Abstract
Seafood is an important source of nutrients for fetal neurodevelopment. Most individuals are exposed to the toxic element mercury through seafood. Due to the neurotoxic effects of mercury, United States government agencies recommend no more than 340 g (12 oz) per week of seafood consumption during pregnancy. However, recent studies have shown that selenium, also abundant in seafood, can have protective effects against mercury toxicity. In this study, we analyzed mercury and selenium levels and selenoprotein mRNA, protein, and activity in placenta of a cohort of women in Hawaii in relation to maternal seafood consumption assessed with dietary surveys. Fish consumption resulted in differences in mercury levels in placenta and cord blood. When taken as a group, those who consumed no fish exhibited the lowest mercury levels in placenta and cord blood. However, there were numerous individuals who either had higher mercury with no fish consumption or lower mercury with high fish consumption, indicating a lack of correlation. Placental expression of selenoprotein mRNAs, proteins and enzyme activity was not statistically different in any region among the different dietary groups. While the absence of seafood consumption correlates with lower average placental and cord blood mercury levels, no strong correlations were seen between seafood consumption or its absence and the levels of either selenoproteins or selenoenzyme activity.
Keywords: methylmercury, placenta, seafood, selenium, selenoprotein
INTRODUCTION
High mercury (Hg) exposures are well known for their toxic effects on human health as seen in events of contamination in Minamata, Japan and in Iraq [1-4]. Exposures to such high amounts of Hg are now extremely uncommon, but the potential effects of low-level MeHg exposures remain controversial. The largest contributor to Hg in the atmosphere is coal-burning power plants [5]. After Hg is released into the air and settles into sources of water, microorganisms convert it to MeHg, which accumulates in small fish and bioaccumulates in predatory species with the highest levels being observed in sharks and toothed whales. For most individuals, primary exposure to MeHg is through seafood [6]. Epidemiological studies in the Faroe Islands and New Zealand report adverse effects associated with increasing maternal seafood MeHg exposures [7, 8] whereas studies in the Seychelle Islands and Bristol, United Kingdom report seafood consumption is associated with beneficial, rather than adverse outcomes in prenatally exposed children [9, 10].
Studies of catastrophically high MeHg exposures in Japan [11, 12] and Iraq [3, 4] and unusually high MeHg exposures from shark meats eaten in New Zealand [13] or pilot whale meats eaten in the Faroe Islands [14, 15] demonstrated that maternal MeHg exposure during pregnancy correspond with neurodevelopmental outcome defects (amounting to ~0.1 IQ points) subsequently observed in the children of exposed mothers. However, studies of ocean fish consumption in the Seychelles [16-18] found beneficial effects on child development associated with higher levels of maternal fish consumption and greater amounts of MeHg than those observed in the Faroe Islands study [19]. The largest, most comprehensive study of this issue found increasing maternal seafood consumption in the United Kingdom was associated with substantial beneficial effects [20] that increased child IQ’s by as much as 5 points [9]. A smaller study conducted in the United States found IQ’s of children whose mothers consumed the most seafood during pregnancy were similarly increased [21]. These effects are not attributed to MeHg exposure, but are instead thought to occur as a result of improved maternal nutritional intakes.
Based largely on the Faroe Islands study, the Federal Drug Administration (FDA) and National Research Council (NRC) in the United States issued seafood consumption advice in 2004 recommending that women who might become pregnant, pregnant women, nursing mothers, and young children should consume no more than 12 oz of low Hg seafood per week and to avoid eating shark, swordfish, king mackerel, or tilefish because of their high MeHg levels [22]. Although meant to protect children of fish consuming populations, mothers that take this advice to the extreme of avoiding seafood altogether could actually harm their own health and diminish child neurodevelopmental outcomes [9, 10, 23]. Seafood is an important source of polyunsaturated fatty acids, which are essential for the neurodevelopment of a growing fetus [8, 9, 24]. Additional risks that have been linked to decreased fish consumption and corresponding low omega-3 fatty acid levels are preeclampsia and premature delivery [9, 25].
Seafood is also an important source of other nutrients including vitamin D and selenium (Se). Selenium is incorporated into the amino acid selenocysteine, which is required in the active sites of enzymes that prevent and/or reverse oxidative damage in brain tissues of all forms of animal life with recognizable nervous systems. These enzymes include the glutathione peroxidases (GPx), thioredoxin reductases (TRx), and methionine R sulfoxide reductase. Dietary Se deficiency or decreased Se bioavailability result in decreases in selenoprotein levels and selenoenzyme activities. Reduced Se levels and placental glutathione peroxidase activity have been observed in preeclamptic versus normotensive placenta [26]. In addition, it is well documented that the mRNA levels for several selenoproteins are regulated by dietary Se [27, 28]. Thus, analysis of selenoprotein mRNAs, proteins, and enzymatic activities provides three independent assessments of dietary Se status and Se bioavailability.
Recent studies signify supplemental Se’s ability to mitigate Hg toxicity [29], and high MeHg exposures can irreversibly inhibit critical Se-dependent enzymes, leaving vulnerable tissues of the brain and neuroendocrine organs inadequately protected against oxidative damage [30]. Therefore, MeHg-dependent inhibition of Se-dependent enzymes is a major contributing cause, and may be the exclusive cause of the adverse child outcomes that occur in mothers that eat pilot whale or shark meats with disproportionately high Hg:Se molar ratios. Supplemental Se is able to replace the Se bound by MeHg, ameliorating or preventing MeHg toxicity in all species of animals, birds, fish and invertebrates that have been tested [29, 31]. Selenium is naturally present in all foods, but is especially abundant in ocean fish, and greatly exceeds MeHg levels in all but a few predatory species, e.g., mako shark [25].
The current pilot study was conducted to investigate the correlations between maternal fish consumption, Hg, Se, and omega-3 fatty acids in cord blood and placentas from a cohort of women in Hawaii [32]. This report is a secondary analysis, with the objective of investigating the correlations between the dietary recall of seafood consumption and placental Se status and antioxidant selenoenzyme protein and activity levels. Our results show that while the absence of seafood consumption correlates with lower placental and cord blood Hg levels, no correlations were seen between seafood consumption or its absence and the levels of either selenoproteins or selenoenzyme activity. This information, along with the known benefits of fish consumption from previous studies, provides crucial insights that should help to dispel the misinformation about the potential risks of seafood consumption during pregnancy.
MATERIALS AND METHODS
Subjects and enrollment
All women who presented to the Kapiolani Medical Center Labor and Delivery suite between June 2010 and March 2011 were assessed for eligibility to participate. Participants were considered eligible if they met the following criteria: age 18 to 45 years old, in labor with a live singleton fetus, gestational age ≥ 37 weeks, and not a cord blood donor. In addition, because specimens had to be processed within 2 hours of delivery, only women who ultimately delivered when laboratory assistants were available were eligible for participation. Women who reported tobacco, alcohol, or illicit drug use or chronic medical conditions were excluded. Patients who met eligibility criteria were approached about participating in the study. The first 100 to consent to participation were included. Patients were concomitantly enrolled in the Hawaii Biospecimen Repository, and all demographic and clinical data obtained via the repository project were available for this study. Either intrapartum or after delivery, patients were asked to complete a dietary survey of their fish and seafood consumption during their pregnancy. Women were asked to quantify the amount of seafood they had eaten by recording how many times they had eaten seafood in the last month and approximately how much they typically ate in each instance (they were informed that 3 oz is approximately the size of a deck of cards). Total fish consumption in the last month was calculated by multiplying the number of times the women reported eating seafood by the amount of seafood they typically ate during each meal.
Sample collection
After delivery, a single tube of approximately 14 mL of whole blood was collected from the umbilical cord in a standard EDTA tube. Samples were processed within 2 hours of delivery and 2 mL was aliquoted and stored at −25°C for Hg and Se assays. The rest of the sample was centrifuged and the red cell fraction was separated into aliquots that were stored at −80°C for omega-3 fatty acid analyses. Samples of placental tissue were obtained from regions proximal and peripheral to the umbilical cord, and frozen in liquid nitrogen within 2 hours of delivery, then transferred to freezers for storage at −80°C.
Mercury and selenium sample analysis
Umbilical cord blood and placental tissue samples collected were shipped to the Energy & Environmental Research Center at the University of North Dakota. Individual samples of blood and placental tissue (~0.25 g) were weighed into single-use, trace element-free 50-mL digestion tubes (Environmental Express, Mt. Pleasant, SC). Samples were prepared in a series of digestion sets (~50 samples per set) with every 10th sample prepared in duplicate along with elemental spike recovery samples. Each set included analysis blanks, certified reference materials (UTAK) and quality control samples.
Samples (and identically treated controls) were uniformly treated with 5 mL of HNO3 (Fisher Trace Metal Grade, Fisher Scientific, www.fishersci.com), capped and heated at 85°C in deep cell hot blocks (Environmental Express) for 24 hours. Samples were digested in capped tubes to protect from environmental background contamination and potential elemental losses from sample volatilization. Samples were cooled, then 1.5 mL of 30% H2O2 (Fisher Certified A.C.S., Fisher Scientific) was added to each prior to being recapped and returned to heating in the dry block at 85°C for 8 hours more. Samples were cooled, and 15 mL of 12 N HCl (Fisher Trace Metal Grade, Fisher Scientific) were added. Samples were heated at 90°C for 90 minutes to reduce SeVI to SeIV. Samples were cooled and diluted to 25 mL with double-distilled water.
Digested and diluted samples were analyzed for total Hg content by cold-vapor atomic absorption spectrophotometry using a CETAC M-6000A (CETAC Technologies, Omaha, NE), and total Se was analyzed by hydride generation atomic absorption spectroscopy using a PS Analytical Dual Millennium Excalibur (PS Analytical, Deerfield Beach, FL). When necessary, samples were further diluted to concentrations that coincided with the instrumental calibration range. Before data from sample analysis runs were entered into the database, Hg and Se concentrations in sample digestion blanks and elemental recoveries in samples of certified reference materials were evaluated to qualify the analysis batch data for inclusion. Analytical blanks and whole blood controls (UTAK Laboratories, Valencia CA) for mercury and selenium were digested and run alongside samples in all batches. The certified values (and expected ranges) for mercury 14.7 μg/kg (12.5 to 16.9) and selenium 214 μg/kg (182-246) for UTAK 2 coincided well with the observed values. Average concentrations of mercury and selenium in these batches were 13.6 ± 1.1 μg Hg/kg and 202 ± 13.1 μg Se/kg, respectively.
Total Hg and Se mass concentrations in samples in μg/kg (parts per billion; ppb) were converted to molar concentrations (μmol/kg). Descriptive statistics and data distributions for mass and molar concentrations of Hg and Se were individually calculated and graphed for each tissue. Preliminary assessments of elemental concentrations in blood and placental tissues were performed on the basis of tissue and in regards to Hg–Se interactions.
Placenta sample preparation for RNA and protein analysis
Placenta lobes proximal and peripheral to the umbilical cord were thawed, washed in 1× PBS, and cut into three ~1 cm2 sections, one each from the maternal side, fetal side and middle region. The reasoning behind RNA extraction from the three different sections is based on information from our colleagues and publications showing that the expression patterns of different regions of the placenta are physiologically very distinct [33, 34]. Frozen tissue sections were ground into powder on dry ice, then divided into separate tubes for RNA or protein extraction and transferred to a freezer at −80°C for storage.
Selenoprotein mRNA quantitation by real time RT-PCR
Pulverized samples (50-100 mg) were thawed and RNA was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA) and RNeasy Mini kit with RNase-free DNase I treatment (all from Qiagen, Valencia, CA). Concentration and purity of extracted RNA was determined using A260/A280 measured on an ND1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE). Synthesis of cDNA was carried out using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems Inc, Foster City, CA), with 1 μg RNA per 20 μl reaction. For real-time PCR, 200 ng of cDNA was used in 5 μl reactions with Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen). Reactions were carried out in a LightCycler 480 II thermal cycler (Roche Applied Biosystems, Indianapolis, IN). Cycling conditions were as suggested in the SYBR Green kit instructions and results analyzed using Absolute Quantification Software (Roche).
Selenoprotein analysis by western blotting of placental protein
Pulverized samples were thawed and protein was extracted by homogenizing 0.5 g of tissue on ice in 0.5 ml of CelLytic MT buffer (Sigma, St. Louis, MO) containing 10× protease inhibitor cocktail (Calbiochem, San Diego, CA), and 7 mM EDTA. Homogenates were centrifuged at 16,000 rpm (Beckman Coulter microcentrifuge) for 20 minutes and supernatant was removed and stored at −80°C. Protein concentrations were determined using the Bradford Reagent (Bio-Rad, Hercules, CA) and 10 μg total protein was combined with reduced Laemmli buffer, boiled at 95°C for 10 min, cooled on ice, and loaded into wells of 4–20% precast polyacrylamide gels (Bio-Rad). Protein was transferred to PVDF membranes (LI-COR Biosciences, Lincoln, NE), which were blocked for one hour with Blocking Buffer (LI-COR) and then probed for one hour with primary antibodies, including goat anti-GPx1 (R&D Systems, Minneapolis, MN), rabbit anti-SelP [22], and mouse monoclonal IgG anti-β-actin (Sigma). Appropriate secondary antibodies (LI-COR) were incubated with the membranes for one hour and the bands were detected and quantified using a LI-COR Odyssey Infrared Imaging System and Image Studio Software (LI-COR). This software is used to measure mean intensity from regions of interest that corresponded to bands to be measured. The intensity of the target bands (e.g. GPx1 band) was normalized to that of the loading control band (e.g. β-actin band) to obtain normalized levels of target proteins.
Selenoenzyme assays on placenta tissue
Glutathione peroxidase (GPx) enzyme activity was assayed in aliquots of the same placenta samples used for western blotting above. Samples of 30 μg per assay were used with a commercial kit (PercipioBiosciences) per manufacturer’s instructions. Reactions were performed in duplicate. Thioredoxin reductase (TrxR) enzyme activity was assayed in aliquots of the same samples. Samples of 60 μg per assay were used with a commercial kit (Sigma) per manufacturer’s instructions.
Statistical analysis
T-test was used for all other analyses, as indicated in the figure legends.
RESULTS
Fish consumption surveys and outcomes
This study included 100 women who met all eligibility requirements [32]. Participants were categorized into three groups based on their seafood consumption during their last month of pregnancy. The maternal fish consumption groupings were: none (no seafood consumption), <12 oz/wk, or ≥12 oz/wk. Eighty six percent of the women reported eating fish during the last month of pregnancy, with 9% eating more than the recommended amount of 12 oz/wk. Types of fish consumed were tabulated, and are given in Supplemental Table 1. Ahi (yellowfin tuna) was by far the most commonly consumed fish, representing 40% of all types of fish eaten, followed by salmon (16%) mahimahi (10%) and canned tuna (10%).
Mercury and Selenium analysis in umbilical cord blood and placentas
Figure 1 illustrates Hg and Se concentrations in umbilical cord blood and placentas, respectively. There were significant increases in umbilical cord blood Hg contents in the groups reporting maternal fish consumption, compared to the maternal group reporting no fish consumption (none versus <12 oz/wk: p-value 0.004, 2.4 fold change; none versus ≥12 oz/wk: p-value 0.001, 2.7 fold change), but no difference between Hg contents of samples from groups with maternal consumption rates of <12 oz/wk versus ≥12 oz/wk. Two outliers with high blood or placental Hg among those in the no fish consumption group suggest that these individuals may have been exposed to other sources of Hg. Two additional samples were omitted from all analyses due to sample processing errors. There were also significant increases in placental Hg levels in the samples from the group with maternal consumption rates of <12 oz/wk compared to those from the no fish group (p-value 0.018, 1.9 fold change) and a non-significant trend in the ≥12 oz/wk group versus the no fish consumption group.
Figure 1. Maternal fish consumption effects on mercury and selenium levels in umbilical cord blood and placentas.
Data represented by average ± SEM. Statistical analysis performed with t-test. None: n=13; <12 oz/wk: n=75; ≥12 oz/wk: n=10. * p-value < 0.05. ** p-value < 0.01.
Increasing seafood consumption resulted in slight, but non-significant changes in Se concentrations in umbilical cord blood or placenta. The lack of effects of seafood consumption on Se concentrations in both cord blood and placenta may be due to homeostatic regulation of optimal tissue Se contents in preferentially supplied compartments such as placental and fetal tissues, as well as the influence of Se intake from additional dietary sources and/or maternal intake of prenatal supplements that contain Se.
Mercury levels do not closely correlate with fish consumption
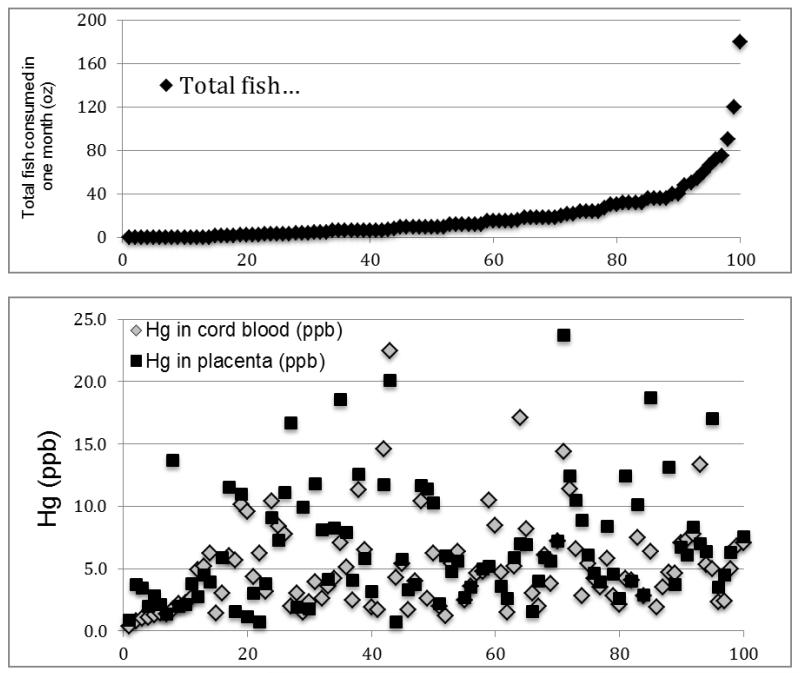
To further assess the relationship between fish consumption and Hg levels in cord blood and placenta, the number of times fish was consumed in the last month of pregnancy was ordered from none to highest and plotted in the upper panel of Figure 2. Placenta and cord blood Hg values were plotted for the samples in the same order in the lower panel. These figures show that even in the no fish group (samples 1-14) there is a considerable range of Hg concentrations, and in the highest fish consumers, there are examples where Hg levels are among the lowest values. Analysis of the fish consumption of the 7 women with the highest placental Hg levels is given in Supplemental Table 2, again showing no distinct pattern or correlation between fish consumption and Hg levels. Note that the individual with the second highest placental Hg had only consumed fish once, and the individual with the seventh highest placental Hg had consumed no fish.
Figure 2. Maternal fish consumption ordered from none to highest and plotted along with mercury levels in umbilical cord blood and placentas.
Fish consumption values in the upper panel are given as the ounces eaten in the last month of pregnancy. Hg levels in the lower panel are given as ppb.
Selenoprotein mRNA levels in placenta
Dietary Se is well documented to affect selenoprotein transcript levels, selenoprotein levels, and selenoenzyme activity [28]. Further, Hg and Se inversely affect each other’s bioavailability [36]. Thus, we carried out assessment of selenoprotein mRNAs, proteins, and enzyme activity levels in placentas of mothers who had not eaten fish compared to those who ate ≥12 oz/wk. We initially carried out preliminary assessments of selenoprotein mRNA levels in a placenta obtained following Caesarean section delivery. RNA was extracted from tissue obtained from the fetal side, middle section, and maternal side of the placenta. cDNA was prepared by reverse transcription and quantitative real time PCR was performed for 22 of the 25 human selenoproteins and two housekeeping genes, ubiquitin C (UBC) and glyceraldehyde-3-phosphate dehydrogenase (GAPD) [35]. Three selenoprotein mRNAs, those encoding glutathione peroxidases 2, 3 and 6 were not assessed as their tissue distribution is known to be highly restricted, and not detectable in this tissue. Expression levels of each selenoprotein mRNA in each of the tissue sections were normalized to both housekeeping genes, with very close agreement between the ratios. Supplemental Figure 1 shows quantitation normalized to UBC.
Our results reveal several important findings. First, these preliminary studies demonstrate that we can easily measure selenoprotein mRNAs from reasonably obtainable amounts of placental tissue. Second, they show significant differences in selenoprotein mRNA expression levels and tissue specific patterns of occurrence in the placenta. For example, 12 selenoprotein mRNAs, TrxR1, TrxR2, TrxR3, Dio3, SelH, SelI, SelK, SelN, SelO, SelS, SelV and SelW exhibit highest expression in the middle section, with lower but approximately equal levels in the fetal and maternal facing placenta samples. Five selenoprotein mRNAs, GPx1, Dio2, SelM, SelT, and Sel15, exhibit the highest expression levels in fetal facing placental tissue. Four selenoprotein mRNAs, GPx4, SelP, SPS2, and Dio1 exhibit a pattern whereby expression is nearly equal in the fetal facing and middle section, and lower in the maternal facing portion.
Selenoprotein mRNA levels for 5 selenoproteins were quantified in placental samples from mothers who had consumed varying levels of fish during the last month of pregnancy. Tissue was obtained from the regions proximal and peripheral to the umbilical cord to assess potential differences in these regions. When comparing gene expression of specific placental transcripts, it is important to consider the sampling site and control for placental inhomogeneity, as placental architecture and blood flow are not uniform across the chorioallantoic human placental disk [33, 34]. In addition, a previous study reported differential expression of selenoproteins in regions with differing proximity to the umbilical cord [26].
Tissue was then sectioned to obtain regions from the fetal side, middle, and maternal side. The mRNAs quantified included the two most abundantly expressed glutathione peroxidases, GPx1 and GPx4, the two iodothyronine deiodinases implicated in thyroid hormone metabolism in placenta, Dio2 and Dio3, and the Se transport protein, selenoprotein P (SelP). Data for GPx1, GPx4 and SelP mRNAs normalized to UBC mRNA are shown in Supplemental Figure 2. Expression of GPx1, GPx4 and SelP mRNAs were not statistically different between the group that ate no fish and the group that ate <12 oz/wk (not shown) or the group that ate ≥12 oz/wk, regardless of placental region, although GPx1 mRNA showed a trend toward higher expression in the ≥12 oz/wk group in tissue from the fetal side, both proximal to the umbilical cord (p-value 0.105) and peripheral (p-value 0.13).
Similarly, no statistical differences were seen for expression of Dio2 and Dio3 in association with maternal fish consumption (not shown). Though not significant, higher fish consumption of ≥12 oz/wk compared to the no fish group resulted in a trend toward higher GPx1 and SelP mRNA expression levels in placenta from the fetal side.
Selenoprotein levels in placenta
As mRNA levels do not always correlate with protein levels or enzyme activity, protein levels of GPx1 and SelP were quantified in the regions indicated above, with the results depicted in Figure 3. As indicated, there were no statistically significant differences of selenoprotein levels in all regions of the placenta in either group.
Figure 3. Western blotting detection of selenoproteins from indicated regions of human placenta.
Tissue homogenates were electrophoresed on 4-20% polyacrylamide gels, transferred to PVDF membranes and probed with antibodies to the indicated proteins. Membranes were washed and incubated with LI-COR secondary antibodies followed by enhanced infrared fluorescence detection. GPx1 and SelP protein expression in placenta lobes proximal (A, C) and peripheral to the umbilical cord (B, D) were normalized to β-actin. Data represented by average ± SEM. Statistical analysis performed with t-test. None: n=13; ≥12 oz/wk: n=10. P-values for GPx1: cord fetal = 0.458, cord middle = 0.904, cord maternal = 0.329, peripheral fetal = 0.754, peripheral middle = 0.604, peripheral maternal = 0.765. P-values for SelP: cord fetal = 0.246, cord middle = 0.819, cord maternal = 0.683, peripheral fetal = 0.397, peripheral middle = 0.360, peripheral maternal = 0.580.
Selenoenzyme activity levels in placenta
Cellular glutathione peroxidase and thioredoxin reductase activities were measured, with no fish consumption-dependent differences in any region of the placenta, although a slight trend toward higher GPx activity was seen in placenta from the fetal side proximal to the umbilical cord in samples from the high fish consumption group, consistent with the increase in GPx1 mRNA in this region (Figure 4).
Figure 4. Selenoenzyme activity assays from indicated regions of human placenta.
Tissue homogenates were used to measure cellular glutathione peroxidase activity and thioredoxin reductase activity in placenta lobes proximal (A, C) and peripheral (B, D) to the umbilical cord using commercial kits. Data represented by average ± SEM. Statistical analysis performed with t-test. None: n=13; ≥12 oz/wk: n=10. P-values for GPx: cord fetal = 0.177, cord middle = 0.174, cord maternal = 0.075, peripheral fetal = 0.226, peripheral middle = 0.917, peripheral maternal = 0.514. P-values for Trx: cord fetal = 0.609, cord middle = 0.282, cord maternal = 0.666, peripheral fetal = 0.163, peripheral middle = 0.463, peripheral maternal = 0.311.
DISCUSSION
The aim of this study was to explore the relationship between fish consumption during pregnancy and the concentrations of Hg, Se and selenoproteins in placenta and cord blood. This report is part of a larger study exploring the relationship between these parameters as well as omega-3 fatty acid levels and infant/child neurodevelopment. Herein, we investigated Hg and Se concentrations in cord blood and placental tissue, as well as selenoprotein expression in the placenta. There were several key findings.
Maternal consumption of seafood during pregnancy resulted in significantly higher Hg concentrations in umbilical cord blood compared to that of the no fish group, while placental Hg contents were significantly higher in samples from the group with seafood consumption <12 oz/wk and trended higher in samples from the group that ate ≥12 oz/wk compared to those that ate no seafood. Because people in Hawaii consume approximately four times more seafood than the U.S. population [24], we hypothesized that this might result in higher Hg concentrations in placenta and cord blood samples with increasing seafood consumption. This was indeed the case, as the group with high seafood consumption exhibited Hg concentrations ~2.1 and ~2.7 fold higher in placenta and cord blood, respectively, compared to those that did not eat seafood. However, analysis of individual levels rather than averages reveals a considerable range in Hg levels, in some cases higher Hg associated with no seafood and lower Hg associated with the highest seafood consumption. No consistent trends were observed between types of fish consumed and Hg levels. Thus, other sources of Hg exposure should also be considered in evaluating risk, and this information is beyond the scope of this study.
In 2009–2010 NHANES data, 2.3±0.41% of 1,786 women ages 16 to 49 had blood Hg concentrations greater than 5.8 μg/L, the concentration in maternal blood that is associated with the 0.1 μg/kg/day RfD [37]. Since 11% of the samples in the present study were above the 9.6 μg/L cord blood Hg concentration associated with the RfD, these children might be assumed to be at accentuated risk of neurodevelopmental impairments. However, the Hg RfD was established prior to recognition that the biochemical mechanism of MeHg toxicity involves irreversible inhibition of brain selenoenzymes [30, 35]. Since considering the biochemical mechanism of MeHg toxicity will reduce the uncertainty related to MeHg toxicodynamics, the 10-fold factor that is currently applied may no longer be required. However, further evaluations will be necessary to actually establish relative risks from MeHg exposures associated with maternal consumption of Se-rich ocean fish versus those from eating pilot whale meats which contain 4-5 times more Hg than Se [31]. This is especially true since outcomes of epidemiological studies that have examined the effects of maternal MeHg exposures from ocean fish consumption do not coincide with findings of studies of populations exposed to MeHg from eating shark or pilot whale meats [9, 10, 21, 38].
Fish are an excellent source of Se, thus we hypothesized that the high seafood group might also exhibit higher placenta and cord blood Se levels. Interestingly, the levels of Se in the placenta and cord blood were not statistically different between the two groups despite the difference in seafood consumption. This may be due to homeostatic regulation of tissue Se in this compartment, as well as relatively high baseline levels of dietary Se and potential use of prenatal vitamin and mineral supplements.
The placenta provides nutrients, gas exchange, and waste elimination for the growing fetus. It also acts as a barrier against toxic elements such as cadmium, but not MeHg. A recent study investigated the role of the placenta in the transfer of toxic elements from mother to fetus by comparing profiles of the elements between placenta and cord [38]. In that study, the placenta was found to be an effective barrier against inorganic Hg. In the present study, the ratio of Hg in placenta to cord blood was higher in women who did not consume seafood than in those who did. More Se is retained in the placenta than cord blood for this group as well.
We also measured selenoprotein expression within the placenta, including a comparison of fetal versus maternal sides. mRNA levels did not change in a statistically significant way between groups, suggesting that despite changes in Hg, selenoprotein transcripts were not affected in those who consumed fish. In placentas of women who consumed ≥12 oz/wk of seafood, there is a trend towards increased GPx1 mRNA expression, especially in samples from the fetal side, although GPx1 protein levels were unchanged. Higher seafood consumption was also associated with a trend towards increased GPx4 mRNA expression with increasing Hg levels throughout the placenta. Total GPx activity tends to increase especially at the fetal side with more seafood consumption, and this may aid to combat the toxicity associated with Hg. This suggests that with increasing Hg concentrations associated with seafood consumption, promoter elements of GPx1 and 4 may be activated which leads to an increase in their mRNA expression.
Our results show that while the absence of seafood consumption correlates with lower placental and cord blood Hg levels, no strong correlations were seen between seafood consumption or its absence and the levels of either selenoproteins or selenoenzyme activity.
Key extracellular selenoproteins are the plasma Se transport protein, SelP, and the plasma glutathione peroxidase, GPx3. Our findings indicate variable SelP mRNA and protein expression with no differences associated with fish consumption. As we reported in 2009, selenoprotein genes have putative metal response elements (MREs), which are regulated by metal responsive transcription factor 1 (MTF-1). In response to metal exposure, MTF-1 is transported from the cytosol to the nucleus to regulate the expression of MRE containing genes [39]. Although, SelP has a putative MRE, SelP mRNA levels were not affected by Hg levels in this study. Measurement of SelP and GPx3 levels in the cord blood would provide insights regarding their roles in Se transport in response to elevated Hg levels.
Since the Hg:Se molar ratios did not approach equimolar stoichiometries, the lack of Hg-dependent effects on selenoenzyme expression coincide with expectations. Future studies assessing DHA and EPA levels and neurodevelopmental outcomes in this same cohort will provide crucial insights into the magnitudes of risk versus benefits of seafood consumption during pregnancy. We expect these findings will help to elucidate the complex interplay between maternal intakes of nutrients from seafood in relation to child neurodevelopment, and will better inform the public in relation to US EPA seafood consumption recommendations.
Supplementary Material
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the participants in this research, Dr. Gillian Bryant-Greenwood and Sandy Yamamoto for invaluable advice on placental physiology, and financial support from National Institutes of Health grants G12-MD007601 and U54-MD007584 from the NIMHD and DK47320 from the NIDDK. Dr. Ralston’s work on this article was funded by U.S. Environmental Protection Agency grant number RD834792-01.
ABBREVIATIONS
- Dio
iodothyronine deiodinase
- EPA
Environmental Protection Agency
- GPx
glutathione peroxidase
- Hg
mercury
- MeHg
Methylmercury
- NRC
National Research Council
- Se
selenium
- Sel
selenoprotein
- TrxR
thioredoxin reductase
- UBC
ubiquitin C
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Amin-Zaki LS, Elhassani S, Majeed MA, Clarkson TW, Doherty RA, Greenwood MR, Giovanoli-Jakubczak T. Perinatal methylmercury poisoning in Iraq. Am. J. Dis. Child. 1976;130:1070–6. doi: 10.1001/archpedi.1976.02120110032004. [DOI] [PubMed] [Google Scholar]
- [2].Karagas MR, Choi AL, Oken E, Horvat M, Schoeny R, Kamai E, Cowell W, Grandjean P, Korrick S. Evidence on the human health effects of low-level methylmercury exposure. Environ. Health Perspect. 2012;120:799–806. doi: 10.1289/ehp.1104494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Marsh DO, Myers GJ, Clarkson TW, Amin-Zaki L, Tikriti S, Majeed MA. Fetal methylmercury poisoning: clinical and toxicological data on 29 cases. Ann. Neurol. 1980;7:348–53. doi: 10.1002/ana.410070412. [DOI] [PubMed] [Google Scholar]
- [4].Marsh DO, Clarkson TW, Cox C, Myers GJ, Amin-Zaki L, Al-Tikriti S. Fetal methylmercury poisoning. Relationship between concentration in single strands of maternal hair and child effects. Arch. Neurol. 1987;44:1017–22. doi: 10.1001/archneur.1987.00520220023010. [DOI] [PubMed] [Google Scholar]
- [5].United States Environmental Protection Agency Mercury. http://www.epa.gov/hg/about.htm.
- [6].Xue J, Zartarian VG, Liu SV, Geller AM. Methyl mercury exposure from fish consumption in vulnerable racial/ethnic populations: probabilistic SHEDS-Dietary model analyses using 1999-2006 NHANES and 1990-2002 TDS data. Sci. Total Environ. 2012;414:373–9. doi: 10.1016/j.scitotenv.2011.10.010. [DOI] [PubMed] [Google Scholar]
- [7].Debes F, Budtz-Jorgensen E, Weihe P, White RF, Grandjean P. Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years. Neurotoxicol. Teratol. 2006;28:536–547. doi: 10.1016/j.ntt.2006.02.005. [DOI] [PubMed] [Google Scholar]
- [8].Strain JJ, Davidson PW, Bonham MP, Duffy EM, Stokes-Riner A, Thurston SW, Wallace JM, Robson PJ, Shamlaye CF, Georger LA, Sloane-Reeves J, Cernichiari E, Canfield RL, Cox C, Huang LS, Janciuras J, Myers GJ, Clarkson TW. Associations of maternal long-chain polyunsaturated fatty acids, methyl mercury, and infant development in the Seychelles Child Development Nutrition Study. Neurotoxicology. 2008;29:776–82. doi: 10.1016/j.neuro.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hibbeln JR, Davis JM, Steer C, Emmett P, Rogers I, Williams C, Golding J. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet. 2007;369:578–85. doi: 10.1016/S0140-6736(07)60277-3. [DOI] [PubMed] [Google Scholar]
- [10].van Wijngaarden E, Thurston SW, Myers GJ, Strain JJ, Weiss B, Zarcone T, Watson GE, Zareba G, McSorley EM, Mulhern MS, Yeates AJ, Henderson J, Gedeon J, Shamlaye CF, Davidson PW. Prenatal methyl mercury exposure in relation to neurodevelopment and behavior at 19 years of age in the Seychelles Child Development Study. Neurotoxicol. Teratol. 2013;39C:19–25. doi: 10.1016/j.ntt.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Takeuchi T, Eto K, Kinjo Y, Tokunaga H. Human brain disturbance by methylmercury poisoning, focusing on the long-term effect on brain weight. Neurotoxicology. 1996;17:187–90. [PubMed] [Google Scholar]
- [12].Takeuchi T, Eto K, Tokunaga H. Mercury level and histochemical distribution in a human brain with Minamata disease following a long-term clinical course of twenty-six years. Neurotox. 1989;10:651–7. [PubMed] [Google Scholar]
- [13].Crump KS, Kjellström T, Shipp AM, Silvers A, Stewart A. Influence of prenatal mercury exposure upon scholastic and psychological test performance: benchmark analysis of a New Zealand cohort. Risk Anal. 1998;18:701–13. doi: 10.1023/b:rian.0000005917.52151.e6. [DOI] [PubMed] [Google Scholar]
- [14].Grandjean P, Weihe P, White RF, Debes F. Cognitive performance of children prenatally exposed to “safe” levels of methylmercury. Environ. Res. 1998;77:165–72. doi: 10.1006/enrs.1997.3804. [DOI] [PubMed] [Google Scholar]
- [15].Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, Murata K, Sørensen N, Dahl R, Jørgensen PJ. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol. Teratol. 1997;19:417–28. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- [16].Davidson PW, Myers GJ, Cox C, Axtell C, Shamlaye C, Sloane-Reeves J, Cernichiari E, Needham L, Choi A, Wang Y, Berlin M, Clarkson TW. Effects of prenatal and postnatal methylmercury exposure from fish consumption on neurodevelopment: outcomes at 66 months of age in the Seychelles Child Development Study. JAMA. 1998;280:701, 7. doi: 10.1001/jama.280.8.701. [DOI] [PubMed] [Google Scholar]
- [17].Myers GJ, Davidson PW. Prenatal methylmercury exposure and children: neurologic, developmental, and behavioral research. Environ. Health Perspect. 1998;106:841–7. doi: 10.1289/ehp.98106841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Myers GJ, Davidson PW, Palumbo D, Shamlaye C, Cox C, Cernichiari E, Clarkson TW. Secondary analysis from the Seychelles Child Development Study: the child behavior checklist. Envir. Res. 84:12–9. doi: 10.1006/enrs.2000.4085. [DOI] [PubMed] [Google Scholar]
- [19].Myers GJ, Davidson PW, Strain JJ. Nutrient and methyl mercury exposure from consuming fish. J. Nutr. 2007;137(2007):2805–8. doi: 10.1093/jn/137.12.2805. [DOI] [PubMed] [Google Scholar]
- [20].Daniels JL, Longnecker MP, Rowland AS, Golding J, ALSPAC Study Team Fish intake during pregnancy and early cognitive development of offspring. Epidemiology. 2004;15:394–402. doi: 10.1097/01.ede.0000129514.46451.ce. [DOI] [PubMed] [Google Scholar]
- [21].SA Lederman, Jones RL, Caldwell KL, Rauh V, Sheets SE, Tang D, Viswanathan S, Becker M, Stein JL, Wang RY, Perera FP. Relation between cord blood mercury levels and early child development in a World Trade Center cohort. Environmental Health Perspectives. 2008;116:1085–1091. doi: 10.1289/ehp.10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].United States Environmental Protection Agency . What you need to know about mercury in fish and shellfish. 2004. http://www.epa.gov/fishadvisories/advice/ [Google Scholar]
- [23].Davidson PW, Cory-Slechta DA, Thurston SW, Huang LS, Shamlaye CF, Gunzler D, Watson G, van Wijngaarden E, Zareba G, Klein JD, Clarkson TW, Strain JJ, Myers GJ. Fish consumption and prenatal methylmercury exposure: Cognitive and behavioral outcomes in the main cohort at 17 years from the Seychelles child development study. Neurotoxicology. 2011;32:711–717. doi: 10.1016/j.neuro.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sato RL, Li GG, Shaha S. Antepartum seafood consumption and mercury levels in newborn cord blood. Am. J. Obstet. Gynecol. 2006;194:1683–1688. doi: 10.1016/j.ajog.2006.03.005. [DOI] [PubMed] [Google Scholar]
- [25].Kaneko JJ, Ralston NV. Selenium and mercury in pelagic fish in the central north pacific near Hawaii. Biol. Trace. Elem. Res. 2007;119:242–254. doi: 10.1007/s12011-007-8004-8. [DOI] [PubMed] [Google Scholar]
- [26].Mistry HD, Kurlak LO, Williams PJ, Ramsay MM, Symonds ME, Broughton Pipkin F. Differential expression and distribution of placental glutathione peroxidases 1, 3 and 4 in normal and preeclamptic pregnancy. Placenta. 2010;5:401–8. doi: 10.1016/j.placenta.2010.02.011. [DOI] [PubMed] [Google Scholar]
- [27].Squires JE, Stoytchev I, Forry EP, Berry MJ. SBP2 binding affinity is a major determinant in differential selenoprotein mRNA translation and sensitivity to nonsense-mediated decay. Mol. Cell. Biol. 2007;27:7848–7855. doi: 10.1128/MCB.00793-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sunde RA, Raines AM, Barnes KM, Evenson JK. Selenium status highly regulates selenoprotein mRNA levels for only a subset of the selenoproteins in the selenoproteome. Biosci. Rep. 2009;29:329–38. doi: 10.1042/BSR20080146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Berry MJ, Ralston NV. Mercury toxicity and the mitigating role of selenium. Ecohealth. 2008;5:456–9. doi: 10.1007/s10393-008-0204-y. [DOI] [PubMed] [Google Scholar]
- [30].Carvalho CM, Chew EH, Hashemy SI, Lu J, Holmgren A. Inhibition of the human thioredoxin system. A molecular mechanism of mercury toxicity. J. Biol. Chem. 2008;283:11913–23. doi: 10.1074/jbc.M710133200. [DOI] [PubMed] [Google Scholar]
- [31].Ralston NV. Selenium health benefit values as seafood safety criteria. Ecohealth. 2008;5:442–455. doi: 10.1007/s10393-008-0202-0. [DOI] [PubMed] [Google Scholar]
- [32].Soon R, Dye TD, Ralston NV, Berry MJ, Sauvage LM. Seafood consumption and umbilical cord blood mercury concentrations in a multiethnic maternal and child health cohort. BMC Pregnancy Childbirth. 2014;14:209. doi: 10.1186/1471-2393-14-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wyatt SM, Kraus FT, Roh CR, Elchalal U, Nelson NM, Sadovsky Y. The correlation between sampling site and gene expression in the term human placenta. Placenta. 2005;26:372–79. doi: 10.1016/j.placenta.2004.07.003. [DOI] [PubMed] [Google Scholar]
- [34].Tzschoppe AA, Struwe E, Dörr HG, Goecke TW, Beckmann MW, Schild RL, Dötsch J. Differences in gene expression dependent on sampling site in placental tissue of fetuses with intrauterine growth restriction. Placenta. 2010;31:178–85. doi: 10.1016/j.placenta.2009.12.002. [DOI] [PubMed] [Google Scholar]
- [35].Dewing AS, Rueli RH, Robles MJ, Nguyen-Wu ED, Zeyda T, Berry MJ, Bellinger FP. Expression and regulation of mouse selenoprotein P transcript variants differing in non-coding RNA. RNA Biol. 2012;9:1361–9. doi: 10.4161/rna.22290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ralston NVC, Raymond LJ. Dietary selenium’s protective effects against methylmercury toxicity. Toxicology. 2010;278:112–23. doi: 10.1016/j.tox.2010.06.004. [DOI] [PubMed] [Google Scholar]
- [37].Bircha RJ, Bigler J, Rogers JW, Zhuang Y, Clickner RP. Trends in blood mercury concentrations and fish consumption among U.S. women of reproductive age, NHANES, 1999–2010. Environmental Research. 2014 doi: 10.1016/j.envres.2014.02.001. http://dx.doi.org/10.1016/j.envres.2014.02.001. [DOI] [PubMed] [Google Scholar]
- [38].Sakamoto M, Yasutake A, Domingo JL, Chan HM, Kubota M, Murata K. Relationships between trace element concentrations in chorionic tissue of placenta and umbilical cord tissue: Potential use as indicators for prenatal exposure. Environment International. 2013;60:106–11. doi: 10.1016/j.envint.2013.08.007. [DOI] [PubMed] [Google Scholar]
- [39].Stoytcheva ZR, Berry MJ. Transcriptional regulation of mammalian selenoprotein expression. Biochimica et Biophysica Acta. 2009;1790:1429–40. doi: 10.1016/j.bbagen.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.