Abstract
Purpose: The present study is aimed to select the suitable method for preparation of camptothecin loaded polymeric nanoparticles by utilizing the multi-criteria decision making method. Novel approaches of drug delivery by formulation using nanotechnology are revolutionizing the future of medicine. Recent years have witnessed unprecedented growth of research and application in the area of nanotechnology. Nanoparticles have become an important area of research in the field of drug delivery because they have the ability to deliver a wide range of drug to varying areas of body.
Methods: Despite of extensive research and development, polymeric nanoparticles are frequently used to improve the therapeutic effect of drugs. A number of techniques are available for the preparation of polymeric nanoparticles. The Analytical Hierarchy Process (AHP) is a method for decision making, which are derived from individual judgements for qualitative factors, using the pair-wise comparison matrix. In AHP, a decision hierarchy is constructed with a goal, criteria and alternatives.
Results: The model uses three main criteria 1) Instrument, 2) Process and Output and 3) Cost. In addition, there are eight sub-criteria’s as well as eight alternatives. Pair-wise comparison matrixes are used to obtain the overall priority weight and ranking for the selection of suitable method. Nanoprecipitation technique is the most suitable method for the preparation of camptothecin loaded polymeric nanoparticles with the highest overall priority weight of 0.297
Conclusion: In particular, the result indicates that the priority weights obtained from AHP could be defined as a multiple output for finding out the most suitable method for preparation of camptothecin loaded polymeric nanoparticles.
Keywords: Analytical hierarchy process, Camptothecin, Multi-criteria, Nanotechnology, Polymeric nanoparticles
Introduction
Camptothecin (CPT) is a naturally occurring quinoline alkaloid isolated from the chinese plant Camptotheca acuminate.1,2 Camptothecin targets the nuclear enzyme topoisomerase I and inhibits the relegation of the cleaved DNA strand, resulting in tumour cell death.2,3 It shows a significant anticancer activity with a broad spectrum of human malignancies.4 However, the aqueous insolubility, low stability at physiological pH and sever systemic toxicity limits the clinical application of camptothecin.5 To overcome the insolubility, instability and toxicity problems of CPT, several approaches have been investigated. These include approaches such as conjugation to polymers, intercalation into liposomes, solubilization in micro emulsions, entrapment in microspheres, solid dispersion, nanotechnology and formation of inclusion complexes with cyclodextrin.1,3 Novel approaches of drug delivery by formulation using nanotechnology are revolutionizing the future of medicine.
Nanotechnology is an area of science and technology, devoted to construct structures in the nanometers scale size range by manipulating the atoms and molecules, which retains its unique properties and is rapidly expanding field due to the multi-disciplinary support from researchers.6-8 The principle of nanotechnology is applied in engineering, electronics and biomedical sciences which includes gene therapy, imaging and novel drug discovery. Despite of numerous scientific efforts, the increased use of nanotechnology is widely anticipated in pharmaceutical and biotechnology industries.7,9
Nanotechnology is a highly important area of research, in pharmaceutical industries, to develop an effective drug delivery system that can transport and deliver a drug precisely and safely to the site of action for treating a variety of diseases and disorders.7,10 The efficiency of drug delivery to various parts of the body is directly affected by particle size.11
Application of nanotechnology for treatment, diagnosis, monitoring and control of biological systems has recently been referred to as nanomedicine by National institutes of health.6 Nanotechnology plays an important role in nanomedicine by increasing the therapeutic indices and safety profile of the drug, by lowering the required doses used for effective therapy.8,12 It has many advantages in the protection the drug from premature degradation and interactions with the biological environment, enhancement of absorption into a selective tissues and improvement of intracellular penetration.13,14
Nanomedicine facilitated the creation of novel nanotherapeutics by using different nanomaterials. Several types of nanoparticulate systems, which including biodegradable polymeric nanoparticles, polymeric micelles, solid nanoparticles, lipid based nanoparticles e.g., solid lipid nanoparticles (SLN), nanostructures lipid carriers (NLC) and lipid drug conjugates (LDC), liposomes, inorganic nanoparticles, dendrimers, magnetic nanoparticles, nanocrystals, nanotubes and quantum dots are still under investigation for convenient drug delivery.15,16
Nanoparticles have become an important area of research in the field of drug delivery because they have the ability to deliver a wide range of drugs to varying area of body for a sustained period of time.17 In recent days, nanoparticles are used for various biomedical applications where they facilitate laboratory diagnosis and therapeutics. More specifically for drug delivery purposes, the use of nanoparticles is attracting, due to their unique capabilities and their negligible side effects in the treatment of various diseases.18
Polymeric nanoparticles are one of the most popular due to their easy production and process diversity into the required characteristics for the design of suitable drug delivery systems.12,19 Polymeric Nanoparticles have attracted the interest of many research groups and have utilized in an increasing number of fields during the last decades. It plays a pivotal role in a wide spectrum in overcoming several limitations of drug delivery system and provides an attractive alternative for long term delivery of therapeutic agents for chronic administration.20
The dispersion of preformed polymers and the polymerization of monomers are generally the two main strategies for preparation of polymeric nanoparticles. However, there are various methods used for the preparation of polymeric nanoparticles such as desolvation, dialysis, ionic gelation, nanoprecipitation, solvent evaporation, salting out, spray drying and supercritical fluid.
However, the choice of an appropriate method depends upon various factors. Hence, the solution of selecting the suitable method was a real concern, because selection of inappropriate method may lead to loss of materials resources, financial resources and time of research.21 To achieve the goal of selecting the suitable method for the preparation of camptothecin loaded polymeric nanoparticles, there is a need of using a scientific approach Multi-Criteria Decision Making (MCDM) method. The Analytical Hierarchy Process (AHP) can help in this regards. The AHP is an operational research model first developed by Saaty in 1980, is a flexible multi-criteria decision making methodology that transforms a complex problem into a hierarchy with respect to one or more criteria.22 One advantage of the AHP is that it is designed to handle situations in which the subjective judgements of individuals constitute an important part of decision process.21
Thus, AHP technique involves structuring multiple choice criteria into hierarchy, assessing the relative importance of criteria, comparing alternatives for each criterion and determining an overall priority weight and ranking of the alternatives.22 The objective of the present study is to select the most suitable method for the preparation of camptothecin loaded polymeric nanoparticles from various methods available using analytical hierarchy process.
Materials and Methods
Analytical Hierarchy Process (AHP)
In this method, a simple hierarchical model consists of goal, criteria and alternatives are constructed. AHP is composed of several previously existing but unassociated concepts and techniques, such as hierarchical structuring, pair-wise comparisons, the eigen-vector method for deriving weights and consistency considerations. According to saaty, the method has three phases: 1) Decomposing, 2) Comparative Judgements, 3) Synthesizing.23
In Decomposing phase, the elements of decision problem are arranged in form of hierarchy. The top elements of hierarchy is overall goal, the next level is the criteria which impact the goal directly, the next level is the operational sub-criteria, against which the decision alternatives of the lowest level of hierarchy can be evaluated and all the elements of a given level are assumed to be mutually independent.23
In Comparative Judgement Phase, elements of one level of a hierarchy are compared pair-wise as to the strength of their influence on an element of the next higher level. Saaty has suggested a scale of 1 to 9 when comparing two elements, with a score of 1 representing indifference between the two elements and 9 representing the overwhelming dominance of that element over the other. These comparison leads to dominance matrices which are called pair-wise comparison matrices.23
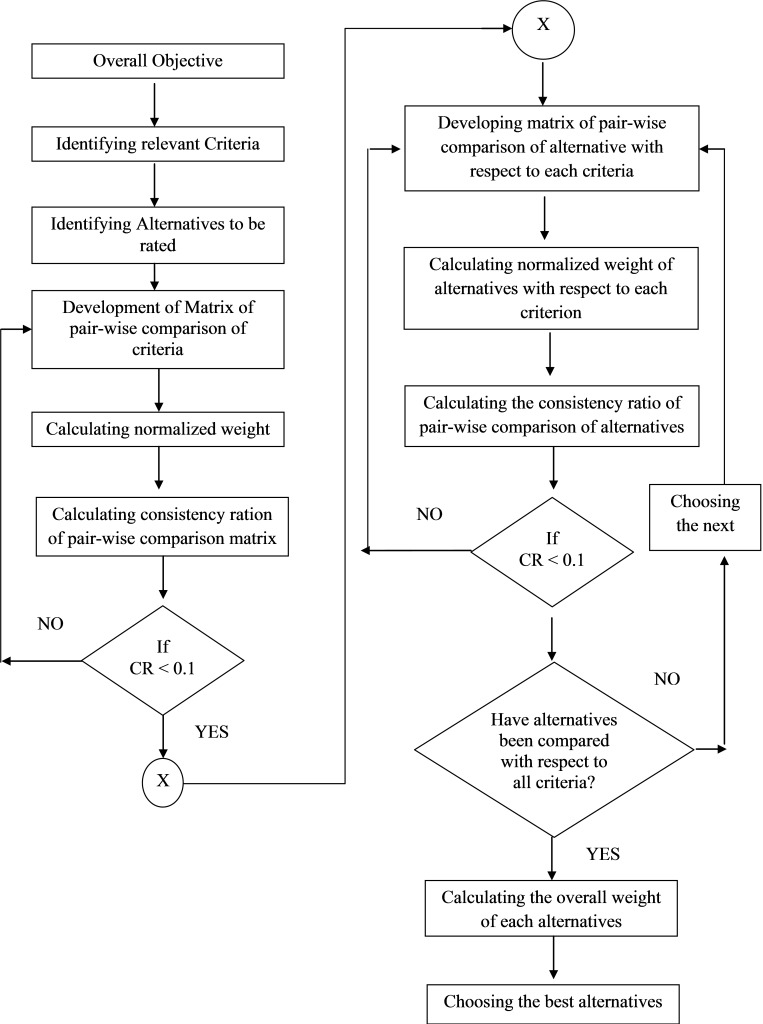
The next phase is to synthesize the priorities, the simple hierarchical model which evaluates alternatives with respects to criteria and sub-criteria of overall goal. The priorities of all alternatives with respect to each criterion are calculated. The overall priorities weights are calculated from pair-wise comparison matrix.23 Figure 1 shows the flow chart of the AHP methodology.
Figure 1 .
Flow chart for AHP methodology
Hierarchy Model
Figure 2 shows a four level hierarchy model for the selection of suitable method for the preparation of camptothecin loaded polymeric nanoparticles. The first level represents the goal of the problem. The objective of the model is divided into three main criteria such as Instrument, Process and Output and Cost in the second level. The third level consists of eight sub-criteria such as Instrument Availability (SC01), Instrument Backup (SC02), Ease of Operation (SC03), Minimum number of excipients (SC04), Minimum average particle size and Poly Dispersibility Index (PDI) (SC05), Maximum yield of Nanoparticles (SC06), Reproducible Results (SC07) and Minimum preparation cost (SC08) related to the main criteria. Also, eight potential alternatives/methods desolvation (DS), dialysis (DI), ionic gelation (IG), nanoprecipitation (NP), salting out (SO), solvent evaporation (SE), spray drying (SD), supercritical fluid (SF) are given in the final level of proposed hierarchical model.24
Figure 2 .
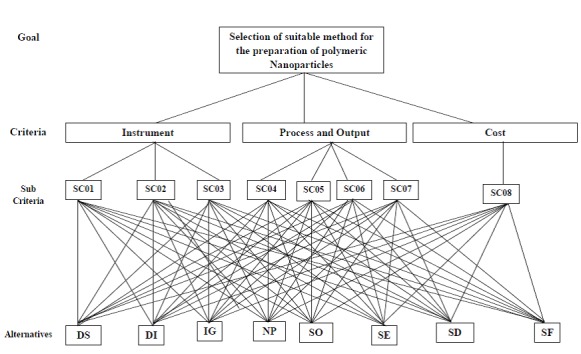
Hierarchy model for the selection of suitable method for the preparation of camptothecin loaded polymeric nanoparticles
Main Criteria and Sub-Criteria
Choosing the potential alternatives for the preparation of camptothecin loaded polymeric nanoparticles as well as selecting the related main criteria and sub-criteria (Table 1) are based on the five principles for the preparation of safe nanoparticles.25 More specifically, the main principles are as follows: 1) Reducing the size of drug in nanometer range without altering the function of drug. 2) Replacing the use of toxic chemicals used in the preparation with suitable nontoxic chemicals. 3) Bonding of atoms and molecules to nanoparticles preserving the desired property of the product, devoid of toxicity. 4) Use of nontoxic polymers to reduce the toxicity of the drug by encapsulating the high toxic drugs. 5) Use of limited quantity of toxic chemicals, when the use of the toxic chemical cannot be avoided.21,25 Eight potential methods or alternatives selected for the preparation of polymeric Nanoparticles are given in Table 2. A brief procedure of each method is being discussed below.
Table 1. Main criteria and sub-criteria for the selection of suitable method.
| Main Criteria | Sub-criteria | |
| Code | Description | |
| Instrument | SC01 | Instrument Availability |
| SC02 | Instrument Backup | |
| SC03 | Ease of Operation | |
| Process and Output | SC04 | Minimum number of excipients |
| SC05 | Minimum average particle size and PDI | |
| SC06 | Maximum yield of Nanoparticles | |
| SC07 | Reproducible Results | |
| Cost | SC08 | Minimum preparation cost |
Table 2. Potential alternatives for the preparation of polymeric Nanoparticles .
| Sl. NO | Techniques | Code |
| 01 | Desolvation Technique | DS |
| 02 | Dialysis Technique | DI |
| 03 | Ionic Gelation Technique | IG |
| 04 | Nanoprecipitation Technique | NP |
| 05 | Salting Out Technique | SO |
| 06 | Solvent Evaporation Technique | SE |
| 07 | Spray Drying Technique | SD |
| 08 | Supercritical Fluid Technique | SF |
Desolvation Technique
Desolvation technique has been widely used in preparation of polymeric nanoparticles. This technology is applicable for a wide range of polymers by changing the charge and pH by addition of a desolvating agent like ethanol or concentrated organic salt solution. Briefly, the hydrogen ion concentration is adjusted by the addition of ethanol drop wise continuously at a controlled rate of 1ml/min to the protein solution containing drug under constant stirring until the solution become turbid. Further the co-acervates formed are hardened by the addition of the cross linking agent glutaraldehyde. For the production of stable nanoparticles, the lowest required glutaraldehyde concentration of about 40% with a reaction time of 24 hrs is used. After the elimination of ethanol by evaporation under pressure, nanoparticles are purified by centrifugation to remove the free drug and excess cross linking agent.26,27 The nanosuspension produced is freeze dried using 5% mannitol as a cryoprotectant to obtain a fine powder of nanoparticles.28
Dialysis Technique
Dialysis technique is a simple and effective technique for preparation of polymeric nanoparticles with narrow distribution. Briefly, drug and polymers are placed inside a dialysis tube / membrane after dissolving with water miscible organic solvents with appropriate molecular weight cut off. The organic phase diffuses out through the dialysis tube / membrane into the aqueous phase which decrease the interfacial tension between them. Subsequently, the homogenous suspension of nanoparticles is formed by the displacement of solvent inside the membrane followed by progressive aggregation of polymer due to the loss of solubility. The nanosuspension produced is freeze dried using 5% mannitol as a cryoprotectant to obtain a fine powder of nanoparticles.20,29,30
Ionic Gelation Technique
Polymers play a major role for designing a oral delivery system, use of natural polymers like chitosan and alginates instead of toxic chemicals polymers in oral delivery system enhances the permeation effect, enzyme inhibitory ability and mucoadhesive property of the drug. Briefly, based on the solubility of the drug and polymer, they are dissolved in weak acidic medium or water and the resultant solution is added to the solution containing counter ions and stabilizer drop wise under constant stirring. Spherical shaped particles are formed due to the complexation of oppositively charged species which results in gelation and precipitation. The particle size is reduced to the nanometric range buy sonicating the resultant solution. The nanosuspension produced is freeze dried using 5% mannitol as a cryoprotectant to obtain a fine powder of Nanoparticles.31,32
Nanoprecipitation Technique
Nanoprecipitation technique was first introduced by fessi for preparation of polymeric nanoparticles. The formation of particles is based on the precipitation and subsequent solidification of the polymers due to the interfacial deposition of polymer after displacement of semi polar solvents miscible with water, from a lipophilic solution. Briefly, drug and polymer are dissolved in a water miscible organic solvent and added to the aqueous phase containing stabilizer under stirring. The decrease in interfacial tension between the aqueous and organic phase results in the rapid diffusion of organic solvent into aqueous phase. Small droplets of nanoparticles with a well defined size characterized by a narrow distribution are formed instantaneously during the solvent flow, diffusion and surface tension at the interface of organic solvent and the aqueous phase causing turbulence. The nanosuspension produced is freeze dried using 5% mannitol as a cryoprotectant to obtain a fine powder of nanoparticles.20,33
Salting Out Technique
Salting out technique is a modified version of emulsion process to overcome the use of organic solvents like surfactants and chlorinated solvents which are hazardous to the environment as well as to physiological system. Briefly, drug and polymer are dissolved in organic solvent miscible in water and the resultant solution is added to aqueous solution containing the salting out agent and stabilizer under constant stirring. Magnesium chloride, calcium chloride and magnesium acetate are the commonly used salting out agents. This salting out agents prevents the miscibility of organic solvent in aqueous phase resulting in the formation of emulsion. A reverse salting out effect obtained by dilution of emulsion with excess amount of water, leads to the precipitation of polymer, which encapsulates the drug in the polymer matrix results in the formation of nanoparticles. The cross flow filtration technique is used to remove the residual solvent and salting out agents.20,34
Solvent Evaporation Technique
Solvent evaporation technique is very commonly used for the preparation of polymeric nanoparticles. Briefly, the polymer is dissolved in an organic solvent into which the drug is dissolved / dispersed. The resultant solution is then added to the aqueous phase containing surfactant / emulsifying agent like poly vinyl alcohol, polysorbate 80, poloxamer 188 etc., under high homogenization to form an emulsion. After the formation of stable emulsion, the organic solvent is evaporated / removed either by increasing the temperature under reduced pressure or by continuous stirring. The nanosuspension produced is freeze dried using 5% mannitol as a cryoprotectant to obtain a fine powder of nanoparticles.35,36
Spray Drying Technique
Spray drying technique is a well-established method commonly used in pharmaceutical industries for producing a drug powder from a liquid phase. Rotary atomizers and pressure nozzles are used in spray dryer for fine droplet generation, utilizing vibrating mesh technology. Briefly, drug and polymer are dissolved in ultrapure water with surfactant (tween 80) and filtered through 0.45 µm syringe filter prior to spray drying to minimize blockage. The resultant solution is then spray dried at a range of outlet temperature varied between 30 and 55°C under aforementioned conditions. Millions of precisely sized droplets are formed by the vibration of mesh upwards and downwards caused by the piezoelectric actuator driven at an ultrasonic frequency (i.e., 60 KHz). The novel electrostatic particle collector, consisting of a grounded star electrode (cathode) and cylindrical particle collecting electrode (anode) are used to collect the fine powder with high efficiency.20,26
Supercritical Fluid Technology
Supercritical fluid technology offers an interesting and effective production of polymeric nanoparticles by avoiding the use of organic solvent. However, environmental friendly solvents, with the potential to produce the polymeric nanoparticles with high purity and without any trace of organic solvent are used. Briefly, drug and polymers are dissolved in a supercritical fluid to form a solution, followed by the rapid expansion of the solution across an orifice or a capillary nozzle into ambient air. High degree of super saturation accompanied by the rapid pressure reduction in the expansion, results in the homogenous nucleation and well dispersed uniform sized nanoparticles.37,38
Determination of Priority Weight and Ranking
Figure 2 shows the proposed hierarchy model for selecting the suitable method, where the first level is the overall objective. The figure also shows the three main criteria in the second, eight sub-criteria in the third and eight alternatives in the last level. Assessment of the criteria weights are developed using the pair-wise comparison.39-41
According to AHP method, the elements of one level are pair-wise compared with the elements of next higher level resulting in a number of pair-wise comparison matrixes. The pair-wise comparisons were made using the saaty’s scale (Table 3). The pair-wise comparison weights of the ith criteria against the jth criteria are assigned as follows
Table 3. The fundamental scale for pair-wise comparison.
| Intensity of Importance | Definition | Explanation |
| 1 | Equal importance | Two activities contribute equally to the objective |
| 2 | Weak or Slight | |
| 3 | Moderate importance | Experience and judgement slightly favour one activity over another |
| 4 | Moderate plus | |
| 5 | Strong importance | Experience and judgement strongly favour one activity over another |
| 6 | Strong plus | |
| 7 | Very strong or demonstrated importance | An activity is favoured very strongly over another; its dominance demonstrated in practice |
| 8 | Very very strong | |
| 9 | Extreme importance | The evidence favouring one activity over another is of the highest possible order of affirmation |
| Reciprocals of above | If activity i has one of the above non-zero numbers assigned to it when compared with activity j, then j has the reciprocal value when compared with i | A reasonable assumption |
If criteria i and j are equally important, then
Aij = wi/wj = 1 and Aji = wj/wi = 1
If criteria i be moderately more important than criteria j, then
Aij = wi/wj = 3 and Aji = wj/wi = 1/3
If criteria i be extremely more important than criteria j, then
Aij = wi/wj = 9 and Aji = wj/wi = 1/9
The AHP method requires the following pair-wise comparison matrix A, which contains the relative weights of the criteria
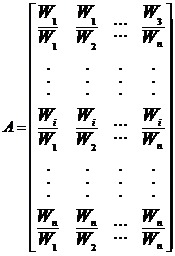
Where wi is the importance weight of the ith criteria with respect to goal, or the importance weight of the ith subcriteria (i = 1, . . . , n) with respect to criteria and so on. Furthermore, the importance weights can be obtained using the following equation.
Aw = λmaxw
Where, λmax is the maximum eigen-value of the matrix and w = (w1, . . . , wn) is the corresponding eigen-vector of A. Expert choice software was used to compute the overall priority weight of each alternative. Among the eight alternatives, an alternative with the highest priority weight is the suitable method for the preparation of camptothecin loaded polymeric nanoparticles.39,40,42
Consistency Ratio (CR)
The Consistency ratio is calculated to determine inconsistencies in the evaluation. The value of all above pair-wise comparison matrix should be lower than 0.1, indicating that the expert’s judgements/weights allotted are reasonable. In order to calculate the consistency ratio, eigen-value λmax is obtained from the matrix A. The degree of consistency (CI) can be estimated as shown in the following expression.42,43
CI = λmax – n / n – 1
Consistency ratio (CR) can be calculated from the relation of the consistency index (CI) and the random consistency index (RI). The RI value is obtained from Table 4 and the value depends on the value of n.42,43
Table 4. Random Index (RI) of analytical hierarchy process .
| N | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| RI | 0 | 0 | 0.58 | 0.9 | 1.12 | 1.24 | 1.32 | 1.41 | 1.45 | 1.49 |
CR = CI / RI
Results and Discussion
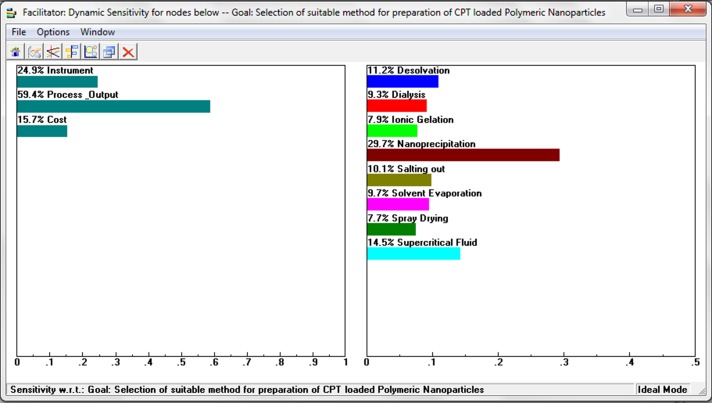
Pair-wise comparison matrix (Figure 3 to 10) was constructed by assigning the weights to all the elements using the saaty’s scale. All the constructed pair-wise comparison matrixes were found to be consistent, as the consistency ratio was < 0.1. Hence, the weights allotted were reasonable. From the pair-wise comparison matrix, priority weights from each sub-criterion are calculated and ranks are assigned based on overall priority weights. Table 5 shows the overall priority weight and ranking of potential eight alternatives obtained from AHP methods. Out of eight alternatives, the fourth alternative nanoprecipitation received a highest overall priority weight of 0.297 followed by supercritical fluid technique with 0.144, desolvation technique with 0.111, salting out technique with 0.100, solvent evaporation technique with 0.097, dialysis technique with 0.093, ion gelation technique with 0.079 priority weights. However, the spray drying technique received the least overall priority weight of 0.077. The sensitivity investigation of the decisions made is shown in the Figure 11.
Figure 3 .
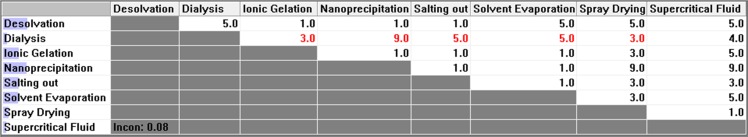
Pair-wise comparison matrix with respect to instrument availability
Figure 10 .
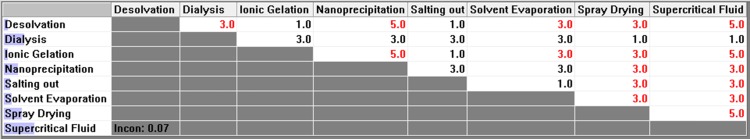
Pair-wise comparison matrix with respect to minimum preparation cost
Table 5. Overall priority weights and ranking of the potential alternatives .
| SC01 | SC02 | SC03 | SC04 | SC05 | SC06 | SC07 | SC08 | Overall Priority Weight | Rank | |
| DS | 0.045 | 0.012 | 0.004 | 0.004 | 0.016 | 0.002 | 0.008 | 0.02 | 0.111 | 3 |
| DI | 0.008 | 0.003 | 0.001 | 0.004 | 0.032 | 0.002 | 0.037 | 0.006 | 0.093 | 6 |
| IG | 0.029 | 0.012 | 0.002 | 0.003 | 0.011 | 0.003 | 0.008 | 0.011 | 0.079 | 7 |
| NP | 0.043 | 0.02 | 0.017 | 0.01 | 0.112 | 0.018 | 0.025 | 0.052 | 0.297 | 1 |
| SO | 0.029 | 0.013 | 0.007 | 0.002 | 0.018 | 0.004 | 0.01 | 0.017 | 0.100 | 4 |
| SE | 0.027 | 0.014 | 0.001 | 0.003 | 0.025 | 0.004 | 0.014 | 0.009 | 0.097 | 5 |
| SD | 0.009 | 0.004 | 0.003 | 0.009 | 0.007 | 0.009 | 0.032 | 0.004 | 0.077 | 8 |
| SF | 0.006 | 0.003 | 0.005 | 0.007 | 0.053 | 0.013 | 0.055 | 0.002 | 0.144 | 2 |
Inconsistency = 0.07
Figure 11 .
Sensitivity analysis
Figure 4 .
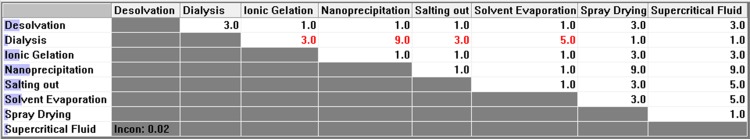
Pair-wise comparison matrix with respect to instrument backup
Figure 5 .
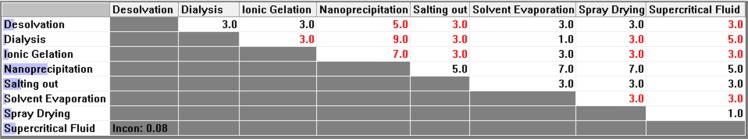
Pair-wise comparison matrix with respect to ease of operation
Figure 6 .
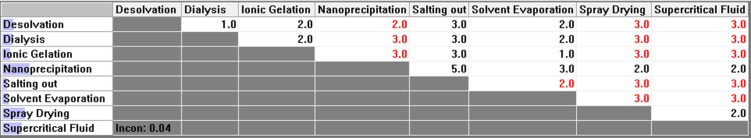
Pair-wise comparison matrix with respect to minimum number of excipients
Figure 7 .
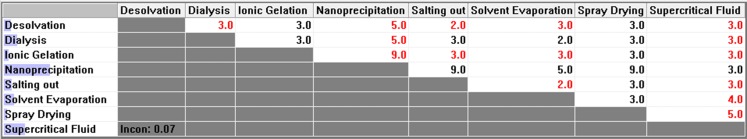
Pair-wise comparison matrix with respect to minimum Average particle size & PDI
Figure 8 .
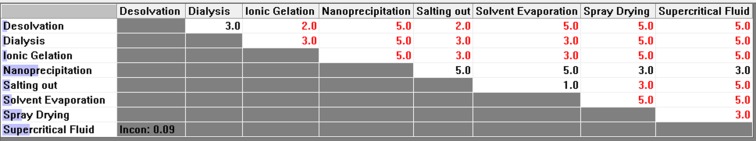
Pair-wise comparison matrix with respect to maximum yield of nanoparticles
Figure 9 .
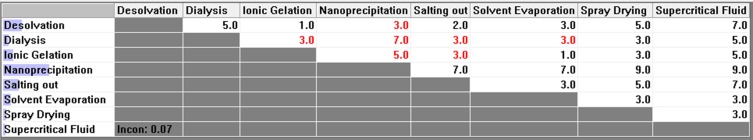
Pair-wise comparison matrix with respect to reproducible results
Conclusion
In the present study, we investigated the problem of selecting the suitable method for the preparation of camptothecin loaded polymeric nanoparticles using a multi-criteria decision making approach implementing the analytical hierarchy process. This paper proposed a hierarchy model consisting of three main criteria with eight sub-criteria and eight alternatives. Expert choice software was used to compute the overall priority weight of each alternative. The results of the study revealed that the nanoprecipitation technique is the most suitable method for the preparation of camptothecin loaded polymeric nanoparticles with the highest overall priority weight of 0.297 than any other methods.
Ethical Issues
Not applicable.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Watanabe M, Kawano K, Yokoyama M, Opanasopit P, Okano T, Maitani Y. Preparation of camptothecin-loaded polymeric micelles and evaluation of their incorporation and circulation stability. Int J Pharm . 2006;308(1-2):183–9. doi: 10.1016/j.ijpharm.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 2.Peng CL, Lai PS, Lin FH, Yueh-Hsiu Wu S, Shieh MJ. Dual chemotherapy and photodynamic therapy in an HT-29 human colon cancer xenograft model using SN-38-loaded chlorin-core star block copolymer micelles. Biomaterials . 2009;30(21):3614–25. doi: 10.1016/j.biomaterials.2009.03.048. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Yang M, Wang Q, Li Y, Guo R, Jiang X. et al. 10-Hydroxycamptothecin loaded nanoparticles: Preparation and antitumor activity in mice. J Control Release . 2007;119(2):153–62. doi: 10.1016/j.jconrel.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Venditto VJ, Simanek EE. Cancer therapies utilizing the camptothecins: a review of the in vivo literature. Mol Pharm . 2010;7(2):307–49. doi: 10.1021/mp900243b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Botella P, Abasolo I, Fernandez Y, Muniesa C, Miranda S, Quesada M. et al. Surface-modified silica nanoparticles for tumor-targeted delivery of camptothecin and its biological evaluation. J Control Release . 2011;156(2):246–57. doi: 10.1016/j.jconrel.2011.06.039. [DOI] [PubMed] [Google Scholar]
- 6.Moghimi SM, Hunter AC, Murray JC. Nanomedicine: current status and future prospects. FASEB J . 2005;19(3):311–30. doi: 10.1096/fj.04-2747rev. [DOI] [PubMed] [Google Scholar]
- 7.Sahoo SK, Dilnawaz F, Krishnakumar S. Nanotechnology in ocular drug delivery. Drug Discov Today . 2008;13(3-4):144–51. doi: 10.1016/j.drudis.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Koo OM, Rubinstein I, Onyuksel H. Role of nanotechnology in targeted drug delivery and imaging: a concise review. Nanomedicine . 2005;1(3):193–212. doi: 10.1016/j.nano.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Sahoo SK, Labhasetwar V. Nanotech approaches to drug delivery and imaging. Drug Discov Today . 2003;8(24):1112–20. doi: 10.1016/s1359-6446(03)02903-9. [DOI] [PubMed] [Google Scholar]
- 10.Faraji AH, Wipf P. Nanoparticles in cellular drug delivery. Bioorg Med Chem . 2009;17(8):2950–62. doi: 10.1016/j.bmc.2009.02.043. [DOI] [PubMed] [Google Scholar]
- 11.Hughes GA. Nanostructure-mediated drug delivery. Nanomedicine . 2005;1(1):22–30. doi: 10.1016/j.nano.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Kilicay E, Demirbilek M, Turk M, Guven E, Hazer B, Denkbas EB. Preparation and characterization of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHX) based nanoparticles for targeted cancer therapy. Eur J Pharm Sci . 2011;44(3):310–20. doi: 10.1016/j.ejps.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric Nanoparticles. Mol Pharm . 2008;5(4):505–15. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces . 2010;75(1):1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Hamidi M, Azadi A, Rafiei P. Hydrogel nanoparticles in drug delivery. Adv Drug Deliv Rev . 2008;60(15):1638–49. doi: 10.1016/j.addr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev . 2002;54(5):631–51. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 17.Hans ML, Lowman AM. Biodegradable nanoparticles for drug delivery and targeting. Curr Opin Solid State Mater Sci . 2002;6(4):319–27. [Google Scholar]
- 18.Mahmoudi M, Sant S, Wang B, Laurent S, Sen T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv Drug Deliv Rev . 2011;63(1-2):24–46. doi: 10.1016/j.addr.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Mohanraj VJ, Chen Y. Nanoparticles - A review. Trop J Pharm Res . 2006;5(1):561–73. [Google Scholar]
- 20.Rao JP, Geckeler KE. Polymer nanoparticles: Preparation techniques and size-control parameters. Prog Polym Sci . 2011;36(7):887–913. [Google Scholar]
- 21.Moorthi C, Kathiresan K. Fabrication of Dual Drug Loaded Polymeric Nanosuspension: Incorporating Analytical Hierarchy Process and Data Envelopment Analysis in the Selection of a Suitable Method. Int J Pharm Pharm Sci . 2013;5(2):499–504. [Google Scholar]
- 22.Mohajeri N, Amin GR. Railway station site selection using analytical hierarchy process and data envelopment analysis. Comput Ind Eng . 2010;59(1):107–114. [Google Scholar]
- 23.Murat BC, Meral S. The analytic hierarchy and Analytic network processes. Hacet J Math Stat . 2003;32:65–73. [Google Scholar]
- 24.Yousefpour M, Rahimi A. Characterization and selection of optimal parameters to achieve the best tribiological performance of the electrodeposited Cr nanocomposite coating. Mater Des . 2014;54:382–9. [Google Scholar]
- 25.Morose G. The 5 principles of design for safer nanotechnology. J Clean Prod . 2010;18(3):285–9. [Google Scholar]
- 26.Pinto Reis C, Neufeld RJ, Ribeiro AJ, Veiga F. Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomedicine. 2006;2(1):8–21. doi: 10.1016/j.nano.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Weber C, Coester C, Kreuter J, Langer K. Desolvation process and surface characterisation of protein nanoparticles. Int J Pharm . 2000;194(1):91–102. doi: 10.1016/s0378-5173(99)00370-1. [DOI] [PubMed] [Google Scholar]
- 28.Elzoghby AO, Samy WM, Elgindy NA. Albumin-based nanoparticles as potential controlled release drug delivery systems. J Control Release . 2012;157(2):168–82. doi: 10.1016/j.jconrel.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 29.Liu M, Zhou Z, Wang X, Xu J, Yang K, Cui Q. et al. Formation of poly (L, D-lactide) spheres with controlled size by direct dialysis. Polymer . 2007;48(19):5767–79. [Google Scholar]
- 30.Choi SW, Kim JH. Design of surface-modified poly(D,L-lactide-co-glycolide) nanoparticles for targeted drug delivery to bone. J Control Release . 2007;122(1):24–30. doi: 10.1016/j.jconrel.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Fan W, Yan W, Xu Z, Ni H. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf B Biointerfaces . 2012;90:21–7. doi: 10.1016/j.colsurfb.2011.09.042. [DOI] [PubMed] [Google Scholar]
- 32.Rajan M, Raj V. Encapsulation, characterisation and in-vitro release of anti-tuberculosis drug using chitosan - poly ethylene glycol nanoparticles. Int J Pharm Pharm Sci . 2012;4(4):255–9. [Google Scholar]
- 33.Fessi H, Puisieux F, Devissaguet JP, Ammoury N, Benita S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int J Pharm . 1989;55(1):R1–4. [Google Scholar]
- 34.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release . 2001;70(1-2):1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 35.Jaiswal J, Gupta SK, Kreuter J. Preparation of biodegradable cyclosporine nanoparticles by high-pressure emulsification-solvent evaporation process. J Control Release . 2004;96(1):169–78. doi: 10.1016/j.jconrel.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 36.Hoa LTM, Chi NT, Nguyen LH, Chien DM. Preparation and characterisation of nanoparticles containing ketoprofen and acrylic polymers prepared by emulsion solvent evaporation method. J Exp Nanosci . 2012;7(2):189–97. [Google Scholar]
- 37.Varshosaz J, Hassanzadeh F, Mahmoudzadeh M, Sadeghi A. Preparation of cefuroxime axetil nanoparticles by rapid expansion of supercritical fluid technology. Powder Technol . 2009;189(1):97–102. [Google Scholar]
- 38.Sekhon BS. Supercritical fluid technology: An overview of pharmaceutical applications. Int J PharmTech Res . 2010;2(1):810–26. [Google Scholar]
- 39.Saaty TL. The analytical hierarchy process: planning, priority setting, resource allocation. New York: McGraw-Hill International Book Co; 1980. [Google Scholar]
- 40.Saaty TL. Relative measurement and its generalization in decision making: Why pair-wise comparisons are central in mathematics for the measurement of intangible factors, the analytic hierarchy/network process. Rev R Acad Cien Serie A Mat . 2008;102(2):251–318. [Google Scholar]
- 41.Perez-Vegaa S, Peter S, Salmeron-Ochoa I, Nieva-de la Hidalga A, Sharratt PN. Analytical hierarchy processes (AHP) for the selection of solvents in early stages of pharmaceutical process Development. Process Saf Environ Prot . 2011;89(4):261–7. [Google Scholar]
- 42.Saaty TL. Decision making with the analytic hierarchy process. Int J Services Sciences . 2008;1(1):83–98. [Google Scholar]
- 43.Velmurugan R, Selvamuthukumar S, Manavalan R. Multi criteria decision making to select the suitable method for the preparation of nanoparticles using an analytical hierarchy process. Pharmazie. 2011;66(11):836–42. [PubMed] [Google Scholar]