Abstract
Aim
Thrombolytic therapy induces faster clot dissolution than anticoagulation in patients with acute pulmonary embolism (PE) but is associated with an increased risk of haemorrhage. We reviewed the risks and benefits of thrombolytic therapy in the management of patients with acute PE.
Methods and results
We systematically reviewed randomized controlled studies comparing systemic thrombolytic therapy plus anticoagulation with anticoagulation alone in patients with acute PE. Fifteen trials involving 2057 patients were included in our meta-analysis. Compared with heparin, thrombolytic therapy was associated with a significant reduction of overall mortality (OR; 0.59, 95% CI: 0.36–0.96). This reduction was not statistically significant after exclusion of studies including high-risk PE (OR; 0.64, 95% CI: 0.35–1.17). Thrombolytic therapy was associated with a significant reduction in the combined endpoint of death or treatment escalation (OR: 0.34, 95% CI: 0.22–0.53), PE-related mortality (OR: 0.29; 95% CI: 0.14–0.60) and PE recurrence (OR: 0.50; 95% CI: 0.27–0.94). Major haemorrhage (OR; 2.91, 95% CI: 1.95–4.36) and fatal or intracranial bleeding (OR: 3.18, 95% CI: 1.25–8.11) were significantly more frequent among patients receiving thrombolysis.
Conclusions
Thrombolytic therapy reduces total mortality, PE recurrence, and PE-related mortality in patients with acute PE. The decrease in overall mortality is, however, not significant in haemodynamically stable patients with acute PE. Thrombolytic therapy is associated with an increase of major and fatal or intracranial haemorrhage.
Keywords: Pulmonary embolism, Thrombolytic therapy, Systematic review
Introduction
Pulmonary embolism (PE) is a common disease occurring in 60–112 per 100 000 people/year.1,2 According to prospective cohort studies, the case fatality rate in the acute phase ranges from 7 to 11%.3–5 Nevertheless, acute PE represents a wide spectrum of clinical presentations with varying clinical outcomes. To guide the management of acute PE, the European Society of Cardiology6 and the American Heart Association7 have proposed a three-level risk stratification scheme based on haemodynamic status and the presence of right ventricular dysfunction (RVD) or myocardial injury. High-risk (or massive) PE is defined as an acute PE with sustained systemic arterial hypotension. Intermediate-risk (or submassive) PE is defined by the presence of RVD or injury in the absence of arterial hypotension. Finally, low-risk PE is defined by the absence of hypotension and of markers of RVD or injury.
In 1970, the first randomized trial comparing urokinase with heparin for patients with PE was published8 and 7 years later, streptokinase was approved by the US Food and Drug Administration (FDA) for the treatment of high-risk PE. Since then, thrombolytic therapy has been shown to induce faster clot dissolution and haemodynamic improvement compared with heparin alone.9–13 However, more than three decades later, the role of thrombolytic therapy in the treatment of patients with non-high-risk PE remains controversial. Previous meta-analyses have failed to demonstrate a positive impact of thrombolytic treatment on total mortality and suggested an increase in major haemorrhage.14–16 These studies were limited by the relatively small number of patients included in randomized trials.
Current guidelines recommend thrombolytic therapy for patients with high-risk PE in the absence of contraindications.6,7,17 Thrombolysis is not recommended on a routine basis for patients with intermediate-risk PE but may be considered following individual risk-to-benefit analysis. More recently, further evidence regarding the role of thrombolytic therapy for patients with acute PE was published,13,18 including the large multicentre PEITHO trial.19
We performed a systematic review and meta-analysis of randomized controlled trials comparing anticoagulation plus systemic thrombolysis with anticoagulation alone for patients with acute PE. We also sought to identify potential subgroups of patients with a favourable risk–benefit ratio and planned to separately analyse studies according to their criteria of PE severity.
Methods
Search strategy, study selection, data extraction, and analysis were performed according to a pre-defined protocol.
Search strategy
Two authors (C.M. and G.J.) systematically searched Medline, Embase and the Cochrane Controlled Trials registry using the following key words: (pulmonary embolism AND [thrombolysis OR thrombolytic therapy OR streptokinase Or urokinase OR tenecteplase OR desmoteplase OR reteplase OR tissue plasminogen activator]) The detailed search strategy is available in Supplementary material online and was last updated on 16 February 2014. To ensure a comprehensive literature search, we examined reference lists from retrieved articles and reference literature (guidelines and systematic reviews) and questioned experts in PE for possible published or unpublished missing studies.
Study selection and data extraction
We included randomized controlled trials comparing a thrombolytic agent [streptokinase, urokinase, recombinant tissue plasminogen activator (alteplase), desmoteplase, reteplase, or tenecteplase] administered systemically by the i.v. route and heparin (unfractionated or low-molecular-weight heparin) with heparin alone in patients with acute PE. Studies comparing two regimens of thrombolytic therapy, and those using mechanical thrombectomy along with thrombolytic treatment or local catheter-delivered thrombolysis, were excluded. Two investigators (C.M. and G.J.) independently evaluated studies for possible inclusion. Non-relevant studies were excluded based on title and abstract. For potentially relevant studies, full-text was obtained and two investigators (C.M. and G.J.) independently assessed study eligibility and extracted the data on study design, patient characteristics, and outcomes. Disagreement about study inclusion or data extraction was resolved by consensus or by discussion with a third author (A.P.).
Outcomes and measurements
The primary efficacy outcome was early all-cause mortality (in hospital or within 30 days of inclusion). Three secondary efficacy outcomes were considered: recurrent PE confirmed by a validated diagnostic examination; death related to PE and the combination of all-cause death or clinical deterioration requiring rescue treatment (vasopressors, mechanical ventilation, cardio-pulmonary resuscitation, systemic rescue thrombolysis, or embolectomy).
Two safety outcomes, major bleeding and fatal or intracranial haemorrhage, were analysed. Major bleeding was defined according to the International Society of Thrombosis and Haemostasis (ISTH)20 when sufficient information was available. In the other cases, definitions from the original studies were used. Two authors (C.M. and G.J.) independently extracted the patient characteristics and outcomes from retrieved studies.
Study quality assessment
Quality of included studies was assessed using quality criteria developed by Jadad et al.21 evaluating the quality of randomization, blinding and handling of exclusion and attrition. Two investigators (C.M. and G.J.) assessed study quality independently. Disagreements were resolved by consensus.
Data analysis
All analyses were performed on data reported according to the intention-to-treat principle. Pooled odds ratios (ORs) were obtained using the Mantel–Haenszel method given the small sample size in some studies and the low prevalence of events.22 A continuity correction of 0.5 was applied for studies without event in one arm. Fixed effect models were used because of the low heterogeneity. The significance level was set at 0.05. The heterogeneity was measured by the I2 statistic.23 Potential heterogeneity factors were explored by pre-specified subgroup analyses including: studies excluding high-risk PE, studies including (not exclusively) high-risk PE, studies limited to intermediate-risk PE (defined by the presence of RVD on imaging studies, and/or by elevated cardiac biomarkers), studies including low- and intermediate-risk PE, exclusion of older patients, and the type of thrombolytic agent used. For the safety outcomes, subgroup analyses were performed according to the type of a diagnostic procedure (invasive vs. non-invasive). Post hoc supplementary subgroup analyses were performed to explore the impact of the length of follow-up (14 days or less vs. 15–30 days) and the time from the onset of symptoms to inclusion (superior or inferior to 1 week). Finally, we performed a meta-regression to explore a potential variation of the odds ratios over time using the logarithm of the odds ratios over years of publication.
Sensitivity analyses were conducted to check the robustness of the pooled ORs by removing each study one-by-one, by excluding lower quality studies (Jadad score <4) and by applying several continuity corrections (correction from 0.01 to 0.5 by step of 0.1) and treatment arm correction.24 Additionally, an exact method was applied. The advantage is to account for studies without event in both arms. Publication bias was assessed using inspection of the funnel plot, Egger's test, and the trim and fill method.25 The R package ‘meta: Meta analysis with R, version 1.6-1’ and StatXact-8.0.0 (Cytel, Inc., Cambridge, MA, USA) was used for these analyses.
Results
Study selection and characteristics
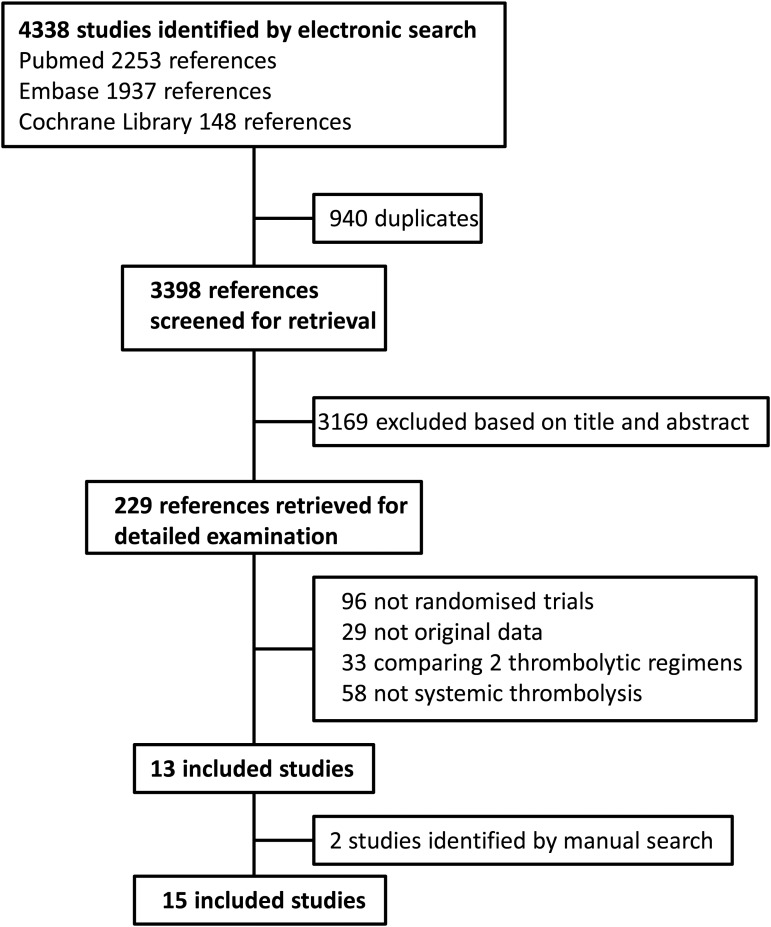
The search retrieved a total of 4338 references, among which 940 duplicates were identified. Of the 3398 remaining articles, 3169 were excluded based on title and abstract (Figure 1). Full-text was obtained for the remaining 229 articles. Of these, 29 did not contain original data, 58 did not study systemic thrombolysis, 96 were non-randomized trials, 33 compared two thrombolytic regimens, and 13 satisfied the inclusion criteria. Two additional studies were identified by manual search.19,26 Inter-readers agreement was high (Kappa coefficient 0.99). The main characteristics of the included studies are displayed in Table 1.
Figure 1.
Study flow chart.
Table 1.
Characteristics of included studies
| First author and year of publication | Number of patients | Eligibility | Severity criteria | High-risk PE included | Thrombolysis | Control | Age limit (years) | Follow-upa | Invasive angiography | Primary endpoint |
|---|---|---|---|---|---|---|---|---|---|---|
| Becattini (2010) | 58 | Acute PE<10 days | RVD | No | Tenecteplase 30–50 mg plus heparin | Heparin | 85 | 30 days | No | 24 h RVDb |
| Dalla Volta (1992) | 36 | Acute PE<10 days | Miller score >11 | No | Alteplase 100 mg/2 h plus heparin | Heparin | 80 | 30 days | 100% | Pulmonary perfusionc |
| Dotter (1979) | 31 | Acute PE | No | Yes | Streptokinase 2–11 MIU 18–72 h | Heparin | No | 7 days | 100% | Pulmonary perfusionc |
| Fasullo (2011) | 72 | Acute PE<6 h | RVD | No | Alteplase 100 mg/2 h plus heparin | Heparin | 75 | 10 days | No | RVDb |
| Goldhaber (1993) | 101 | Acute PE<14 days | No | No | Alteplase 100 mg/2 h plus heparin | Heparin | No | 14 days | 21% | RVDb |
| Jerjes-Sanchez (1995) | 8 | Acute PE<14 days | Massive | Yes | Streptokinase 1.5 MIU/2 h | Heparin | No | In-hospital | No | RVD, pulmonary perfusiond |
| Kline (TOPCOAT) (2013) | 83 | Acute PE | RVD or hypoxaemia | No | Tenecteplase 30–50 mg/2 h plus enoxaparin | LMWH | No | 5 days | NA | Composite clinical outcome |
| Konstantinides (MAPPET) (2002) | 256 | Acute PE<4 days | RVD or pHTA | No | Alteplase 100 mg/2 h plus heparin | Heparin | 80 | 30 days/in-hospital | 16% | Death or treatment escalation |
| Levine (1990) | 58 | Acute PE<14 days | No | No | Alteplase 0.6 mg/kg/2 min | Heparin | No | 10 days | 67% | Pulmonary perfusiond |
| Ly (1978) | 20 | Acute PE<5 days | >1 lobed | Yes | Streptokinase 72 h | Heparin | 70 | 10 days | 100% | Pulmonary perfusionc |
| Marini (1988) | 30 | Acute PE<7 days | >9 segmentsd | No | Urokinase 2.4–3.3 MIU /12–72 h | Heparin | 72 | 7 days | 100% | Pulmonary perfusiond |
| Meyer (PEITHO) (2014) | 1005 | Acute PE<15 days | RVD and elevated troponin | No | Tenecteplase 30–50 mg plus heparin | Heparin | No | 7 days | 1.4% | Death or haemodynamic collapse |
| Sharifi (2013) | 121 | Acute PE<10 days | ≥2 lobesd | No | Alteplase 50 mg/2 h + heparin | Heparin or LMWH | No | In-hospital | No | Pulmonary hypertensionb |
| Stein (PIOPED) (1990) | 13 | Acute PE<7 days | ≥ 1 lobe or ≥ 2 segmentsd | No | Alteplase 40–80 mg/40–90 min + heparin | Heparin | No | 7 days | 100% | Pulmonary perfusionc |
| UPET (1970) | 160 | Acute PE<5 days | No | Yes | Urokinase 12 h | Heparin | No | 14 days | 100% | Pulmonary perfusionc,d |
PE, pulmonary embolism; RVD, right ventricular dysfunction; LMWH, low-molecular-weight heparin; MIU: million units, NA, not available, pHTA, pulmonary hypertension.
aFollow-up for overall mortality.
bEchocardiography.
cPulmonary angiography.
dBased on ventilation-perfusion scintigraphy.
Study quality/risk of bias
The quality of randomization was considered adequate in nine studies (Supplementary material online, Table S1). Both patients and investigators were blinded to the treatment arm in eight studies. Among the 15 included studies, 11 were considered of good quality (Jadad score 4–5), one of fair quality, and three of poor quality. Inclusion criteria differed among studies: four studies8,19,27–29 included patients with high-risk PE, and three specifically included patients with intermediate-risk PE10,13,19(Table 1). Six studies used an upper age limit as an exclusion criteria and the mean age of included patients varied from 5330 to 68 years.10 Bleeding complications could be extracted according to the ISTH definition in all studies but three8,29,31 in which data were extracted according to the authors' definition of major haemorrhage. Finally, the outcome ‘death or treatment escalation’ was extracted from the case description available in the published studies, but this outcome was specifically pre-defined in only five studies.8,10,19,26,31
Overall mortality
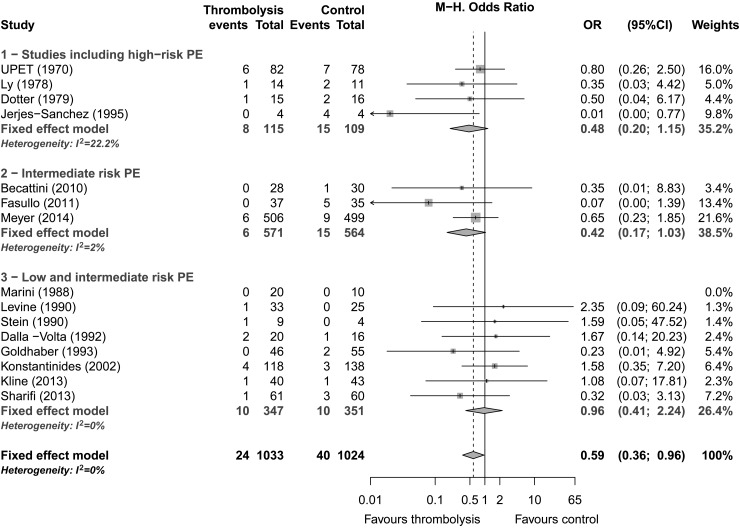
All 15 studies including 2057 patients reported early (≤30 days) all-cause mortality. The reported mortality was 2.3% (24/1033) in the thrombolysis group and 3.9% (40/1024) in the control group. Thrombolytic therapy was associated with a significant reduction of early mortality (pooled OR: 0.59; 95% CI: 0.36–0.96, P = 0.03; Figure 2). No heterogeneity was observed among studies (I2 = 0%). After exclusion of studies including high-risk PE, treatment effect was similar, but statistical significance was lost (OR: 0.64; 95% CI: 0.35–1.17). The pooled ORs were similar in studies including high-risk PE (OR: 0.48; 95% CI: 0.2–1.15) and studies including only intermediate-risk PE (0.42; 95% CI: 0.17–1.03), whereas the pooled OR was close to one in studies including both low- and intermediate-risk PE (0.96; 95% CI: 0.41–2.24) (Table 2 and Figure 2). Subgroup analysis based on the thrombolytic regimen did not show any significant differences between alteplase (OR: 0.64; 95% CI: 0.29–1.41), tenecteplase (OR: 0.65; 95% CI: 0.26–1.64) or older thrombolytics (OR: 0.48; 95% CI: 0.20–1.15) (P = 0.86).
Figure 2.
Early mortality by pulmonary embolism severity, Forest plot.
Table 2.
Efficacy outcomes, subgroup analyses
| All studies |
Studies includinga High-risk PE |
Intermediate-risk PE |
Low and intermediate-risk PE |
Group difference |
|||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | I2 (%) | OR (95% CI) | OR (95% CI) | OR (95% CI) | P-value | |
| Mortality | 0.59 (0.36 to 0.96) | 0.034 | 0 | 0.48 (0.20 to 1.15) | 0.42 (0.17 to 1.03) | 0.96 (0.41 to 2.24) | 0.36 |
| PE mortality | 0.29 (0.14 to 0.60) | <0.001 | 0 | 0.15 (0.03 to 0.78) | 0.17 (0.05 to 0.67) | 0.63 (0.20 to 1.97) | 0.23 |
| Death or treatment escalation | 0.34 (0.22 to 0.52) | <0.001 | 0 | 0.18 (0.04 to 0.79) | 0.37 (0.20 to 0.69) | 0.35 (0.18 to 0.66) | 0.67 |
| PE recurrence | 0.50 (0.27 to 0.94) | 0.031 | 0 | 0.97 (0.31 to 2.98) | 0.25 (0.06 to 1.03) | 0.46 (0.17 to 1.21) | 0.33 |
aNot exclusively.
Pulmonary embolism-related mortality
Pulmonary embolism -related mortality was reported in 13 studies,8,10–13,19,26–32 including 1776 patients (Supplementary material online, Figure S1). The reported PE-related mortality was 0.6% (6/890) among patients allocated to thrombolytic therapy and 3.0% (27/886) in the control group. Thrombolytic treatment was associated with a significant reduction of PE-related mortality (OR: 0.29; 95% CI: 0.14–0.60, P < 0.001) and no heterogeneity was detected (I2 0%). No significant difference was found according to the thrombolytic regimen (P = 0.61) or the severity of PE (P = 0.23).
Death or need for treatment escalation
This outcome was available in nine studies including 1639 patients (Supplementary material online, Figure S2). It was pre-defined in five studies (1474 patients)10,13,19,26,31 and obtained based on the detailed description of complications in the remaining four studies (185 patients).12,27–29 Death or treatment escalation were reported in 3.6% (29/808) of the patients allocated to thrombolytic therapy and 10.2% (85/831) in the control group. Thrombolytic therapy was associated with a significant reduction of this outcome (OR: 0.34; 95% CI: 0.22–0.52, P < 0.001). The result was similar when the analysis was restricted to the five studies in which the outcome was pre-defined10,13,19,26,31 (OR: 0.37; 95% CI: 0.24–0.59, P < 0.0001). No heterogeneity was detected (I2 = 0%). No significant difference was found according to the thrombolytic regimen (P = 0.54) or the severity of PE (P = 0.67).
Pulmonary embolism recurrence
Pulmonary embolism recurrence was reported in 11 studies,8,10–13,18–19,28,30–32 including 1928 patients (Supplementary material online, Figure S3). The reported incidence of PE recurrence was 1.3% (13/966) among patients allocated to thrombolytic therapy and 2.9% (28/962) in the control group. Thrombolytic therapy was associated with a statistically significant reduction of PE recurrence (OR; 0.50; 95% CI: 0.27–0.94, P = 0.03). No heterogeneity was detected (I2 = 0%). No significant difference was found according to the thrombolytic regimen (P = 0.39) or the severity of PE (P = 0.33).
Major bleeding
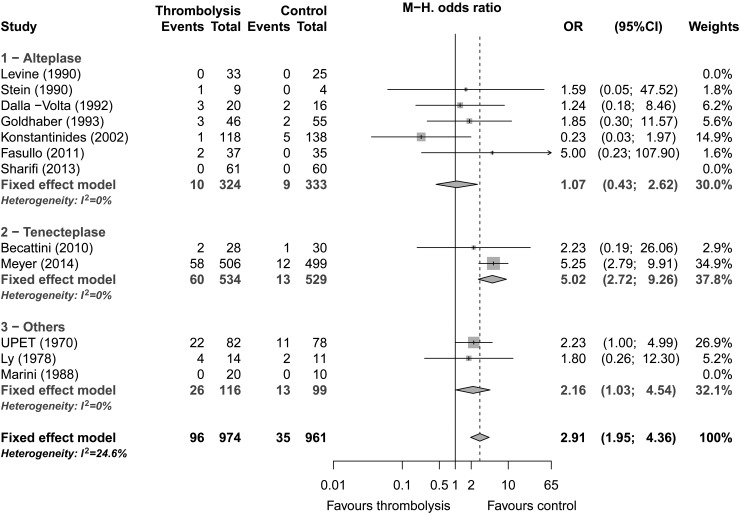
Major bleeding was reported in 12 studies (1935 patients) and was present in 9.9% (96/974) of patients allocated to thrombolytic therapy and 3.6% (35/961) of the control group. Thrombolytic treatment was associated with a significantly increased risk of major bleeding (OR: 2.91; 95% CI: 1.95–4.36, P < 0.0001; Figure 3). Low amount of heterogeneity was detected (I2 = 25%). Subgroup analysis did not show any differences between studies using invasive vs. non-invasive diagnostic procedures. Subgroup analysis based on thrombolytic agent suggested a lower risk of major bleeding in studies using alteplase compared with tenecteplase (Table 3). Nevertheless, this subgroup difference was not significant after exclusion of the study by Konstantinides et al.31 which used a more restrictive definition of major haemorrhage. Finally, the association between thrombolytic therapy and the risk of major bleeding was lower in studies using an upper age limit (OR: 1.13; 95% CI: 0.47–2.71) than in studies including older patients (OR: 3.71; 95% CI: 2.32–5.92) (P = 0.02).
Figure 3.
Major bleeding by drug, Forest plot.
Table 3.
Safety outcomes, subgroup analyses
| All studies |
Alteplase |
Tenecteplase |
Other thrombolytics |
Group difference |
|||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | I2 (%) | OR (95% CI) | OR (95% CI) | OR (95% CI) | P-value | |
| Major bleeding | 2.91 (1.95 to 4.36) | <0.001 | 25 | 1.07 (0.43 to 2.62) | 5.02 (2.72 to 9.26) | 2.16 (1.03 to 4.54) | 0.02 |
| Fatal/intracranial haemorrhage | 3.18 (1.25 to 8.11) | 0.008 | 0 | 1.09 (0.27 to 4.40) | 7.32 (1.64 to 32.63) | NA | 0.07 |
Intracranial or fatal haemorrhage
Intracranial or fatal haemorrhage was reported in 12 studies including 1864 patients (Supplementary material online, Figure S4). The reported incidence of fatal or intracranial haemorrhage was 1.7% (n = 16/933) in the thrombolysis group and 0.3% (n = 3/931) in the control group. Thrombolytic treatment was associated with a significantly increased risk of fatal or intracranial haemorrhage (OR: 3.18; 95% CI: 1.25–8.11, P = 0.008). Subgroup analysis based on thrombolytic agent suggested a lower risk of intracranial or fatal haemorrhage in studies using alteplase compared with tenecteplase (Table 3). The risk of fatal or intracranial haemorrhage was lower in studies with an upper age limit (OR: 1.82; 95% CI: 0.37–8.93) than those without (OR: 4.11; 95% CI: 1.25–13.5), but this difference was not statistically significant (P = 0.42).
Other potential sources of heterogeneity
There were no significant differences between studies using a time from the onset of symptoms superior or inferior to 1 week except for the outcome major bleeding. The risk of major bleeding was higher in studies using a longer time of symptoms before inclusion. Similarly, no significant differences were observed according to the length of follow-up except for the outcome major bleeding. The risk of major bleeding was higher in studies using a shorter follow-up. The results of these analyses are provided on Supplementary material online, Tables S4 and S5. Finally, the meta-regression of the logarithms of the odds ratios over years of publication did not detect any significant interaction between these variables for any of the outcomes (Supplementary material online, Table S6).
Sensitivity analyses
Excluding studies one by one did not significantly alter the effect of thrombolysis with regard to the overall mortality, but statistical significance was lost after exclusion of the study by Jerjes-Sanchez et al.27 (OR: 0.66, 95% CI: 0.40–1.11, P = 0.09) or the study by Fasullo et al.13 (OR: 0.65, 95% CI: 0.39–1.08, P = 0.12). Similarly, statistical significance was lost after deletion of a single study12,13,18,19 for the outcome PE recurrence without major alteration of the treatment effect size (OR; ∼0.55). The outcomes of PE-related mortality and death or treatment escalation were not sensitive to a specific study. The study by Meyer et al.19 contributed importantly to the outcomes of major bleeding and fatal or intracranial haemorrhage: the ORs were reduced (1.66; 95% CI: 0.96–2.88 and 1.53; 95% CI: 0.48–4.87) and statistical significance was lost after omission of this study.
The pooled ORs were robust regarding the continuity correction for all outcomes except for intracranial or fatal haemorrhage: the OR reached 4.67 (95% CI: 1.46–14.92, P = 0.009) for a continuity correction of 0.1. The use of an exact method to combine odds ratios across studies provided similar estimates except for intracranial or fatal haemorrhage: the OR reached 5.18 (95% CI: 1.47–27.88, P < 0.004).
When only studies with good quality (Jadad score 4 or 5) were combined, the OR for the outcome overall mortality was unchanged (0.61; 95% CI: 0.36–1.06) but the statistical significance was lost (P = 0.08). The results were similar for the secondary outcomes.
Publication bias
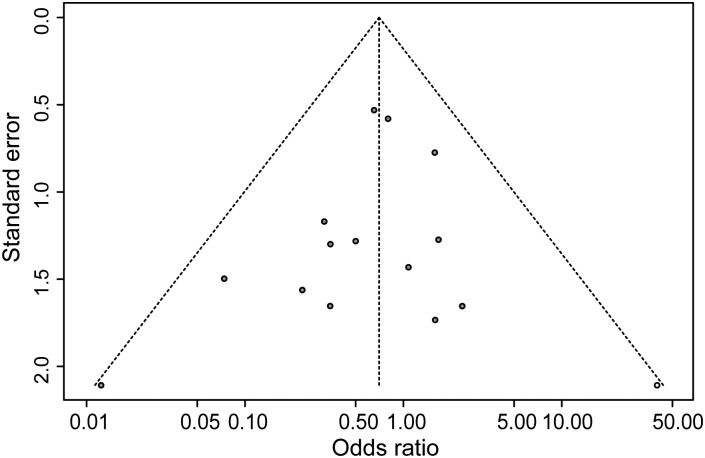
The inspection of funnel plots revealed potential publication bias, especially for PE recurrence and death or treatment escalation (P = 0.02 for both, Egger's test). Missing studies for the funnel plots to be symmetrical (trim and fill method) would be studies with low precision and ORs in favour of controls. One missing study was detected for the outcome overall mortality (Figure 4) (study symmetrical to Jerjes-Sanchez) and three studies for the outcome PE recurrence. If these missing studies were added, the ORs for the outcomes overall mortality and PE-related mortality remained in favour of thrombolytic treatment but were not significantly different from 1 (0.69, 95% CI: 0.40–1.19 and 0.84, 95% CI: 0.44–1.60). For the other outcomes, the ORs were slightly modified and remained significantly different from 1.
Figure 4.
Early mortality, Funnel plot of included studies.
Discussion
Evidence from currently available randomized trials suggests that thrombolytic treatment reduces overall mortality of patients with acute PE. Although the estimated treatment effect corresponded to a 41% relative risk reduction of overall mortality, the benefit of thrombolytic therapy for patients with acute PE could not be definitely established. This observation deserves several comments:
First, the robustness of our primary result was limited, since statistical significance was lost after exclusion of the study by Jerjes-Sanchez et al.27 This small size, open-label trial included only patients with high-risk PE and was limited by gross imbalance between-groups, the four patients allocated to heparin treatment having been admitted for an acute episode of unstable PE despite previous therapeutic anticoagulation. Nevertheless, even after exclusion of this trial, a trend towards decreased all-cause mortality was observed (OR: 0.65; 95% CI: 0.39–1.08). Second, early mortality is relatively uncommon among patients with acute PE, particularly those with non-high-risk PE. Therefore, considering a mortality of 3.9% in the control group, a 41% relative risk reduction would lead to an absolute mortality risk reduction of only 1.6% in our overall population. Our subgroup analyses suggest a possible correlation between PE severity and treatment effect. The pooled ORs were similar in studies including (not exclusively) high-risk PE and studies restricted to intermediate-risk PE with an estimated mortality risk reduction ∼55%, whereas the OR was suggestive of an absence of benefit in studies including only low- and intermediate-risk PE. However, these subgroup analyses were limited by a lack of statistical power and some between-groups overlap in terms of PE severity.
On the other hand, the mortality benefit of thrombolytic therapy may be underestimated because of the possibility to administer rescue thrombolysis to clinically deteriorating patients allocated to anticoagulant therapy; it is likely that some of these patients might have died without rescue treatment. Rescue thrombolysis was actually administered in 4.6 and 23% of control patients in the two largest randomized trials in this meta-analysis,19,31 and its use was restricted to patients with secondary haemodynamic collapse in PEITHO.19 However and despite reasonable equipoise on the efficacy of thrombolysis in intermediate-risk PE, a randomized trial not allowing for emergency escalation of treatment (such as rescue thrombolysis) would certainly be considered ethically unacceptable. When treatment escalation was combined with all-cause mortality, the pooled OR was slightly lower and statistically significant in all PE severity subgroups and sensitivity analyses.
The benefits of thrombolytic therapy must be weighed against the risk of haemorrhagic complications. The rate of major bleeding complications reported in our meta-analysis in patients allocated to thrombolytic therapy (9.9%) is similar to that reported by Wan et al.15 (9.1%) and Dong et al.14 (10.4%). In contrast to our meta-analysis, these previous reports failed to demonstrate a significant association between thrombolytic therapy and major bleeding. This difference appears to result mainly from the inclusion of the PEITHO trial in our meta-analysis, which included more patients than all other studies together and a higher proportion of elderly patients with a higher bleeding risk. Interestingly, our subgroup analysis suggested a higher risk for both major bleeding and fatal or intracranial haemorrhage in patients treated by tenecteplase compared with those treated with alteplase, probably because two of the three studies using tenecteplase10,19 had the oldest populations of all 15 studies included in this meta-analysis. As also previously reported by others,33 the inclusion of older patients was associated with an increased risk of bleeding in our analysis (Supplementary material online, Table S3).
Given the non-negligible risks of systemic thrombolytic treatment and the relatively low risk of early mortality among patients with acute PE, research has recently focused on minimization of thrombolysis-related risks by dose reduction,18 a strategy of catheter-delivered, ultrasound-assisted administration of thrombolytic treatment,34 and the identification of intermediate-risk patients with a potentially favourable risk-to-benefit ratio for thrombolytic therapy. Identification of intermediate-risk patients based on RVD and/or laboratory biomarkers was performed in three studies10,13,19 with a trend towards reduced mortality of borderline significance. Right ventricular dysfunction and laboratory markers of myocardial injury have been widely validated as adverse prognostic markers in patients with PE.35–39 However, the observed mortality rate in the control group (2.6%) in studies including intermediate-risk PE10,13,19 was lower than reported in large cohort studies of unselected PE patients3,40 which may have limited the conclusiveness of this subgroup analysis. This low mortality rate might be explained by the selection criteria of randomized trials and probably also by the close monitoring of the patients included in the trials on intermediate-risk PE. Since most of the studies included PE patients with a wide spectrum of severity, an individual data meta-analysis is warranted to specifically assess treatment benefit in patients with intermediate risk PE, or to identify a subgroup of patients with a favourable risk–benefit ratio.
The present study has several potential limitations. As previously discussed, and despite the inclusion of large recent randomized trials, the power of our analysis remains low because of the low incidence of the primary outcome leading to the loss of statistical significance in some subgroup and sensitivity analyses and to a loss of precision of treatment effect estimate in subgroup analyses Nevertheless, the present meta-analysis included more than twice as many patients as previous reports allowing for a more precise estimate of treatment effect and the selection of a robust primary outcome. Previous meta-analyses frequently reported combined outcomes of variable clinical significance,15 complicating the risk-to-benefit estimation of thrombolytic therapy. Systematic reviews and meta-analyses depend on the quality of included studies. Although the quality of the majority of the studies included in this review was considered good, important differences in inclusion criteria, thrombolytic regimens and dosing, and PE severity were present among studies, limiting the generalization of our conclusions.
Conclusion
Systemic thrombolytic therapy is associated with a significant reduction of overall mortality in patients with PE, but this reduction is not statistically significant after exclusion of studies including high-risk PE. Thrombolytic therapy significantly reduces PE-related mortality, the combined outcome of death or treatment escalation and symptomatic PE recurrence, but is associated with an increase in fatal, intracranial, or major haemorrhage. Owing to this narrow risk–benefit ratio, further analysis based on individual data is warranted to identify which subgroup of patients with non-high-risk PE might benefit from thrombolytic therapy.
Authors' contributions
A.P. conceived the study and participated to the analysis interpretation and article redaction by critically revising the study protocol and the article draft. C.C. participated to the study conception and article redaction by critically revising the study protocol and article draft and performed the statistical analysis. C.M. conceived the study, wrote the protocol draft, performed the literature search, extracted the data, made the statistical analysis and wrote the article draft. G.J. performed the literature search and extracted the data and participated to the study conception, analysis interpretation, and article redaction by critically revising the study protocol and article draft. G.M. participated to the study conception, analysis interpretation, and article redaction by critically revising the study protocol and the article draft. M.L. participated to the study conception, analysis interpretation, and article redaction by critically revising the study protocol and the article draft. O.S. participated to the study conception, analysis interpretation, and article redaction by critically revising the study protocol and the article draft. S.K. participated to the study conception, analysis interpretation, and article redaction by critically revising the study protocol and the article draft. All authors approved the final version of the article.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
Funding to pay the Open Access publication charges for this article was provided by the division of internal medicine of the Geneva University Hospitals.
Conflict of interest: G.M. reports grants and non-financial support from Boehringer Ingelheim, during the conduct of the study; grants from Sanofi aventis, grants and non-financial support from Leo Pharma, grants and non-financial support from Bayer Haelthcare, non-financial support from Daichii Sankyo, outside the submitted work; O.S. reports personal fees from Actelion, grants and personal fees from Bayer Health care, non-financial support from CHIESI, non-financial support from GSK, grants from Daichii Sankyo, grants from Portola Pharmaceuticals, outside the submitted work. Other authors have no conflict of interest to declare.
References
- 1.Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med. 2011;171:831–837. doi: 10.1001/archinternmed.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kroger K, Moerchel C, Moysidis T, Santosa F. Incidence rate of pulmonary embolism in Germany: data from the federal statistical office. J Thromb Thrombolysis. 2010;29:349–353. doi: 10.1007/s11239-009-0396-1. [DOI] [PubMed] [Google Scholar]
- 3.Laporte S, Mismetti P, Decousus H, Uresandi F, Otero R, Lobo JL, Monreal M. Clinical predictors for fatal pulmonary embolism in 15,520 patients with venous thromboembolism: findings from the Registro Informatizado de la Enfermedad TromboEmbolica venosa (RIETE) Registry. Circulation. 2008;117:1711–1716. doi: 10.1161/CIRCULATIONAHA.107.726232. [DOI] [PubMed] [Google Scholar]
- 4.Aujesky D, Hughes R, Jimenez D. Short-term prognosis of pulmonary embolism. J Thromb Haemost. 2009;7(Suppl. 1):318–321. doi: 10.1111/j.1538-7836.2009.03408.x. [DOI] [PubMed] [Google Scholar]
- 5.Stein PD, Kayali F, Olson RE. Estimated case fatality rate of pulmonary embolism, 1979 to 1998. Am J Cardiol. 2004;93:1197–1199. doi: 10.1016/j.amjcard.2004.01.058. [DOI] [PubMed] [Google Scholar]
- 6.Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galie N, Pruszczyk P, Bengel F, Brady AJ, Ferreira D, Janssens U, Klepetko W, Mayer E, Remy-Jardin M, Bassand JP. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) Eur Heart J. 2008;29:2276–2315. doi: 10.1093/eurheartj/ehn310. [DOI] [PubMed] [Google Scholar]
- 7.Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, Jenkins JS, Kline JA, Michaels AD, Thistlethwaite P, Vedantham S, White RJ, Zierler BK. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123:1788–1830. doi: 10.1161/CIR.0b013e318214914f. [DOI] [PubMed] [Google Scholar]
- 8.Urokinase pulmonary embolism trial. Phase 1 results: a cooperative study. JAMA. 1970;214:2163–2172. [PubMed] [Google Scholar]
- 9.Konstantinides S, Tiede N, Geibel A, Olschewski M, Just H, Kasper W. Comparison of alteplase versus heparin for resolution of major pulmonary embolism. Am J Cardiol. 1998;82:966–970. doi: 10.1016/s0002-9149(98)00513-x. [DOI] [PubMed] [Google Scholar]
- 10.Becattini C, Agnelli G, Salvi A, Grifoni S, Pancaldi LG, Enea I, Balsemin F, Campanini M, Ghirarduzzi A, Casazza F. Bolus tenecteplase for right ventricle dysfunction in hemodynamically stable patients with pulmonary embolism. Thromb Res. 2010;125:e82–e86. doi: 10.1016/j.thromres.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Dalla-Volta S, Palla A, Santolicandro A, Giuntini C, Pengo V, Visioli O, Zonzin P, Zanuttini D, Barbaresi F, Agnelli G, Morpurgo M, Giulia Marini M, Visani L. PAIMS 2: alteplase combined with heparin versus heparin in the treatment of acute pulmonary embolism. Plasminogen activator Italian Multicenter Study 2. J Am Coll Cardiol. 1992;20:520–526. doi: 10.1016/0735-1097(92)90002-5. [DOI] [PubMed] [Google Scholar]
- 12.Goldhaber SZ, Haire WD, Feldstein ML, Miller M, Toltzis R, Smith JL, Taveira da Silva AM, Come PC, Lee RT, Parker JA, Mogtader A, McDonough TJ, Braunwald E. Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion. Lancet. 1993;341:507–511. doi: 10.1016/0140-6736(93)90274-k. [DOI] [PubMed] [Google Scholar]
- 13.Fasullo S, Scalzo S, Maringhini G, Ganci F, Cannizzaro S, Basile I, Cangemi D, Terrazzino G, Parrinello G, Sarullo FM, Baglini R, Paterna S, Di Pasquale P. Six-month echocardiographic study in patients with submassive pulmonary embolism and right ventricle dysfunction: comparison of thrombolysis with heparin. Am J Med Sci. 2011;341:33–39. doi: 10.1097/MAJ.0b013e3181f1fc3e. [DOI] [PubMed] [Google Scholar]
- 14.Dong BR, Hao Q, Yue J, Wu T, Liu GJ. Thrombolytic therapy for pulmonary embolism. Cochrane Database Syst Rev. 2009:CD004437. doi: 10.1002/14651858.CD004437.pub3. doi:10.1002/14651858.CD004437.pub3. [DOI] [PubMed] [Google Scholar]
- 15.Wan S, Quinlan DJ, Agnelli G, Eikelboom JW. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials. Circulation. 2004;110:744–749. doi: 10.1161/01.CIR.0000137826.09715.9C. [DOI] [PubMed] [Google Scholar]
- 16.Thabut G, Thabut D, Myers RP, Bernard-Chabert B, Marrash-Chahla R, Mal H, Fournier M. Thrombolytic therapy of pulmonary embolism: a meta-analysis. J Am Coll Cardiol. 2002;40:1660–1667. doi: 10.1016/s0735-1097(02)02381-1. [DOI] [PubMed] [Google Scholar]
- 17.Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, Nelson ME, Wells PS, Gould MK, Dentali F, Crowther M, Kahn SR. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e419S–e494S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M. Moderate pulmonary embolism treated with thrombolysis (from the ‘MOPETT’ Trial) Am J Cardiol. 2013;111:273–277. doi: 10.1016/j.amjcard.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 19.Meyer G, vicaut E, Danays T, Agnelli G, Becattini C, Beyer-Westendorf J, Bluhmki E, Bouvaist H, Brenner B, Couturaud F, Dellas C, Empen K, Franca A, Galie N, Geibel A, Goldhaber SZ, Jimenez D, Kozak M, Kupatt C, Kucher N, Lang IM, Lankeit M, Meneveau N, Pacouret G, Palazzini M, Petris A, Pruszczyk P, Rugolotto M, Salvi A, Schellong S, Sebanne M, Sobkowicz B, Stefanovic BS, Thiele H, torbicki A, Verschuren F, Konstantinides SV. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370:1402–1411. doi: 10.1056/NEJMoa1302097. [DOI] [PubMed] [Google Scholar]
- 20.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 21.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 22.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23:1351–1375. doi: 10.1002/sim.1761. [DOI] [PubMed] [Google Scholar]
- 25.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 26.Kline J, Hernandez J, Kabrehl C, Courtney D, Jones A, Nordenholtz K, Diercks D, Klinger J. Randomized trial of tenecteplase or placebo with low-molecular weight heparin for acute submassive pulmonary embolism: assessment of patient-oriented cardiopulmonary outcomes at three months. J Am Coll Cardiol. 2013;61 E2074. [Google Scholar]
- 27.Jerjes-Sanchez C, Ramirez-Rivera A, Lourdes Garcia M, Arriaga-Nava R, Valencia S, Rosado-Buzzo A, Pierzo JA, Rosas E. Streptokinase and heparin versus heparin alone in massive pulmonary embolism: a randomized controlled trial. J Thromb Thrombolysis. 1995;2:227–229. doi: 10.1007/BF01062714. [DOI] [PubMed] [Google Scholar]
- 28.Dotter CT, Seaman AJ, Rosch J, Porter JM. Streptokinase and heparin in the treatment of major pulmonary embolism: a randomised comparison. Vascu Surg. 1979;13:42–52. [Google Scholar]
- 29.Ly B, Arnesen H, Eie H, Hol R. A controlled clinical trial of streptokinase and heparin in the treatment of major pulmonary embolism. Acta Med Scand. 1978;203:465–470. doi: 10.1111/j.0954-6820.1978.tb14909.x. [DOI] [PubMed] [Google Scholar]
- 30.Marini C, Di Ricco G, Rossi G, Rindi M, Palla R, Giuntini C. Fibrinolytic effects of urokinase and heparin in acute pulmonary embolism: a randomized clinical trial. Respiration. 1988;54:162–173. doi: 10.1159/000195517. [DOI] [PubMed] [Google Scholar]
- 31.Konstantinides S, Geibel A, Heusel G, Heinrich F, Kasper W. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002;347:1143–1150. doi: 10.1056/NEJMoa021274. [DOI] [PubMed] [Google Scholar]
- 32.Levine M, Hirsh J, Weitz J, Cruickshank M, Neemeh J, Turpie AG, Gent M. A randomized trial of a single bolus dosage regimen of recombinant tissue plasminogen activator in patients with acute pulmonary embolism. Chest. 1990;98:1473–1479. doi: 10.1378/chest.98.6.1473. [DOI] [PubMed] [Google Scholar]
- 33.Stein PD, Matta F, Steinberger DS, Keyes DC. Intracerebral hemorrhage with thrombolytic therapy for acute pulmonary embolism. Am J Med. 2012;125:50–56. doi: 10.1016/j.amjmed.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 34.Kucher N, Boekstegers P, Muller O, Kupatt C, Beyer-Westendorf J, Heitzer T, Tebbe U, Horstkotte J, Muller R, Blessing E, Greif M, Lange P, Hoffmann RT, Werth S, Barmeyer A, Hartel D, Grunwald H, Empen K, Baumgartner I. Randomized controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014;129:479–486. doi: 10.1161/CIRCULATIONAHA.113.005544. [DOI] [PubMed] [Google Scholar]
- 35.Stein PD, Matta F, Janjua M, Yaekoub AY, Jaweesh F, Alrifai A. Outcome in stable patients with acute pulmonary embolism who had right ventricular enlargement and/or elevated levels of troponin I. Am J Cardiol. 2010;106:558–563. doi: 10.1016/j.amjcard.2010.03.071. [DOI] [PubMed] [Google Scholar]
- 36.Schoepf UJ, Kucher N, Kipfmueller F, Quiroz R, Costello P, Goldhaber SZ. Right ventricular enlargement on chest computed tomography: a predictor of early death in acute pulmonary embolism. Circulation. 2004;110:3276–3280. doi: 10.1161/01.CIR.0000147612.59751.4C. [DOI] [PubMed] [Google Scholar]
- 37.Sanchez O, Trinquart L, Colombet I, Durieux P, Huisman MV, Chatellier G, Meyer G. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J. 2008;29:1569–1577. doi: 10.1093/eurheartj/ehn208. [DOI] [PubMed] [Google Scholar]
- 38.Becattini C, Vedovati MC, Agnelli G. Prognostic value of troponins in acute pulmonary embolism: a meta-analysis. Circulation. 2007;116:427–433. doi: 10.1161/CIRCULATIONAHA.106.680421. [DOI] [PubMed] [Google Scholar]
- 39.Lankeit M, Friesen D, Aschoff J, Dellas C, Hasenfuss G, Katus H, Konstantinides S, Giannitsis E. Highly sensitive troponin T assay in normotensive patients with acute pulmonary embolism. Eur Heart J. 2010;31:1836–1844. doi: 10.1093/eurheartj/ehq234. [DOI] [PubMed] [Google Scholar]
- 40.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353:1386–1389. doi: 10.1016/s0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]