Abstract
Drug-induced liver injury (DILI) is a major problem for pharmaceutical industry and drug development. Mechanisms of DILI are many and varied. Elucidating the mechanisms of DILI will allow clinicians to prevent liver failure, need for liver transplantation, and death induced by drugs. Methimazole and propylthiouracil (PTU) are two convenient antithyroid agents which their administration is accompanied by hepatotoxicity as a deleterious side effect. Although several cases of antithyroid drugs-induced liver injury are reported, there is no clear idea about the mechanism(s) of hepatotoxicity induced by these medications. Different mechanisms such as reactive metabolites formation, oxidative stress induction, intracellular targets dysfunction, and immune-mediated toxicity are postulated to be involved in antithyroid agents-induced hepatic damage. Due to the idiosyncratic nature of antithyroid drugs-induced hepatotoxicity, it is impossible to draw a specific conclusion about the mechanisms of liver injury. However, it seems that reactive metabolite formation and immune-mediated toxicity have a great role in antithyroids liver toxicity, especially those caused by methimazole. This review attempted to discuss different mechanisms proposed to be involved in the hepatic injury induced by antithyroid drugs.
Keywords: Drug-Induced Liver Injury (DILI), Endocrinology, Hepatotoxicity, Mechanistic toxicology, Methimazole, Propylthiouracil
Introduction
Drugs-induced liver injury (DILI) is an important side effect for many pharmaceuticals.1 Some drugs are known to cause hepatic injury, where in most cases the mechanism of hepatotoxicity is not fully understood.2-5 The pathogenesis of DILI is usually involves the participation of the parent drug and/or its metabolite(s). Mechanisms of DILI are many and varied.6 Elucidating the mechanisms of DILI, will help scientists to design safer pharmaceuticals and suggest new ways to treat and/or prevent liver injury induced by different medications.
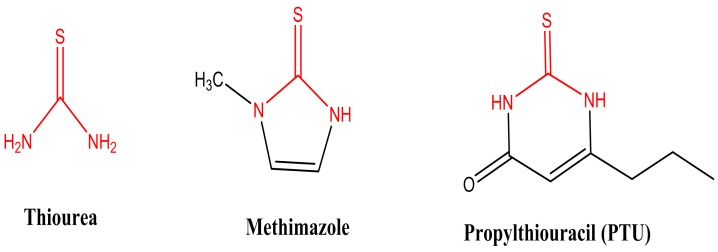
Antithyroid drugs are chemically thionamide compounds (Figure 1), which are used in the management of hyperthyroidism in humans more than 60 years.7 Administration of these drugs is associated with different adverse effects including deleterious ones such as agranulocytosis8,9 and hepatotoxicity.10,11 Other well known complications of antithyroid drugs include skin rash,12 teratogenicity,13 abnormalities of smell and taste,14 and lupus erythmatosus.15,16
Figure 1 .
Commonly used antithyroid drugs and thiourea as their parent compound.
Methimazole, 2-Mercapto-1-methylimidazole (Figure 1), is an anti-thyroid drug from thiono-sulfur chemical class that developed in 1950.7,17 Administration of this drug is associated with hepatotoxicity.11 Several cases of drug-induced hepatic damage have been reported after methimazole administration.10,18-20, However, the mechanism(s) of methimazole-induced hepatotoxicity is not fully understood so far.
The antithyroid drug, propylthiouracil (PTU) (Figure 1), was introduced for clinical use 60 years ago and is estimated to be used in many children and adolescents.7,17 Hepatotoxicity is a dangerous side effect associated with PTU administration.21 There are some reports of PTU-induced liver failure and death.22-25 This drug seems to has a more severe hepatotoxic profile in pediatrics.26 To date there has not been any mechanistic evaluation of the hepatotoxicity induced by PTU. Some investigations suggested to withdraw PTU from the market because of its dangerious and fatal hepatotoxic reactions.23,24 Hence, understanding the mechanism(s) of hepatotoxicity induced by PTU will provide new ways to prevent/treat liver injury induced by this drug.
This study attempts to review different mechanisms proposed to be involved in the hepatic injury induced by antithyroid medications. Some postulated mechanisms of antithyroids hepatotoxicity including reactive metabolites formation, oxidative stress induction, intracellular targets dysfunction, and immune-mediated toxicity are reviewed in current investigation.
Antithyroid drugs’reactive metabolites
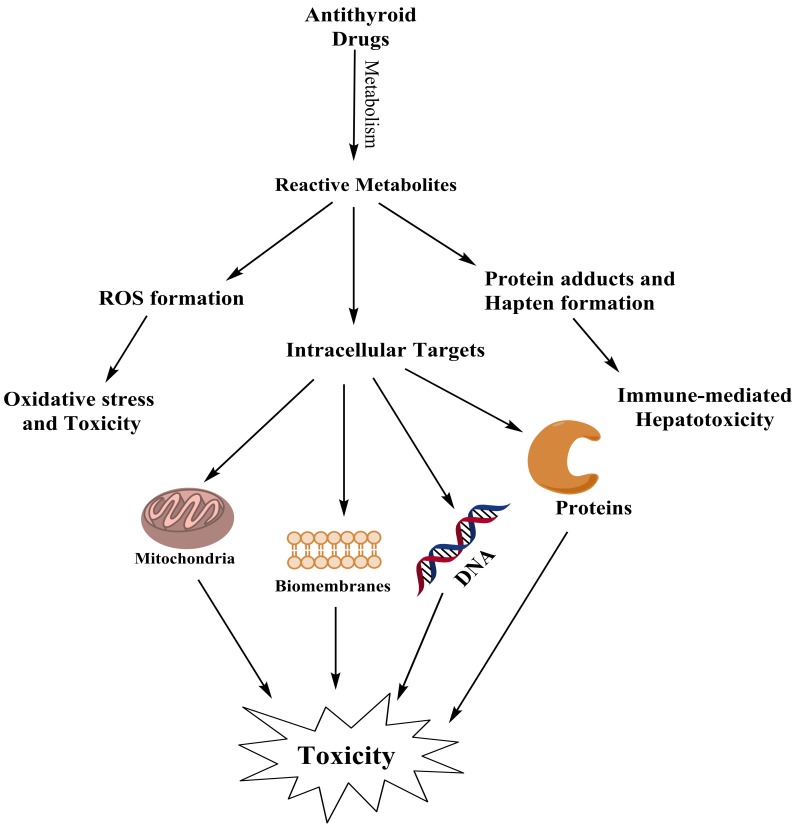
Many investigations has been performed on the significance of reactive metabolites in the pathogenesis of drug-induced hepatotoxicity.1,27 Drugs’ reactive metabolites might be detoxified by cellular defense mechanisms, and/or invade different intracellular targets, which finally encounter cytotoxicity and cell death.
The primary metabolic pathway for the majority of xenobiotics entails the cytochrome P450 (CYP450) system.28 Other hepatic enzyme systems such as flavine-dependent monoxygenase (FMO) and/or phase ΙΙ xenobiotics metabolizing enzymes, might also be involved in drug bioactivation and hepatotoxicity.29,30 Reactive metabolites have the capability to induce cellular injury via several mechanisms such as covalent binding to cellular macromolecules.31 The covalent adduction of reactive metabolites to critical cellular targets might has many consequences such as disruption in cellular calcium (Ca2+), which is a critical ion to preserve cell homeostasis.32 Recently, efforts to further understanding of the involvement of metabolic activation of a drug and following covalent binding to cellular macromolecules in adverse drug reactions are growing.33
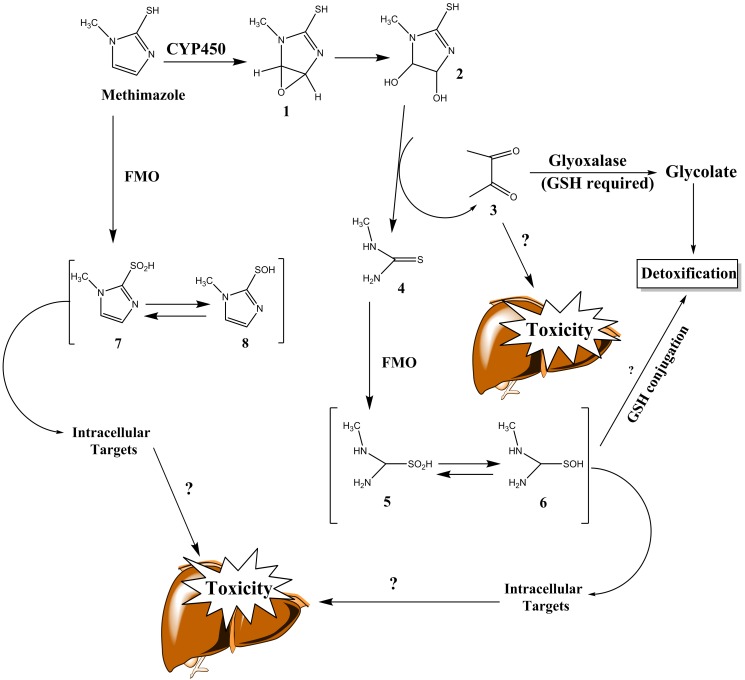
The evidences of metabolic bioactivation of methimazole has been proven in different experiments.34,35 Furthermore, it has been shown that enzyme-induction enhanced methimazole-induced hepatotoxicity,36 which is an indicator implying the critical role of reactive metabolites in the liver injury. Methimazole reactive metabolites are proposed to be involved in different side effects associated with this drug, including olfactory mucosal damage37,38 or agranulocytosis.39 N-methylthiourea and glyoxal are two suspected methimazole, reactive intermediates, which their probable role in liver injury is reviewed in current study (Figure 2).35,40,41
Figure 2 .
Proposed methimazole metabolites, and their role in hepatic injury. Reactive intermediates formed during methimazole metabolism may bound to macromolecule targets (e.g proteins), and cause toxicity or might be detoxified by nucleophilic molecules such as glutathione (GSH). FMO: Flavine-containing monooxygenase, CYP: cytochrome P450, GSH: reduced glutathione. Adapted from references.35,40
Cytochrome P450 enzyme (CYP450) and flavoprotein-mixed-function oxidase (FMO) are founded to be responsible for methimazole metabolism.37,38,42-45 The proposed bioactivation pathways of methimazole in liver have been explained in previous studies. In one of these investigations, this pathway consisted of CYP-mediated epoxidation of the double bond in methimazole to give compound (1) (Figure 2), subsequent ring opening to the dihydrodiol (2) with release of glyoxal (3) and N-methyl thiourea (4) (Figure 2).35,40 Following further flavin monooxygenase-mediated bioactivation (Figure 2), the suggested proximate toxicant, N-methylthiourea (4)35 is converted to the putative ultimate toxicants, sulfenic acid (5) and sulfinic acid (6) (Figure 2).40 Sulfenic acids are reactive nucleophilic agents, capable of interacting with different intracellular targets.46 Hence, these reactive metabolites might play a role in methimazole-induced injury toward hepatocytes. Another presented metabolic pathway for methimazole is the direct S-oxidation of this drug by FMO enzyme (Figure 2).47 S-Oxidation products of methimazole, includes some other sulfenic (7) and sulfinic acid species (8) (Figure 2), which might have a role in the adverse effects induced by this drug.44 Several events such as the loss of rat liver microsomal P450 during methimazole metabolism,45 and the olfactory toxicity induced by this drug37 are attributed to these reactive intermediates.
As mentioned, some studies showed the metabolic activation by direct oxidation of the thiol group of methimazole,47 which might be responsible for the toxicity induced by this drug. It has been shown that the major metabolic pathways of a variety of cyclic thiocarbamides other than methimazole; including 2-mercapto-4,5-dihydroimidazole and 2-mercaptobenzimidazole compounds,48 are also known to involve oxidation at their thiol groups, giving the corresponding sulfenates.48 Nevertheless, 2-mercapto-4,5-dihydroimidazole and 2-mercaptobenzimidazole were totally ineffective in inducing hepatotoxicity.48 This strongly suggests that the direct S-oxidation pathway may not be responsible for the toxicity of methimazole.48 Some experiments has been shown that, the generation of reactive intermediates is involved in covalent binding to olfactory mucosa, as assessed by autoradiography, following administration of 3H-labelled methimazole.38 Some studies showed the lack of olfactory toxicity of the sulphur-lacking methimazole analogues.37,49 These valuable studies may shed light on the mechanisms of methimazole-induced toxicity toward hepatocytes and other organs rather than liver.
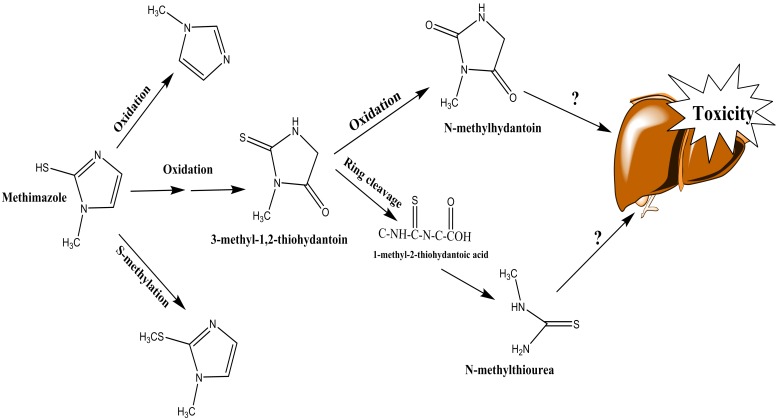
In another study, it has been observed that methimazole will oxidized to N-methylhydantoin and also N-methylthiourea (Figure 3).47 Again, the methyl thiourea is formed through this metabolic pathway, and might be responsible for methimazole toxicity in liver (Figure 3). Nevertheless, the other metabolite, N-methylhydantoin has not been evaluated for its toxicity toward hepatocytes yet (Figure 3).
Figure 3 .
N-methylthiourea and hydantoin ring formation during methimazole metabolism.
The other methimazole reactive metabolite, glyoxal (3) (Figure 2), is a well-known cytotoxic agent with capability of inducing oxidative stress and cellular dysfunction.50,51 It has been found that in addition to N-methylthiourea, as the proposed toxic metabolite of methimazole,35 glyoxal might also has a great role in methimazole-induced cytotoxicity (Figure 2).52 Glyoxal detoxification process is involved the effect of glyoxalase enzyme, which is a glutathione (GSH)-required process (Figure 2).53 GSH-depleted cells and/or liver are reported to be very susceptible to methimazole adverse effects.34,52 The higher susceptibility of GSH-depleted cells to methimazole might be expectable by considering the role of GSH in detoxification of methimazole reactive metabolites such as glyoxal (Figure 2).53,54 However, the role of GSH in conjugating/deactivating other methimazole intermediates cannot be ruled out (Figure 2). The exact reactive metabolite(s) and/or its proportion in liver injury induced by methimazole needs further investigations to be completely revealed, but it seems that bioactivation of this drug in liver is a proposed mechanism by which methimazole caused liver damage in contribution with other potential factors.
Although deleterious and even fatal cases of liver injury have been reported after PTU administration,55,56 there is no mechanistic investigation on the hepatotoxicity induced by this drug. Liver failure induced by PTU, appears to be different in several aspects in children and adults.52 For the past decade, health care professionals have worried that children treated with PTU might be at a higher risk of liver injury.57,58
To date, there is no report on the PTU reactive metabolite(s) formation in liver, and the role of such intermediates in the hepatotoxicity induced by this drug is ambiguous. Some investigations proposed that reactive metabolites are produced during myeloperoxidase (MPO) action on PTU in neutrophils, which might be related to agranulocytosis as a side effect of this drug.59,60 However, the production of such metabolite in liver has not been proven yet (Figure 4). Some studies suggested the role of glucoronidation as a metabolic pathway for PTU detoxification (Figure 4).61 Since a significant difference between uridine diphosphoglucoronosyl transferases (UGTs) activity in adults and children has been proved,62,63 the different profile of PTU-induced hepatotoxicity might be attributed to the UGTs activity in pediatrics (Figure 4).
Figure 4 .
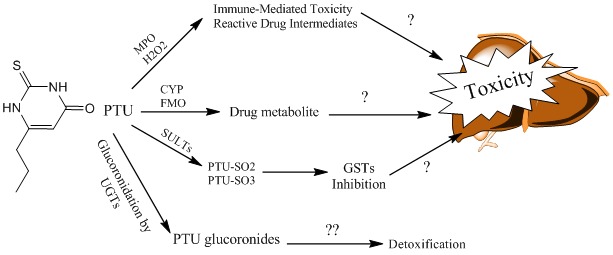
Proposed PTU metabolic pathways and its relation to hepatic injury.
MPO: Myeloperoxidase, H2O2: Hydrogen peroxide, CYP: Cytochrome P450, FMO: Flavine-containing monooxygenase, SULTs: Sulfotransferases, GST: Glutathione-S-Transferase, UGT: Uridine diphospho glucoronosyl transferase, PTU-SO: Sulfate conjugate of PTU.
Another finding which might be attributed to PTU-induced liver injury is the effects of this drug and/or its metabolites on intracellular targets such as vital enzymes. Kimio et al. have reported that PTU and its sulafted metabolites (Figure 4), inhibited glutathione transferase (GSTs) and glutathione peroxidase (GPx), concentration dependently.64 Since GSTs and GPx play a critical role as intracellular defense mechanisms against toxic insult,65 their inhibition might be relevent to PTU-induced hepatic injury. However more investigation is needed to prove such mechanism.
As mentioned, no mechanistic evaluation is available about PTU-induced hepatotoxicity to date. Hence, more future experiments are needed to elucidate the mechanism(s) of PTU-induced liver injury to prevent the fatal hepatic damage caused by this drug. Overall, it can be concluded that the exact reactive metabolite(s) by which antithyroid drugs cause toxicity in liver is not clear completely yet. However, drug bioactivation and reactive intermediates formation seems to have a great role in antithyroid drugs-induced hepatic injury, at least for those caused by methimazole.
Antithyroid drugs and intracellular targets
Mitochondria
Different investigations mentioned the role of intracellular targets in antithyroid agents-induce cytotoxicity.34,36 Among these, is mitochondrion as a critical intracellular target for xenobiotics.66,67 Mitochondria are major potential targets for many xenobiotics-induced toxicity.65 It has been shown that some chemicals including different drugs caused mitochondrial damage in hepatocytes.6 Previous investigations revealed that antithyroid drugs such as methimazole might affect hepatocytes mitochondria as revealed by collapse in mitochondrial membrane potential (ΔΨm).68 The effects of methimazole on cellular mitochondria might be attributed to its reactive metabolites such as glyoxal.41,52 Glyoxal has been shown to be a mitochondrial toxin.69 Methimazole-induced mitochondrial injury is more severe in glutathione-depleted cells.52 This indicates the critical role of glutathione in preventing the adverse effects of methimazole on intracellular targets and its consequent toxicity.
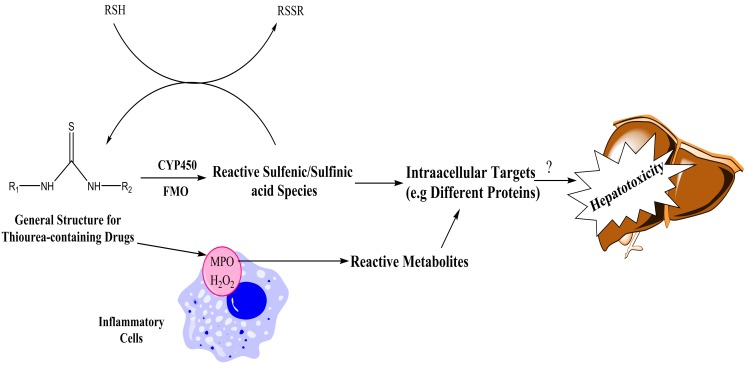
Cellular mitochondria seems to be a target for PTU to induce cellular damage and toxicity.66 It has been found that shape and size of mitochondria was changed to giant mitochondria (megamitochondria) in PTU-induced hepatic injury.66 In addition it has been observed that the inner and outer membrane of mitochondria were fragmented and their matrices were lytic in PTU-induced hepatotoxicity.66 Due to the critical role of cellular mitochondria in regulating cell function, apoptosis and cell death,67 the effects of PTU on this organelle might has a role in PTU-induced hepatic injury (Figure 5).
Figure 5 .
The proposed mechanisms for bioactivation of drugs with thiourea moiety.
RSH: Thiol-containing targets (e.g glutathione and proteins), CYP450: Cytochrome P450, FMO: Flavin-dependent monooxygenase, MPO: Myeloperoxidase, H2O2: Hydrogen peroxide.
Proteins
Due to their abundance in cells, proteins are major targets of attack by xenobiotics.70 In contrast to binding of xenobiotics to intracellular targets such as DNA, the toxicological significance of protein binding is less clear. Not all protein bindings are toxicologically relevant, however when critical proteins such as different enzymes are attacked by xenobiotics, the toxicity might occur.71 Enzymes are sensitive proteins, which might be a target for xenobiotics to induce hepatotoxicity. Catalase (CAT), glutathione peroxidase (GPx), glutathione transferase (GST), and superoxide dismutase (SOD) are enzymes, which seems to be a target for antithyroid drugs to induce cellular dysfunction and toxicity.72 It has been shown that, glyoxal as a methimazole metabolite and as a reactive aldehyde produced in many biological processes,73 deactivated cellular enzymatic antioxidants.74-76 Moreover, it has been found that PTU and its sulfate conjugates inhibited glutathione transferase (GST) and glutathione peroxidase (GPx) enzymes, concentration dependently.64
Antioxidant enzymes deactivation by xenobiotics might lead to imbalance in production and deletion of reactive oxygen species (ROS) and finally oxidative stress. The production of reactive oxygen species (ROS) has been implicated in hepatotoxicity induced by many chemicals.77 The increase in cellular ROS can lead to state of oxidative stress that consequently damage cells, especially in those with weak defense mechanisms. Lipid peroxidation can be the consequences of ROS or reactive metabolites formation.78 The role of ROS formation and lipid peroxidation in methimazole-induced hepatotoxicity is investigated in different studies.36,52,72 It has been shown that methimazole-induced cytotoxicity was accompanied with ROS formation, lipid peroxidation, and glutathione reservoirs depletion41,52, which are signs of oxidative stress in biological systems.
In conclusion, it can be stated that cellular antioxidant defense mechanisms impairment and oxidative stress induction seem to have a role in antithyroid drugs-induced hepatotoxicity, since these drugs deactivated antioxidant enzymes.64,72 Further investigation is needed to reveal such mechanisms, especially in PTU cases.
Role of inflammation and immune system in antithyroid drugs-induced liver injury
A number of investigations have suggested a variety of factors, which may not linked to drug metabolism, could also affect DILI. Among these, are immunological reactions. Immunological reactions and inflammatory process have been implicated in the development of liver injury induced by many drugs.1,79
Different investigations reported the release of autoantibodies and cytokines in antithyroid-treated patients and/or animals.80,81 Kobayashi et al. found that cytokine-mediated immune response could has a great role in methimazole-induced hepatic injury in mice.79 These findings might suggest a role for immune system in mediating hepatic injury induced by antithyroid medications. Weiss et al. have reported cases of PTU-induced hepatic damage in which auto-antibodies were demonstrated.82 Hyashida et al. have shown lymphocyte sensitization in a patient with neonatal liver injury probably by placental transfer of PTU.83 All these reports are in line with the hypothesis that immune system plays a role in the pathogenesis of liver damage associated with PTU therapy.
An intriguing theory for immune-mediated DILI is the hapten hypothesis.84 According to this theory, the drug reactive metabolites are undergoes covalent binding with different proteins. The drug-protein complex is then recognized by immune system, consequently the activation of immune system might lead to toxicity.84 As mentioned, antithyroid drugs’reactive metabolites are capable of interacting with different intracellular targets, including proteins (Figure 6). Hence, these modified proteins might act as haptens and stimulate immune system.
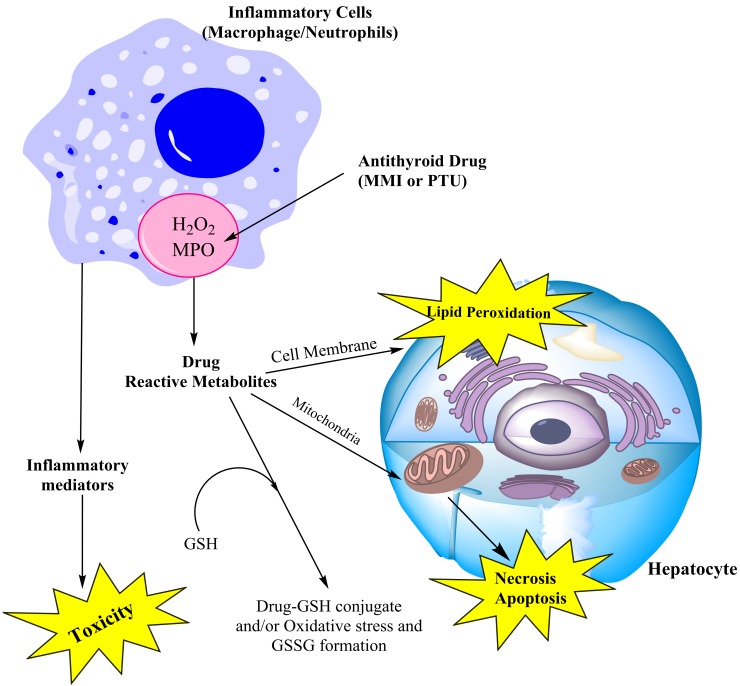
Figure 6 .
Postulated role of inflammatory cells in antithyroid drugs bioactivation and its consequent liver injury. MMI: Methimazole, PTU: Propylthiouracil, MPO: Myeloperoxidase, GSH: Glutathione, GSSG: Oxidized glutathione.
There is much to learn about the role and mechanism of immune-mediated DILI. Recently the models for studying hepatotoxicity has been greatly altered and new experimental tools for DILI are developed. These new strategies include drug-inflammation interaction model (Figure 6).85,86 In Drug-inflammation interaction model it is postulated that a slight, non-toxic inflammation stress will exacerbate drugs-induced liver injury.87 It has been postulated that inflammatory cells aggregated in liver and their inflammatory mediators, have a pivotal role in mediating liver injury (Figure 6).87 On the other hand, neutrophils and macrophages (kupffer cells in liver) contain myeloperoxidase (MPO) enzyme (Figure 6).88 Peroxidases might have a major role in drug metabolism.88 Hence, in addition to the role of immunological reactions and inflammatory mediators in drug-inflammation interaction model, the ability of inflammatory cells in mediating drug metabolism via MPO, might also be considered in toxic reactions of drugs in this model (Figure 6).
It has been shown that methimazole was metabolized by myeloperoxidase enzyme in an in vitro experiment to produce reactive metabolites and oxidized glutathione.89 In another study by Waldhauser et al., it has been found that PTU converted to reactive intermediates by neutrophils MPO.58 It has been suggested that this reaction might be attributed to the agranulocytosis associated with PTU administration.58 Since inflammatory cells aggregate in liver at drug-inflammation interaction model, the chance of reactive metabolites formation might increase and consequently the hepatotoxicity ensue (Figure 6). The hepatotoxicity induced by antithyroid medications could be the subject of future studies in these novel experimental models, to improve our understanding of the mechanisms of liver injury induced by these agents.
Hepatotoxicity induced by conventional drug/chemicals with thiourea structure
Methimazole and PTU are thiourea-containing structures (Figure 1). Moreover, N-methylthiourea is one of the suspected hepatotoxic metabolite of methimazole.35 This section tried to review the toxicity of thiourea-containing chemicals to get a better insight into the hepatotoxicity induced by antithyroid drugs.
Thiourea (Figure 1), is the parent compound for many drugs and industrial agents. Some antituberculosis agents,90 centrally acting histamine H3 antagonists,91 and anti HIV reverse transcriptase (RT) inhibitors,92 are among thiourea-containing drugs.
Different adverse effects toward biological systems are attributed to thiourea-based chemicals. Genotoxicity,93,94 hepatotoxicity,95,96 pulmonary toxicity,97 and contact dermatitis98 are adverse events associated with thiourea-containing compounds. Derivatives of thiourea are among the early drugs identified to cause hepatic injury.99,100
Different forms of flavine-dependent monoxygenase enzymes (FMOs) believed to have a great role in mediating thiourea-containing chemicals metabolism and converting them to reactive intermediates.101,102 The thiourea metabolism is believed to occur via S-oxidation of the thionocarbonyl functional group (Figure 7)44,101 to give reactive sulfenic acid species.103 In an interesting finding on thiourea-containing chemicals toxicity, it has been demonstrated that GSH depletion hastened thiocarbamates toxicity.95,104 As mentioned, it has been revealed that glutathione-depleted cells are very susceptible to methimazole.34,52 These finding might suggest a role for thiourea toxicity (N-methylthiourea as methimazole metabolite), in such conditions. However, as previously stated, the other methimazole metabolite, glyoxal, needs GSH for its detoxification.
Figure 7 .
The possible pathways for antithyroid drugs to induce cytotoxicity
The metabolites produced during thiourea-containing chemicals biotransformation are capable of reacting with protein sulfhydryls and/or GSH (Figure 7). If this adduction makes a mixed disulfide that affect protein (Enzymes) function adversely, then toxicity would ensue (Figure 7).46,101 The olfactory toxicity of drugs such as methimazole, might be attributed to its reactive metabolites produced during FMO enzymes activity in nasal epithelium.37
The exact enzyme responsible for converting methimazole and/or PTU to reactive intermediates in liver is not clearly understood, but further investigation on the role of FMO3 (as the most abundant FMO enzyme isoform in human liver),29 in antithyroid drugs metabolism might enhance our understanding of liver injury induced by these drugs.
Although it is apparent that sulfhydryl reactivity and binding is a common event after thiourea containing drugs biotransformation, the toxicological significance of this fact is less obvious. Hence, evaluating the fate of sulfenic acid species in liver might elucidate the mechanisms of hepatotoxicity induced by thiourea-containing chemicals.
Conclusion remarks
Although, much more investigation are needed for rigorous conclusion to be drawn on the mechanisms of hepatic injury induced by antithyroid drugs, but it seems that a combination of drug reactive metabolite formation and immunological reactions are responsible for the situation. Elucidating the precise mechanisms of hepatotoxicity induced by antithyroid agents, will allow clinicians to prevent fulminant liver failure, need for liver transplantation, and death induced by these medications.
All mentioned proposed mechanisms for antithyroid drugs to induce liver injury are summarized in Figure 7.
Ethical Issues
Not applicable.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Bissell DM, Gores GJ, Laskin DL, Hoofnagle JH. Drug-induced liver injury: Mechanisms and test systems. Hepatology. 2001;33(4):1009–13. doi: 10.1053/jhep.2001.23505. [DOI] [PubMed] [Google Scholar]
- 2.Kaplowitz N. Idiosyncratic drug hepatotoxicity. Nat Rev Drug Discov . 2005;4(6):489–99. doi: 10.1038/nrd1750. [DOI] [PubMed] [Google Scholar]
- 3.Abdoli N, Heidari R, Azarmi Y, Eghbal MA. Mechanisms of the statins cytotoxicity in freshly isolated rat hepatocytes. J Biochem Mol Toxicol. 2013;27(6):287–94. doi: 10.1002/jbt.21485. [DOI] [PubMed] [Google Scholar]
- 4.Heidari R, Babaei H, Eghbal MA. Cytoprotective effects of taurine against toxicity induced by isoniazid and hydrazine in isolated rat hepatocytes. Arh Hig Rada Toksikol . 2013;64(2):15–24. doi: 10.2478/10004-1254-64-2013-2297. [DOI] [PubMed] [Google Scholar]
- 5.Heidari R, Babaei H, Eghbal MA. Amodiaquine-induced toxicity in isolated rat hepatocytes and the cytoprotective effects of taurine and/or N-acetyl cysteine. Res Pharm Sci . 2014;9(2):97–105. [PMC free article] [PubMed] [Google Scholar]
- 6.Russmann S, Kullak-Ublick GA, Grattagliano I. Current concepts of mechanisms in drug-induced hepatotoxicity. Curr Med Chem . 2009;16(23):3041–53. doi: 10.2174/092986709788803097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper DS. Antithyroid drugs. N Engl J Med . 1984;311(21):1353–62. doi: 10.1056/NEJM198411223112106. [DOI] [PubMed] [Google Scholar]
- 8.Cooper DS, Goldminz D, Levin AA, Ladenson PW, Daniels GH, Molitch ME. et al. Agranulocytosis associated with antithyroid drugs. Effects of patient age and drug dose. Ann Intern Med. 1983;98(1):26–9. doi: 10.7326/0003-4819-98-1-26. [DOI] [PubMed] [Google Scholar]
- 9.Yang J, Zhong J, Zhou LZ, Hong T, Xiao XH, Wen GB. Sudden onset agranulocytosis and hepatotoxicity after taking methimazole. Intern Med . 2012;51(16):2189–92. doi: 10.2169/internalmedicine.51.7845. [DOI] [PubMed] [Google Scholar]
- 10.Vitug AC, Goldman JM. Hepatotoxicity from antithyroid drugs. Horm Res . 1985;21(4):229–34. doi: 10.1159/000180054. [DOI] [PubMed] [Google Scholar]
- 11.Woeber KA. Methimazole-induced hepatotoxicity. Endocr Pract . 2002;8(3):222–4. doi: 10.4158/EP.8.3.222. [DOI] [PubMed] [Google Scholar]
- 12.Wing SS, Fantus IG. Adverse immunologic effects of antithyroid drugs. CMAJ . 1987;136(2):121–7. [PMC free article] [PubMed] [Google Scholar]
- 13.Diav-Citrin O, Ornoy A. Teratogen update: antithyroid drugs-methimazole, carbimazole, and propylthiouracil. Teratology . 2002;65(1):38–44. doi: 10.1002/tera.1096. [DOI] [PubMed] [Google Scholar]
- 14.Bandyopadhyay U, Biswas K, Banerjee RK. Extrathyroidal actions of antithyroid thionamides. Toxicol Lett . 2002;128(1-3):117–27. doi: 10.1016/s0378-4274(01)00539-2. [DOI] [PubMed] [Google Scholar]
- 15.Lee SL, PH CH. Drug-induced systemic lupus erythematosus: a critical review. Semin Arthritis Rheum . 1975;5(1):83–103. doi: 10.1016/0049-0172(75)90024-4. [DOI] [PubMed] [Google Scholar]
- 16.Takuwa N, Kojima I, Ogata E. Lupus-like syndrome--a rare complication in thionamide treatment for Graves' disease. Endocrinol Jpn . 1981;28(5):663–7. doi: 10.1507/endocrj1954.28.663. [DOI] [PubMed] [Google Scholar]
- 17.Rivkees SA, Stephenson K, Dinauer C. Adverse events associated with methimazole therapy of graves' disease in children. Int J Pediatr Endocrinol . 2010;2010:176970. doi: 10.1155/2010/176970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez-Lopez JI, Greenberg SE, Kling RR. Drug-induced hepatic injury during methimazole therapy. Gastroenterology . 1962;43:84–7. [PubMed] [Google Scholar]
- 19.Grzywa M, Orlowska-Florek R, Grzywa-Celinska A. Two cases of serious hepatic injury caused by antithyroid drugs. Endokrynol Pol . 2009;60(5):396–400. [PubMed] [Google Scholar]
- 20.Shen C, Zhao CY, Liu F, Wang YD, Yu J. Acute-on-chronic liver failure due to thiamazole in a patient with hyperthyroidism and trilogy of Fallot: case report. BMC Gastroenterol . 2010;10:93. doi: 10.1186/1471-230X-10-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deidiker R, Demello DE. Propylthiouracil-induced fulminant hepatitis: case report and review of the literature. Pediatr Pathol Lab Med . 1996;16(5):845–52. [PubMed] [Google Scholar]
- 22.Cooper DS, Rivkees SA. Putting propylthiouracil in perspective. J Clin Endocrinol Metab . 2009;94(6):1881–2. doi: 10.1210/jc.2009-0850. [DOI] [PubMed] [Google Scholar]
- 23.Rivkees SA, Mattison DR. Propylthiouracil (PTU) Hepatoxicity in Children and Recommendations for Discontinuation of Use. Int J Pediatr Endocrinol . 2009;2009:132041. doi: 10.1155/2009/132041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Limaye A, Ruffolo PR. Propylthiouracil-induced fatal hepatic necrosis. Am J Gastroenterol . 1987;82(2):152–4. [PubMed] [Google Scholar]
- 25.Safani MM, Tatro DS, Rudd P. Fatal propylthiouracil-induced hepatitis. Arch Intern Med . 1982;142(4):838–9. [PubMed] [Google Scholar]
- 26.Rivkees SA, Mattison DR. Ending propylthiouracil-induced liver failure in children. N Engl J Med . 2009;360(15):1574–5. doi: 10.1056/NEJMc0809750. [DOI] [PubMed] [Google Scholar]
- 27.Srivastava A, Maggs JL, Antoine DJ, Williams DP, Smith DA, Park BK. Role of reactive metabolites in drug-induced hepatotoxicity. Handb Exp Pharmacol. 2010;196:165–94. doi: 10.1007/978-3-642-00663-0_7. [DOI] [PubMed] [Google Scholar]
- 28.Baillie TA, Rettie AE. Role of biotransformation in drug-induced toxicity: Influence of intra- and inter-species differences in drug metabolism. Drug Metab Pharmacokinet . 2011;26(1):15–29. doi: 10.2133/dmpk.dmpk-10-rv-089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell SC, Smith RL. A physiological role for flavin-containing monooxygenase (FMO3) in humans? Xenobiotica. 2010;40(5):301–5. doi: 10.3109/00498251003702753. [DOI] [PubMed] [Google Scholar]
- 30.Leung L, Kalgutkar AS, Obach RS. Metabolic activation in drug-induced liver injury. Drug Metab Rev . 2012;44(1):18–33. doi: 10.3109/03602532.2011.605791. [DOI] [PubMed] [Google Scholar]
- 31.Park BK, Kitteringham NR, Maggs JL, Pirmohamed M, Williams DP. The role of metabolic activation in drug-induced hepatotoxicity. Annu Rev Pharmacol Toxicol . 2005;45:177–202. doi: 10.1146/annurev.pharmtox.45.120403.100058. [DOI] [PubMed] [Google Scholar]
- 32.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol . 2003;4(7):552–65. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 33.Takakusa H, Masumoto H, Yukinaga H, Makino C, Nakayama S, Okazaki O. et al. Covalent binding and tissue distribution/retention assessment of drugs associated with idiosyncratic drug toxicity. Drug Metab Dispos . 2008;36(9):1770–9. doi: 10.1124/dmd.108.021725. [DOI] [PubMed] [Google Scholar]
- 34.Mizutani T, Murakami M, Shirai M, Tanaka M, Nakanishi K. Metabolism-dependent hepatotoxicity of methimazole in mice depleted of glutathione. J Appl Toxicol . 1999;19(3):193–8. doi: 10.1002/(sici)1099-1263(199905/06)19:3<193::aid-jat553>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 35.Mizutani T, Yoshida K, Murakami M, Shirai M, Kawazoe S. Evidence for the involvement of N-methylthiourea, a ring cleavage metabolite, in the hepatotoxicity of methimazole in glutathione-depleted mice: structure-toxicity and metabolic studies. Chem Res Toxicol . 2000;13(3):170–6. doi: 10.1021/tx990155o. [DOI] [PubMed] [Google Scholar]
- 36.Heidari R, Babaei H, Roshangar L, Eghbal MA. Effects of enzyme induction and/or glutathione depletion on methimazole-induced hepatotoxicity in mice and the protective role of N-acetylcysteine. Adv Pharm Bull . 2014;4(1):21–8. doi: 10.5681/apb.2014.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Genter MB, Deamer NJ, Blake BL, Wesley DS, Levi PE. Olfactory toxicity of methimazole: dose-response and structure-activity studies and characterization of flavin-containing monooxygenase activity in the Long-Evans rat olfactory mucosa. Toxicol Pathol . 1995;23(4):477–86. doi: 10.1177/019262339502300404. [DOI] [PubMed] [Google Scholar]
- 38.Bergman U, Brittebo EB. Methimazole toxicity in rodents: covalent binding in the olfactory mucosa and detection of glial fibrillary acidic protein in the olfactory bulb. Toxicol Appl Pharmacol . 1999;155(2):190–200. doi: 10.1006/taap.1998.8590. [DOI] [PubMed] [Google Scholar]
- 39.Tesfa D, Keisu M, Palmblad J. Idiosyncratic drug-induced agranulocytosis: possible mechanisms and management. Am J Hematol . 2009;84(7):428–34. doi: 10.1002/ajh.21433. [DOI] [PubMed] [Google Scholar]
- 40.Erve JC. Chemical toxicology: reactive intermediates and their role in pharmacology and toxicology. Expert Opin Drug Metab Toxicol . 2006;2(6):923–46. doi: 10.1517/17425255.2.6.923. [DOI] [PubMed] [Google Scholar]
- 41.Heidari R, Babaei H, Eghbal MA. Ameliorative effects of taurine against methimazole-induced cytotoxicity in isolated rat hepatocytes. Sci Pharm . 2012;80(4):987–99. doi: 10.3797/scipharm.1205-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee PW, Neal RA. Metabolism of methimazole by rat liver cytochrome P-450-containing monoxygenases. Drug Metab Dispos . 1978;6(5):591–600. [PubMed] [Google Scholar]
- 43.Xie F, Zhou X, Genter MB, Behr M, Gu J, Ding X. The tissue-specific toxicity of methimazole in the mouse olfactory mucosa is partly mediated through target-tissue metabolic activation by CYP2A5. Drug Metab Dispos . 2011;39(6):947–51. doi: 10.1124/dmd.110.037895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poulsen LL, Hyslop RM, Ziegler DM. S-oxidation of thioureylenes catalyzed by a microsomal flavoprotein mixed-function oxidase. Biochem Pharmacol . 1974;23(24):3431–40. doi: 10.1016/0006-2952(74)90346-3. [DOI] [PubMed] [Google Scholar]
- 45.Kedderis GL, Rickert DE. Loss of rat liver microsomal cytochrome P-450 during methimazole metabolism. Role of flavin-containing monooxygenase. Drug Metab Dispos. 1985;13(1):58–61. [PubMed] [Google Scholar]
- 46.Mansuy D, Dansette PM. Sulfenic acids as reactive intermediates in xenobiotic metabolism. Arch Biochem Biophys . 2011;507(1):174–85. doi: 10.1016/j.abb.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 47.Skellern GG, Steer S. The metabolism of [2-14C]methimazole in the rat. Xenobiotica . 1981;11(9):627–34. doi: 10.3109/00498258109045874. [DOI] [PubMed] [Google Scholar]
- 48.Decker CJ, Doerge DR, Cashman JR. Metabolism of benzimidazoline-2-thiones by rat hepatic microsomes and hog liver flavin-containing monooxygenase. Chem Res Toxicol . 1992;5(5):726–33. doi: 10.1021/tx00029a021. [DOI] [PubMed] [Google Scholar]
- 49.Brittebo EB. Metabolism-dependent toxicity of methimazole in the olfactory nasal mucosa. Pharmacol Toxicol . 1995;76(1):76–9. doi: 10.1111/j.1600-0773.1995.tb00107.x. [DOI] [PubMed] [Google Scholar]
- 50.Shangari N, O'Brien PJ. The cytotoxic mechanism of glyoxal involves oxidative stress. Biochem Pharmacol . 2004;68(7):1433–42. doi: 10.1016/j.bcp.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 51.Banach MS, Dong Q, O'Brien PJ. Hepatocyte cytotoxicity induced by hydroperoxide (oxidative stress model) or glyoxal (carbonylation model): prevention by bioactive nut extracts or catechins. Chem Biol Interact . 2009;178(1-3):324–31. doi: 10.1016/j.cbi.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 52.Heidari R, Babaei H, Eghbal M. Mechanisms of methimazole cytotoxicity in isolated rat hepatocytes. Drug Chem Toxicol . 2013;36(4):403–11. doi: 10.3109/01480545.2012.749272. [DOI] [PubMed] [Google Scholar]
- 53.Thornalley PJ. Glutathione-dependent detoxification of alpha-oxoaldehydes by the glyoxalase system: involvement in disease mechanisms and antiproliferative activity of glyoxalase I inhibitors. Chem Biol Interact . 1998;111-112:137–51. doi: 10.1016/s0009-2797(97)00157-9. [DOI] [PubMed] [Google Scholar]
- 54.Thornalley PJ. Glyoxalase I--structure, function and a critical role in the enzymatic defence against glycation. Biochem Soc Trans . 2003;31(Pt 6):1343–8. doi: 10.1042/bst0311343. [DOI] [PubMed] [Google Scholar]
- 55.Ichiki Y, Akahoshi M, Yamashita N, Morita C, Maruyama T, Horiuchi T. et al. Propylthiouracil-induced severe hepatitis: a case report and review of the literature. J Gastroenterol . 1998;33(5):747–50. doi: 10.1007/s005350050167. [DOI] [PubMed] [Google Scholar]
- 56.Malozowski S, Chiesa A. Propylthiouracil-induced hepatotoxicity and death. Hopefully, never more. J Clin Endocrinol Metab. 2010;95(7):3161–3. doi: 10.1210/jc.2010-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rivkees SA. Pediatric Graves' disease: controversies in management. Horm Res Paediatr . 2010;74(5):305–11. doi: 10.1159/000320028. [DOI] [PubMed] [Google Scholar]
- 58.Rivkees SA. 63 years and 715 days to the "boxed warning": unmasking of the propylthiouracil problem. Int J Pediatr Endocrinol 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee E, Miki Y, Hosokawa M, Sayo H, Kariya K. Oxidative metabolism of propylthiouracil by peroxidases from rat bone marrow. Xenobiotica . 1988;18(10):1135–42. doi: 10.3109/00498258809042236. [DOI] [PubMed] [Google Scholar]
- 60.Waldhauser L, Uetrecht J. Oxidation of propylthiouracil to reactive metabolites by activated neutrophils. Implications for agranulocytosis. Drug Metab Dispos. 1991;19(2):354–9. [PubMed] [Google Scholar]
- 61.Karras S, Memi E, Kintiraki E, Krassas GE. Pathogenesis of propylthiouracil-related hepatotoxicity in children: present concepts. J Pediatr Endocrinol Metab . 2012;25(7-8):623–30. doi: 10.1515/jpem-2012-0059. [DOI] [PubMed] [Google Scholar]
- 62.Anderson GD. Children Versus Adults: Pharmacokinetic and Adverse-Effect Differences. Epilepsia . 2002;43(Suppl 3):53–9. doi: 10.1046/j.1528-1157.43.s.3.5.x. [DOI] [PubMed] [Google Scholar]
- 63.de Wildt SN, Kearns GL, Leeder JS, Van Den Anker JN. Glucuronidation in humansPharmacogenetic and developmental aspects. Clin Pharmacokinet. 1999;36(6):439–52. doi: 10.2165/00003088-199936060-00005. [DOI] [PubMed] [Google Scholar]
- 64.Kariya K, Sawahata T, Okuno S, Lee E. Inhibition of hepatic glutathione transferases by propylthiouracil and its metabolites. Biochem Pharmacol . 1986;35(9):1475–9. doi: 10.1016/0006-2952(86)90112-7. [DOI] [PubMed] [Google Scholar]
- 65.Hayes JD, McLellan LI. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic Res. 1999;31(4):273–300. doi: 10.1080/10715769900300851. [DOI] [PubMed] [Google Scholar]
- 66.Mete UO, Kaya M, Colakoglu S, Polat S, Tap O, Ozbilgin MK. et al. Ultrastructure of the liver in the propylthiourcil induced hepatitis. J Islamic Acad Sci . 1993;6(4):268–76. [Google Scholar]
- 67.Lee HC, Wei YH. Mitochondrial role in life and death of the cell. J Biomed Sci . 2000;7(1):2–15. doi: 10.1007/BF02255913. [DOI] [PubMed] [Google Scholar]
- 68.Heidari R, Babaei H, Eghbal MA. Cytoprotective effects of organosulfur compounds against methimazole-induced toxicity in isolated rat hepatocytes. Adv Pharm Bull . 2013;3(1):135–42. doi: 10.5681/apb.2013.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mehta R, Shangari N, O'Brien PJ. Preventing cell death induced by carbonyl stress, oxidative stress or mitochondrial toxins with vitamin B anti-AGE agents. Mol Nutr Food Res . 2008;52(3):379–85. doi: 10.1002/mnfr.200600190. [DOI] [PubMed] [Google Scholar]
- 70.Zhou S, Chan E, Duan W, Huang M, Chen YZ. Drug bioactivation covalent binding to target proteins and toxicity relevance. Drug Metab Rev . 2005;37(1):41–213. doi: 10.1081/dmr-200028812. [DOI] [PubMed] [Google Scholar]
- 71.Pumford NR, Halmes NC. Protein targets of xenobiotic reactive intermediates. Annu Rev Pharmacol Toxicol . 1997;37:91–117. doi: 10.1146/annurev.pharmtox.37.1.91. [DOI] [PubMed] [Google Scholar]
- 72.Amara IB, Hakim A, Troudi A, Soudani N, Makni FA, Zeghal KM. et al. Protective effects of selenium on methimazole-induced anemia and oxidative stress in adult rats and their offspring. Hum Exp Toxicol . 2011;30(10):1549–60. doi: 10.1177/0960327110392403. [DOI] [PubMed] [Google Scholar]
- 73.Thorpe SR, Baynes JW. Maillard reaction products in tissue proteins: new products and new perspectives. Amino acids . 2003;25(3-4):275–81. doi: 10.1007/s00726-003-0017-9. [DOI] [PubMed] [Google Scholar]
- 74.Grimsrud PA, Xie H, Griffin TJ, Bernlohr DA. Oxidative stress and covalent modification of protein with bioactive aldehydes. J Biol Chem . 2008;283(32):21837–41. doi: 10.1074/jbc.R700019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vander Jagt DL, Hunsaker LA, Vander Jagt TJ, Gomez MS, Gonzales DM, Deck LM. et al. Inactivation of glutathione reductase by 4-hydroxynonenal and other endogenous aldehydes. Biochem Pharmacol . 1997;53(8):1133–40. doi: 10.1016/s0006-2952(97)00090-7. [DOI] [PubMed] [Google Scholar]
- 76.Choudhary D, Chandra D, Kale RK. Influence of methylglyoxal on antioxidant enzymes and oxidative damage. Toxicol Lett . 1997;93(2–3):141–52. doi: 10.1016/s0378-4274(97)00087-8. [DOI] [PubMed] [Google Scholar]
- 77.Muriel P. Role of free radicals in liver diseases. Hepatology International . 2009;3(4):526–36. doi: 10.1007/s12072-009-9158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol . 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 79.Kaplowitz N. Drug-induced liver injury. Clin Infect Dis . 2004;38(Suppl 2):S44–8. doi: 10.1086/381446. [DOI] [PubMed] [Google Scholar]
- 80.Thong HY, Chu CY, Chiu HC. Methimazole-induced antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis and lupus-like syndrome with a cutaneous feature of vesiculo-bullous systemic lupus erythematosus. Acta Derm Venereol . 2002;82(3):206–8. doi: 10.1080/00015550260132523. [DOI] [PubMed] [Google Scholar]
- 81.Kobayashi M, Higuchi S, Ide M, Nishikawa S, Fukami T, Nakajima M. et al. Th2 cytokine-mediated methimazole-induced acute liver injury in mice. J Appl Toxicol . 2012;32(10):823–33. doi: 10.1002/jat.2731. [DOI] [PubMed] [Google Scholar]
- 82.Weiss M, Hassin D, Bank H. Propylthiouracil-induced hepatic damage. Arch Intern Med . 1980;140(9):1184–5. [PubMed] [Google Scholar]
- 83.Hayashida CY, Duarte AJ, Sato AE, Yamashiro-Kanashiro EH. Neonatal hepatitis and lymphocyte sensitization by placental transfer of propylthiouracil. J Endocrinol Invest . 1990;13(11):937–41. doi: 10.1007/BF03349663. [DOI] [PubMed] [Google Scholar]
- 84.Ju C, Uetrecht JP. Mechanism of idiosyncratic drug reactions: reactive metabolites formation, protein binding and the regulation of the immune system. Curr Drug Metab . 2002;3(4):367–77. doi: 10.2174/1389200023337333. [DOI] [PubMed] [Google Scholar]
- 85.Roth RA, Ganey PE. Intrinsic versus idiosyncratic drug-induced hepatotoxicity--two villains or one? J Pharmacol Exp Ther. 2010;332(3):692–7. doi: 10.1124/jpet.109.162651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roth RA, Luyendyk JP, Maddox JF, Ganey PE. Inflammation and drug idiosyncrasy--Is there a connection? J Pharmacol Exp Ther. 2003;307(1):1–8. doi: 10.1124/jpet.102.041624. [DOI] [PubMed] [Google Scholar]
- 87.Ganey PE, Luyendyk JP, Maddox JF, Roth RA. Adverse hepatic drug reactions: inflammatory episodes as consequence and contributor. Chem Biol Interact . 2004;150(1):35–51. doi: 10.1016/j.cbi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 88.Tafazoli S, O'Brien PJ. Peroxidases: a role in the metabolism and side effects of drugs. Drug DiscovToday. 2005;10(9):617–25. doi: 10.1016/S1359-6446(05)03394-5. [DOI] [PubMed] [Google Scholar]
- 89.McGirr LG, Jatoe SD, O'Brien PJ. Myeloperoxidase catalysed cooxidative metabolism of methimazole: oxidation of glutathione and NADH by free radical intermediates. Chem Biol Interact . 1990;73(2-3):279–95. doi: 10.1016/0009-2797(90)90009-c. [DOI] [PubMed] [Google Scholar]
- 90.Nishida CR, Ortiz De Montellano. Bioactivation of antituberculosis thioamide and thiourea prodrugs by bacterial and mammalian flavin monooxygenases. Chem Biol Interact . 2011;192(1):21–5. doi: 10.1016/j.cbi.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vollinga RC, Menge WMPB, Leurs R, Timmerman H. New analogs of burimamide as potent and selective histamine H3 receptor antagonists: the effect of chain length variation of the alkyl spacer and modifications of the N-thiourea substituent. J Med Chem . 1995;38(12):2244–50. doi: 10.1021/jm00012a025. [DOI] [PubMed] [Google Scholar]
- 92.Vig R, Mao C, Venkatachalam TK, Tuel-Ahlgren L, Sudbeck EA, Uckun FM. Rational design and synthesis of phenethyl-5-bromopyridyl thiourea derivatives as potent non-nucleoside inhibitors of HIV reverse transcriptase. Bioorg Med Chem . 1998;6(10):1789–97. doi: 10.1016/s0968-0896(98)00108-4. [DOI] [PubMed] [Google Scholar]
- 93.Ziegler-Skylakakis K, Nill S, Pan JF, Andrae U. S-oxygenation of thiourea results in the formation of genotoxic products. Environ Mol Mutagen . 1998;31(4):362–73. [PubMed] [Google Scholar]
- 94.Dearfield KL. Ethylene thiourea (ETU). A review of the genetic toxicity studies. Mutat Res. 1994;317(2):111–32. doi: 10.1016/0165-1110(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 95.Onderwater RC, Commandeur JN, Groot EJ, Sitters A, Menge WM, Vermeulen NP. Cytotoxicity of a series of mono-and di-substituted thiourea in freshly isolated rat hepatocytes: a preliminary structure-toxicity relationship study. Toxicology . 1998;125(2-3):117–29. doi: 10.1016/s0300-483x(97)00169-8. [DOI] [PubMed] [Google Scholar]
- 96.Onderwater RC, Commandeur JN, Vermeulen NP. Comparative cytotoxicity of N-substituted N'-(4-imidazole-ethyl)thiourea in precision-cut rat liver slices. Toxicology . 2004;197(2):81–91. doi: 10.1016/j.tox.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 97.Scott AM, Powell GM, Upshall DG, Curtis CG. Pulmonary toxicity of thioureas in the rat. Environ Health Perspect . 1990;85:43–50. doi: 10.1289/ehp.85-1568340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kanerva L, Estlander T, Jolanki R. Occupational allergic contact dermatitis caused by thiourea compounds. Contact Dermatitis . 1994;31(4):242–8. doi: 10.1111/j.1600-0536.1994.tb01996.x. [DOI] [PubMed] [Google Scholar]
- 99.Gabrilove Jl KMJ. Sensitivity to thiouracil: Report of three cases. J Am Med Assoc . 1944;124(8):504–5. [Google Scholar]
- 100.Eisen MJ. Fulminant hepatitis during treatment with propylthiouracil. N Engl J Med . 1953;249(20):814–6. doi: 10.1056/NEJM195311122492007. [DOI] [PubMed] [Google Scholar]
- 101.Smith PB, Crespi C. Thiourea toxicity in mouse C3H/10T1/2 cells expressing human flavin-dependent monooxygenase 3. Biochem Pharmacol . 2002;63(11):1941–8. doi: 10.1016/s0006-2952(02)00978-4. [DOI] [PubMed] [Google Scholar]
- 102.Onderwater RC, Rettie AE, Commandeur JN, Vermeulen NP. Bioactivation of N-substituted N'-(4-imidazole-ethyl)thioureas by human FMO1 and FMO3. Xenobiotica . 2006;36(7):645–57. doi: 10.1080/00498250500354329. [DOI] [PubMed] [Google Scholar]
- 103.Miller AE, Bischoff JJ, Pae K. Chemistry of aminoiminomethanesulfinic and -sulfonic acids related to the toxicity of thioureas. Chem Res Toxicol . 1988;1(3):169–74. doi: 10.1021/tx00003a007. [DOI] [PubMed] [Google Scholar]
- 104.Lee PW, Arnau T, Neal RA. Metabolism of alpha-naphthylthiourea by rat liver and rat lung microsomes. Toxicol Appl Pharmacol . 1980;53(1):164–73. doi: 10.1016/0041-008x(80)90393-2. [DOI] [PubMed] [Google Scholar]