Abstract
Purpose: The purpose of this study was to investigate the regular moderate exercise effect on the miR-192 expression changes in kidney of Streptozotocin- induced diabetic rats.
Methods: Forty adult male Wistar rats were divided into four groups of 10, including Sedentary Control group, Healthy 60 days Exercise group, diabetic group and Diabetic 60 days Exercise. Diabetes was induced by injection of 60 mg/kg Streptozotocin and after 48 hour blood glucose levels higher than 250 mg/dl were included to diabetic rats. After 48 hour of induction diabetes, exercise protocol was begun. Animals performed 5 days of consecutive treadmill exercise (60 min/day) with 22 m/min speeds for 60 days. Kidney of the rats has removed and MicroRNA was extracted from kidney using miRCURYTM RNA isolation kit.
Results: Exercise upregulated miR-192 expression level significantly in the kidney of diabetic rats in comparison to healthy group. There is not any significant change in miR-192 expression in diabetic 60 days exercise compared to control group.
Conclusion: These results may indicate that exercise can help to prevent the progression of diabetic nephropathy.
Keywords: Diabetes, MiR-192, Kidney, Exercise
Introduction
MicroRNAs (miRs) are a group of small, non-coding RNAs that are approximately 22 nucleotides long.1 They are endogenously produced transcripts have been shown to play key roles in gene regulation.1 MiRNAs generally decrease expression of their mRNA targets via translational repression or enhanced mRNA decay.2 New findings have revealed important functions for specific miRs in many cellular and biological processes, oncogenes and insulin secretion.3-5 Research in the last few years ago has focused on the analysis of differential miRNA expression in renal disease.6 Several miRNAs were enriched in human kidney, including miR-192, miR-194, miR-204 and miR-215.7 MiR-192 enriched in rat kidney cortex relative to medulla.8 Sun et al7 identified a conserved putative Ets-1 transcription factor binding site upstream of miR-192 and others have reported that Ets-1 is important for kidney development and function.9 The involvement of miRNAs in many other renal diseases is under intense investigation, including diabetic nephropathy,10 immunologic renal diseases such as allograft rejection and autoimmune renal diseases.11
Diabetes mellitus (DM) is a serious chronic metabolic disease.12 It is the seventh leading cause of death in the United States.13 Type 1 diabetes mellitus (T1DM) comprises 5 to 10% of all causes of diabetes.14 The existence of chronic hyperglycemia in individuals with type 1 DM is associated with dyslipidemia,12 cardiovascular diseases15 and, macrovascular and microvascular disorders.12 Therefore, amplification of glycemic improvement of lipid profile and immune cells function in patients with type 1DM are essential features in reducing morbidity and mortality in this kind of patients.16 Currently, regular exercise, along with insulin therapy and diet planning, has been considered one of the three main approaches to the treatment of type1 DM.15
The main benefits of exercise for people with type 1 DM are: improvement of insulin sensitivity and lipid profile,17 reduction of insulin substitution,17 and attenuation of autonomic and cardiovascular dysfunction.17 In addition, physical activity has been shown to prevent or delay the development of other diabetes related complications, including nephropathy,18 retinopathy19 and neuropathy.20 Also reduce risk of other chronic diseases16,21 and reduce the functional decline that occurs with aging.22
For decades, exercise has been considered as a cornerstone of diabetes management, along with meal and medication.23 There is at present very little research on how exercise impacts epigenetic phenomena such as miRNAs but several studies have determined the impact of acute exercise on circulating miRNA24 and some studies have reported the effects of exercise on miRNAs in skeletal muscle in humans,25,26 and neutrophils.27 Authors study is the first positive steps in determining whether exercise play a role on expression changes miR-192 in the kidney diabetic.
Diabetic nephropathy is the main cause of end stage renal disease in the western world.28,18 Recent studies have shown that miR (microRNA)-192 plays key roles in renal pathological and physiological responses.29 MiR-192 expression was displayed to be associated with diabetic nephropathy.1,29-32 It was described that miR-192 was increased in glomeruli of streptozotocin- induced diabetic mice, as well as db/db diabetic mice, along with TGFβ and collagen 1 α2 levels.32,33 However, some studies have shown that decreased miR-192 expression in proximal tubular epithelial cells in advanced diabetic nephropathy.29,30 Due to the important miRNAs in complications of diabetes and given the current evidence regarding the beneficial effects of exercise in patients with diabetes, the aim of the present experiments was to describe of the effect of regular moderate exercise on expression changes miRNA192 in the kidney of streptozotocin- induced diabetic rats.
Materials and Methods
Subjects
Forty adult male Wistar rats (Raze Institute, Tehran, Iran) with weighing (300±50 g) were housed at room temperature (22-25 °C) with 12:12 h light/dark cycles and free access to food and water and housed as three animals to a cage. They were randomly divided into four groups of 10, including Sedentary Control group (SC), Healthy Control with 60-day Exercise group (H60E(, Sedentary Diabetic group (SD (and Diabetic with 60-day Exercise (D60E). Diabetes was induced by injection of 60 mg/kg Streptozotocin(STZ).19 STZ dissolved in 0.1 M of citrate buffer (pH 4.5) in 12 h fasted rats. Rats in the control groups received an intraperitoneal injection of an equal volume of citrate buffer instead of STZ. After 48 h, blood samples were obtained from the tail vein and blood glucose levels were measured using glucometer (Arkray, Kyoto, Japan). The rats with blood glucose levels higher than 250 mg/dl were included to the protocol as diabetic rats.19
Exercise protocol
Before beginning the formal 60 day exercise protocol, animals were familiarized to treadmill running (5-20 min/day) for 5 consecutive days. After this period of habituation, the exercised animals performed 5 days of consecutive treadmill exercise (60 min/day) with 22 m/min speeds.34 At the beginning of 60 min exercise, to warm up the rats, treadmill speed had been set at 5 m/min and progressively increased to 22 m/min. At the final of 60 minute exercise, the speed progressively decreased to 5 m/min to cool down. Mild electrical shock was used the negligible amount to motivate animals to run. Control animals did not carry out treadmill exercise but were placed on a nonmoving treadmill for 60 min/day for 5 days a week. Exercised animals were studied 24 h after their last exercise session.
Measurement of blood glucose level
The blood glucose concentrations of diabetic rats were measured in blood collected from the tail vein in the morning, at the first, middle and end of each experimental period. The body weight of all rats was measured.
Sample collection
After the experimental periods, all of the rats were anesthetized by intraperitoneal injection of 100mg/kg ketamine and 5 mg/kg xylazine.35 Kidney of rats were immediately removed and washed with cold 9% normal saline, and weights were measured.
Total RNA extraction and real time PCR
MicroRNA was extracted from kidney using miRCURYTM RNA isolation kit (Exiqon, Vedbaek, Denmark) according to the manufacturer’s protocol.36,37 PCR primer sequence for miR-192 was CUGCCAAUUCCAUAGGUCACAG (www.mirbase.org). The procedure was performed based on spin column using a proprietary resin as a separation matrix for RNA from other cell components. RNA content and purity were measured using Nanodrop 1000 spectrophotometer (Thermo scientific, Wilmington DE 19810 USA). The expression profile of miR-192 was performed on total RNA extracts by using universal cDNA synthesis kit. Briefly, total RNA containing microRNA was polyadenylated and cDNA was synthesized using a poly(T) primer with a 30 degenerate anchor and a 50 universal tag (Exiqon, Vedbaek, Denmark) Each cDNA was used as a template for separate assay for microRNA and mRNA quantitative real-time PCR by using SYBR Green master mix (Exiqon, Vedbaek, Denmark). Real-time PCR reactions were fulfilled on a Bio-Rad iQ5 detection System (Bio-Rad, Richmond, CA, USA).The amount of PCR products were normalized with housekeeping beta-glucuronidase gene for miR-192.38 The 2-(DDCt) method was used to determine relative quantitative levels of miR-192. The results were expressed as the fold-difference to the relevant controls.
Data analysis and statistics
Data were analyzed by using one analysis of variance (ANOVA). The post hoc LSD was performed to determine which condition differed significantly from each other.
Results
Glucose results
Blood glucose level was significantly higher in sedentary diabetic rats compared with those from sedentary control group (P<0.05).
Measuring of blood glucose level in diabetes with 60-day exercise group indicated a significant difference on 30th and 60th days in 60-day exercise protocol (Table 1). Also the means of final blood glucose level showed a significant decrease in comparison with initial blood glucose level in diabetes with 60-day exercise group (P<0.05) (Table 1).
Table 1. General characteristics of STZ- induced diabetic and healthy rats.
| Parameter | SC | SD | H60E | D60E |
| Initial Blood Glucose (mg/dl) | 85.3±4.3# | 378.8±39.1*$ | 91.3±5.2# | 324.8±17.7*$ |
| Final Blood Glucose (mg/dl) | 88.3±5.2# | 455.5±33.8*$ | 81.5±3.4# | 175.8±34.8*#$ |
| Final Body Weight(g) | 280.1±8.1# | 183.0±10.9*$ | 259.1±9.7# | 288.8±23.5# |
Data are means±SEM. SC= Sedentary Control, SD= Sedentary Diabetic, H60E= Healthy 60 days Exercise, D60E= Diabetic 60 days Exercise.
* Significant difference compared with SC group (P<0.05)
# Significant difference compared with SD group (P<0.05)
$ Significant difference compared with H60E group (P<0.05)
Also the final blood glucose level showed a significant decrease in comparison with initial blood glucose level in diabetes with 60-day exercise group (P<0.05) (Table 1).
60-day exercise declined the blood glucose level in diabetes rats in comparison with sedentary diabetic group (P<0.05) (Table 1).
The expression of kidney miR-192 has been presented in Figure 1. MiR-192 expression level showed a no significant decrease in the kidney of sedentary diabetic rats in comparison to sedentary control group (Figure 1) (p<0.05). Treatment with 60 days exercise showed an increase in expression of miR-192 in the kidney of diabetic exercise group as compared to diabetic control group, although it not significant (Figure 1). But miR-192 expression level upregulated significantly in the kidney of diabetic rats with 60 days exercise in comparison to healthy exercise group (Figure 1) (p<0.05). Another way, exercise down regulated miR-192 expression significantly in the kidney of healthy exercise group in comparison to control group (Figure 1) (p<0.05).
Figure 1.
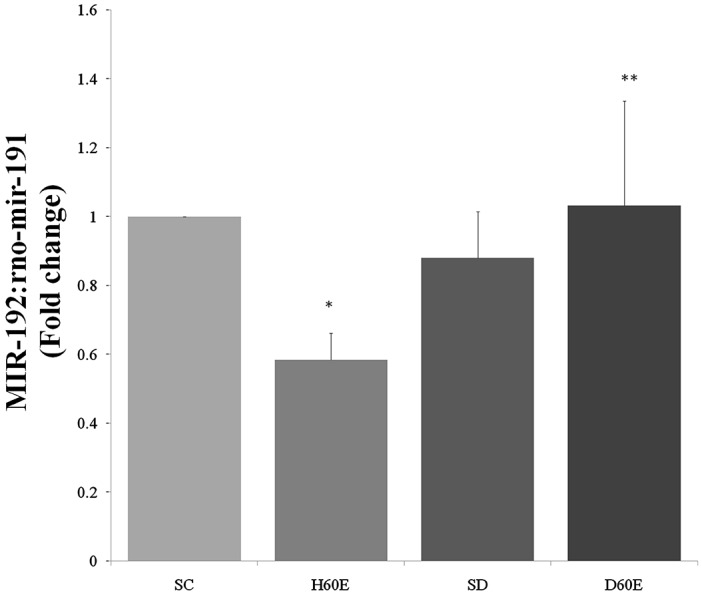
Effect of exercise on miRNA-192 expression in kidney diabetic rats (Means±SE). SC: Sedentary Control group, H60E: healthy with 60-day Exercise group, SD: Sedentary Diabetic group, D60E: Diabetes with 60-day Exercise group.
* Significant difference compare with Sedentary Control group (P<0.05 (and ** significant difference compare with healthy 60-day Exercise group (p<0.05).
Discussion
This study indicated that diabetes decreased miR-192 expression level in the kidney of sedentary diabetic rats in comparison to control group, which was not statistically significant. In spite of, this slight decline is in line with Krupa et al30 and Robert et al29 studies that showed decreased miR-192 expression in proximal tubular epithelial cells in advanced diabetic nephropathy.
The main finding of current study, it was which exercise did not cause changes in expression of miR-192 in the kidney of diabetic exercise group as compared to sedentary control group. Changes in expression of miR-192 in the kidney of diabetic exercise group as compared to healthy group with 60 days exercise showed a statistically significant increase.
This study showed that treatment with moderate regular exercise can lead to statistically significant decrease in the expression of miR-192 in the kidney of healthy group as compared to control group.
It is believed that hyperglycemia activates protein kinase C,39,40 polyol and hexosamine pathway fluxes,39,40 enhances advanced glycation end products and induces oxidative/ nitrosative stress in several cells, among their leukocytes and renal cells.41,42 Many of the above mentioned factors converge to activate NF-κB to produce its downstream pro-inflammatory cytokines.41,43 In this situation, elevated levels of the pro-inflammatory cytokines in blood and renal tissue cooperate to develop and progress of diabetic nephropathy.41,43 Of these, the cytokine transforming growth factor (TGF-b) has emerged as having a key role in the development of renal hypertrophy and accumulation of extracellular matrix in diabetes.44 Our data confirmed previous studies and we observed a significant increase in the means of fasting blood glucose level in sedentary diabetic rats during the examination period. Exercise can lead to desirable changes in circulating levels of glucose,45 increased blood flow and delivery of glucose to the skeletal muscle(insulin facilitated and insulin independent).45 The effect of increased glucose uptake by skeletal muscle decreases the amount of circulating glucose and hyperglycemia that is seen with diabetes, favoring overall glycemic control45 Present study also are in line with mentioned studies in which, we observed a significant decrease in the means of blood glucose level in diabetic 60 days exercise rats during the examination period. Several miRNAs were enriched in human kidney, including miR-192, miR-194, miR-204, miR-215 and miR-216.7 Some studies showed that the expression of miR-192 is increased in diabetic kidney glomeruli in mouse models,1,32 Whereas other studies showed that the renal levels of miR-192 were decreased in patients with severe diabetic nephropathy,29,30,46 Present study showed a decrease on miR-192 expression in kidney diabetic, although this decrease was not significant but that is in line with studies by Krupa et al,30 Robert et al29 and Wang et al.46
Although some studies have shown that physical activity accelerate diabetic nephropathy progression,47 several randomized trials in diabetic animals with proteinuria displayed that aerobic exercise training decreased urine protein excretion.48,49 Several studies also have determined the impact of acute exercise on circulating miRNAs,24,50 and they have presented the effects of exercise on miRNAs in skeletal muscle in humans.26 Until now, no study has evaluated the expression changes miRNA profile after exercise in renal diabetic. The authors, for the first time, examined the effects of exercise on miRNA192 expression changes in kidney diabetic. In overall, miR-192 expression level in the kidney of sedentary diabetic rats than the control group, Showed a decrease which was not statistically significant.miR-192 expression level showed a significant increase in the kidney of diabetic rats with 60 days exercise than the Healthy exercise group. In spite of, exercise did not cause changes in expression of miR-192 in the kidney of diabetic exercise group as compared to control group. The lack of change on miR-192 expression probably reflects the protective effect of exercise in expression changes miR-192 in diabetic kidney. Exercise lead to decrease in miR-192 expression in the kidney of healthy exercise group than the control group. Due to the positive effects of probably exercise in the prevention of miR-192 expression changes, we could be argued that long-term exercise probably leads to significant expression changes of miR-192 in kidney diabetic rats.
Conclusion
These results indicate that exercise in diabetic renal can prevent from the expression changes in miR-192 and may be help to prevent the progression of diabetic nephropathy.
Ethical Issues
The study protocol was designed in accordance with NIH guidelines and ethics committee for the use of animals in research at Tabriz University of Medical Sciences.
Acknowledgments
This study was financially supported by Drug Applied Research Center of Tabriz University of Medical Sciences. Our data in this work were derived from the thesis of Ms. Hajar Oghbaei for a Master of Science degree in physiology (thesis serial number: 91/2-3/3).
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Kato M, Arce L, Natarajan R. MicroRNAs and their role in progressive kidney diseases. Clin J Am Soc Nephrol. 2009;4(7):1255–66. doi: 10.2215/CJN.00520109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Djuranovic S, Nahvi A, Green R. A parsimonious model for gene regulation by miRNAs. Science. 2011;331(6017):550–3. doi: 10.1126/science.1191138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang TC, Mendell JT. microRNAs in vertebrate physiology and human disease. Annu Rev Genomics Hum Genet. 2007;8:215–39. doi: 10.1146/annurev.genom.8.080706.092351. [DOI] [PubMed] [Google Scholar]
- 4.Poy MN, Eliasson L, Krutzfeldt J, Kuwajima S, Ma X, Macdonald PE. et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. 2004;432(7014):226–30. doi: 10.1038/nature03076. [DOI] [PubMed] [Google Scholar]
- 5.Zhang B, Pan X, Cobb GP, Anderson TA. MicroRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302(1):1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 6.Ho J, Kreidberg JA. The long and short of microRNAs in the kidney. J Am Soc Nephrol. 2012;23(3):400–4. doi: 10.1681/ASN.2011080797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Y, Koo S, White N, Peralta E, Esau C, Dean NM. et al. Development of a micro-array to detect human and mouse microRNAs and characterization of expression in human organs. Nucleic Acids Res. 2004;32(22):e188. doi: 10.1093/nar/gnh186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian Z, Greene AS, Pietrusz JL, Matus IR, Liang M. MicroRNA-target pairs in the rat kidney identified by microRNA microarray, proteomic, and bioinformatic analysis. Genome Res. 2008;18(3):404–11. doi: 10.1101/gr.6587008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Razzaque MS, Naito T, Taguchi T. Proto-oncogene Ets-1 and the kidney. Nephron. 2001;89(1):1–4. doi: 10.1159/000046034. [DOI] [PubMed] [Google Scholar]
- 10.Kaucsar T, Racz Z, Hamar P. Post-transcriptional gene-expression regulation by micro RNA (miRNA) network in renal disease. Adv Drug Deliv Rev. 2010;62(14):1390–401. doi: 10.1016/j.addr.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Anglicheau D, Sharma VK, Ding R, Hummel A, Snopkowski C, Dadhania D. et al. MicroRNA expression profiles predictive of human renal allograft status. Proc Natl Acad Sci U S A. 2009;106(13):5330–5. doi: 10.1073/pnas.0813121106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu J, Pang W, Chen J, Bai S, Zheng Z, Wu X. Hypoglycemic effect of polysaccharides with different molecular weight of Pseudostellaria heterophylla. BMC Complement Altern Med. 2013;13:267. doi: 10.1186/1472-6882-13-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrato EH, Hill JO, Wyatt HR, Ghushchyan V, Sullivan PW. Physical activity in US adults with diabetes and at risk for developing diabetes, 2003. Diabetes care. 2007;30(2):203–9. doi: 10.2337/dc06-1128. [DOI] [PubMed] [Google Scholar]
- 14.Hu J, Yu X, Wang Z, Wang F, Wang L, Gao H. et al. Long term effects of the implantation of Wharton's jelly-derived mesenchymal stem cells from the umbilical cord for newly-onset type 1 diabetes mellitus. Endocr J. 2013;60(3):347–57. doi: 10.1507/endocrj.ej12-0343. [DOI] [PubMed] [Google Scholar]
- 15.De Angelis K, Schaan BD, Maeda CY, Dall'ago P, Wichi RB, Irigoyen MC. Cardiovascular control in experimental diabetes. Braz J Med Biol Res. 2002;35(9):1091–100. doi: 10.1590/s0100-879x2002000900010. [DOI] [PubMed] [Google Scholar]
- 16.Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001;286(10):1218–27. doi: 10.1001/jama.286.10.1218. [DOI] [PubMed] [Google Scholar]
- 17.Kivela R, Silvennoinen M, Touvra AM, Lehti TM, Kainulainen H, Vihko V. Effects of experimental type 1 diabetes and exercise training on angiogenic gene expression and capillarization in skeletal muscle. FASEB J. 2006;20(9):1570–2. doi: 10.1096/fj.05-4780fje. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh S, Khazaei M, Moien-Afshari F, Ang LS, Granville DJ, Verchere CB. et al. Moderate exercise attenuates caspase-3 activity, oxidative stress, and inhibits progression of diabetic renal disease in db/db mice. Am J Physiol Renal Physiol. 2009;296(4):F700–8. doi: 10.1152/ajprenal.90548.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozkaya YG, Agar A, Hacioglu G, Yargicoglu P. Exercise improves visual deficits tested by visual evoked potentials in streptozotocin-induced diabetic rats. Tohoku J Exp Med. 2007;213(4):313–21. doi: 10.1620/tjem.213.313. [DOI] [PubMed] [Google Scholar]
- 20.Balducci S, Iacobellis G, Parisi L, Di Biase N, Calandriello E, Leonetti F. et al. Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complications. 2006;20(4):216–23. doi: 10.1016/j.jdiacomp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Bassuk SS, Manson JE. Epidemiological evidence for the role of physical activity in reducing risk of type 2 diabetes and cardiovascular disease. J Appl Physiol (1985) 2005;99(3):1193–204. doi: 10.1152/japplphysiol.00160.2005. [DOI] [PubMed] [Google Scholar]
- 22.Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC. et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1435–45. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- 23.Kurdak H, Sandikci S, Ergen N, Dogan A, Kurdak SS. The effects of regular aerobic exercise on renal functions in streptozotocin induced diabetic rats. J Sports Sci Med. 2010;9(2):294–9. [PMC free article] [PubMed] [Google Scholar]
- 24.Sawada S, Kon M, Wada S, Ushida T, Suzuki K, Akimoto T. Profiling of circulating microRNAs after a bout of acute resistance exercise in humans. PLoS One. 2013;8(7):e70823. doi: 10.1371/journal.pone.0070823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drummond MJ, Mccarthy JJ, Fry CS, Esser KA, Rasmussen BB. Aging differentially affects human skeletal muscle microRNA expression at rest and after an anabolic stimulus of resistance exercise and essential amino acids. Am J Physiol Endocrinol Metab. 2008;295(6):E1333–40. doi: 10.1152/ajpendo.90562.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nielsen S, Scheele C, Yfanti C, Akerstrom T, Nielsen AR, Pedersen BK. et al. Muscle specific microRNAs are regulated by endurance exercise in human skeletal muscle. J Physiol. 2010;588(Pt 20):4029–37. doi: 10.1113/jphysiol.2010.189860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radom-Aizik S, Zaldivar F Jr, Oliver S, Galassetti P, Cooper DM. Evidence for microRNA involvement in exercise-associated neutrophil gene expression changes. J Appl Physiol (1985) 2010;109(1):252–61. doi: 10.1152/japplphysiol.01291.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giacchetti G, Sechi LA, Rilli S, Carey RM. The renin-angiotensin-aldosterone system, glucose metabolism and diabetes. Trends Endocrinol Metab. 2005;16(3):120–6. doi: 10.1016/j.tem.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Jenkins RH, Martin J, Phillips AO, Bowen T, Fraser DJ. Transforming growth factor beta1 represses proximal tubular cell microRNA-192 expression through decreased hepatocyte nuclear factor DNA binding. Biochem J. 2012;443(2):407–16. doi: 10.1042/BJ20111861. [DOI] [PubMed] [Google Scholar]
- 30.Krupa A, Jenkins R, Luo DD, Lewis A, Phillips A, Fraser D. Loss of MicroRNA-192 promotes fibrogenesis in diabetic nephropathy. J Am Soc Nephrol. 2010;21(3):438–47. doi: 10.1681/ASN.2009050530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung AC, Huang XR, Meng X, Lan HY. miR-192 mediates TGF-beta/Smad3-driven renal fibrosis. J Am Soc Nephrol. 2010;21(8):1317–25. doi: 10.1681/ASN.2010020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Putta S, Lanting L, Sun G, Lawson G, Kato M, Natarajan R. Inhibiting microRNA-192 ameliorates renal fibrosis in diabetic nephropathy. J Am Soc Nephrol. 2012;23(3):458–69. doi: 10.1681/ASN.2011050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kato M, Putta S, Wang M, Yuan H, Lanting L, Nair I. et al. TGF-beta activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nat Cell Biol. 2009;11(7):881–9. doi: 10.1038/ncb1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor RP, Ciccolo JT, Starnes JW. Effect of exercise training on the ability of the rat heart to tolerate hydrogen peroxide. Cardiovasc Res. 2003;58(3):575–81. doi: 10.1016/s0008-6363(03)00285-2. [DOI] [PubMed] [Google Scholar]
- 35.Atalay M, Oksala NK, Laaksonen DE, Khanna S, Nakao C, Lappalainen J. et al. Exercise training modulates heat shock protein response in diabetic rats. J Appl Physiol (1985) 2004;97(2):605–11. doi: 10.1152/japplphysiol.01183.2003. [DOI] [PubMed] [Google Scholar]
- 36.Lässer C, Eldh M, Lötvall J. Isolation and characterization of RNA-containing exosomes. J Vis Exp. 2012;(59):e3037. doi: 10.3791/3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biyashev D, Veliceasa D, Topczewski J, Topczewska JM, Mizgirev I, Vinokour E. et al. miR-27b controls venous specification and tip cell fate. Blood. 2012;119(11):2679–87. doi: 10.1182/blood-2011-07-370635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peltier HJ, Latham GJ. Normalization of microRNA expression levels in quantitative RT-PCR assays: identification of suitable reference RNA targets in normal and cancerous human solid tissues. RNA. 2008;14(5):844–52. doi: 10.1261/rna.939908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luis-Rodriguez D, Martinez-Castelao A, Gorriz JL, De-Alvaro F, Navarro-Gonzalez JF. Pathophysiological role and therapeutic implications of inflammation in diabetic nephropathy. World J Diabetes. 2012;3(1):7–18. doi: 10.4239/wjd.v3.i1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou X, Wang B, Zhu L, Hao S. A novel improved therapy strategy for diabetic nephropathy: targeting AGEs. Organogenesis. 2012;8(1):18–21. doi: 10.4161/org.19332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palsamy P, Subramanian S. Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2-Keap1 signaling. Biochim Biophys Acta. 2011;1812(7):719–31. doi: 10.1016/j.bbadis.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 42.Wu CC, Sytwu HK, Lin YF. Cytokines in diabetic nephropathy. Adv Clin Chem. 2012;56:55–74. doi: 10.1016/b978-0-12-394317-0.00014-5. [DOI] [PubMed] [Google Scholar]
- 43.Patel S, Santani D. Role of NF-kappa B in the pathogenesis of diabetes and its associated complications. Pharmacol Rep. 2009;61(4):595–603. doi: 10.1016/s1734-1140(09)70111-2. [DOI] [PubMed] [Google Scholar]
- 44.Reeves WB, Andreoli TE. Transforming growth factor beta contributes to progressive diabetic nephropathy. Proc Natl Acad Sci U S A. 2000;97(14):7667–9. doi: 10.1073/pnas.97.14.7667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warren M. Physical activity: exercise prescription for the older adult with type 2 diabetes. Top Geriatr Rehabil. 2010;26(3):221–32. [Google Scholar]
- 46.Wang B, Herman-Edelstein M, Koh P, Burns W, Jandeleit-Dahm K, Watson A. et al. E-cadherin expression is regulated by miR-192/215 by a mechanism that is independent of the profibrotic effects of transforming growth factor-beta. Diabetes. 2010;59(7):1794–802. doi: 10.2337/db09-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsuoka K, Nakao T, Atsumi Y, Takekoshi H. Exercise regimen for patients with diabetic nephropathy. J Diabet Complications. 1991;5(2-3):98–100. doi: 10.1016/0891-6632(91)90032-k. [DOI] [PubMed] [Google Scholar]
- 48.Ward KM, Mahan JD, Sherman WM. Aerobic training and diabetic nephropathy in the obese Zucker rat. Ann Clin Lab Sci. 1994;24(3):266–77. [PubMed] [Google Scholar]
- 49.Chiasera JM, Ward-Cook KM, Mccune SA, Wardlaw GM. Effect of aerobic training on diabetic nephropathy in a rat model of type 2 diabetes mellitus. Ann Clin Lab Sci. 2000;30(4):346–53. [PubMed] [Google Scholar]
- 50.Baggish AL, Hale A, Weiner RB, Lewis GD, Systrom D, Wang F. et al. Dynamic regulation of circulating microRNA during acute exhaustive exercise and sustained aerobic exercise training. J Physiol. 2011;589(Pt 16):3983–94. doi: 10.1113/jphysiol.2011.213363. [DOI] [PMC free article] [PubMed] [Google Scholar]


