Abstract
Purpose: Breast cancer is the second leading cancer type among people of advanced countries. Various methods have been used for cancer treatment such as chemotherapy and radiotherapy. In the present study we have designed and synthesized a new group of drug delivery systems (DDS) containing a new class of Cell Penetrating Peptides (CPPs) named Peptide Amphiphiles (PAs).
Methods: Two PAs and anionic peptides were synthesized using solid phase peptide synthesis (SPPS), namely [KW]4, [KW]5, E4 and E8. Then nano-peptides were synthesized by non-covalent binding between PAs and poly anions as [KW]4-E4, [KW]4-E8, [KW]5-E4 and [KW]5-E8.
Results: Flow cytometry studies showed that increased chain length of PAs with a higher ratio between hydrophobicity and net charge results in increased intracellular uptake by MCF7 cells after 2h incubation. Moreover, nano-peptides showed greater intracellular uptake compared to PAs. Anti-proliferative assay revealed that by increasing chain length of PAs, the toxicity effect on MCF7 cells is reduced, however nano-peptides did not show significant toxicity on MCF7 cells even at high concentration levels.
Conclusion: These data suggest that due to the lack of toxicity effect at high concentration levels and also high cellular uptake, nano-peptides are more suitable carrier compared to PAs for drug delivery.
Keywords: Cell Penetrating Peptides, Amphiphilic Peptides, Solid Phase Peptide Synthesis, Fluorescein Isothiocyanate, Drug Delivery System, Cellular Uptake
Introduction
Cancer is among the most prevalent causes of death in the world. We often neglect that cancer is not a single disease,1,2 but a set of diseases.3 There are several methods for cancer treatment, one of these methods is chemotherapy.4,5 In cancer chemotherapy the cell is affected by cytotoxic materials more in the process of division than in rest attitude.5-7 Chemotherapy can cause many different side effects. Some are more likely to occur than others, a major one of which is resistance to a specific dose of anticancer drugs.8-11 In order to improve the intracellular delivery of cargo molecules in the cellular membrane such as drugs and biologically important molecules, novel drug delivery systems (DDS) are used.12,13 One of these systems is peptide-mediated drug delivery which has been extensively applied to a wide range of cargo molecules.14 Cell-Penetrating Peptides (CPPs) provides a promising solution to the problems commonly related to the drug delivery of conventional cancer chemotherapeutics as well as oligonucleotide based treatments. CPPs are short peptides which have the ability to move through the cellular plasma membrane; they can move on their own or together with cargoes. Normally CPPs have a length of 5–30 amino acids; they generally have basic amino acid side chains; and they are often amphipathic.15,16 The reason which makes CPPs a promising class of drug delivery vehicles is the ability to carry cargoes over the cellular plasma membrane. This method has some certain advantages including the relatively easy preparation, and also that a single peptide sequence can be exploited for a range of different cargoes without chemical modification. Moreover the peptides are potentially able to protect the cargo from exposure to serum proteins and therefore they can extend the length of blood circulation in vivo. The ability to carry cargoes into the cell makes CPP-based delivery a promising method for delivery of cancer drugs.15,17 Among various types of CPPs, Peptide Amphiphies (PAs) appear to be efficient means for drug transporting.12,18 PAs generally consist of hydrophobic and charged segments.12,19 It was found out that PAs are promising vehicles for drug and gene delivery because of their biocompatibility and bioactivity.20-22 Using linear CPPs which are positively charged as drug carriers for biologically active cargos has been mentioned already.23-26 It was reported that Polyarginines, TAT (trans-acting activator of transcription), and Penetratin improve the cellular uptake of various drugs.27,28 Therefore, the determining factor for the efficiency and application of the delivery system is the proper combination of amino acids in the structure of peptide.22 We presented a new range of amphiphilic peptides containing lysine (K) and tryptophan (W) such as [KW]4, (Lys-Trp- Lys- Trp- Lys- Trp- Lys- Trp) and [KW]5, (Lys- Trp- Lys- Trp- Lys- Trp- Lys- Trp- Lys- Trp); these peptides were suitable carriers for different applications. Addition of negatively charged poly glutamates (E4, E8 ) (Glu- Glu- Glu- Glu), (Glu- Glu- Glu- Glu- Glu- Glu- Glu- Glu)to the positively charged [KW]4 or [KW]5 led to the formation of nanostructures through intermolecular interactions.
Materials and Methods
Solid-phase peptide synthesis
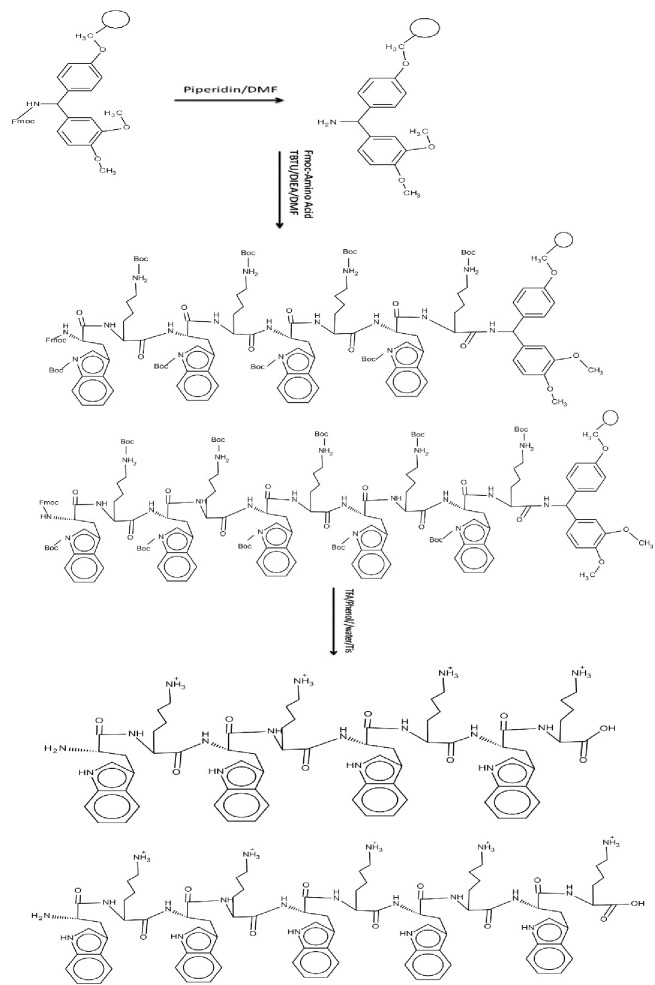
Classical glass reaction vessels made by glassblowers or purchased from manufacturers are suitable for performing Solid-phase peptide synthesis (SPPS). CPPs were synthesized by solid-phase synthesis applying N-(9-fluorenyl) methoxycarbonyl (Fmoc)-based chemistry and employing Fmoc-L-amino acid building blocks. N, N, N′, N′-Tetramethyl-O-(benzotriazol-1-yl)uronium tetrafluoroborate (TBTU) and N,N-diisopropylethylamine (DIPEA) in N,N-dimethylformamide (DMF) were employed as coupling and activating reagents, respectively. At each step Fmoc deprotection was performed using piperidine in DMF (20%). To wash the resin at each step N, N-dimethylformamide (DMF) and Dichloromethane (DCM) were used. Trifluoroacetic acid (TFA), Phenol and triisopropylsilane (TIS) were used for cleavaging of peptides from resin. All amino acids and Rink amidresine were purchased from Aapptec, USA. TBTU and fluorescein isothiocyanate (FITC), DIEA, DCM, and DMF were purchased from Sigma-Aldrich Chemical Co. USA. KCN, Phenol, Ninhydrin, and Piridin were purchased from Merck, Germany. The chemical structures of Solid-Phase synthesis of [KW]4 and [KW]5 are shown in Figure 1.
Figure 1 .
Chemical structures of Solid-Phase synthesis of [KW]4 and [KW]5
Linear peptide synthesis
The linear peptide was assembled on Fmoc-Rink amide resin by solid-phase peptide synthesis strategy applying Fmoc-protected amino acids [FMOC-Trp(Boc)-OH and Fmoc-Lys(Boc)-OH] on a classical glass reaction vessels at room temperature.8 FMOC-Rink amide resin (0.049 mmol, 0.3 mmol/g ) was swelled in DMF (4ml) for about 1h under dry nitrogen. The excess solvent was filtered off . The steps of swelling and filteration were repeated for two more times and then coupling reactions were performed. After removing the Fmoc group at the N-terminal of resin linear peptide sequence was assembled on the resin using piperidine in DMF (20% v/v, 2 mL, 30 min). Fmoc-Lys (Boc)-OH (0.148 mmol) was coupled to the N-terminal of Rink amide resin in the presence of TBTU (0.148 mmol) and DIPEA (0.148 mmol) in DMF (2 mL) by mixing for 1.5 h. Then the coupling was completed (which was confirmed by Kaiser test), the reaction solution was filtered off, and then the resin was collected by filtration and washed with DMF (3×2 mL) and DCM (3×2 mL, followed by N-terminal Fmoc-deprotection using piperidine in DMF (20% v/v, 2 mL, 30min). The resin was washed with DMF (3×2 mL) and DCM (3×2 mL). The subsequent amino acids, Fmoc-Lys(Boc -OH (0.148 mmol) and Fmoc-Trp (Boc)-OH (0.148 mmol), were coupled for four times alternatively, and for five times respectively, in a similar manner. To support the linear peptide on SPPS, Fmoc-deprotection at the N-terminal was carried out in the presence of piperidine in DMF (20% v/v, 10 mL, 2 × 30min). The resin was washed with DMF and DCM, respectively (each 3 × 2 mL). After that the resins were dried in vacuum for 24 h.29 Fresh cleavage cocktail composed of TFA/phenol/ /water/Tis (88:5:5:2 v/v/v/v, 10 mL), was added to the resins.30 The mixture was shaken at room temperature for 2 h. Through filtration the resin was collected and then washed with another 2 mL of cleavage cocktail. Using dry nitrogen, combined filtrates were evaporated and reduced into a minimum volume. The crude peptide was precipitated by adding cold diethyl ether (Et2O).29 Therefore the attained crude mixture was centrifuged, and the ether was removed by decantation.31 Finally the product was lyophilized.29
Synthesis of Peptide Nano-particles
In this study, for synthesis of peptide nano-particles, synthesized peptide sequences of [KW]4 and [KW]5 were conjugated to poly glutamate [E]4 and [E]8 with self assembly manner, through which a new structure with new and different characteristics and size was created. For preparing the nano-particle, after synthesis and cleavage processes, the PAs [KW]4 and [KW]5 were dissolved in DMSO with 1:1, 1:5, and 1:10 proportions and added to the polyglutamate dissolved in DMSO. It was then sonicated so that the sequences congucated as self assembly and the nano-particles was synthesized.
Fluorescently-Labeled Conjugate of peptides
The linear peptides, (KWKWKWKW) and (KWKWKWKWKW), were assembled on SPPS using Fmoc/tBu SPPS methodology. Appropriately protected amino acids were assembled on Fmoc-Rink amide resin (0.124 g, 0.049 mmol, and 0.3 mmol/g) according to the solid-phase synthesis strategy described above. In order to label the peptide, the Fmoc protecting group was removed from end of the peptide using piperidine in DMF (20%, 2 mL, 30min), then FITC was attached to peptide.14 This was achieved by adding resin-bound linear peptide (0.049 mmol) into a solution of 1:1 equivalent of FITC that was provided in pyridine/DMF/DCM (12:7:5). The solution was allowed to be blended overnight. The reaction completion was investigated using ninhydrin test. If the coupling of FITC to the amino group is not complete, ninhydrin test will give a blue color. The coupling with FITC would have been repeated if needed. The resin was washed with DMF (2x), isopropanol (2x), and DCM (2x).32 After that, as stated above, the prepared Fluorescently-Labeled conjugate of peptides was cleavaged from resin by shaking the resins with a mixture of TFA/Phenol/ /water/Tis (88:5:5:2 v/v/v/v, 10 mL). Then different concentrations of peptides (10, 25 and 50μm) were prepared. The chemical structures of preparing Fluorescently-labeled conjugates of [KW]4 and [KW]5 are shown in Figure 2.
Figure 2 .
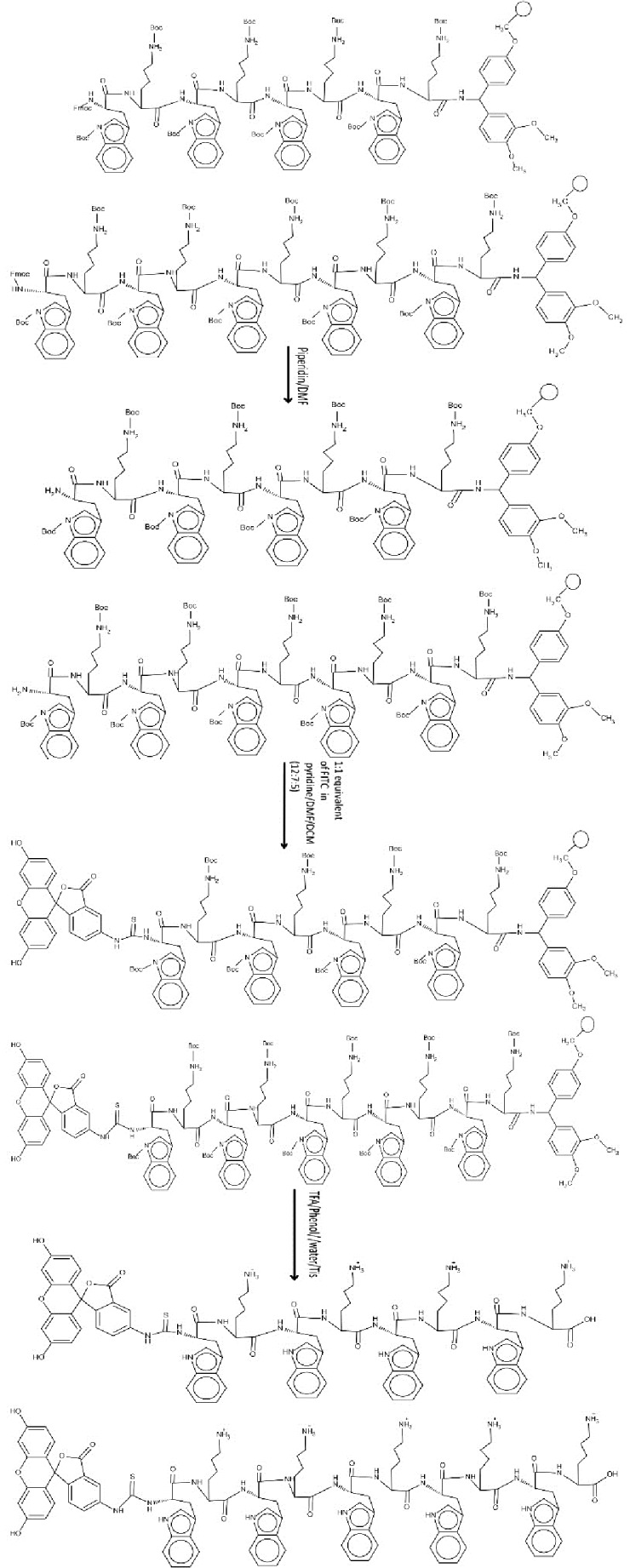
Chemical structures of preparing Fluorescently-labeled conjugates of [KW]4 and [KW]5.
Cell Culture
MCF-7 (human breast adenocarcinoma) cells were obtained from the Institute of Hematology and Blood Diseases Hospital (Tehran, Iran). The cells were grown on 25 or 75 cm2 cell culture flasks with RPMI-1640 medium supplemented with 10% FBS, 100 units/ml penicillin, and 100 µg/mL streptomycin. Cells were cultured in an incubator maintained at 37°C with 5% CO2 under fully humidified conditions. All experiments were performed on cells in the exponential growth phase.33
Antiproliferative Assay
MCF-7 cells (2 × 10 4) were seeded in 96-well plates 24 h prior to the experiment. The old medium (RPMI containing 10% FBS was replaced with medium containing different oncentrations of [KW]4, [KW]5, [KW]4-E8, [KW]4-E4, [KW]5 -E8, and [KW]5 -E4 (10, 25, and 50 μM) and incubated for 24 or 48 h at 37 ° C in a humidified atmosphere of 5% CO2.29 Then 20 µL of MTT solution (2 mg/mL) was added to each well, and the plates were incubated for another 4 hours. After that the MTT medium was replaced by mixture of 200 µL of dimethyl sulfoxide (DMSO) and 25 µl Sorensen’s phosphate buffer in each well, and the mixture was shaken at room temperature to dissolve the reacting dye. Employing a microplate reader (Bio-Tek) optical density was measured at 570 nm.31 The cell survival rate was measured using the following equation.29
Fluorescence Microscopy
The cellular uptake studies of FITC-[KW]4 and FITC-[KW]5 were imaged using an Olympus florescence microscopy. MCF-7 cells were grown on a coverslip in a 6-well plate (2 × 105 cells per well) for 24 hours.29 Phosphate-buffered saline (PBS; 0.1 M, pH 7.4) were used to wash the cells. Cells were preincubated with different concentrations of FITC-conjugated peptide (10, 25 and 50 μm) in 3 mL of blank culture medium for 2h at 37°C.33 Subsequently the cells were washed with PBS three times, and then were fixed with 2% formaldehyde followed by washing with PBS three times. Finally the cells were observed under a fluorescence microscopy.29
Cell Uptake Quantification by Flow Cytometry
MCF-7 cells were seeded in 6-well plates (2 × 105 cells per well) and were allowed to grow for 24 hours. Cells were washed with phosphate-buffered saline (PBS; 0.1 M, pH 7.4) and preincubated with different concentrations of FITC-conjugated [KW]4 and [KW]5 (10, 25, and 50 μm) in 3ml of blank culture medium for 2h at 37 °C. The cells were washed three times with PBS after the incubation. A total volume of 1.5 mL of 0.25% trypsin solution was added to each six-well plate, and then by incubation for 3 minutes at 37°C, the cells were detached from cell culture plate. The cell suspensions were centrifuged for 5 minutes and resuspended in 0.5 mL PBS. Finally, the cells were resuspended in PBS and subjected to flow cytometry analysis using a FACS caliber.33
Results and Discussion
Fmoc/tBu solid-phase peptide synthesis on the Rink amide resin was used for synthesizing linear peptide [KW]4, [KW]5 with hydrophobic (W) and charged (K) residues. After synthesizing the peptide sequences, the last Fmoc group on the N-terminal of tryptophan was deprotected by piperidine (20% v/v, DMF). To produce the linear peptides [KWKWKWKW], [KWKWKWKWKW] with the average size of 43-100 nm which were used for the next steps of experiment, the resin-peptide was dried, and the cleavage of the peptide from the resin was carried out using the cleavage cocktail containing TFA/Phenol/ /water/Tis (88:5:5:2 v/v/v/v, 10 mL). After synthesis and cleavage processes peptide, nano-particles were synthesized by dissolving the CPPs [KW]4, [KW]5 in DMSO, and then were added to the polyglutamate dissolved in DMSO. The average size of nanopeptides was 47-54nm.
Cytotoxicity
Evaluation of cytotoxic effects of different concentrations (5, 10, and 25 µM) of peptides and nanoparticles were performed after 24 and 48 h exposure. As shown in Figure 3 and Figure 4, the results indicated that both of PAs didn’t show any toxicity after 24 h, but [KW]4 had toxicity effect after 48 h at concentration of 25µM, while [KW]5 didn’t show any toxicity effect. The interesting result was obtained that after connecting E8 and E4 to establish [KW]4 and [KW]5, and synthesize nano-particles, toxicity markedly decrease. PAs –E8 showed less toxicity compared to PAs –E4. In total increased chain length of PAs and nanoparticles results in decreased toxicity.
Figure 3 .
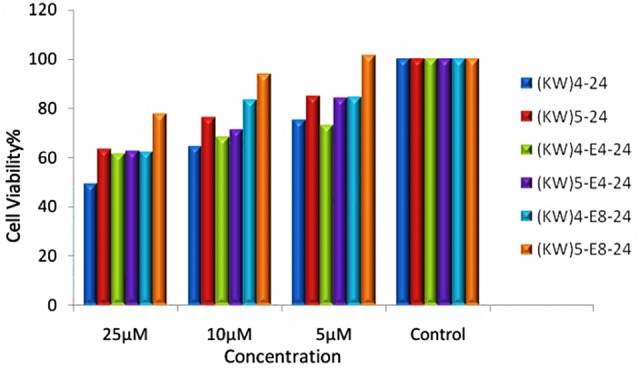
Effect of synthesized peptides on MCF-7 cells after 24h exposure (Data are expressed as the mean of 3 measurements)
Figure 4 .

Effect of synthesized peptides on MCF-7 cells after 48h exposure (Data are expressed as the mean of 3 measurements)
Flow Cytometry
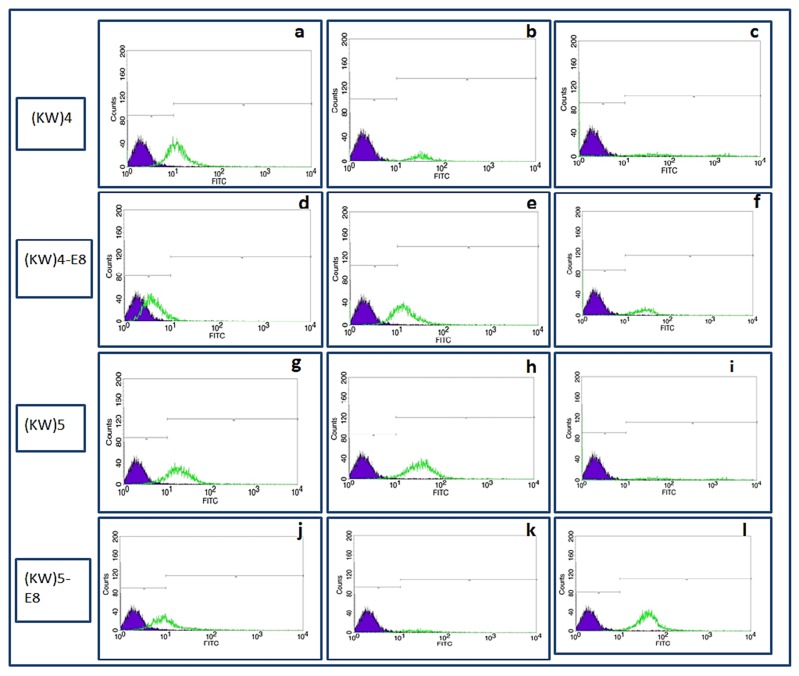
The fluorescent-labeled compounds were synthesized according to the procedure described above. Cultured MCF-7 cells were treated by FITC-[KW]4, FITC-[KW]5, FITC-[KW]4-E8, and FITC-[KW]5-E8 at concentrations of 10 µM, 25 µM and 50 µM for 2h at 37° in a humidified atmosphere of 5% CO2. Then the cells were detached using trypsin and intracellular uptake was measured by FACS device. Results indicated that cellular uptake for both CPPs, [KW]4 and [KW]5 ,is higher at concentration of 25 µM than other concentrations. For nanoparticles, at concentration of 50 µM, cellular uptake was significantly increased. Whereas at concentrations of 10 µM and 25µM fluorescent signal was declined (Figure 5 and Figure 6). Comparing the two CCPs, [KW]5 showed higher cellular uptake at all concentrations' levels.
Figure 5 .
Intracellular uptake of [KW]4 (a: 10µM, b: 25µM, c: 50µM ), [KW]4-E8 (d: 10µM, e: 25µM, f: 50µM ), [KW]5 (g: 10µM, h: 25µM, i: 50µM) [KW]5-E8 (j: 10µM, k: 25 µM, l: 50µM) in MCF-7 cells
Figure 6 .
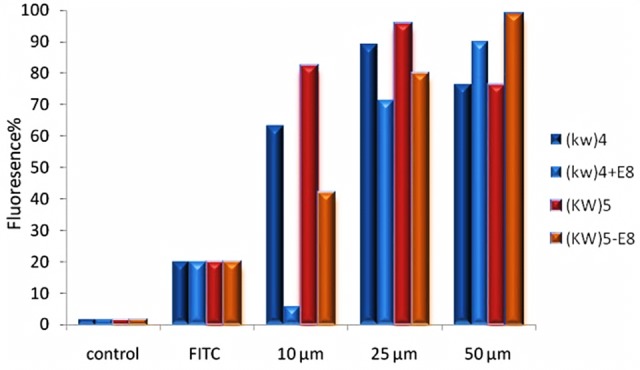
The results of flow cytometry studies in MCF-7 cells for [KW]5, [KW]4 (10, 25and 50 μM) and [KW]5-E8, [KW]4–E8 (10,25 and 50 μM) for 2h (Data are expressed as the mean of 3 measurements)
Fluorescent microscopy
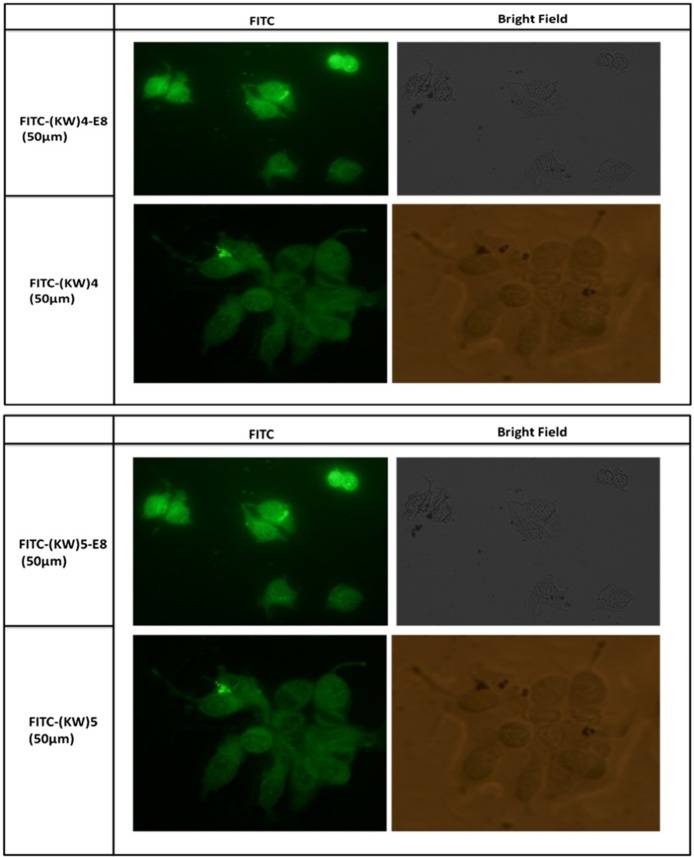
Cellular uptake is detectable using fluorescent microscopy. Just like Flow cytometrics, MCF-7 cells were preincubated with [KW]4, [KW]5, [KW]4-E8, and [KW]5 -E8 at concentrations of 10, 25, and 50 µM for 2 hours at 37°. Fluorescent imaging data illustrated that compared to lower concentrations, high level concentrations (25 and 50 µM) treatment by [KW]4,[KW]4-E8, [KW]5 and [KW]5-E8 increased cell permeability. As shown in Figure 7, [KW]4, [KW]4-E8, [KW]5 and [KW]5-E8 were penetrated into the cytoplasm and nuclear. These data showed that treatment by [KW]4 and [KW]4-E8, [KW]5 and [KW]5 -E8 increased cellular permeability and thereby [KW]4 and [KW]4-E8, [KW]5, [KW]5 -E8 can be suitable carriers for insertion of macromolecules into cytoplasm and nuclear.
Figure 7 .
Fluorescent microscopy images of [KW]4, [KW]5, [KW]4-E8, and [KW]5-E8 uptake by MCF-7 cells after 2h incubation.
Conclusion
In summary, linear amphiphilic peptides were synthesized both separately and conjugated to polyglutamate as DDS. The effect of different peptides and nano-peptides types against breast cancer cell lines including [KW]4, [KW]5, [KW]4-E4, [KW]4-E8, [KW]5-E4 and [KW]5-E8 were evaluated. Nano-peptides and amphiphilic peptides offered several advantages including low cytotoxicity, capability to conjugate to the macromolecules and improving cellular uptake. Nano-peptides showed greater intracellular uptake and less toxicity in higher concentration levels compared to PAs. These results suggest that nano-peptides are more suitable carriers for cellular delivery in high concentration levels compared to PAs.
Ethical Issues
Not applicable.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer . 2004;4(11):839–49. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 2.Mae M, Andaloussi SE, Lehto T, Langel U. Chemically modified cell-penetrating peptides for the delivery of nucleic acids. Expert Opin Drug Deliv . 2009;6(11):1195–205. doi: 10.1517/17425240903213688. [DOI] [PubMed] [Google Scholar]
- 3.Bidwell GL, 3rd, Raucher D. Cell penetrating elastin-like polypeptides for therapeutic peptide delivery. Adv Drug Deliv Rev . 2010;62(15):1486–96. doi: 10.1016/j.addr.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang W, Zalcberg JR, Cosolo W. Interaction of epirubicin with other cytotoxics and anti-emetic drugs. Anticancer Drugs . 1992;3(6):593–7. doi: 10.1097/00001813-199212000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Arican GÖ, Soy NN. Effects of epirubicin and daunorubicin on cell proliferation and cell death in hela cells. J Cell Mol Biol . 2005;4(1):47–52. [Google Scholar]
- 6.Topçul M, Arican GÖ, Erensoy N, Özalpan A. Effect of epirubicin and tamoxifen on labelling index in fm3a cells. J Cell Mol Biol . 2002;1(2):81. [Google Scholar]
- 7.Robert J, Gianni L. Pharmacokinetics and metabolism of anthracyclines. Cancer Surveys . 1992;17:219–52. [PubMed] [Google Scholar]
- 8.Nasrolahi Shirazi A, Tiwari R, Chhikara BS, Mandal D, Parang K. Design and biological evaluation of cell-penetrating peptide-doxorubicin conjugates as prodrugs. Mol Pharm . 2013;10(2):488–99. doi: 10.1021/mp3004034. [DOI] [PubMed] [Google Scholar]
- 9.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med . 2002;53:615–27. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 10.Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol . 2005;205(2):275–92. doi: 10.1002/path.1706. [DOI] [PubMed] [Google Scholar]
- 11.Stavrovskaya AA. Cellular mechanisms of multidrug resistance of tumor cells. Biochemistry (Mosc) . 2000;65(1):95–106. [PubMed] [Google Scholar]
- 12.Nasrolahi Shirazi A, Oh D, Tiwari RK, Sullivan B, Gupta A, Bothun GD. et al. Peptide amphiphile containing arginine and fatty acyl chains as molecular transporters. Mol Pharm . 2013;10(12):4717–27. doi: 10.1021/mp400539r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterfreund RA, Philip JH. Critical parameters in drug delivery by intravenous infusion. Expert Opin Drug Deliv . 2013;10(8):1095–108. doi: 10.1517/17425247.2013.785519. [DOI] [PubMed] [Google Scholar]
- 14.Nasrolahi Shirazi A, Tiwari RK, Oh D, Sullivan B, Mccaffrey K, Mandal D. et al. Surface decorated gold nanoparticles by linear and cyclic peptides as molecular transporters. Mol Pharm . 2013;10(8):3137–51. doi: 10.1021/mp400199e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Regberg J, Srimanee A, Langel U. Applications of cell-penetrating peptides for tumor targeting and future cancer therapies. Pharmaceuticals (Basel) . 2012;5(9):991–1007. doi: 10.3390/ph5090991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Derossi D, Joliot AH, Chassaing G, Prochiantz A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J Biol Chem . 1994;269(14):10444–50. [PubMed] [Google Scholar]
- 17.Sarko D, Beijer B, Garcia Boy R, Nothelfer EM, Leotta K, Eisenhut M. et al. The pharmacokinetics of cell-penetrating peptides. Mol Pharm . 2010;7(6):2224–31. doi: 10.1021/mp100223d. [DOI] [PubMed] [Google Scholar]
- 18.Kim JK, Anderson J, Jun HW, Repka MA, Jo S. Self-assembling peptide amphiphile-based nanofiber gel for bioresponsive cisplatin delivery. Mol Pharm . 2009;6(3):978–85. doi: 10.1021/mp900009n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bulut S, Erkal TS, Toksoz S, Tekinay AB, Tekinay T, Guler MO. Slow release and delivery of antisense oligonucleotide drug by self-assembled peptide amphiphile nanofibers. Biomacromolecules . 2011;12(8):3007–14. doi: 10.1021/bm200641e. [DOI] [PubMed] [Google Scholar]
- 20.Dheur S, Dias N, Van Aerschot A, Herdewijn P, Bettinger T, Remy JS. et al. Polyethylenimine but not cationic lipid improves antisense activity of 3'-capped phosphodiester oligonucleotides. Antisense Nucleic Acid Drug Dev . 1999;9(6):515–25. doi: 10.1089/oli.1.1999.9.515. [DOI] [PubMed] [Google Scholar]
- 21.Jeong JH, Kim SH, Kim SW, Park TG. Intracellular delivery of poly(ethylene glycol) conjugated antisense oligonucleotide using cationic lipids by formation of self-assembled polyelectrolyte complex micelles. J Nanosci Nanotechnol . 2006;6(9-10):2790–5. doi: 10.1166/jnn.2006.414. [DOI] [PubMed] [Google Scholar]
- 22.Nasrolahi Shirazi A, Tiwari RK, Oh D, Banerjee A, Yadav A, Parang K. Efficient delivery of cell impermeable phosphopeptides by a cyclic peptide amphiphile containing tryptophan and arginine. Mol Pharm . 2013;10(5):2008–20. doi: 10.1021/mp400046u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Andaloussi S, Holm T, Langel U. Cell-penetrating peptides: mechanisms and applications. Curr Pharm Des . 2005;11(28):3597–611. doi: 10.2174/138161205774580796. [DOI] [PubMed] [Google Scholar]
- 24.Fotin-Mleczek M, Fischer R, Brock R. Endocytosis and cationic cell-penetrating peptides--a merger of concepts and methods. Curr Pharm Des . 2005;11(28):3613–28. doi: 10.2174/138161205774580778. [DOI] [PubMed] [Google Scholar]
- 25.Deshayes S, Morris MC, Divita G, Heitz F. Cell-penetrating peptides: tools for intracellular delivery of therapeutics. Cell Mol Life Sci . 2005;62(16):1839–49. doi: 10.1007/s00018-005-5109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandez-Carneado J, Kogan MJ, Pujals S, Giralt E. Amphipathic peptides and drug delivery. Biopolymers . 2004;76(2):196–203. doi: 10.1002/bip.10585. [DOI] [PubMed] [Google Scholar]
- 27.Torchilin VP, Rammohan R, Weissig V, Levchenko TS. TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proc Natl Acad Sci U S A . 2001;98(15):8786–91. doi: 10.1073/pnas.151247498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silhol M, Tyagi M, Giacca M, Lebleu B, Vives E. Different mechanisms for cellular internalization of the HIV-1 Tat-derived cell penetrating peptide and recombinant proteins fused to Tat. Eur J Biochem . 2002;269(2):494–501. doi: 10.1046/j.0014-2956.2001.02671.x. [DOI] [PubMed] [Google Scholar]
- 29.Nasrolahi Shirazi A, Mandal D, Tiwari RK, Guo L, Lu W, Parang K. Cyclic peptide-capped gold nanoparticles as drug delivery systems. Mol Pharm . 2013;10(2):500–11. doi: 10.1021/mp300448k. [DOI] [PubMed] [Google Scholar]
- 30.Solé NA, Barany G. Optimization of solid-phase synthesis of [ala8]-dynorphin a. J Org Chem . 1992;57(20):5399–403. [Google Scholar]
- 31.Wender PA, Mitchell DJ, Pattabiraman K, Pelkey ET, Steinman L, Rothbard JB. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proc Natl Acad Sci U S A . 2000;97(24):13003–8. doi: 10.1073/pnas.97.24.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pathak S, Chauhan VS. Rationale-based, de novo design of dehydrophenylalanine-containing antibiotic peptides and systematic modification in sequence for enhanced potency. Antimicrob Agents Chemother . 2011;55(5):2178–88. doi: 10.1128/AAC.01493-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi N-Q, Gao W, Xiang B, Qi X-R. Enhancing cellular uptake of activable cell-penetrating peptide–doxorubicin conjugate by enzymatic cleavage. Int J Nanomed . 2012;7:1613. doi: 10.2147/IJN.S30104. [DOI] [PMC free article] [PubMed] [Google Scholar]