ablation for even the earliest stage of AF (paroxysmal AF) is disappointing (event-free survival <50% at 5 years) (4,5). As the population ages, the AF population in the United States is expected to reach 15 million by 2050 (6), leading to a quest for alternative nonpharmacological, nonablative therapies for managing patients with drug-refractory AF.
Neuromodulation is a novel therapy that has been used successfully in various diseases, including epilepsy (7) and heart failure (HF) (8). We (9-13) and others (14) have shown that low-level cervical vagus nerve stimulation (LLVNS), at voltages substantially below that associated with slowing the sinus rate or atrioventricular (AV) nodal conduction, significantly suppresses AF inducibility and shortens AF duration. More recently, we demonstrated in canines that AF inducibility was suppressed by LLVNS using a completely noninvasive approach by transcutaneous low-level stimulation of the tragus (LLTS), the anterior protuberance of the ear, where the auricular branch of the vagus nerve is accessible (15). In the present study, we evaluated the antiarrhythmic effects of 1-hour LLTS in patients referred for catheter ablation of paroxysmal AF. In addition, we examined the anti-inflammatory effects of LLTS in these patients.
METHODS
STUDY POPULATION
Patients with paroxysmal AF referred to the electrophysiological laboratory for AF ablation were eligible for enrollment. Patients were excluded if they had left ventricular dysfunction (defined as left ventricular ejection fraction <40%), significant valvular disorder (i.e., prosthetic valve or hemodynamically relevant valvular diseases) or recent (<6 months) stroke. All patients underwent baseline transthoracic and transesophageal echocardiography within 2 days prior to ablation. Antiarrhythmic medications were discontinued 5 days before the procedure except amiodarone, which was discontinued for at least 6 weeks. All patients received pre-procedure anticoagulation with either warfarin, to maintain an international normalized ratio between 2.0 and 3.0, or a new oral anticoagulant agent (NOAC). Warfarin was continued throughout the procedure, whereas NOACs were discontinued for 24 hours pre-procedure. The study was approved by the Institutional Review Board of the University of Oklahoma Health Sciences Center. After providing written informed consent, patients were randomized to either LLTS for 1 hour or sham LLTS (control).
EXPERIMENTAL PROTOCOL
Our laboratory routinely performs the ablation procedure under general anesthesia, using desflurance, vecuronium, and propofol. Before induction of general anesthesia, incremental voltages were applied to the right tragus (20 Hz, 1 ms square wave) through a Grass S88 stimulator (Natus Neurology Incorporated, Warwick, Rhode Island), connected to an isolation unit, until the patient experienced discomfort. The discomfort threshold was defined as the lowest voltage that resulted in any discomfort at the stimulation site. After induction of general anesthesia, sheaths were inserted in both femoral veins and coronary sinus. An octapolar catheter was positioned at the His bundle to monitor the atrial-His (AH) interval and a duodecapolar catheter was positioned in the coronary sinus for atrial pacing. In the baseline state, the atrial effective refractory period (AERP) was measured using programmed stimulation (S1-S1 = 600 ms) at 10x diastolic threshold from the right atrial appendage and the distal coronary sinus electrodes using 10 ms S1-S2 decrements. Subsequently, burst atrial pacing (cycle length decreasing from 250 ms to 200 ms) was delivered through the coronary sinus catheter to induce AF. This process was repeated until AF was induced. AF inducibility, defined as the number of attempts required to induce AF, was recorded as was the duration of AF induced by burst atrial pacing. The AF atrial cycle length was determined manually by averaging 30 consecutive beats at a paper speed 100 mm/s, using screen calipers, as previously described (16). Additionally, interelectrogram intervals of <100 ms and continuous electrical activity were counted as a single interval (16).
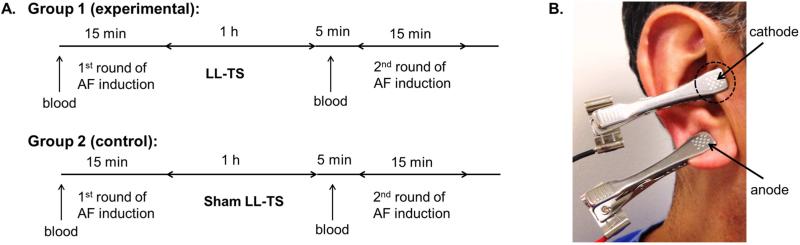
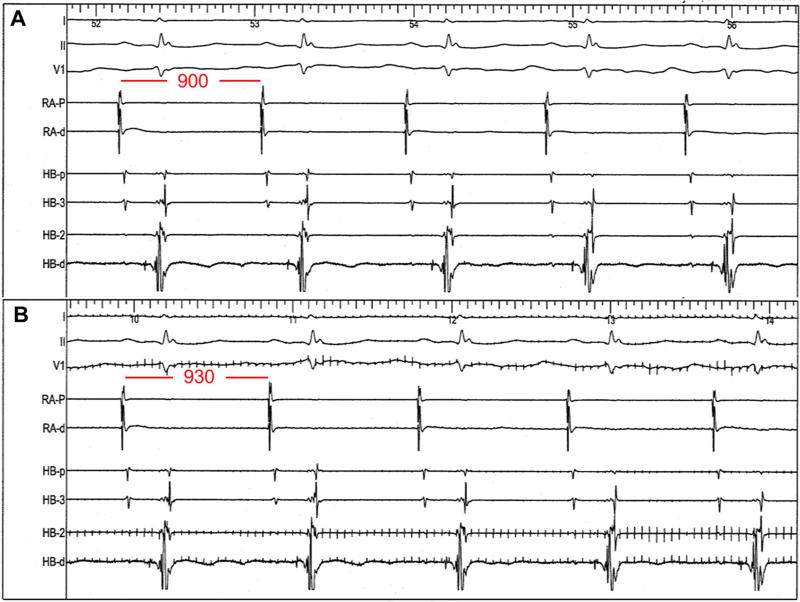
After acquiring the baseline parameters, LLTS or sham was initiated, regardless of AF continuation (Figure 1A). Stimulation of the right ear tragus was accomplished by attaching a flat metal clip onto the tragus, which served as the cathode, with another clip at an adjacent site for the anode (Figure 1B). Incremental voltages were applied to the tragus (20 Hz, 1 ms duration, square wave) using a Grass S88 stimulator until slowing of the sinus rate or AV nodal conduction (AH prolongation) was achieved (Figure 2). As soon as the sinus rate decreased and/or the AH interval prolonged, stimulation was turned off to avoid excessive bradycardia and/or heart block. The lowest voltage to slow the sinus rate or prolong the AH interval was defined as the threshold for setting the LLTS in each patient. LLTS was set at 50% below the voltage required to slow the sinus rate or prolong the AH interval. In the experimental group, LLTS was applied continuously for 1 hour; in the control group, the threshold was measured but no LLTS was delivered. To prevent inadvertent supra-threshold vagus nerve stimulation, the sinus rate and AH interval were monitored continuously to ensure that they were not altered by LLTS. Bispectral index (BIS) was continuously monitored, too, during the procedure. BIS was maintained between 40 and 60 during the maintenance phase of general anesthesia. During the 1-hour period of LLTS or sham stimulation, transseptal puncture and electroanatomical mapping, but not ablation, were performed. If the patient remained in AF at the end of the hour, cardioversion was performed to restore sinus rhythm. Within 5 minutes following the end of the LLTS application, programmed stimulation and burst atrial pacing were repeated using the same protocol as before, to measure the AERP and induce AF, respectively. Figure 1A illustrates the study protocol.
FIGURE 1. Study Protocol.
(A) Following the first induction of atrial fibrillation (AF) the groups underwent low-level electrical stimulation of the auricular branch of the right vagus nerve at the tragus (LLTS) or a sham procedure for 1 hour, followed by a second round of AF induction (B) To achieve electrical stimulation, a flat metal clip was attached to the right tragus (dashed circle), which served as the cathode. Another clip on the ear lobe served as the anode.
FIGURE 2. Voltage Threshold for Heart Rate Slowing.
(A) Before stimulation, sinus cycle length is 900 ms. (B) During stimulation at 20 V (note stimulation artifact), there is an increase in the sinus cycle length to 930 ms.
During baseline sinus rhythm, 5 ml of blood was simultaneously obtained from the peripheral femoral venous sheath (systemic sample) and the coronary sinus sheath (cardiac sample). Simultaneous cardiac and systemic samples were drawn again following 1 hour of LLTS or sham (Figure 1A). For each blood draw, the first 10 ml were discarded, and the blood was immediately transferred into tubes containing no anticoagulant agent. After incubation in an upright position at room temperature for 45 minutes to allow clotting, the blood was centrifuged for 15 minutes at 2,000 g to obtain serum. Patients’ serum was saved frozen at -80° C and processed in batches of 6 to 8. The investigators performing the biomarker assays were blinded to group assignment. Pro-inflammatory cytokines, including tumor necrosis factor (TNF)-α, C-reactive protein (CRP), interleukin (IL)-6, and IL-10 were measured using a commercially available assay analyzed on a flow cytometer (multiplex assay; R&D Systems, Minneapolis, Minnesota). All immunoassays were run in duplicate and read according to manufacturer's instructions.
STATISTICAL ANALYSIS
Data are presented as mean ± SD or percentages for continuous and categorical variables, respectively. Comparisons in baseline characteristics between groups were performed using Student t test or chi-square test for continuous and categorical variables, respectively. The differences in burst atrial pacing-induced AF duration, number of attempts, AERP, and inflammatory cytokines before and after 1 hour of LLTS or sham were compared between the groups with 2-way analysis of variance (ANOVA). The ANOVA modeling assumptions were evaluated by plotting the residuals by the predicted values (for the constant variance assumption) and comparing the normal QQ plot with the QQ plot of the residuals (for the normality assumption). The assumptions of constant variance and normality were reasonable for all ANOVA models based on the residual plots. The difference in AF recurrence at follow-up between the 2 groups was evaluated using the log-rank test. Statistical significance was declared at p < 0.05. All statistical analyses were performed using SAS 9.2 software (SAS Institute, Inc., Cary, North Carolina).
Based on experimental data (11), the present study was powered to detect a 50% reduction in the duration of burst atrial pacing-induced AF after LLTS compared to control. A sample size of 40 patients (20 in each group) would provide at least 80% power to detect this difference, at a 2-sided significance α level of 0.05.
RESULTS
We randomized 40 patients to either LLTS (n = 20) or sham control (n = 20). No statistically significant differences were observed in the baseline clinical and echocardiographic characteristics between the 2 groups (Table 1); the discomfort threshold and threshold for slowing the sinus rate or AV conduction were also similar. During threshold determination, the sinus rate decreased by 2.6 ± 1.0 beats/min in the LLTS group and by 2.7 ± 1.3 in the controls. The AH interval increased by 1.4 ± 0.5 ms in the LLTS group and by 1.5 ± 0.5 ms in the controls. There was no difference in the subtle changes of sinus rate and AH interval between the 2 groups. During LLTS or sham stimulation, there was no appreciable effect on either the sinus rate or AH interval. Under general anesthesia, we found no change in BIS levels when LLTS was applied, indicating no effect of LLTS on level of awareness.
TABLE 1.
Baseline Patient Characteristics
| LLTS (n = 20) | Control (n = 20) | p Value | |
|---|---|---|---|
| Age, yrs | 60.9 ± 7.8 | 62.9 ± 9.8 | 0.48 |
| Male | 15 (75) | 11 (55) | 0.18 |
| Duration of AF, yrs | 6.6 ± 4.9 | 5.5 ± 4.3 | 0.48 |
| Hypertension | 15 (75) | 15 (75) | 1.00 |
| Diabetes | 1 (5) | 5 (25) | 0.08 |
| Coronary artery disease | 3 (15) | 4 (20) | 0.68 |
| Obstructive sleep apnea | 8 (40) | 7 (35) | 0.74 |
| Left ventricular ejection fraction, % | 61.7 ± 7.3 | 60.8 ± 6.1 | 0.66 |
| Mean CHA2DS2-VASc score | 1.5 ± 0.9 | 2.2 ± 1.3 | 0.06 |
| CHA2DS2-VASc score | 0.43 | ||
| 0 | 3 (15) | 2 (10) | |
| 1 | 7 (35) | 4 (20) | |
| ≥2 | 10 (50) | 14 (70) | |
| Beta-blockers | 11 (55) | 12 (60) | 0.75 |
| ACE-I/ARB | 7 (35) | 11 (55) | 0.20 |
| Statin | 6 (30) | 8 (40) | 0.51 |
| New oral anticoagulant agents | 12 (60) | 15 (75) | 0.31 |
| Amiodarone | 1 (5) | 1 (5) | 1.00 |
| Other antiarrhythmic agents | 12 (60) | 14 (70) | 0.49 |
| Left atrial diameter, mm | 42.5 ± 9.1 | 43.3 ± 6.9 | 0.77 |
| Left atrial volume index, ml/m2 | 27.7 ± 12.9 | 23.9 ± 6.3 | 0.46 |
| Left ventricular hypertrophy | 13 (65) | 14 (70) | 0.74 |
| Heart rate, beats/min | 63.1 ± 13.0 | 63.8 ± 10.7 | 0.86 |
| AH interval, ms | 99.1 ± 30.1 | 99.5 ± 30.9 | 0.97 |
| Direct current cardioversion | 11 (55) | 9 (45) | 0.75 |
| Discomfort threshold, V | 29.1 ± 10.4 | 28.3 ± 9.0 | 0.87 |
| Threshold for slowing heart rate, V | 39.8 ± 25.7 | 33.5 ± 20.2 | 0.42 |
Values are n (%) or mean SD.
ACE-I = angiotensin-converting enzyme inhibitor; AF = atrial fibrillation; AH = atrial His; ARB = angiotensin receptor blocker; LLTS = low-level tragus stimulation.
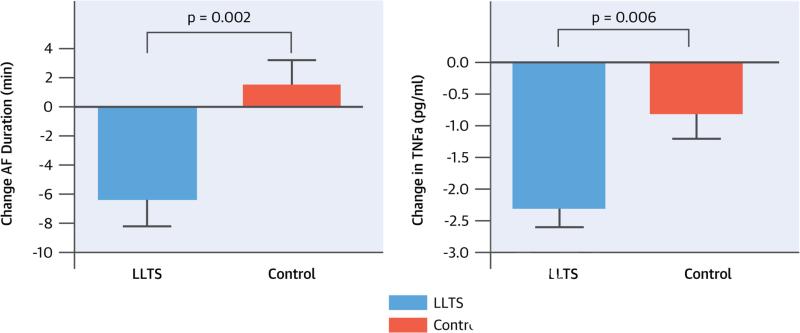
Summarized in Table 2, there were no statistically significant differences in the baseline electrophysiological parameters between the 2 groups. AF was inducible in all but 1 patient in whom, after LLTS, AF was not inducible after 15 attempts. Pacing-induced AF duration decreased significantly by 6.3 ± 1.9 min in the LLTS group compared to baseline and increased by 1.4 ± 1.8 min in the control group (between-group comparison p = 0.002; Central Illustration). Likewise, pacing-induced AF cycle length increased significantly (28.8 ± 6.5 ms) in the LLTS group compared to baseline, but decreased (8.7 ± 6.5 ms) in the controls (between-group comparison p = 0.0002; Figure 3). More attempts were required to induce AF in the LLTS group compared to baseline, whereas fewer attempts were required in the control group compared to baseline (LLTS: 2 attempts before vs. 4 attempts after; control: 2 attempts before vs. 1 attempt after; between-group comparison p = 0.005). The AERP at the right atrium and the distal coronary sinus increased in the LLTS group and decreased in the control group (both p = 0.04 for comparison between groups; Figure 4).
TABLE 2.
Electrophysiologic Changes
| LLTS (n = 20) | Control (n = 20) | p Value* | |||
|---|---|---|---|---|---|
| Baseline | 1 hour | Baseline | 1 hour | ||
| AF duration, min | 16.7 ± 6.2 | 10.4 ± 5.2 | 17.1 ± 5.7 | 18.5 ± 5.6 | 0.002 |
| AF cycle length, ms | 189.1 ± 37.6 | 217.9 ± 33.0 | 200.6 ± 42.3 | 191.9 ± 49.1 | 0.0002 |
| Number of attempts (median, interquartile range, min and max) | 2 (1 to 4) (1 to 10) | 4 (2 to 6) (1 to 15) | 2 (1 to 4) (1 to 10) | 1 (1 to 2) (1 to 6) | 0.005 |
| RA AERP, ms | 222.3 ± 31.1 | 232.3 ± 31.1 | 236.4 ± 46.5 | 227.8 ± 43.0 | 0.04 |
| CS AERP, ms | 222.7 ± 41.0 | 230.8 ± 41.0 | 253.3 ± 53.7 | 240.7 ± 50.6 | 0.04 |
Values are mean ± SD unless otherwise indicated.
Comparison of the change in each parameter before and after LLTS or sham between the 2 groups.
AERP = atrial effective refractory period; CS = coronary sinus; RA = right atrial; other abbreviations as in Table 1.
FIGURE 3. Effect of LLTS on Cycle Length.
There was a significant increase in AF cycle length compared to baseline in the LLTS group, but not in the control group. Abbreviations as in Figure 1.
FIGURE 4. Effect of LLTS on AERP.
Both the right atrial (RA) and coronary sinus (CS) atrial effective refractory period (AERP) increased in the LLTS group and decreased in the control group. Other abbreviations as in Figure 1.
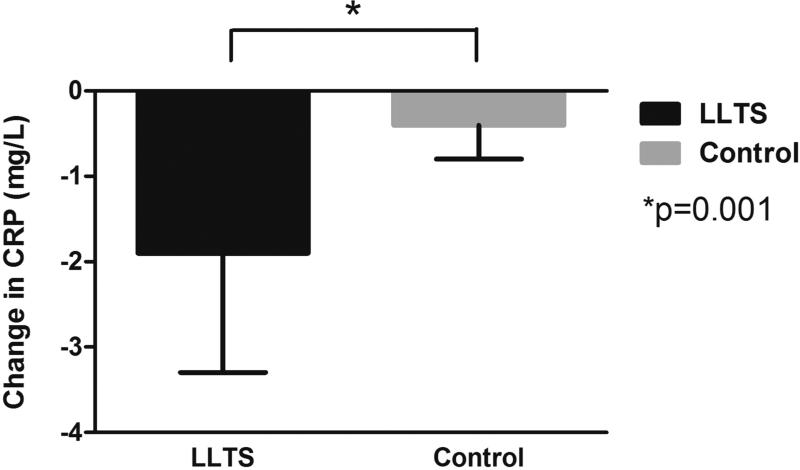
There were no significant differences in any of the cytokines measured at baseline between the 2 groups (Table 3). Systemic TNF-α levels were suppressed significantly (2.3 ± 0.3 pg/ml) compared to baseline in the LLTS group but not in the control group (between-group comparison p = 0.006; Central Illustration). Importantly, the magnitude of decrease in TNF-α levels by LLTS was comparable with the difference between patients with active versus inactive inflammatory diseases (17). Systemic CRP levels decreased significantly in the LLTS group (1.9 ± 1.4 ng/l) but not in the controls (between-group comparison p = 0.001; Figure 5). Of note, the decrease in TNF-α and CRP levels was similar in patients who did or did not undergo cardioversion. On the contrary, no difference was observed in the coronary sinus TNF-α or CRP levels between the 2 groups, indicating that the effect of LLTS was mediated through the systemic circulation. Systemic and coronary sinus IL-6 and IL-10 levels did not differ significantly between the 2 groups.
TABLE 3.
Cytokine Changes
| LLTS (n = 20) | Control (n = 20) | |||||
|---|---|---|---|---|---|---|
| Baseline | 1 hour | Baseline | 1 hour | p Value* | ||
| Femoral vein | TNF-α, pg/ml | 9.2 ± 4.8 | 6.9 ± 4.4 | 7.6 ± 3.0 | 6.8 ± 3.0 | 0.006 |
| Il-6, pg/ml | 3.2 ± 2.0 | 4.3 ± 2.1 | 3.9 ± 2.9 | 5.7 ± 3.0 | 0.18 | |
| CRP, mg/l | 9.8 ± 10.2 | 7.9 ± 8.2 | 6.6 ± 3.4 | 6.2 ± 3.9 | 0.001 | |
| IL-10, pg/ml | 0.41 ± 0.15 | 0.67 ± 0.55 | 0.47 ± 0.21 | 0.84 ± 0.49 | 0.61 | |
| Coronary sinus | TNF-α, pg/ml | 9.1 ± 5.5 | 7.4 ± 5.0 | 7.8 ± 3.3 | 6.3 ± 2.8 | 0.83 |
| IL-6, pg/ml | 5.5 ± 2.7 | 6.3 ± 3.3 | 5.7 ± 2.9 | 5.9 ± 2.7 | 0.49 | |
| CRP, mg/l | 8.3 ± 4.7 | 9.7 ± 9.5 | 8.0 ± 5.4 | 7.1 ± 3.6 | 0.27 | |
| IL-10, pg/ml | 0.38 ± 0.16 | 0.60 ± 0.59 | 0.49 ± 0.17 | 0.79 ± 0.49 | 0.76 | |
Values are mean ± SD.
Comparison of the change in each parameter before and after LLTS or sham between the 2 groups.
CRP = C-reactive protein; IL = interleukin; TNF-α = tumor necrosis factor-alpha; other abbreviations as in Table 1.
FIGURE 5. Effect of LLTS on Systemic CRP Levels.
There was a significant decrease in systemic C-reactive protein (CRP) levels compared to baseline in the LLTS group, but not in the control group. Other abbreviations as in Figure 1.
No major adverse events were noted in the study, including no effect on blood pressure or heart rate during stimulation. In 2 patients, a mild burn on the right ear was observed at the site of the stimulating electrode, which resolved with conservative measures. This adverse effect was not observed after adjusting the tension of the metal clip delivering LLTS.
Patients were followed at 1 month, 3 months, and approximately every 3 months thereafter. Median follow-up was 6 months (interquartile range 4 to 10 months). During follow-up, 5 (25%) patients in each group experienced recurrence of AF or atrial tachycardia (p = 0.93 by log-rank test).
DISCUSSION
In this study, transcutaneous electrical stimulation of the auricular branch of the right vagus nerve at the tragus suppressed AF and decreased inflammatory cytokines. This proof-of-concept and first-in-man study raises the possibility that noninvasive autonomic neuromodulation may be used to treat patients with paroxysmal AF. Notably, the average stimulation voltage used in this study (50% below the threshold for slowing the sinus rate or AV conduction) was less than the average discomfort threshold, suggesting that this treatment modality may be tolerated by ambulatory patients. Enhanced activity of the cardiac autonomic nervous system (CANS) and AF create a vicious cycle, in which high CANS activity can initiate AF and AF further augments CANS activity (13). A noninvasive neuromodulatory therapy such as LLTS that can break this cycle in early AF may be useful for a large population of AF patients. Additionally, by shortening the AF duration and suppressing the inflammatory process (Central Illustration), this therapy may prevent AF from progressing to more advanced stages, thereby decreasing the morbidities associated with longstanding AF, such as stroke, dementia, and HF.
ANTIARRHYTHMIC EFFECTS OF LLTS
CANS is a neural network that controls the heart's vascular, contractile, and electrophysiological functions. The neural signals in this network are integrated and processed at the ganglionated plexi (GP), which may contain hundreds to thousands of autonomic neurons and are typically located in the epicardial fat pads (18,19). Hyperactivity of the major atrial GP plays a critical role in AF initiation and maintenance, while ablating these GP provides additional benefits to the standard circumferential pulmonary vein (PV) isolation procedure (20). Prior experimental studies demonstrated that LLVNS and LLTS are antiadrenergic and anticholinergic (10,11,14,15). When LLTS was delivered to the cranial end of the transected vagal trunk, all the antiarrhythmic effects of LLTS disappeared, while LLVNS applied at the distal end of the transected vagal trunks retained its antiarrhythmic effects (9,15). Although LLVNS and LLTS probably activate both the afferent and efferent vagal fibers, these observations suggest that the efferent fibers of the vagus nerves are crucial to the antiarrhythmic effects. The seemingly “paradoxical” anticholinergic effects of LLVNS and LLTS, which require efferent vagal fibers for their actions, are mediated by inhibiting the activity of the major atrial GP, as evidenced by direct neural recordings within canine GP (10,12,13). Likewise, LLVNS decreases the activity of the left stellate ganglion, the gateway of sympathetic innervation to the heart (14). In the present study, similar mechanisms are likely underlying the antiarrhythmic effects of LLTS. The anticholinergic effects prolong the AERP and the antiadrenergic effects suppress the myocyte calcium transients, leading to the inhibition of both re-entry and triggered firing (21). Moreover, suppressing CANS activity also breaks the vicious cycle formed by hyperactivity of the CANS and atrial remodeling (13).
Our results can be interpreted in light of the currently accepted mechanism for AF initiation and maintenance, which requires both trigger and substrate (3). The trigger for paroxysmal AF often lies in the PV myocardium, whereas atrial and autonomic remodeling provides the substrate for AF to sustain once initiated (3). We have provided evidence that LLTS modified the substrate for AF by prolonging the AERP and AF cycle length. Although this study offers no direct evidence that LLTS could inhibit triggered activity, this effect has been demonstrated in animal studies, in which LLVNS inhibited induction of rapid firing and AF from the PVs (9,10). Moreover, the antiarrhythmic effects of LLVNS and LLTS were very similar in various animal models of AF, including rapid atrial pacing, acetylcholine-induced AF, and high-frequency stimulation-induced triggered firing (9,13,15). In this study, we used widely accepted parameters, such as AF duration, AF cycle length, and AERP. Although AF inducibility lacks accuracy and its clinical significance is debatable (22), the favorable effects of LLTS on other endpoints, such as AF duration and AERP, lend credence to our results.
ANTI-INFLAMMATORY EFFECT OF LLTS
Our findings’ significance is highlighted by substantial evidence linking AF with inflammation (23-26). In a large population-based study, CRP levels predicted both the presence of AF at baseline and AF development during follow-up, even after adjusting for other known cardiovascular risk factors (23). Risk of AF was also progressively higher with increasing CRP quartiles (23). In another case-control study, TNF-α levels were significantly increased in patients with AF compared to controls, and there was a graded increase in TNF-α among patients with paroxysmal, persistent, and permanent AF(25). In the present study, it is noteworthy that blood levels of inflammatory cytokines in both the LLTS and control groups were elevated at baseline (27) and similar to those reported in large population-based cohorts and case-control studies (23,25). Moreover, the magnitude of decrease in TNF-α and CRP levels by LLTS is consistent with the difference between controls and prerheumatoid arthritis patients (27) as well as for patients with active versus inactive inflammatory diseases, suggesting a significant biological effect. Extrapolating from the post-myocardial infarction literature, a decrease in CRP and/or TNF-α of a similar magnitude to that observed in our study, if sustained, would predict a lower incidence of recurrent cardiovascular events (28,29). Nonetheless, given that the sustainability of inflammatory cytokine reduction and the long-term effects of LLTS remain unknown, these results should be interpreted cautiously.
More than 80% of the nerve fibers in the vagal trunks are afferent vagal fibers carrying neural input from nearly the entire body, and the tonic activity of the vagus nerves is essential to maintain immune homeostasis (30). Our results are consistent with the recently recognized anti-inflammatory properties of the vagus nerve (30,31). Stimulation of the cholinergic anti-inflammatory pathway, which is comprised of efferent vagus nerve signals, leads to acetylcholine-dependent activation of the alpha7 nicotinic acetylcholine receptor subunit on monocytes and macrophages, resulting in reduced production of the inflammatory cytokines TNF-α, IL-1β, and IL-6, but not IL-10 (31,32). Reduction in inflammatory markers with vagus nerve stimulation was also observed in a canine model of heart failure (33). The finding that LLTS suppresses systemic but not coronary sinus levels of TNF-α and CRP suggests that the reduction of inflammatory cytokines resulted from activating the cholinergic anti-inflammatory pathway and not just a local cardiac vagal effect. The cholinergic anti-inflammatory pathway involves efferent vagus nerve fibers, travelling to the spleen through the celiac ganglion and the splenic nerve to inhibit inflammatory responses (30). If the anti-inflammatory effects observed resulted from a local cardiac effect, the coronary sinus cytokine levels would have been decreased before the systemic cytokine levels. Importantly, we have provided evidence that the cholinergic anti-inflammatory pathway can be activated without heart rate slowing, consistent with a previous experimental study, which demonstrated that transcutaneous vagus nerve stimulation inhibited inflammatory cytokine production in mice with endotoxemia (34).
CLINICAL IMPLICATIONS
In this study, we demonstrated that neuromodulation by LLTS is a promising, noninvasive therapy to treat AF and AF-related inflammation. Given the recently demonstrated beneficial effects of vagus nerve stimulation in patients with HF (8,35), neuromodulation may provide a means of treating patients with both HF and AF with a single treatment modality. Moreover, these results may provide the basis to potentially expand the therapeutic targets of this treatment modality into other inflammatory conditions, including rheumatoid arthritis and systemic lupus erythematosus (30). In the present study, the stimulation voltage (50% below the cardiac threshold) was lower than the discomfort threshold in the average patient. Importantly, LLTS with the stimulation strength 80% below the cardiac threshold exerted similar antiarrhythmic effects as 50% below the cardiac threshold (15), indicating that this noninvasive approach can be tolerated by ambulatory patients. Moreover, transcutaneous stimulation of the auricular branch of the vagus nerve in patients with epilepsy was safe and tolerable in a recent pilot study (36).
LIMITATIONS
The optimal stimulation parameters for LLTS have not been determined. In this study we used 20 Hz based on our previous animal experience (9-12). Further studies are necessary to evaluate the shortest time period and the lowest stimulation strength of LLTS that would have a beneficial effect. Intermittent stimulation may be more efficacious than continuous, based on recent experimental evidence (37). Although any LLTS-caused discomfort may have been masked by general anesthesia, the average stimulation level (<50% lower than the voltage threshold) was lower than the average discomfort threshold. In every patient, the discomfort threshold was higher than the LLTS voltage level. Thus, it is unlikely that discomfort is a major contributing factor to the effect of LLTS. There was a trend towards an increased prevalence of diabetes in the control group, which appears to be clinically significant. However, the 2 groups were otherwise well matched; thus, it is unlikely that these small differences influenced the results. In this study we examined the acute effects of LLTS; further trials are warranted to evaluate the long-term clinical importance of our findings.
CONCLUSIONS
In this study, we demonstrated for the first time in humans that the duration and inducibility of AF as well as inflammatory cytokines were suppressed noninvasively by low-level transcutaneous electrical stimulation of the auricular branch of the vagus nerve at the tragus. Our results support the notion that autonomic neuromodulation may emerge as an alternative nonpharmacological, nonablative modality to treat paroxysmal AF. Further studies in ambulatory patients are warranted.
PERSPECTIVES.
Competency in Medical Knowledge: Atrial fibrillation and associated inflammatory cytokines can be suppressed by delivering low-level transcutaneous vagus nerve stimulation at the tragus. Translational outlook: Further studies are needed to explore the safety, efficacy and long-term outcomes of non-pharmacologic, non-ablative neuromodulatory therapy for patients with paroxysmal atrial fibrillation.
Figure. CENTRAL ILLUSTRATION Neuromodulation Suppresses Inflammation and AF.
This study examined the antiarrhythmic and anti-inflammatory effects of low-level electrical stimulation of the auricular branch of the right vagus nerve at the tragus (LLTS) in patients referred for atrial fibrillation (AF) ablation. We demonstrated for the first time in humans that LLTS compared to control (A) decreased AF duration and (B) suppressed inflammatory cytokines. TNF-α = tumor necrosis factor-alpha.
Acknowledgments
Funding source: Funded by NIH/NIGMS #8P20GM103447 to Stavros Stavrakis
ABBREVIATIONS
- AERP
atrial effective refractory period
- AF
atrial fibrillation
- AV
atrioventricular
- CANS
cardiac autonomic nervous system
- CRP
C-reactive protein
- GP
ganglionated plexi
- IL
interleukin
- LLTS
low-level tragus stimulation
- LLVNS
low-level vagus nerve stimulation
- TNF-α
tumor necrosis factor-alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
REFERENCES
- 1.Benjamin EJ, Wolf PA, D'Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 3.Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation). J Am Coll Cardiol. 2006;48:854–906. doi: 10.1016/j.jacc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Ouyang F, Tilz R, Chun J, et al. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation. 2010;122:2368–77. doi: 10.1161/CIRCULATIONAHA.110.946806. [DOI] [PubMed] [Google Scholar]
- 5.Weerasooriya R, Khairy P, Litalien J, et al. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow-up? J Am Coll Cardiol. 2011;57:160–6. doi: 10.1016/j.jacc.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 6.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–25. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Menachem E. Vagus-nerve stimulation for the treatment of epilepsy. Lancet Neurol. 2002;1:477–82. doi: 10.1016/s1474-4422(02)00220-x. [DOI] [PubMed] [Google Scholar]
- 8.De Ferrari GM, Crijns HJ, Borggrefe M, et al. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J. 2011;32:847–55. doi: 10.1093/eurheartj/ehq391. [DOI] [PubMed] [Google Scholar]
- 9.Li S, Scherlag BJ, Yu L, et al. Low-level vagosympathetic stimulation: a paradox and potential new modality for the treatment of focal atrial fibrillation. Circ Arrhythm Electrophysiol. 2009;2:645–51. doi: 10.1161/CIRCEP.109.868331. [DOI] [PubMed] [Google Scholar]
- 10.Sha Y, Scherlag BJ, Yu L, et al. Low-level right vagal stimulation: anticholinergic and antiadrenergic effects. J Cardiovasc Electrophysiol. 2011;22:1147–53. doi: 10.1111/j.1540-8167.2011.02070.x. [DOI] [PubMed] [Google Scholar]
- 11.Sheng X, Scherlag BJ, Yu L, et al. Prevention and reversal of atrial fibrillation inducibility and autonomic remodeling by low-level vagosympathetic nerve stimulation. J Am Coll Cardiol. 2011;57:563–71. doi: 10.1016/j.jacc.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 12.Yu L, Scherlag BJ, Li S, et al. Low-level vagosympathetic nerve stimulation inhibits atrial fibrillation inducibility: direct evidence by neural recordings from intrinsic cardiac ganglia. J Cardiovasc Electrophysiol. 2011;22:455–63. doi: 10.1111/j.1540-8167.2010.01908.x. [DOI] [PubMed] [Google Scholar]
- 13.Yu L, Scherlag BJ, Sha Y, et al. Interactions between atrial electrical remodeling and autonomic remodeling: how to break the vicious cycle. Heart Rhythm. 2012;9:804–9. doi: 10.1016/j.hrthm.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 14.Shen MJ, Shinohara T, Park HW, et al. Continuous low-level vagus nerve stimulation reduces stellate ganglion nerve activity and paroxysmal atrial tachyarrhythmias in ambulatory canines. Circulation. 2011;123:2204–12. doi: 10.1161/CIRCULATIONAHA.111.018028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu L, Scherlag BJ, Li S, et al. Low-level transcutaneous electrical stimulation of the auricular branch of the vagus nerve: a noninvasive approach to treat the initial phase of atrial fibrillation. Heart Rhythm. 2013;10:428–35. doi: 10.1016/j.hrthm.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Haissaguerre M, Sanders P, Hocini M, et al. Changes in atrial fibrillation cycle length and inducibility during catheter ablation and their relation to outcome. Circulation. 2004;109:3007–13. doi: 10.1161/01.CIR.0000130645.95357.97. [DOI] [PubMed] [Google Scholar]
- 17.Evereklioglu C, Er H, Turkoz Y, Cekmen M. Serum levels of TNF-alpha, sIL-2R, IL-6, and IL-8 are increased and associated with elevated lipid peroxidation in patients with Behcet's disease. Mediators Inflamm. 2002;11:87–93. doi: 10.1080/09629350220131935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armour JA, Murphy DA, Yuan BX, Macdonald S, Hopkins DA. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec. 1997;247:289–98. doi: 10.1002/(SICI)1097-0185(199702)247:2<289::AID-AR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 19.Pauza DH, Skripka V, Pauziene N, Stropus R. Morphology, distribution, and variability of the epicardiac neural ganglionated subplexuses in the human heart. Anat Rec. 2000;259:353–82. doi: 10.1002/1097-0185(20000801)259:4<353::AID-AR10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 20.Katritsis DG, Pokushalov E, Romanov A, et al. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. J Am Coll Cardiol. 2013;62:2318–25. doi: 10.1016/j.jacc.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 21.Patterson E, Lazzara R, Szabo B, et al. Sodium-calcium exchange initiated by the Ca2+ transient: an arrhythmia trigger within pulmonary veins. J Am Coll Cardiol. 2006;47:1196–206. doi: 10.1016/j.jacc.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 22.Richter B, Gwechenberger M, Filzmoser P, et al. Is inducibility of atrial fibrillation after radio frequency ablation really a relevant prognostic factor? Eur Heart J. 2006;27:2553–9. doi: 10.1093/eurheartj/ehl307. [DOI] [PubMed] [Google Scholar]
- 23.Aviles RJ, Martin DO, Apperson-Hansen C, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–10. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 24.Friedrichs K, Klinke A, Baldus S. Inflammatory pathways underlying atrial fibrillation. Trends Mol Med. 2011;17:556–63. doi: 10.1016/j.molmed.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Solus J, Chen Q, et al. Role of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm. 2010;7:438–44. doi: 10.1016/j.hrthm.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim HS, Willoughby SR, Schultz C, et al. Effect of atrial fibrillation on atrial thrombogenesis in humans: impact of rate and rhythm. J Am Coll Cardiol. 2013;61:852–60. doi: 10.1016/j.jacc.2012.11.046. [DOI] [PubMed] [Google Scholar]
- 27.Masi AT, Rehman AA, Elmore KB, Aldag JC. Serum acute phase protein and inflammatory cytokine network correlations: comparison of a pre-rheumatoid arthritis and non-rheumatoid arthritis community cohort. J Innate Immun. 2013;5:100–13. doi: 10.1159/000345700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation. 1998;97:2007–11. doi: 10.1161/01.cir.97.20.2007. [DOI] [PubMed] [Google Scholar]
- 29.Ridker PM, Rifai N, Pfeffer M, et al. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101:2149–53. doi: 10.1161/01.cir.101.18.2149. [DOI] [PubMed] [Google Scholar]
- 30.Huston JM, Tracey KJ. The pulse of inflammation: heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. J Intern Med. 2011;269:45–53. doi: 10.1111/j.1365-2796.2010.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 32.Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–8. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Popovic ZB, Bibevski S, et al. Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ Heart Fail. 2009;2:692–9. doi: 10.1161/CIRCHEARTFAILURE.109.873968. [DOI] [PubMed] [Google Scholar]
- 34.Huston JM, Gallowitsch-Puerta M, Ochani M, et al. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit Care Med. 2007;35:2762–8. doi: 10.1097/01.CCM.0000288102.15975.BA. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz PJ, De Ferrari GM, Sanzo A, et al. Long term vagal stimulation in patients with advanced heart failure: first experience in man. Eur J Heart Fail. 2008;10:884–91. doi: 10.1016/j.ejheart.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 36.Stefan H, Kreiselmeyer G, Kerling F, et al. Transcutaneous vagus nerve stimulation (t-VNS) in pharmacoresistant epilepsies: a proof of concept trial. Epilepsia. 2012;53:e115–8. doi: 10.1111/j.1528-1167.2012.03492.x. [DOI] [PubMed] [Google Scholar]
- 37.Shinlapawittayatorn K, Chinda K, Palee S, et al. Low-amplitude, left vagus nerve stimulation significantly attenuates ventricular dysfunction and infarct size through prevention of mitochondrial dysfunction during acute ischemia-reperfusion injury. Heart Rhythm. 2013;10:1700–7. doi: 10.1016/j.hrthm.2013.08.009. [DOI] [PubMed] [Google Scholar]