Abstract
Due to the vast and inappropriate use of the antibiotics, microorganisms have begun to develop resistance to the commonly used antimicrobial agents. So therefore, development of the new and effective antimicrobial agents seems to be necessary. According to some recent reports, carbon-based nanomaterials such as fullerenes, carbon nanotubes (CNTs) (especially single-walled carbon nanotubes (SWCNTs)) and graphene oxide (GO) nanoparticles show potent antimicrobial properties. In present review, we have briefly summarized the antimicrobial activity of carbon-based nanoparticles together with their mechanism of action. Reviewed literature show that the size of carbon nanoparticles plays an important role in the inactivation of the microorganisms. As major mechanism, direct contact of microorganisms with carbon nanostructures seriously affects their cellular membrane integrity, metabolic processes and morphology. The antimicrobial activity of carbon-based nanostructures may interestingly be investigated in the near future owing to their high surface/volume ratio, large inner volume and other unique chemical and physical properties. In addition, application of functionalized carbon nanomaterials as carriers for the ordinary antibiotics possibly will decrease the associated resistance, enhance their bioavailability and provide their targeted delivery.
Keywords: Carbon nanotubes, Fullerene, Graphene oxide, Antimicrobial activity, Antimicrobial mechanism, Metal–carbon nanocomposites
Introduction
The increasing resistance of the microorganisms towards antibiotics has been led to serious health problems in the recent years. Most infection-causing bacteria are resistant to at least one of the antibiotics that are generally used to eradicate the infection.1 This problem encourages the researchers to study the new agents which can effectively inhibit microbial growth.
Nanomaterials have been considered for use in the optical devices, superconductors, fuel cells, catalysts, biosensors, drug and gene delivery and so on.2-5 Nanomaterials as the novel drug delivery systems have been also applied to improve the physicochemical and therapeutic effectiveness of the drugs.6-8 Likewise, nanotechnology in pharmaceuticals and microbiology showed promising applications to overcome the problem of antibiotic resistance.2,9-11 Over the past few years, various nano-sized antibacterial agents such as metal and metal oxide nanoparticles have been evaluated by researchers. Several types of metal and metal oxide nanoparticles such as silver (Ag), silver oxide (Ag2O), titanium dioxide (TiO2), zinc oxide (ZnO), gold (Au), calcium oxide (CaO), silica (Si), copper oxide (CuO), and magnesium oxide (MgO) have been known to show antimicrobial activity.12-18
It has been known that carbon-based nanoparticles exhibit high antimicrobial activity as well. Early studies indicated that fullerenes, single-walled carbon nanotubes (SWCNTs) and graphene oxide (GO) nanoparticles showed potent microcidal properties. These new allotropic types of carbon have been discovered in the last two decades, and, since then, they have used in many field of science.19-21
It has also been revealed that, the size and surface area of carbon nanomaterials are important parameters affecting their antibacterial activity; that is, increasing the nanoparticles surface area by decreasing their size lead to improving their activity for interaction with bacteria.22,23
Generally, the antimicrobial activity of the nanoparticles depend on their composition, surface modification, intrinsic properties, and the type of microorganism.23,24 It has been proposed that carbon-based nanomaterials cause membrane damage in bacteria due to an oxidative stress.25-29 According to recent studies the physical interaction of carbon-based nanomaterials with bacteria, rather than oxidative stress, is the primary antimicrobial activity of these nanostructures.28,30 In fact, the interactions between bacterial cells and carbon-based nanomaterials play an important role in their antimicrobial mechanism.31 There is some evidence in the literature that the aggregation between bacterial cells and carbon nanomaterials cause direct contact between the cells and carbon nanomaterials which in turn lead to cell death.30-32
It is clear that, prior to biomedical application of the carbon-based nanostructures; some important issues related to their toxicity should be clearly elucidated. In point of fact, they need to be purified and functionalized23,33 and their solubility in physiological media should be improved as well.34,35 In this article the antimicrobial activity of carbon-based nanoparticles and their mechanism of action were briefly reviewed.
Carbon nanotubes
CNTs are nano-sized hollow cylindrical form of carbon which has been synthesized by Lijima in 1991.36 Since then, CNTs have been applied in many fields of science and technology. Kang et al (2007) provided the first document that showed SWCNTs had strong antimicrobial activity on Escherichia coli (E. coli). They demonstrated that SWCNTs could cause severe membrane damage and subsequent cell death.30 In other study (2008) they presented the first evidence that the size of carbon nanotubes was an important factor affecting their antibacterial activity. They prepared SWCNTs and multi-walled carbon nanotubes (MWCNTs) and investigated their antibacterial effect against E. coli. Their results indicated that SWCNTs were much more toxic to bacteria than MWCNTs. The authors also reported that, direct cell contact with CNTs influenced the cellular membrane integrity, metabolism processes and morphology of E. coli. According to the authors, SWCNTs could penetrate into the cell wall better than MWCNTs due to their smaller nanotube diameter. Furthermore, the superior surface area of SWCNTs initiated better interaction with the cell surface.22
Arias and Yang (2009) investigated the antimicrobial activities of SWCNTs and MWCNTs with different surface groups towards rod-shaped or round-shaped gram-negative and gram-positive bacteria. According to their results, SWCNTs with surface groups of -OH and -COOH indicated improved antimicrobial activity to both gram-positive and gram-negative bacteria while MWCNTs with the same surface groups did not exhibit any significant antimicrobial effect. Their results showed that, formation of cell-CNTs aggregates caused to damage the cell wall of bacteria and then release of their DNA content.37
In a study by Yang et al (2010), the effect of SWCNTs length on their antimicrobial activity was investigated. Upon their findings the longer SWCNTs indicated stronger antimicrobial activity due to their improved aggregation with bacterial cells.31
Dong et al (2012) investigated the antibacterial properties of SWCNTs dispersed in different surfactant solutions (sodium holate, sodium dodecyl benzenesulfonate, and sodium dodecyl sulfate) against Salmonella enteric (S. enteric), E. coli, and Enterococcus faecium. According to their results, SWCNTs exhibited antibacterial activity against both S. enterica and E. coli which was improved with the increase of nanotube concentrations. The combination of SWCNTs with surfactant solutions was also found to be low toxic to 1321N1 human astrocytoma cells, so they can be employed in biomedical applications especially for drug-resistant and multidrug-resistant microorganisms.38 Figure 1 shows the schematic mechanism of antimicrobial activity of carbon nanotubes.
Figure 1 .
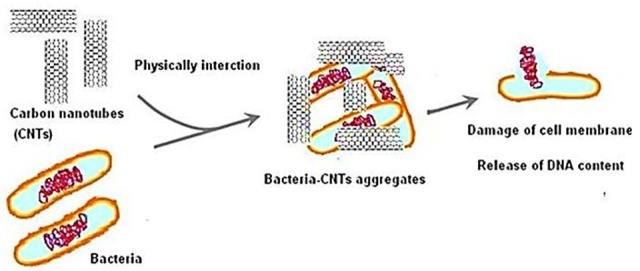
Mechanism of antimicrobial activity of carbon nanotubes.
Fullerenes
Fullerenes are soccer ball-shaped molecules composed of carbon atoms.39 Fullerenes showed antimicrobial activity against various bacteria, such as E. coli, Salmonella and Streptococcus spp.39 The antibacterial effect was probably due to inhibition of energy metabolism after internalization of the nanoparticles into the bacteria.26,33 It has also been suggested that fullerene derivatives can inhibit bacterial growth by impairing the respiratory chain.19,40 In the beginning, a decrease of oxygen uptake (at low fullerene derivative concentration) and then an increase of oxygen uptake (followed by an enhancement of hydrogen peroxide production) are occurred.19,40 Another bactericidal mechanism which has been proposed was the induction of cell membrane disruption.19 As stated by the literature, the hydrophobic surface of the fullerenes can easily interact with membrane lipids and intercalate into them.19,39,40 The discovery of fullerenes ability to interact with biological membranes has encouraged many researchers to evaluate their antimicrobials applications.41
Among three different classes of fullerene compounds (positively charged, neutral, and negatively charged), cationic derivatives showed the maximum antibacterial effect on E. coli and Shewanella oneidensis; while the anionic derivatives were almost ineffective.19,42 This could be owing to the strong interactions of negatively charged bacteria with the cationic fullerenes.42
Deryabin et al (2014) compared the antibacterial activity of two water-soluble derivatives of the fullerene. In their work, protonated amine (AF) and deprotonated carboxylic (CF) groups were added to the fullerene cage via organic linkers. The former positively charged derivative bounded effectively to the E. coli cells; however, the later negatively charged one did not exhibit any significant antibacterial activity. They concluded that the water-soluble cationic fullerene derivative could be used in the preparation of chemical disinfectants.40
Fullerenes can also be applied as photosensitizers in photodynamic therapy (PDT) when their solubility is increased via functionalizing with hydrophilic groups.39 In fact, water soluble fullerenes in the presence of biological reducing agents produce superoxide and this process is relatively more cytotoxic towards microbial cells than mammalian cells.43,44 Tegos et al (2005) tested antibacterial activity of fulleropyrrolidinium salts after photoirradiation and their results showed that more than 99.9% of bacterial and fungal cells were killed.
In the other study, Yu et al (2005) evaluated the antibacterial activity of a sulfobutyl fullerene derivative on environmental bacteria. They found that, the employed derivative was able to inhibit environmental bacteria after photoirradiation.19 Moreover, Mizuno and et al (2011) reported that cationic-substituted fullerene derivative were highly effective in killing a broad spectrum of microbial cells after irradiation with white light. They evaluated a new group of synthetic fullerene derivatives, which possessed either basic or quaternary amino groups, against gram-positive (Staphylococcus aureus (S. aureus)), gram-negative bacteria (E. coli) and fungi (Candida albicans (C. albicans)). They reported that the most important affecting factor was an increased number of quaternary cationic groups that were widely dispersed around the fullerene cage to minimize aggregation. According to their results, S. aureus was found to be most susceptible; E. coli was intermediate, while C. albicans was the most resistant species. The authors suggested that, the quaternized fullerenes could effectively be applied in treatment of superficial infections, e.g. in wounds and burns, where light penetration into tissue is not problematic.45
Graphene oxide (GO)
A monolayer of carbon atoms which are tightly packed into a two-dimensional crystal is normally called Graphene.44 GO nanosheets which could be readily dispersed in water are produced by chemically modification of the graphene with suspended hydroxyl, epoxyl, and carboxyl groups. It is documented that, membrane stress resulted from direct contact with sharp nanosheets is the major antimicrobial mechanism of GO.46
Both graphene and GO were shown inhibitory effect on the growth of E. coli. Akhavan and Ghaderi (2010) tested the antibacterial activity of graphene sheets and verified that direct interaction of the related extremely sharp edges with bacteria caused RNA effluxes through the damaged cell membranes of both gram-negative (E.) and gram-positive (S. aureus) bacteria.47 Gurunathan et al (2012) also studied the antimicrobial activity of GO and reduced graphene oxide nanowalls. Their results proved that the direct contact of bacteria (S. aureus) with the very sharp edge of the applied nanowalls led to the cell membrane damage. According to the authors, the antibacterial effect of the reduced graphene nanowires was comparable with SWCNTs.34 The similar antibacterial activity against E. coli were reported by Hu et al (2010) for GO and reduced graphene oxide nanosheets.44
In another work, Azimi et al (2014) testified the antimicrobial effect of two functionalized GO nanostructures (grapheme oxide-chlorophyllin and graphene oxide-chlorophyllin-Zn) against E. coli. The authors proposed that the functionalized GO led to cell membrane damage of E. coli. Furthermore, their results signified that the surface chemistry and metal toxicity played a major role in antibacterial activity of graphene oxide-chlorophyllin-Zn. The physical interaction of GO with the cell membrane, hydrogen bonding of colorless tetrapyrroles with a specific outer cellular component and generation of intercellular hydroxyl radicals through aqueous leaching of Zn2+ were suggested as the possible antibacterial mechanisms.46
Carbon nanocomposites composed of carbon nanostructures and metal nanoparticles (e.g. CNT-Ag and GO-Ag nanocomposites) have recently been revealed the appropriate antibacterial activity against both gram-negative and positive bacteria. However, the antibacterial activity of CNT-Ag was superior to GO-Ag nanocomposites which could be due to good dispersion of the Ag nanoparticles into the CNT.48
Ag-carbon nanocomplexes also showed efficient inhibitory activity against some important pathogens such as Burkholderia cepacia, methicillin-resistant S. aureus, multidrug-resistant Acinetobacter baumannii and Klebsiella pneumoniae. These nanostructures could inhibit the growth of bio-defense bacteria such as Yersinia pestis as well.49
Different types of carbon-based nanoparticles used as antimicrobial agent; their mechanisms of action as well as the associated characteristics have been summarized in Table 1.
Table 1. Types of carbon-based nanoparticles as antimicrobial agent, their mechanisms of action and characteristics .
| Type of nanoparticles | Proposed mechanism of antimicrobial action | Main characteristics as antimicrobial agent | The main factors that influence antimicrobial activity | References |
| Fullerene | Inhibit bacterial growth by impairing the respiratory chain; inhibition of energy metabolism. | Stability; Photodynamic therapy activity; high ability to functionalization; high surface/volume ratio; large inner volume | Particle size; type of functional group; surface charge. | 19,21,39-41,44 |
| SWTNs | Physical interaction with cell membrane; formation of cell-CNTs aggregates; induction the cell membrane disruption. | Stability; high ability to functionalization; high surface/volume ratio; large inner volume | Particle size; particle length; type of functional group; type of buffer; concentration; surface charge. | 27,30,31,38 |
| GO | Physical interaction with cell membrane; formation of cell-GO aggregates; induction the cell membrane disruption. | Stability; high ability to functionalization, high surface/volume ratio; sharp edges of nanowalles. | Particle size; type of functional group. | 25,34,35,44,46 |
Conclusion
A number of studies have reported effective antimicrobial activity of the carbon nanostructures. The size of these nanoparticles plays an important role in the inactivation of microorganisms. Among carbon nanostructures, fullerenes, SWCNTs and GO nanoparticles and their derivatives were found to be more efficient as antibacterial agents. The probable mechanisms of their antibacterial activity were proposed as follow: inhibition of bacterial growth by impairing the respiratory chain; inhibition of energy metabolism; physical interaction with cell membrane; formation of cell-CNTs/ cell-GO aggregates; induction the cell membrane disruption. In order to biological and medicinal applications, carbon nanostructures should be purified and functionalized. Their solubility should also be enhanced in physiological media. Finally, application of carbon nanocomposites composed of carbon nanostructures and metal nanoparticles could be considered as a hopeful approach for disinfection purposes. However, further studies are necessary to understand the exact mechanisms of the antimicrobial activity of carbon nanostructures.
Ethical Issues
Not applicable.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Allahverdiyev AM, Abamor ES, Bagirova M, Rafailovich M. Antimicrobial effects of TiO(2) and Ag(2)O nanoparticles against drug-resistant bacteria and leishmania parasites. Future Microbiol . 2011;6(8):933–40. doi: 10.2217/fmb.11.78. [DOI] [PubMed] [Google Scholar]
- 2.Adibkia K, Omidi Y, Siahi MR, Javadzadeh AR, Barzegar-Jalali M, Barar J. et al. Inhibition of endotoxin-induced uveitis by methylprednisolone acetate nanosuspension in rabbits. J Ocul Pharmacol Ther . 2007;23(5):421–32. doi: 10.1089/jop.2007.0039. [DOI] [PubMed] [Google Scholar]
- 3.Tiwari PM, Vig K, Dennis VA, Singh SR. Functionalized gold nanoparticles and their biomedical applications. Nanomater . 2011;1(1):31–63. doi: 10.3390/nano1010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zinjarde SS. Bio-inspired nanomaterials and their applications as antimicrobial agents. Chronic Young Sci . 2012;3(1):74–81. [Google Scholar]
- 5.Bahrami K, Nazari P, Nabavi M, Golkar M, Almasirad A, Shahverdi AR. Hydroxyl capped silver-gold alloy nanoparticles: characterization and their combination effect with different antibiotics against Staphylococcus aureus. Nanomed J . 2014;1(3):155–61. [Google Scholar]
- 6.Ravishankar Rai V, Jamuna Bai A. Nanoparticles and their potential application as antimicrobials. A Méndez-Vilas A, editor. Mysore: Formatex; 2011.
- 7.Marambio-Jones C, Hoek EM. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J Nanopart Res . 2010;12(5):1531–51. [Google Scholar]
- 8.Adibkia K, Barzegar-Jalali M, Nokhodchi A, Siahi Shadbad M, Omidi Y, Javadzadeh Y. et al. A review on the methods of preparation of pharmaceutical nanoparticles. Pharm Sci . 2010;15(4):303–14. [Google Scholar]
- 9.Adibkia K, Javadzadeh Y, Dastmalchi S, Mohammadi G, Niri FK, Alaei-Beirami M. Naproxen-eudragit RS100 nanoparticles: preparation and physicochemical characterization. Colloids Surf B Biointerfaces . 2011;83(1):155–9. doi: 10.1016/j.colsurfb.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Mohammadi G, Nokhodchi A, Barzegar-Jalali M, Lotfipour F, Adibkia K, Ehyaei N. et al. Physicochemical and anti-bacterial performance characterization of clarithromycin nanoparticles as colloidal drug delivery system. Colloids Surf B Biointerfaces . 2011;88(1):39–44. doi: 10.1016/j.colsurfb.2011.05.050. [DOI] [PubMed] [Google Scholar]
- 11.Kannan RR, Jerley AJA, Ranjani M, Prakash VSG. Antimicrobial silver nanoparticle induces organ deformities in the developing Zebrafish (Danio rerio) embryos. J Biomed Sci Engine. 2011;4:248–54. [Google Scholar]
- 12.Azam A, Ahmed AS, Oves M, Khan MS, Habib SS, Memic A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study. Int J Nanomedicine . 2012;7:6003–9. doi: 10.2147/IJN.S35347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Besinis A, De Peralta T, Handy RD. The antibacterial effects of silver, titanium dioxide and silica dioxide nanoparticles compared to the dental disinfectant chlorhexidine on Streptococcus mutans using a suite of bioassays. Nanotoxicology . 2014;8(1):1–16. doi: 10.3109/17435390.2012.742935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emami-Karvani Z, Chehrazi P. Antibacterial activity of ZnO nanoparticle on grampositive and gram-negative bacteria. Afr J Microbiol Res . 2011;5(12):1368–73. [Google Scholar]
- 15.Usman MS, El Zowalaty ME, Shameli K, Zainuddin N, Salama M, Ibrahim NA. Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int J Nanomedicine . 2013;8:4467–79. doi: 10.2147/IJN.S50837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Q, Xue Y, Sun J. Kupfer cell-mediated hepatic injury induced by silica nanoparticles in vitro and in vivo. Int J Nanomedicine . 2013;8:1129–40. doi: 10.2147/IJN.S42242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl Environ Microb . 2007;73(6):1712–20. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zarei M, Jamnejad A, Khajehali E. Antibacterial Effect of Silver Nanoparticles Against Four Foodborne Pathogens. Jundishapur J Microb . 2014;7(1):E8720. doi: 10.5812/jjm.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cataldo F, Da Ros T. Medicinal chemistry and pharmacological potential of fullerenes and carbon nanotubes. Trieste: Springer; 2008. [Google Scholar]
- 20.Wang JT, Chen C, Wang E, Kawazoe Y. A new carbon allotrope with six-fold helical chains in all-sp2 bonding networks. Sci Rep . 2014;4:4339. doi: 10.1038/srep04339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sokolov VI, Stankevich IV. The fullerenes-new allotropic forms of carbon: molecular and electronic structure, and chemical properties. Russ Chem Rev . 1993;62(5):419. [Google Scholar]
- 22.Kang S, Herzberg M, Rodrigues DF, Elimelech M. Antibacterial effects of carbon nanotubes: size does matter! . Langmuir. 2008;24(13):6409–13. doi: 10.1021/la800951v. [DOI] [PubMed] [Google Scholar]
- 23.Buzea C, Pacheco Ii, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases . 2007;2(4):MR17–71. doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- 24.Hajipour MJ, Fromm KM, Ashkarran AA, Jimenez De Aberasturi D, Rojo T. et al. Antibacterial properties of nanoparticles. Trends Biotechnol . 2012;30(10):499–511. doi: 10.1016/j.tibtech.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Gurunathan S, Han JW, Dayem AA, Eppakayala V, Kim JH. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int J Nanomedicine . 2012;7:5901–14. doi: 10.2147/IJN.S37397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shvedova AA, Pietroiusti A, Fadeel B, Kagan VE. Mechanisms of carbon nanotube-induced toxicity: focus on oxidative stress. Toxicol Appl Pharmacol . 2012;261(2):121–33. doi: 10.1016/j.taap.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vecitis CD, Zodrow KR, Kang S, Elimelech M. Electronic-structure-dependent bacterial cytotoxicity of single-walled carbon nanotubes. ACS nano . 2010;4(9):5471–9. doi: 10.1021/nn101558x. [DOI] [PubMed] [Google Scholar]
- 28.Manke A, Wang L, Rojanasakul Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed Res Int . 2013;2013:942916. doi: 10.1155/2013/942916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pacurar M, Qian Y, Fu W, Schwegler-Berry D, Ding M, Castranova V, Guo NL. Cell permeability, migration, and reactive oxygen species induced by multiwalled carbon nanotubes in humanmicrovascular endothelial cells. J Toxicol Environ Health A . 2012;75(3):129–47. doi: 10.1080/15287394.2012.625549. [DOI] [PubMed] [Google Scholar]
- 30.Kang S, Pinault M, Pfefferle LD, Elimelech M. Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir . 2007;23(17):8670–3. doi: 10.1021/la701067r. [DOI] [PubMed] [Google Scholar]
- 31.Yang C, Mamouni J, Tang Y, Yang L. Antimicrobial activity of single-walled carbon nanotubes: length effect. Langmuir . 2010;26(20):16013–9. doi: 10.1021/la103110g. [DOI] [PubMed] [Google Scholar]
- 32.Murray AR, Kisin ER, Tkach AV, Yanamala N, Mercer R, Young SH. et al. Factoring-in agglomeration of carbon nanotubes and nanofibers for better prediction of their toxicity versus asbestos. Part Fibre Toxicol . 2012;9:10. doi: 10.1186/1743-8977-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellucci S. Nanoparticles and Nanodevices in Biological Applications. Germany: Springer-Verlag, Berlin-Heidelberg; 2009. [Google Scholar]
- 34.Dinadayalane TC, Leszczynska D, Leszczynski J. Towards Efficient Designing of Safe Nanomaterials. Leszczynski J, Puzyn T, Kroto H, editors. Cambridge, UK: Royal society of chemistry; 2012.
- 35.Yang K, Wan J, Zhang S, Zhang Y, Lee ST, Liu Z. In vivo pharmacokinetics, long-term biodistribution, and toxicology of PEGylated graphene in mice. ACS nano . 2011;5(1):516–22. doi: 10.1021/nn1024303. [DOI] [PubMed] [Google Scholar]
- 36.Han J. Carbon Nanotubes: Science and Application. Meyyappan M, editor. Boca Raton: CRC Press LLC; 2005.
- 37.Arias LR, Yang L. Inactivation of bacterial pathogens by carbon nanotubes in suspensions. Langmuir . 2009;25(5):3003–12. doi: 10.1021/la802769m. [DOI] [PubMed] [Google Scholar]
- 38.Dong L, Henderson A, Field C. Antimicrobial activity of single-walled carbon nanotubes suspended in different surfactants. J Nanotechnol . 2012;2012:1–7. [Google Scholar]
- 39.Tegos GP, Demidova TN, Arcila-Lopez D, Lee H, Wharton T, Gali H. et al. Cationic fullerenes are effective and selective antimicrobial photosensitizers. Chem Biol . 2005;12(10):1127–35. doi: 10.1016/j.chembiol.2005.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deryabin DG, Davydova OK, Yankina ZZ, Vasilchenko AS, Miroshnikov SA, Kornev AB. et al. The Activity of [60] Fullerene Derivatives Bearing Amine and Carboxylic Solubilizing Groups against Escherichia coli: A Comparative Study. J Nanomater . 2014;2014:1–9. [Google Scholar]
- 41.Yang X, Ebrahimi A, Li J, Cui Q. Fullerene-biomolecule conjugates and their biomedicinal applications. Int J Nanomedicine . 2014;9:77–92. doi: 10.2147/IJN.S52829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakamura S, Mashino T. Biological activities of water-soluble fullerene derivatives. J Phys Conf Ser . 2009;159:012003. [Google Scholar]
- 43.Sharma SK, Chiang LY, Hamblin MR. Photodynamic therapy with fullerenes in vivo: reality or a dream? Nanomedicine (Lond) 2011;6(10):1813–25. doi: 10.2217/nnm.11.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu Z, Dai T, Huang L, Kurup DB, Tegos GP, Jahnke A. et al. Photodynamic therapy with a cationic functionalized fullerene rescues mice from fatal wound infections. Nanomedicine (Lond) . 2010;5(10):1525–33. doi: 10.2217/nnm.10.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mizuno K, Zhiyentayev T, Huang L, Khalil S, Nasim F, Tegos GP. et al. Antimicrobial Photodynamic Therapy with Functionalized Fullerenes: Quantitative Structure-activity Relationships. J Nanomed Nanotechnol . 2011;2(2):1–9. doi: 10.4172/2157-7439.1000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Azimi S, Behin J, Abiri R, Rajabi L, Derakhshan AA, Karimnezhad H. Synthesis, Characterization and Antibacterial Activity of Chlorophyllin Functionalized Graphene Oxide Nanostructures. Sci Adv Mater . 2014;6(4):771–81. [Google Scholar]
- 47.Akhavan O, Ghaderi E. Toxicity of Graphene and Graphene Oxide Nanowalls Against Bacteria. Acs nano . 2010;4:5731. doi: 10.1021/nn101390x. [DOI] [PubMed] [Google Scholar]
- 48.Yun H, Kim JD, Choi HC, Lee CW. Antibacterial Activity of CNT-Ag and GO-Ag Nanocomposites Against Gram-negative and Gram-positive Bacteria. Bull Korean Chem Soc . 2013;34(11):3261. [Google Scholar]
- 49.Leid J, Ditto A, Knapp A, Shah P, Wright B, Blust R. et al. In vitro antimicrobial studies of silver carbine complexes: activity of free and nanoparticle carbene formulations against clinical isolates of pathogenic bacteria. J Antimicrob Chemother . 2012;67:138–48. doi: 10.1093/jac/dkr408. [DOI] [PMC free article] [PubMed] [Google Scholar]


