Abstract
Purpose: Polycaprolactone (PCL) is a biodegradable polyester and has attracted attention as a suitable carrier for development of controlled drug delivery due to its non-toxicity and biocompatibility. It has been reported that the biodegradability of PCL can be enhanced by copolymerization with PEG. Molecular weight (Mw) and CL block lengths optimization in a series of synthesized PCEC copolymers was the main purpose of this study.
Methods: The composition of copolymers was designed using full factorial methodology. Molecular weight of used PEG (4 levels) and weight ratio of epsilon-caprolactone/PEG (3 levels) were selected as independent variables. The PCEC copolymers were synthesized by ring opening polymerization. Formation of copolymers was confirmed by FT-IR spectroscopy as well as H-NMR. The Mn of PCEC copolymers was calculated from HNMR spectra. The thermal behavior of copolymers was characterized on differential scanning calorimeter.
Results: Molecular weight of twelve synthesized copolymers was ranged from 1782 to 9264. In order to evaluate the effect of selected variables on the copolymers composition and Mw, a mathematical model for each response parameter with p-value less than 0.001were obtained. Average percent error for prediction of total Mn of copolymers and Mn of CL blocks were 13.81% and 14.88% respectively.
Conclusion: In conclusion, the proposed model is significantly valid due to obtained low percent error in Mn prediction of test sets.
Keywords: PCEC, Copolymer, Full factorial methodology, Molecular weight
Introduction
Poly caprolactone (PCL) is a semi-crystalline, hydrophobic polymer with a glass transition temperature (Tg) of -60 °C and melting point ranging from 59 to 64 °C.1 PCL is synthesized via ring-opening polymerization method with using ε-caprolactone monomer and a variety of anionic, cationic and co-ordination catalysts.2
Suitable properties of PCL including excellent biocompatibility, good solubility and low melting point, make it as an attractive carrier for development of controlled drug delivery systems. However biodegradation of PCL is slow which restricts its clinical application. Thus preparation of PCL copolymers is proposed.3-5 Block and random copolymers of PCL can be synthesized by using monomers such as ethyleneoxide, polyvinylchloride, chloroprene, polyethylene glycol (PEG), polystyrene, diisocyanates (urethanes), tetrahydrofuran (THF), diglycolide, dilactide, δ-valerlactone, substituted caprolactones, 4-vinyl anisole, styrene, methyl methacrylate and vinyl acetate.2,6 Among these monomers, PEG is suitable to construct caprolactone block copolymers because of its hydrophilicity, nontoxicity and absence of antigenicity and immunogenicity.7 Two or three block copolymers of CL-PEG were utilized to develop delivery systems for various drugs.5 PCL–PEG diblock copolymers can be synthesized by ring-opening polymerization from monomethoxy-PEG (MPEG) and ε-CL in presence of a catalyst.8,9 There are two types of PCL/PEG triblock copolymers: PCL–PEG–PCL (PCEC) and PEG–PCL–PEG (PECE) copolymers. Preparation of PCEC is similar to the synthesis of diblock copolymers, with the exception of using dihydroxy PEG as initiator instead of MPEG.10,11 Two steps methods were employed for obtaining PECE copolymers. These copolymers were synthesized by coupling reaction with using PCL diol and PEG in presence of l-lysine methyl ester diisocyanate (LDI) as the chain extender.12-14 Preparation of PECE from MPEG–PCL diblock copolymer by using isophorone diisocyanate (IPDI) and hexamethylene diisocyanate as coupling agent is possible too.7
Molecular weight (Mw) is one of the most important properties of polymers. Most of the physical properties of polymers such as glass transition temperature, strength, stiffness, and viscosity are depended on their Mw.15 Furthermore degradation kinetics of polymers is highly depending on their Mw. Degradation of High molecular weight polyesters such as long chain PCL takes longer time as a result of slow erosion.16 In order to obtain suitable properties on the basis of its application, synthesis of PCL and its copolymers with various block lengths and molecular weight is desirable.
Experimental design methodology is defined as a useful strategy to optimize processes variables and conditions.17 Obtaining valuable required information from the fewest experiment is the main advantages of this approach.18 Optimization of polymerization condition by means of factorial design methodologies has been successfully applied in polymer synthesis.19-21 Molecular weight and CL block lengths optimization in a series of synthesized poly caprolactone-ethylene glycol–caprolactone (PCEC) copolymers was the main purpose of this study. Full factorial methodology was used to determine the impact of two independent variables on the molecular weight of synthesized copolymers.
Materials and Methods
Materials
PEG with average molecular weight of 1, 2, 3 and 4 KDa, ε-caprolactone monomer, dichloromethane and n-hexane was purchased from Merck chemical company (Germany), stannous octoate was obtained from Alfa Aesar, A Johnson Matthey Company (Germany).
Factorial design
The composition of copolymers was designed by full factorial methodology using Minitab 15 software. Molecular weight of used PEG (4 levels) and weight ratio of ε-caprolactone/PEG (CL/PEG) before reaction (3 levels) were selected as independent variables (Table 1). The design consisted of 12 experimental runs. The number average molecular weight (Mn) of copolymers and CL blocks were selected as output factors.
Table 1. Values of independent variable levels .
| variables | levels | |||
| MW of PEG | 1000 | 2000 | 3000 | 4000 |
| Weight ratio of CL/PEG | 0.5 | 1.5 | 2 | - |
Synthesis of copolymers
PCEC copolymers were synthesized by a ring opening polymerization of ε- caprolactone and PEG in the presence of stannous octoate as catalyst. Polymerization of PCEC was carried out in a two necked vessel equipped with a stirrer, a thermometer and a gas inlet tube. Epsilon-caprolactone, PEG with various Mw and stannous octoate were introduced into two necked vessel. The reaction mixture was stirred under dry nitrogen for 6 hours at 130 C. After completion of polymerization, the vessel was connected to a vacuum for 30 minutes at 180 °C.22 After cooling of reaction mixture to the room temperature, the synthesized copolymer was dissolved in dichloromethane and then isolated by precipitation with n-hexane. The obtained solid material was filtered and the residual solvent was removed under reduce pressure.
Characterization of copolymers
FTIR spectroscopy
Fourier-transform infrared spectroscopy of PEG and synthesized copolymers were obtained on a Bomem 2000 FT-IR system (Bomem, Quebec, Canada). Copolymers were dissolved in dichloromethane and thin film of this solution was casted on NaCl plate.
Nuclear Magnetic Resonance (HNMR)
HNMR spectra of copolymers in CDCl3 were obtained with a Bruken-Spectrospin 400 MHz spectrometer (Varian, Switzerland). Mn of copolymers was calculated from HNMR spectrums.
Differential scanning calorimetery
Thermal behavior of copolymers was recorded on a DSC-60 (Shimadzu, Kyoto, Japan). Thermogram of the samples was obtained at a scanning rate of 10°C/min covering temperature range of 25–200°C.
Results
Synthesis and characterization results of PCEC
PCEC copolymers were synthesized by ring-opening polymerization of ε-caprolactone in the presence of PEG with different MW as initiator. Using PEG polymers with different molecular weight and varying the initial CL/PEG weight ratio, 12 copolymers with different Mn, composition and average length of the blocks were obtained. Synthesis of these copolymers was confirmed by FTIR as well as HNMR. FTIR spectrum of PEG and synthesized PCEC triblock copolymers are shown in Figure 1. An absorption band is appeared at 1731 in PCEC copolymers spectrum which is not observed in PEG spectrum. This band could be related to stretching vibration of carbonyl group which confirms formation of copolymer.23,24 Aliphatic CH stretching band of PEO and caprolactone were appeared at 2867 and 2925 cm-1 respectively. As can be seen intensity of these two absorption bands is dependent on the weight ratio of CL/PEG in copolymers. Aliphatic CH stretching band of PEO at 2867 was decreased by increasing weight ratio of CL/PEG. While the absorption band of CH stretching vibration in CL block at 2925 was increased. Another absorption bands at 1104 and 1163 cm-1 are related to C-O-C stretching vibration. Figure 2 demonstrates HNMR of synthesized PCEC triblock copolymer. According to this spectrum, the singlet peak at 3.61 ppm is related to the methylene protons of the –CH2CH2O- units in PEG segment of copolymers. Also two triplets at 4.03 and 2.3 ppm and two multiplets at 1.63 and 1.40 ppm are related to methylene protons in PCL units. Moreover a very weak peak was observed at 4.2 which related to the methylene protons of –CH2CH2O- in PEG end units.
Figure 1 .
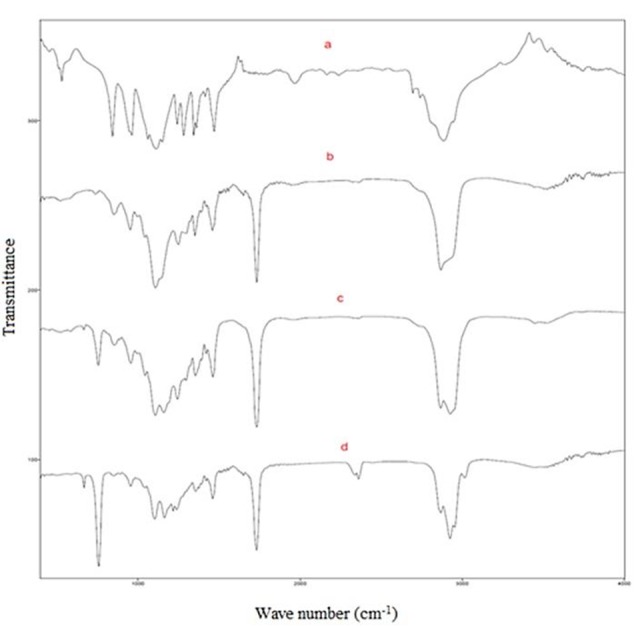
FTIR spectra of PEG(a) and PCEC copolymers prepared by using PEG 3000 and with different CL/PEG weight ratio; 0.5(b), 1.5(c) and 2(d)
Figure 2 .
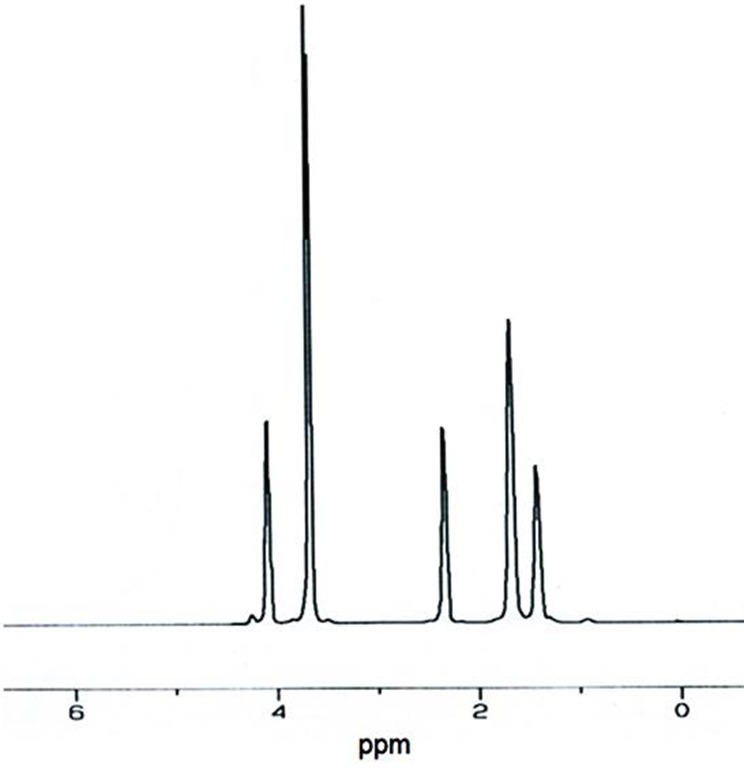
HNMR of PCEC triblock copolymer (Mw of PEG: 3000D, CL/PEG weight ratio: 2)
DSC thermograms of PEG and PCEC copolymers with different CL/PEG weight ratio are shown in Figure 3 and 4. Melting peak at 61.64°C is observed in thermal curve of PEG. DSC thermograms of PCEC copolymers (CL/PEG weight ratio: 1.5 and 2) exhibit two endotherms: The higher temperature endotherms were related to the melting of PEG block and the lower temperature endotherms were attributed to caprolactone blocks. Melting point of PEG segment in these copolymers is lower than that of the PEG homopolymer. Melting point shift of PEG segment to lower temperature in PCEC copolymers was reported by Bogdanov et al. previously.25 Copolymers with lower CL/PEG weight ratio (0.5) exhibited one endothermic peak which is related to melting point of PEG blocks.
Figure 3 .
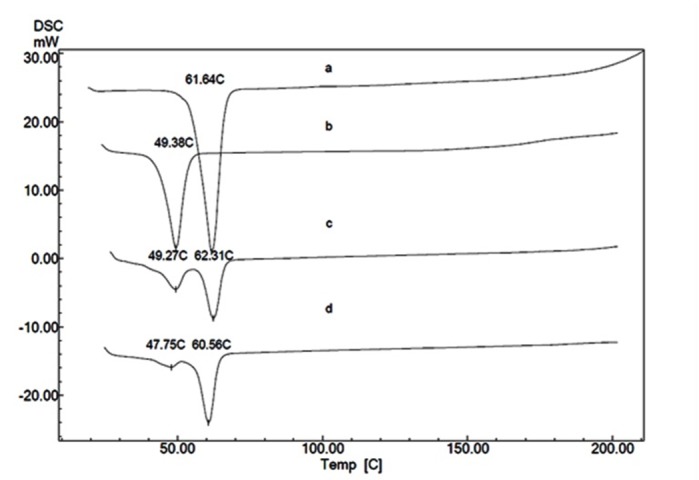
DSC thermogram of PEG 4000 D (a) and PCEC copolymers prepared by using PEG 4000 and with different CL/PEG weight ratio; 0.5(b), 1.5(c) and 2(d)
Figure 4 .
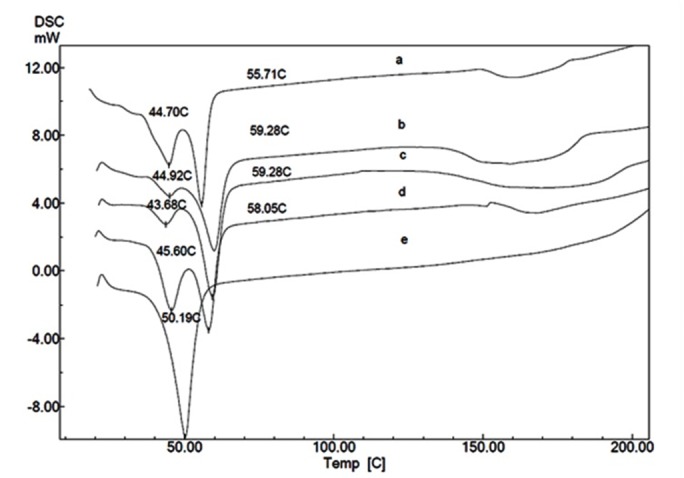
DSC thermogram of PCEC copolymers (various Mw of PEG; different CL/PEG weight ratio: 2000; 1.5(a), 2000; 2(b), 3000; 2(c), 3000; 1.5(d) and 3000; 0.5 (e)
Molecular weight calculation of copolymers from HNMR spectra
Total Mn of copolymers and Mn of CL blocks which were calculated from HNMR spectra are presented in Table 2. Number of CL and PEG repeating unit were determined from integral intensities of methylene protons at 4.03 and 3.61 ppm respectively. As integral intensity of the peak at 4.2 ppm is attributed to four protons in PEG end unit, it can be possible to calculate the number of oxyethylen repeating unit in PEG block and also number of oxycarbonyl 1,5-pentamethylene repeating unit derived from CL ring opening. Therefore Mn of each block and the total Mn of copolymers were obtained from equation (1) in which x is the number of repeating unit in PEG block and y is the number of repeating unit in CL blocks.22
Table 2. Mn of copolymers determinedusing HNMR spectra .
| Mw (PEG) | CL/PEG weight ratio | Mn(PCL) | Mn (total) |
| 4000 | 1.5 | 5009.9200 | 7987.2533 |
| 3000 | 1.5 | 3599.9351 | 6022.3135 |
| 2000 | 0.5 | 1009.5721 | 2974.9694 |
| 3000 | 2 | 5879.9184 | 8917.1156 |
| 1000 | 2 | 1952.4190 | 2865.9842 |
| 3000 | 0.5 | 1376.8258 | 4259.5355 |
| 2000 | 2 | 3669.5094 | 5520.6309 |
| 1000 | 1.5 | 1585.4067 | 2530.2273 |
| 2000 | 1.5 | 3039.4823 | 5117.5531 |
| 4000 | 2 | 6170.7097 | 9264.1935 |
| 4000 | 0.5 | 2447.7241 | 7884.0000 |
| 1000 | 0.5 | 599.4689 | 1782.6589 |
Mn(PCEC)=Mn(PEG) +Mn(PCL) = 44x +114y (1)
Full factorial analysis results
To identify significant factors affecting the composition and Mn of synthesized copolymers, full factorial design methodology was applied to obtain a mathematical model. The obtained data were analyzed to fit the following polynomial equation where Y is the measured responses, C is an intercept, A and B are constant regression coefficients, X1 & X2 are Mw of PEG and weight ratio of CL/PEG respectively.
Y=C+AX1+BX2 (2)
Results of full factorial analysis are presented in Table 3. PCL length and Mn of produced copolymers were very significantly related to Mw of PEG and CL/PEG weight ratio (p<0.001). In order to study the simultaneous effects of variables on each response, three-dimensional surface plots for two responses versus independent variables were plotted (Figure 5). As is evident, both Mw of PEG and weight ratio of CL/PEG had positive impact on the Mn of CL blocks and total Mn of copolymers. Data of synthesized copolymers were randomly divided into training and test sets. Data of test set was used to evaluate the external predictive performance of the proposed model (Figure 6 and 7). Average percent error for prediction of total Mn of copolymers and Mn of CL blocks were 13.81% and 14.88% respectively.
Table 3. Estimated regression coefficient for selected responses .
| Response | Intercept | regression coefficient (Mw of PEG) | regression coefficient (weight ratio of CL/PEG) | p-value | R |
| Y1: Mn(PCL) | -2013.6 | 1.08431 | 1732.92 | < 0.001 | 0.947 |
| Y2 : Mn (total) | -1384.48 | 2.00949 | 1327.34 | < 0.001 | 0.970 |
Figure 5 .

Surface Plot of Mn of PCL (a) and Total Mn (b) vs. CL/PEG (w/w) and Mw (PEG)
Figure 6 .
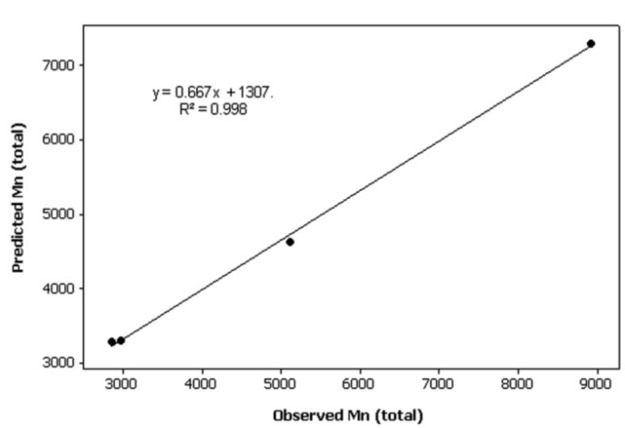
Scatterplot of Predicted Mn (total) vs. Observed Mn (total)
Figure 7 .
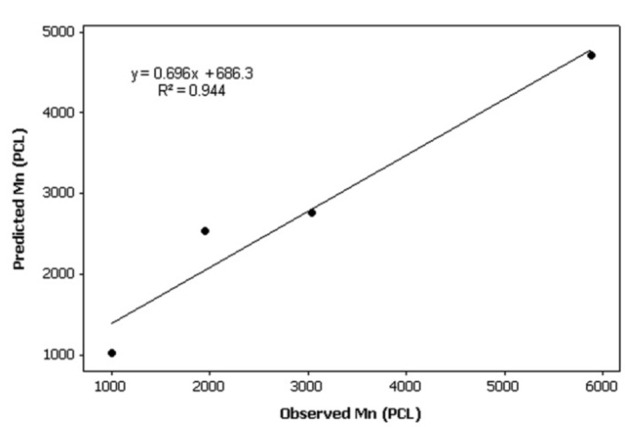
Scatterplot of Predicted Mn (PCL) vs. Observed Mn (PCL)
Discussion
Series of PCEC copolymers with different composition and Mw was synthesized by ring opening polymerization. In this method an active hydrogen atom of the preformed PEG causes a selective acyl-oxygen cleavage of an ester group in the monomer ring and lead to formation of bishydroxy-diester intermediate. A step-by-step addition of monomer units occurs with formation of two external polyester blocks following formation of this intermediate. The length of added blocks depends on the amount of cyclic ester monomer used in the polymerization process.26 Molecular weight and CL block lengths optimization in a series of synthesized PCEC copolymers was carried out using full factorial methodology. Obtaining a mathematical model for each response parameter with p-value less than 0.001 indicated that the models were statistically valid. Moreover considering the low percent error for prediction of total Mn of copolymers and Mn of CL blocks, it might be proposed that this model has a good predictive power in the studied rang of variables with ability to be generalized for Unknown cases.
Conclusion
Based on obtained results it can be concluded that application of full factorial design methodologies can be considered as a useful strategy for molecular weight and block lengths optimization in synthesis process of triblock PCEC copolymers.
Acknowledgments
The authors would like to thank research center for pharmaceutical nanotechnology. This article was a part of a thesis submitted for PhD degree (No.51) in Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran.
Ethical Issues
Not applicable.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Nair LS, Laurencin CT. Biodegradable polymers as biomaterials. Progr Polym Sci . 2007;32:762–98. [Google Scholar]
- 2.Okada M. Chemical syntheses of biodegradable polymers. Progr Polym Sci . 2002;27(1):87–133. [Google Scholar]
- 3.Freiberg S, Zhu XX. Polymer microspheres for controlled drug release. Int J Pharm . 2004;282(1-2):1–18. doi: 10.1016/j.ijpharm.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Merkli A, Tabatabay C, Gurny R, Heller J. Biodegradable polymers for the controlled release of ocular drugs. Progr Polym Sci . 1998;23(3):563–80. [Google Scholar]
- 5.Sinha VR, Bansal K, Kaushik R, Kumria R, Trehan A. Poly-epsilon-caprolactone microspheres and nanospheres: an overview. Int J Pharm . 2004;278(1):1–23. doi: 10.1016/j.ijpharm.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 6.Woodruff MA, Hutmacher DW. The return of a forgotten polymer-Polycaprolactone in the 21st century. Prog Polym Sci . 2010;35:1217–56. [Google Scholar]
- 7.Wei X, Gong C, Gou M, Fu S, Guo Q, Shi S. et al. Biodegradable poly(epsilon-caprolactone)-poly(ethylene glycol) copolymers as drug delivery system. Int J Pharm . 2009;381(1):1–18. doi: 10.1016/j.ijpharm.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 8.Jedliński Z, Kurcok P, Walach W, Janeczek H, Radecka I. Polymerization of lactones, 17. Synthesis of ethylene glycol-L-lactide block copolymers. Die Makromolekulare Chemie. 1993;194(6):1681–9. [Google Scholar]
- 9.Youxin L, Kissel T. Synthesis and properties of biodegradable ABA triblock copolymers consisting of poly (l-lactic acid) or poly (l-lactic-co-glycolic acid) A-blocks attached to central poly (oxyethylene) B-blocks. J Control Release . 1993;27(3):247–57. [Google Scholar]
- 10.Cohn D, Stern T, Gonzalez MF, Epstein J. Biodegradable poly(ethylene oxide)/poly(epsilon-caprolactone) multiblock copolymers. J Biomed Mater Res . 2002;59(2):273–81. doi: 10.1002/jbm.1242. [DOI] [PubMed] [Google Scholar]
- 11.Kim JH, Bae YH. Albumin loaded microsphere of amphiphilic poly(ethylene glycol)/ poly(alpha-ester) multiblock copolymer. Eur J Pharm Sci . 2004;23(3):245–51. doi: 10.1016/j.ejps.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Zhuo RX. Synthesis and in vitro drug release behavior of amphiphilic triblock copolymer nanoparticles based on poly (ethylene glycol) and polycaprolactone. Biomaterials . 2005;26(33):6736–42. doi: 10.1016/j.biomaterials.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 13.Storey RF, Wiggins JS, Mauritz KA, Puckett AD. Bioabsorbable composites. II: Nontoxic, L-lysine-based poly(ester-urethane) matrix composites. Polym Composite. 1993;14(1):17–25. [Google Scholar]
- 14.Skarja GA, Woodhouse KA. Structure-property relationships of degradable polyurethane elastomers containing an amino acid-based chain extender. J Appl Polym Sci . 2000;75(12):1522–34. doi: 10.1163/156856298x00659. [DOI] [PubMed] [Google Scholar]
- 15.Nunes RW, Martin JR, Johnson JF. Influence of molecular weight and molecular weight distribution on mechanical properties of polymers. Polym Eng Sci . 1982;22(4):205–28. [Google Scholar]
- 16.Woodward SC, Brewer PS, Moatamed F, Schindler A, Pitt CG. The intracellular degradation of poly(epsilon-caprolactone) J Biomed Mater Res . 1985;19(4):437–44. doi: 10.1002/jbm.820190408. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez-Lafuente C, Furlanetto S, Fernandez-Arevalo M, Alvarez-Fuentes J, Rabasco AM, Faucci MT. et al. Didanosine extended-release matrix tablets: optimization of formulation variables using statistical experimental design. Int J Pharm . 2002;237(1-2):107–18. doi: 10.1016/s0378-5173(02)00028-5. [DOI] [PubMed] [Google Scholar]
- 18.Chang LC, Wu SC, Tsai JW, Yu TJ, Tsai TR. Optimization of epirubicin nanoparticles using experimental design for enhanced intravesical drug delivery. Int J Pharm . 2009;376(1-2):195–203. doi: 10.1016/j.ijpharm.2009.04.045. [DOI] [PubMed] [Google Scholar]
- 19.Rosa F, Bordado J, Casquilho M. Influence of polymerization conditions on the viscosity of polyacrylamide via experimental design. J Appl Polym Sci . 2006;102(6):5719–24. [Google Scholar]
- 20.Mojarrad JS, Nemati M, Valizadeh H, Ansarin M, Bourbour S. Preparation of glucosamine from exoskeleton of shrimp and predicting production yield by response surface methodology. J Agric Food Chem . 2007;55(6):2246–50. doi: 10.1021/jf062983a. [DOI] [PubMed] [Google Scholar]
- 21.Valizadeh H, Pourmahmood M, Mojarrad JS, Nemati M, Zakeri-Milani P. Application of artificial intelligent tools to modeling of glucosamine preparation from exoskeleton of shrimp. Drug Dev Ind Pharm . 2009;35(4):396–407. doi: 10.1080/03639040802422088. [DOI] [PubMed] [Google Scholar]
- 22.Liu CB, Gong CY, Huang MJ, Wang JW, Pan YF, Zhang YD. et al. Thermoreversible gel-sol behavior of biodegradable PCL-PEG-PCL triblock copolymer in aqueous solutions. J Biomed Mater Res B Appl Biomater . 2008;84(1):165–75. doi: 10.1002/jbm.b.30858. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen THA, Nguyen VC. Formation of nanoparticles in aqueous solution from poly (ε-caprolactone)-poly (ethylene glycol)-poly (ε-caprolactone) Adv Nat Sci: Nanosci Nanotechnol . 2010;1(2):025012. [Google Scholar]
- 24.Ryu JG, Jeong YI, Kim YH, Kim IS, Kim DH, Kim SH. Preparation of core-shell type nanoparticles of poly (ε-caprolactone)/poly (ethylene glycol)/poly (ε-caprolactone) triblock copolymers. Bull Korean Chem Soc . 2001;22(5):467–75. [Google Scholar]
- 25.Bogdanov B, Vidts A, Van Den Buicke A, Verbeeck R, Schacht E. Synthesis and thermal properties of poly(ethylene glycol)-poly(ε-caprolactone) Polymer . 1998;39:1631–6. [Google Scholar]
- 26.Arias JL. Novel strategies to improve the anticancer action of 5-fluorouracil by using drug delivery systems. Molecules . 2008;13(10):2340–69. doi: 10.3390/molecules13102340. [DOI] [PMC free article] [PubMed] [Google Scholar]


