Abstract
Purpose: The purpose of our study was to evaluate the effect of oral taurine on the incidence of febrile episodes during chemotherapy in young adults with acute lymphoblastic leukemia.
Methods: Forty young adults with acute lymphoblastic leukemia, at the beginning of maintenance course of their chemotherapy, were eligible for this study. The study population was randomized in a double blind manner to receive either taurine or placebo (2 gram per day orally). Life quality and side effects including febrile episodes were assessed using questionnaire. Data were analyzed using Pearson’s Chi square test.
Results: Of total forty participants, 43.8% were female and 56.3 % were male. The mean age was 19.16±1.95 years (ranges: 16-23 years). The results indicated that the levels of white blood cells are significantly (P<0.05) increased in taurine treated group. There was no elevation in blasts count. A total of 70 febrile episodes were observed during study, febrile episodes were significantly (P<0.05) lower in taurine patients in comparison to the control ones.
Conclusion: The overall incidence of febrile episodes and infectious complications in acute lymphoblastic leukemia patients receiving taurine was lower than placebo group. Taurine’s ability to increase leukocyte count may result in lower febrile episodes.
Keywords: Acute lymphoblastic leukemia, Taurine, Febrile episode, Antioxidant, Neutrophile, Infection
Introduction
Acute lymphoblastic leukemia (ALL), or acute lymphocytic leukemia, is one of the hematopoietic cells malignancies which are categorized as myeloproliferative disorders.1 It is a malignant neoplasm which is characterized by exaggerated and uncontrolled proliferation of immature bone marrow-derived lymphocyte precursor cells (lymphoblasts). Leukemic lymphoblasts are not able to prepare a normal immune response and fight against infections. The exaggerated growth of lymphoblasts cause a diminution in normal bone marrow cells production hence it leads to a deficiency of peripheral blood circulating cells including red blood cells (anemia), leukocytes other than lymphocytes especially neutrophils (neutropenia), and platelets (thrombocytopenia).2 Clinical presentations of ALL differ from mild and nonspecific symptoms to sever life threatening signs depending on the levels of bone marrow involvement and extramedullary distribution of the disease. Fatigue can be the only sign of ALL-induced anemia but higher degrees of anemia can be featured by dyspnea, angina, dizziness, and lethargy. Fever may be caused by infection reflecting decreased immune defense, by pyrogenic cytokines made by leukemic cells, or both. Petechia, and ecchymosis (in both skin and mucous membrane), pallor, spontaneous bleeding and disseminated intravascular coagulation (DIC) may be caused as a result of thrombocytopenia. Bone and joint pain (arthralgia) can be observed due to the bone marrow expansion by lymphoblasts. ALL also may present with malaise, weight loss, diminished food intake, night sweats, headache, nausea and/or vomiting.3,4
Chemotherapy remains the main choice of cancer treatments. It can cause different kinds of complications due to the cancer itself, toxic effects of chemotherapy and reduction of peripheral blood cells.5 Toxic adverse effects of chemotherapy differ from mild nonspecific symptoms to sever and/or organ specific (hematological, gastrointestinal, dermatological, pulmonary, neurological, cardiac, renal, hepatic and gonadal) complications.6,7 Finding ways to prevent or decrease these side effects is an important part of cancer chemotherapy.8
Chemotherapy induced gastrointestinal problems includes xerostomia and stomatitis, mucositis, nausea and vomiting, taste changes, diarrhea or constipation and anorexia.6,9
Fever is one of the chemotherapy induced complications in cancer patients. In ALL patients fever may be caused by the disease itself or may be a sign of chemo-induced complications specially neutropenia.10 There was well established relationship between the malignancies, immunosupression and infections.11,12 However, little information was provided on the frequency and the type of febrile episodes during chemotherapy excluding the induction phase.13,14
Taurine (2-aminoethane sulfonic acid), a β amino acid, is structurally distinguished from other amino acids by having a sulfonic acid group instead of the carboxylic acid group. Taurine represents one of the most abundant free amino acids in mammalian tissues. It is neither included into protein structure nor metabolized. It is known as a conditionally essential amino acid which is present in several mammalian tissues including: brain, heart, liver, neutrophils, retina, and kidneys in high concentrations.15,16 Antioxidation, xenobiotic conjugation, osmoregulation, intracellular calcium flux regulation, bile acid conjugation as well as neuromodulation, cell membrane stabilzation, cell proliferation and viability are known biological activities in which taurine is taking part.15-18 Plasma taurine concentration decreases significantly after chemotherapy (chemotherapy treated patients exhibited serum taurine levels less than half of those in control group). Since chemotherapy induces taurine depletion, taurine supplementation is believed valuable for patients undergoing chemotherapy.19
The purpose of the present study was to evaluate the influences of taurine co-administration on the incidence of febrile episodes during chemotherapy in young adults with acute lymphoblastic leukemia.
Materials and Methods
Data Collection
Data collection for the study was performed by a medical team in an oncology clinic. As patients entered the study, baseline information was established about their perceptions of life quality and chemotherapy associated symptoms by a questionnaire. The same questionnaire was used and answers were recorded at scheduled visits alongside their chemotherapeutic treatment. Blood samples were taken at the initiation of the study and also every visit to evaluate hematologic laboratory tests.
Study Design and Setting
This double-blind, placebo controlled trial study was conducted in the clinic of oncology, Shahid Ghazi hospital, Tabriz, Iran, following taking written and signed informed consents from all patients. The study was approved by research ethics committee of Tabriz University of Medical Sciences.
Simple randomization method with an allocation ratio of 1:1 was used for this study. Patients were randomized to either placebo or taurine groups at the beginning of their maintenance chemotherapy.
Taurine (Aviforme, UK) was provided as pure powder. Both taurine and placebo were provided as 500 mg capsules by department of pharmaceutics, faculty of pharmacy, Tabriz University of medical sciences, Tabriz, Iran.
Patients in both groups received two 500 mg capsules each time (with a total dosage of 2g/day) twice a day. This supplementation was given throughout the chemotherapy, for 6 months and responses were evaluated by questionnaire and direct physical examination at monthly intervals. Fever was defined as a single axillary temperature over 38.5 °C or two over 38 °C during a 12 hour period. Blood Samples were collected into heparinized tubes and assayed for hematological analysis. Total and differentiate leukocyte count, hemoglobin, hematocrit, platelets and red blood cells were measured using an automatic cell counter. Neutropenia was defined as granulocyte count lower than 1000/µl. Questionnaires were then evaluated based on occurrence of febrile episodes, signs and symptoms of infection or a documented focus for infection.
Study Population
Patients were selected from outpatient chemotherapy clinic in Tabriz. Forty young adults suffering from acute lymphoblastic leukemia were enrolled in the trial. Patients were eligible for inclusion if they aged over 16 years. The study was open to patients being treated according to Cancer and Leukemia Group B (CALGB) 8811 chemotherapy regimen for ALL in adults. The applied regimen described by Larson et.al consisted of five courses: “the induction phase (course I) consisted of a single dose of cyclophosphamide on first day, 3 consecutive days of daunorubicin, weekly vincristine, biweekly subcutaneous (SC) L-asparaginase, and 3 weeks of prednisone. Early intensification (course II) included 2 months of treatment using cyclophosphamide, SC cytarabine, oral 6-mercaptopurine (6-MP), vincristine, SC L-asparaginase and also intrathecal (IT) methotrexate. In course III, the CNS prophylaxis was completed with cranial irradiation (2,400 cGy) and 5 weekly doses of IT methotrexate with daily 6-MP, followed by a maintenance period of daily oral 6-MP and weekly oral methotrexate. Course IV was a late intensification course lasting 8 weeks, followed by prolonged maintenance treatment (course V) with daily 6-MP and weekly methotrexate plus monthly pulses of vincristine and prednisone”.20
Patients were recruited from oncology clinic at the initiation of maintenance course of their chemotherapy treatment. During the study four subjects were dismissed from each group. One patient was lost to be followed up, one patient died during the observation period; six patients initially consented and then refused to continue as a participant in our study because of the following reasons: (1) their symptoms were get worsening, (2) chose not to continue chemotherapy and (3) they could not tolerate the daily dosage of taurine.
Data Analysis
Statistical analysis was carried out using Statistical Package for the Social Science for Windows (SPSS, version 13.0). Patient’s demographic data and differences in the proportion of ALL patients in taurine and control groups were analyzed using Pearson’s Chi square test (Fisher’s exact test).
Data was expressed as the mean ± S.D. Independent sample t-test or Mann Whitney U test were used for comparison of differences between the groups. P-value <0.05 was accepted to indicate statistical significance.
Results
A total of 40 ALL patients were enrolled in the study during their maintenance therapy and 32 patients (80%) were finished the study. The study population was divided into taurine and placebo groups, using simple randomization method. Of the 32 randomized patients, 56.3% were male and 43.8% were female, with median age of 19.16±1.95 years (ranged from 16 to 23); the mean age of the control patients was 19.12±1.82 years and that of the taurine supplemented patients was 19.19±2.14 years. Most of them (93.8%) were single.
The majority of these patients (68.8%) were students, 50% were high school students and 18.8% were studying at the university, 25.1% were unemployed and the remaining 6.1% were self-employed.
In total, 70 febrile episodes were recorded within the duration of the study. Twelve patients (37.5%) had only one episode; 3 patients had 2 episodes (9.4%) and 15 patients (45.9%) had 3 or more episodes. Febrile episodes were lower in taurine patients during whole study period in comparison to the control ones and there was statistically significant differences between two groups (P= 0.049, 0.012, 0.014, 0.022, 0.013 & 0.012 during 6 months of study respectively).
The levels of hemoglobin (Hb), hematocrit (Hct), red blood cells (RBC), and platelets were slightly increased in the taurine supplemented patients during study compared to control patients (Figures 1 and 2) but there were no significant differences between patients of two groups (all P values were higher than 0.05 as shown in Figures 1 and 2). Total white blood cell (WBC) counts, were increased in taurine treated group compared to the control group (Figure 3). The elevation in WBC counts was statistically significant between two study groups within the study period (P= 0.003, 0.000, 0.000, 0.000, 0.000 & 0.000 during 6 months of study respectively). There was no statistically significant (P= 0.325, 0.410, 0.325, 0.700, 0.462 & 1 during 6 months of study respectively) elevation in blasts count occurred in whole study duration. Neutropenia was detected in 31 (44.3%) episodes, neutropenic febrile episodes were higher at control patients but there were no significant differences between taurine and control groups (P= 0.626, 0.626, 0.144, 0.365, 0.465 & 1 during 6 months of study respectively).
Figure 1 .
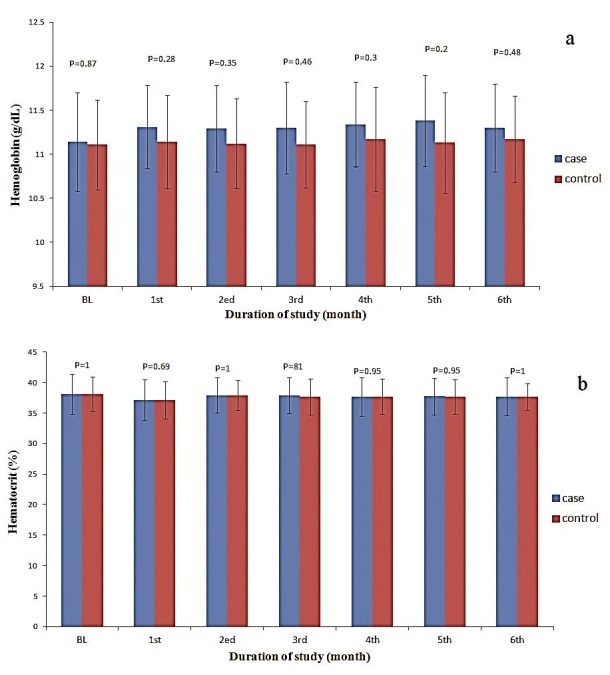
The levels of hemoglobin (a) and hematocrit (b) in the taurine supplemented and control patients during the study.
Figure 2.
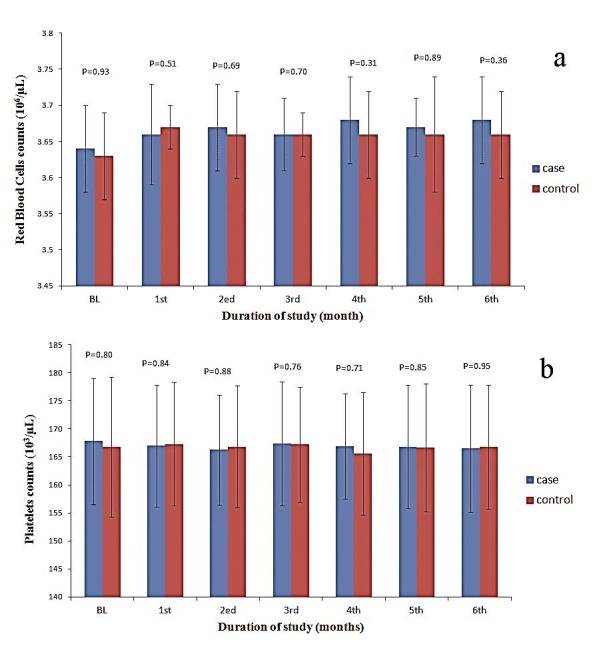
The levels of red blood cells (a) and platelets (b) in the taurine supplemented and control patients during the study.
Figure 3 .
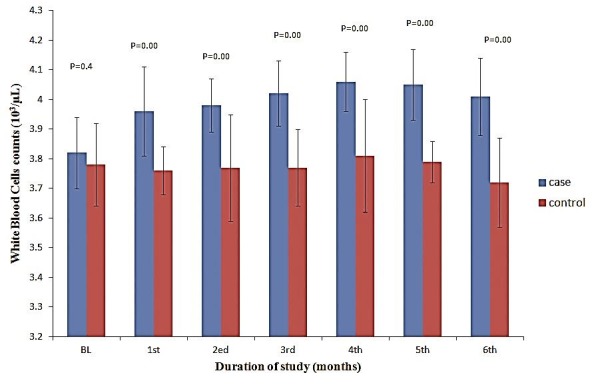
The levels of white blood cells in the taurine supplemented and control patients during the study.
Clinical manifestations of infections were available in 40 (57.1%) occasions of febrile episodes. Of these febrile episodes which had a documented focus of infection, 34 were in control group and the remaining 6 were in the taurine co-treated group. The respiratory system was the most frequently affected system followed by gastrointestinal and genitourinary systems (Table 1). Infectious febrile episodes occurred significantly more frequently in control patients than in taurine supplemented patients excluding first and second months of the study (P= 0.070, 0.070, 0.033, 0.033, 0.022 & 0.033 during 6 months of study respectively).
Table 1. Causes of 70 Febrile Episodes in ALL patients .
| Number of episodes | |
| No causes found | 35 |
| Respiratory tract infections | 15 |
| Acute gastroenteritis | 9 |
| Urinary tract infections | 5 |
| Other infections | 6 |
Discussion
No previous study has evaluated the effect of taurine co-treatment in ALL patients during their chemotherapy regimen. We found that taurine supplementation, as compared with placebo, resulted in a lower numbers of febrile episodes, increased total WBCs counts, decreased neutropenic febrile episodes and also less infectious episodes during maintenance therapy in adolescents with acute lymphoblastic leukemia.
Chemotherapy induced myelosupression and immunosupression may result in fever and infections because of decreased number and function of leucocytes especially neutrophils. Previous studies suggested that taurine could inhibit some kind of tumors and decreased their numbers.21,22 Additionally it was reported that taurine in combination with chemotherapeutic agents could improve their tumor inhibiting ability.23 But it has not been approved as a combination therapy for cancers.
It was reported that taurine had a protecting effect on human lymphocytes.24 Further studies showed that exogenous taurine maintaind human lymphocytes viability through its membrane stabilizing capacity.25 Taurine deficiency may cause significant leucopenia, functional defects of the neutrophils and decreased phagocytosis of microorganisms like Staphylococcus epidermis.26,27 Previous studies showed that taurine supplementation could decrease the immune suppressing adverse effects of cyclophosphamide therapy by increasing the phagocytic activity of macrophages, neutrophiles and monocytes and also increasing the leucocytes function.23
Another study reported that taurine could regulate the balance between protective and microbicidal activities of neutrophil on one hand and damaging effect of the inflammatory cells on the other hand.28 Circulating neutrophils are committed to apoptosis by reactive oxygen species which is produced by activated cells. It is recognized that taurine could protect neutrophils from apoptosis by its antioxidant activity.29 Their findings indicated that taurine could contribute to an increased number and viability of lymphocytes; additionally it could reduce the immune suppression and increase the apoptotic activity of neutrophils. Likewise, in our study, the co-administration of taurine along with chemotherapeutical agents significantly increased total leukocyte counts.
On the other hand taurine chloramine and taurine bromamine (haloamines of MPO-halide system), are produced and released by the activated neutrophils during inflammation. Taurine haloamines could protect the surrounding cells from the inflammation and oxidative stress.30 It has also been suggested that taurine haloamines had antimicrobial properties and could be used as local antiseptics and valuable in treatment of local mucosal and skin infections.31 Additionally our previous study showed that in vitro taurine could attenuate antibacterial activity of gentamycine against Staphylococcus epidermis.32 These findings mentioned the ability of taurine to defend against reactive oxygen species and also act as microbicidal agent which may result in lowering the incidence of febrile episodes among taurine treated patients in the present study.
Conclusion
In conclusion the overall incidence of febrile episodes, nutropenia and infectious complications in ALL patients receiving taurine was lower than the control group. Taurine’s ability to improve immunity by increasing WBC count or viability may result in lower febrile episodes. Future studies with large number of patients should be implemented to evaluate our results.
Acknowledgments
The authors would like to thank the authorities of Hematology and Oncology Research Center of Tabriz University of Medical Science, for their financial support. We also thank the patients and their families, who were participated in this trial. The nurses, laboratory, and other staffs of Shahid Ghazi Hospital of Tabriz are also acknowledged for their cooperation. This article is based on a thesis submitted for PhD degree (No.49) in the faculty of pharmacy, Tabriz University of Medical Sciences in Tabriz, Iran.
Ethical Issues
Not applicable.
Conflict of Interest
There was no conflict of interest in this study.
References
- 1.Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med . 2004;350(15):1535–48. doi: 10.1056/NEJMra023001. [DOI] [PubMed] [Google Scholar]
- 2.El-Sabagh ME, Ramadan KS, El-Slam IMA, Ibrahim AM. Antioxidants status in acute lymphoblastic leukemic patients. Am J Med Med Sci . 2011;1(1):1–6. [Google Scholar]
- 3.Estey EH, Faderl SH, Kantarjian HM. Hematologic Malignancies: Acute Leukemias. Berlin: Springer Berlin Heidelberg; 2008; Available from: http://dx.doi.org/10.1007/978-3-540-72304-2. [Google Scholar]
- 4.Advani AS, Lazarus HM. Adult Acute Lymphocytic Leukemia (Biology and Treatment). Berlin: Springer Berlin Heidelberg; 2011; Available from: http://dx.doi.org/10.1007/978-1-60761-707-5. [Google Scholar]
- 5.Bergkvist K, Wengstrom Y. Symptom experiences during chemotherapy treatment--with focus on nausea and vomiting. Eur J Oncol Nurs . 2006;10(1):21–9. doi: 10.1016/j.ejon.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Carr C, Ng J, Wigmore T. The side effects of chemotherapeutic agents. Curr Anaesth Crit Care . 2008;19(2):70–9. [Google Scholar]
- 7.Vagace JM, Gervasini G. Chemotherapy Toxicity in Patients with Acute Leukemia. In: Antica M, editor. Acute Leukemia: The Scientist's Perspective and Challenge. 1st ed. Rijeka, Croatia: InTech; 2011. p. 391-414. [Google Scholar]
- 8.Reiss M, Reiss G. Drug-induced smell and taste disorders. Med Monatsschr Pharm . 1999;22(12):388–92. [PubMed] [Google Scholar]
- 9.Catane R, Cherny N, Kloke M, Tanneberger S, Schrijvers D. Handbook of advanced cancer care. London: Taylor & Francis; 2006. [Google Scholar]
- 10.Koskenvuo M. Febrile Infections in Children with Leukemia with Special Reference to Respiratory Viral Infections [Doctoral dissertation]. Turku: University hospital of Turku; 2008.
- 11.Te Poele EM, De Bont ES, Marike Boezen H, Revesz T, Bokkerink JP, Beishuizen A. et al. Dexamethasone in the maintenance phase of acute lymphoblastic leukaemia treatment: is the risk of lethal infections too high? . Eur J Cancer. 2007;43(17):2532–6. doi: 10.1016/j.ejca.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Chong CY, Tan AM, Lou J. Infections in acute lymphoblastic leukaemia. Ann Acad Med Singapore . 1998;27(4):491–5. [PubMed] [Google Scholar]
- 13.Castagnola E, Fontana V, Caviglia I, Caruso S, Faraci M, Fioredda F. et al. A prospective study on the epidemiology of febrile episodes during chemotherapy-induced neutropenia in children with cancer or after hemopoietic stem cell transplantation. Clin Infect Dis . 2007;45(10):1296–304. doi: 10.1086/522533. [DOI] [PubMed] [Google Scholar]
- 14.Paksu MS, Paksu S, Akbalik M, Ozyurek E, Duru F, Albayrak D. et al. Comparison of the approaches to non-febrile neutropenia developing in children with acute lymphoblastic leukemia. Fundam Clin Pharmacol . 2012;26(3):418–23. doi: 10.1111/j.1472-8206.2011.00938.x. [DOI] [PubMed] [Google Scholar]
- 15.Huxtable RJ. Physiological actions of taurine. Physiol Rev . 1992;72(1):101–63. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- 16.Oja SS, Saransaari P. Taurine. In: Oja SS, Schousboe A, Saransaari P, editors. Handbook of neurochemistry and molecular neurobiology. 3rd ed. New York: Springer; 2007. p. 155–206.
- 17.Taurine - monograph. Altern Med Rev 2001;6(1):78-82. [PubMed] [Google Scholar]
- 18.Harada H, Kitazaki K, Tsujino T, Watari Y, Iwata S, Nonaka H. et al. Oral taurine supplementation prevents the development of ethanol-induced hypertension in rats. Hypertens Res . 2000;23(3):277–84. doi: 10.1291/hypres.23.277. [DOI] [PubMed] [Google Scholar]
- 19.Desai TK, Maliakkal J, Kinzie JL, Ehrinpreis MN, Luk GD, Cejka J. Taurine deficiency after intensive chemotherapy and/or radiation. Am J Clin Nutr . 1992;55(3):708–11. doi: 10.1093/ajcn/55.3.708. [DOI] [PubMed] [Google Scholar]
- 20.Larson RA, Dodge RK, Burns CP, Lee EJ, Stone RM, Schulman P. et al. A five-drug remission induction regimen with intensive consolidation for adults with acute lymphoblastic leukemia: cancer and leukemia group B study 8811. Blood . 1995;85(8):2025–37. [PubMed] [Google Scholar]
- 21.Okamoto K, Sugie S, Ohnishi M, Makita H, Kawamori T, Watanabe T. et al. Chemopreventive effects of taurine on diethylnitrosamine and phenobarbital-induced hepatocarcinogenesis in male F344 rats. Jpn J Cancer Res . 1996;87(1):30–6. doi: 10.1111/j.1349-7006.1996.tb00196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reddy BS, Rao CV, Rivenson A, Kelloff G. Chemoprevention of colon carcinogenesis by organosulfur compounds. Cancer Res . 1993;53(15):3493–8. [PubMed] [Google Scholar]
- 23.Wang L, Zhao N, Zhang F, Yue W, Liang M. Effect of taurine on leucocyte function. Eur J Pharmacol . 2009;616(1-3):275–80. doi: 10.1016/j.ejphar.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 24.Pasantes-Morales H, Wright CE, Gaull GE. Protective effect of taurine, zinc and tocopherol on retinol-induced damage in human lymphoblastoid cells. J Nutr . 1984;114(12):2256–61. doi: 10.1093/jn/114.12.2256. [DOI] [PubMed] [Google Scholar]
- 25.Redmond HP, Stapleton PP, Neary P, Bouchier-Hayes D. Immunonutrition: the role of taurine. Nutrition . 1998;14(7-8):599–604. doi: 10.1016/s0899-9007(98)00097-5. [DOI] [PubMed] [Google Scholar]
- 26.Schuller-Levis GB, Park E. Taurine and its chloramine: modulators of immunity. Neurochem Res . 2004;29(1):117–26. doi: 10.1023/b:nere.0000010440.37629.17. [DOI] [PubMed] [Google Scholar]
- 27.Sapronov NS, Khnychenko LK, Polevshchikov AV. Effects of new taurine derivatives on primary immune response in rats. Bull Exp Biol Med . 2001;131(2):142–4. doi: 10.1023/a:1017587727533. [DOI] [PubMed] [Google Scholar]
- 28.Marcinkiewicz J, Grabowska A, Bereta J, Bryniarski K, Nowak B. Taurine chloramine down-regulates the generation of murine neutrophil inflammatory mediators. Immunopharmacology . 1998;40(1):27–38. doi: 10.1016/s0162-3109(98)00023-x. [DOI] [PubMed] [Google Scholar]
- 29.Capuozzo E, Pecci L, Baseggio Conrado A, Fontana M. Thiotaurine prevents apoptosis of human neutrophils: a putative role in inflammation. Adv Exp Med Biol . 2013;775:227–36. doi: 10.1007/978-1-4614-6130-2_19. [DOI] [PubMed] [Google Scholar]
- 30.Kang IS, Kim C. Taurine chloramine administered in vivo increases NRF2-regulated antioxidant enzyme expression in murine peritoneal macrophages. Adv Exp Med Biol . 2013;775:259–67. doi: 10.1007/978-1-4614-6130-2_22. [DOI] [PubMed] [Google Scholar]
- 31.Marcinkiewicz J, Strus M, Walczewska M, Machul A, Mikolajczyk D. Influence of taurine haloamines (TauCl and TauBr) on the development of Pseudomonas aeruginosa biofilm: a preliminary study. Adv Exp Med Biol . 2013;775:269–83. doi: 10.1007/978-1-4614-6130-2_23. [DOI] [PubMed] [Google Scholar]
- 32.Islambulchilar M, Sattari MR, Sardashti M, Lotfipour F. Effect of Taurine on the antimicrobial efficiency of Gentamicin. Adv Pharm Bull . 2011;1(2):69–74. doi: 10.5681/apb.2011.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


