Abstract
Purpose: The purpose of this study was to evaluate the effect of intrahippocampal injection of vitamin C and progesterone, alone or in combination, on passive avoidance learning (PAL) in multiple sclerosis.
Methods: Sixty- three male wistar rats were divided into nine groups (n=7) as following: control (saline), lesion, vitamin C (0.2, 1, 5 mg/kg), progesterone (0.01, 0.1, 1 µg/µl) and combination therapy. Lesion was induced by intrahippocampal injection of ethidium bromide. In combination therapy, animals were treated with vitamin C (5 mg/kg) plus progesterone (0.01 mg/kg). Animals in experimental groups received different treatments for 7 days, and then all groups were tested for step through latency (STL).
Results: Our results showed that intrahippocampal injection of ethidium bromide destroys PAL significantly (p<0.001). Treatment with vitamin C (5mg/kg) significantly (p<0.05) improved PAL. Lower doses of progesterone did not affect latency but dose of 1 µg/µl significantly (p<0.05) increased STL. In combination therapy group STL was significantly (p<0.05) more than in the lesion group, although it was not significantly different from the vitamin C group.
Conclusion: Based on our results, we concluded that intrahippocampal injection of vitamin C improves memory for PAL, but progesterone alone or in combination with vitamin C had no improving effects on memory.
Keywords: MS, Vitamin C, Progesterone, Passive avoidance, Intrahippocampal
Introduction
Multiple sclerosis is a chronic inflammatory disease of the central nervous system (CNS) in which the myelin sheet of the nerve fibers is destroyed.1 Myelin is a unique component of the nervous system which increases the efficiency and speed of action potential through the nerve cells.2 Without myelin to protect neurons, the brain and spinal cord signals that allow us to interact with the environment malfunction.3
Hippocampus is the center of learning and memory in the central nervous system. This area is very sensitive and vulnerable in neurological diseases and ischemia4 and severely affected by oxidative damage.5 Dentate gyrus (DG) of the hippocampus, because of its neural stem cells has the ability of restoration and proliferation of cells in rodents and humans throughout life.6 Cognitive impairment occurs in more than 65% of patients with MS, and usually their ability to recall previously learned information reduces.3 Due to the high vulnerability of the hippocampus and the capability of reconstruction, it is the appropriate area for studying the mechanisms involved in this process.
Antioxidants have a protective role in neurons (inhibition of apoptosis) and are involved in nerve regeneration.7 Ascorbic acid (Vitamin C) is a low molecular weight antioxidant that scavenges the reactive oxygen species (ROS) through electron transfer rapidly and prevents lipid peroxidation.8 While it reduces lipid peroxidation, it increases catalase enzymatic activity in the brain, which has the compensatory mechanisms in the formation of free radicals.9 The important role of oxidative stress of the hippocampus is shown in passive avoidance memory deficits.10 Vitamin C is also an essential element to promote myelination.11 Asymmetric distribution of vitamin C in different regions of the brain indicates its vital role in the brain. Vitamin C is transmitted through sodium-dependent vitamin C transporter type 2 (SVCT2) into neurons and the brain12 and the neuronal protective role of vitamin C is in relation to the essential role of this transporter.13 SVCT 2 is found more frequently in dense regions of the neurons in the brain such as the hippocampus, cortex and cerebellum where the issue is related to the regional distribution of ascorbate in the brain. Ascorbate participates in the synthesis of collagen, which is associated with the formation of myelin.12 Vitamin C also can boost learning, memory, and impede memory deficiencies in different experimental conditions.14 It has positive effects on acquisition and retrieval procedure of passive avoidance learning and memory in rats.15
Sex hormones affect the MS disease process.16 Progesterone is a possible factor to boost myelination by oligodendrocytes in the CNS.2 Progesterone has different effects on neurogenesis, regeneration, myelination, recovery from traumatic brain injury and inflammation. Progesterone receptors are located in regions that are involved in fear, stress and anxiety17 such as the hippocampus.18 In regards to progesterone receptors, they are located in the dorsal hippocampus, location of DG, which may have an important role in different aspects of memory.19
Steroid hormones affect different functions of the brain such as mood, cognition, reproductive and motor behavior in a sex-dependent process, and this gender difference exists in cognitive areas like neocortex, hippocampus and amygdala. On the other hand, it is reported that progesterone has variant effects such as being ineffective, worsening or improving of disease.20 Progesterone modulates the improvement effect of estradiol-induced on memory retention.21 Steroid hormones also attenuate peroxide production and leakage of free radicals from mitochondria of brain and reduce oxidative stress.22 Sex steroids decrease ROS via scavenging these products in the brain.2
To further substantiate the effects of vitamin C and progesterone on memory, intrahippocampal administration of vitamin C or progesterone alone or in combination was investigated in ethidium bromide-induced MS in rats.
Materials and Methods
Animals
The Regional Ethics Committee of Tabriz University of Medical Sciences approved all experimental procedures. Every effort was made to minimize the number of animals used and their suffering. Animals were obtained from the colony of Tabriz University of Medical Sciences. The experiments were performed on adult male wistar rats weighting 220-300g and 3-4 months old at the start of the experiments. The animals were housed in a temperature (23±1°C) and humidity-controlled room. The animals were maintained under a 12:12 h light/ dark cycle, with lights off at 8:00 p.m. Food and water was provided ad libitum except for the periods of behavioral testing. The behavioral testing was done during the light phase.
The experiments were performed on sixty -three rats randomly divided into 9 groups (n=7) as follows:
Control group: that received intrahippocampal saline (as solvent)
Lesion group: that received 3 μl intrahippocampal ethidium bromide 0.01%
Vitamin C groups: that received different doses (0.2, 1, 5 mg/kg) of vitamin C after lesion
Progesterone groups: that received different doses (0.01, 0.1, 1 μg/μl) of progesterone after lesion
Combination therapy group: that received vitamin C (5 mg/kg) and progesterone (0.01 μg/μl) after lesion
Surgery
Before surgery, animals were anesthetized with i.p. injections of ketamine (60 mg/kg body weight) and xylazine (12 mg/kg body weight)23 and placed on rat stereotaxic instrument in the skull-flat position. Demyelination was induced bilaterally by direct single injection of 3 μl of 0.01% ethidium bromide in sterile 0.9% saline24 at the rate of 1 μl/min into the DG of hippocampal formation, using appropriate stereotaxic coordinates (AP = -3.8; ML = +2; DV = +3.6).25 Animals in experimental groups received different concentration of vitamin C (0.2, 1, 5 mg/kg)26 or progesterone (0.01, 0.1, 1 μg/μl)19 for 7 days post lesion.
Step-through passive avoidance apparatus
The apparatus used for PA training and procedure were fundamentally the same as described in our previous studies.27 It consisted of a two compartment box. An illuminated chamber (30 × 21 × 20 cm3) made of transparent plastic was connected by a guillotine door to the dark compartment (30 × 21 × 20 cm3) that had black opaque walls and ceiling. The floors of the two compartments were constructed of stainless steel rods (3 mm in diameter, 10 mm apart) through which footshock could be delivered from a constant current source.
Retention test
After 24 h of PA training, the rat was placed in the illuminated chamber and 10s later the guillotine door was raised and the latency of entering the dark compartment (STL: step-through latency) was recorded during 10 mins.
Analysis
Data are expressed, as means ± SEM. The statistical analysis of the data (latency 24 h after training) was carried out by one-way ANOVA-followed by Tukey's test. In all comparisons, P < 0.05 was considered significant.
Results
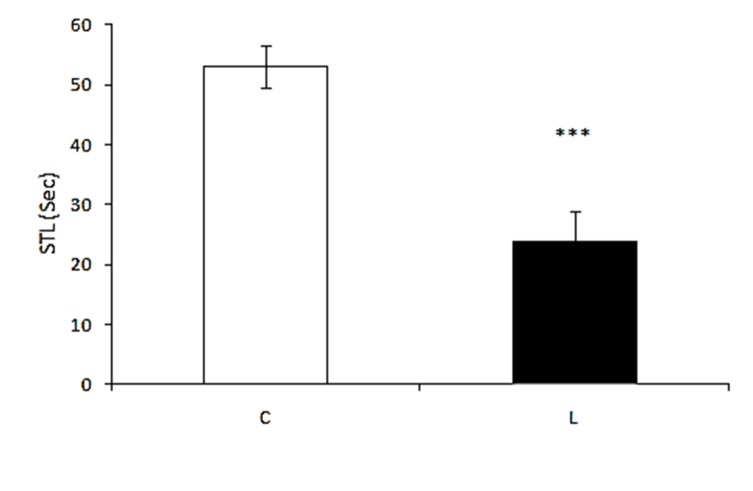
As shown in Figure 1, an independent t-test indicated that there is a significant difference (F=1.520, p<0.001) in STL between the control (53.38±12.64) and lesion (23.71±1.88) groups. This result suggests that injection of ethidium bromide in DG of hippocampus effectively reduces STL and deteriorates passive avoidance learning.
Figure 1 .
The effect of pretraining intrahippocampal injection of ethidium bromide on STL. C: control, L: lesion. ***p<0.001 when compared with control group. Data are expressed as mean ± SEM for n = 7 animals per group.
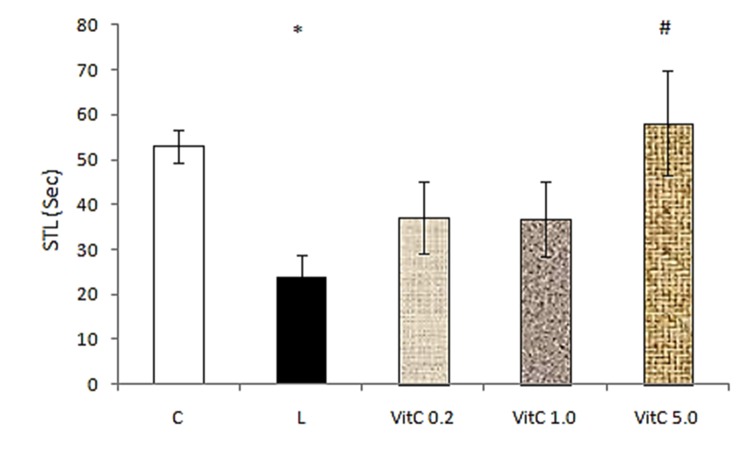
A one-way ANOVA comparison between groups also showed that there is a significant difference (F= 3.946, p<0.05) between lesion group (23.71±1.88) and the group that received 5 mg/kg vitamin C intrahippocampally (58.14±27.96) (Figure 2).
Figure 2 .
The effect of pretraining intrahippocampal injection of different doses of vitamin C on STL. C: control, L: lesion, Vit C: vitamin C with doses of 0.2, 1.0 and 5.0 mg/kg. *p<0.05 when compared with control group and #p<0.05 when compared with lesion group. Data are expressed as mean ± SEM for n = 7 animals per group.
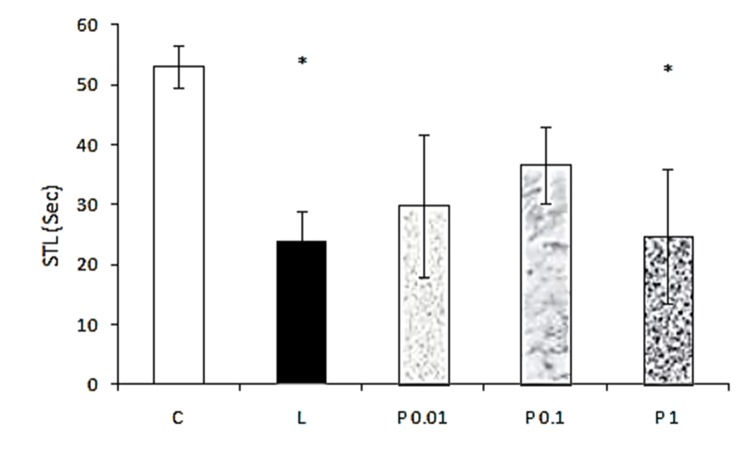
Statistical analysis of STL in progesterone groups (which is shown in Figure 3) showed that induction of lesion with ethidium bromide in the hippocampus and injection of high dose of progesterone (1 μg/μl) significantly (F=3.391, p<0.05) decreased passive avoidance learning.
Figure 3 .
The effect of pretraining intrahippocampal injection of different doses of progesterone on STL. C: control, L: lesion, P: progesterone with doses of 0.01, 0.1 and 1.0 μg/μl. *p<0.05 when compared with control group. Data are expressed as mean ± SEM for n = 7 animals per group.
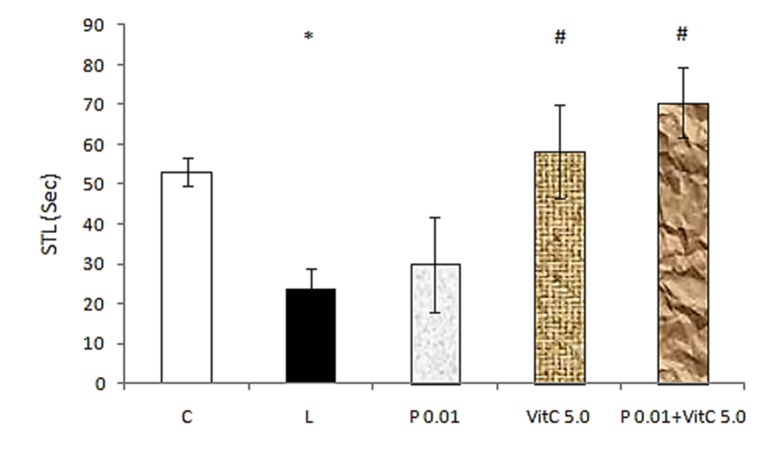
As illustrated in Figure 4, a one-way ANOVA revealed that the STL in combination therapy group (0.01 μg/μl of progesterone + 5 mg/kg of vitamin C) was significantly (F=5.691, p<0.05) more than the lesion group (23.71±1.88) and combination therapy could promote passive avoidance learning. However, there was no difference between the effective dose of vitamin C and combination group (Figure 4). These results together showed that progesterone could not potentiate the effect of vitamin C on memory.
Figure 4 .
The effect of pretraining intrahippocampal injection of vitamin C (5mg/kg) plus progesterone (0.01 μg/μl) on STL. C: control, L: lesion, P 0.01: progesterone 0.01 μg/μl, Vit C vitamin C 5 mg/kg. *p<0.05 when compared with control group and #p<0.05 when compared with lesion group. Data are expressed as mean ± SEM for n = 7 animals per group.
Discussion
In the present study, the effect of vitamin C and progesterone was evaluated on memory in MS. Their effect was assessed on a passive avoidance task through the intrahippocampal administration in male wistar rats. In order to induce MS, direct injection of ethidium bromide in DG region of hippocampus was performed and then the effect of vitamin C and progesterone on passive avoidance memory investigated. Vitamin C had a promoting effect on memory task and increased STL in comparison with the vehicle group. Progesterone in lower doses had no effect on STL, but in a high dose significantly decreased PAL. This experience does not appear to be due to a nonspecific effect of intrahippocampal injection, while saline injection had no effect on the learning capability of rats.
Intrahippocampal microinjection of vitamin C (5 mg/kg in) in our study increased STL significantly and our results are compatible with the results of other studies.15,28-31 A study of the oral supplementation of vitamin C indicated that it could attenuate the risk of dementia in aged mice.28 Shahidi et al. (2008) showed that i.p. administration of vitamin C could improve acquisition and retention in passive avoidance process in intact rats.15 Two different studies also showed that i.p. injection of vitamin C could be useful in retention of memory in the scopolamine treated rats29 and impede amnesia in homocysteine administered rats.30
One of the possible mechanisms of vitamin C on learning and memory is the modulatory role on neurotransmitter systems such as cholinergic14,29 and serotonergic29 systems. In addition, vitamin C restores acetyl cholinesterase activity, which has an essential role in learning and memory processes.31
Hippocampus has an important role in learning and memory processes32 and oxidative stress is considered to be a probable caused due to impairment in hippocampal function.10 Vitamin C is an antioxidant14,33,34 that scavenges the ROS and prevents lipid per oxidation;8 therefore, intrahippocampal injection of vitamin C probably has mediated its function through one or some of these mechanisms in this study.
Intrahippocampal administration of progesterone in this study had different effects on passive avoidance learning and memory. Progesterone has different effects in memory including enhancement, no effect or decrease of memory formation.35,36 El-Bakri et al. (2004) indicated that progesterone treatment in ovariectomized rats did not show significant learning compared to the vehicle treated groups in a Morris Water Maze task.37 On the other hand, some studies demonstrated that subcutaneous injection of progesterone impairs social recognition memory,18 spatial working and reference memory performance, cognition function and avoidance tasks in young rats and mice,38 but it does not impair novel object recognition task.36 Our results are consistent with El-Bakri et al. (2004) study.
Progesterone has different mechanisms depending on the type of task. Intraperitoneal injection of progesterone in mice can impair retention of memory through facilitation and reinforcement of GABAergic activity, which attenuates arousal levels.35,39 Some studies indicated that reduction of neuronal activity through activation of GABA receptors by progesterone may produce analgesic or anxiolytic that undermines cognitive function.19,36 It is also important to consider that as males have higher levels of steroid receptor co-activators, which enhance steroid hormone action in many brain regions,40 it is likely that lower levels of progesterone are sufficient to elicit a physiological response within the male brain.18 These data are consistent with that in female mice that have lower plasma levels of progesterone in diestrus, which helps them to learn to avoid footshock faster than females in estrus.39 In our study dose, 1 µg/µl, progesterone deteriorated the passive avoidance learning that is consistent with the findings of Bychowski 2012 and Bimonte-Nelson 2004. Given the observed different effects of progesterone, it indicates that various factors affected the results such as: model of administration, behavioral test kind, gender, age of animal, time of hormone treatment, and dose of hormone.
Conclusion
In conclusion, this study showed that intrahippocampal injection of vitamin C (5 mg/kg) improves passive avoidance learning in ethidium bromide model of MS, whereas progesterone alone or in combination with vitamin C does not improve or affect the memory.
Acknowledgments
This study was financially supported by the Neuroscience Research Centre of Tabriz University of Medical Sciences. The article is derived from the MSc dissertation of Ms Faezeh Mehrvash entitled ‘‘The effect of progesterone and Vitamin C on passive avoidance learning, cell death and demyelination induced by ethidium bromide in hippocampus of male wistar rats’’.
Ethical Issues
Not applicable.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Cudrici C, Niculescu T, Niculescu F, Shin ML, Rus H. Oligodendrocyte cell death in pathogenesis of multiple sclerosis: Protection of oligodendrocytes from apoptosis by complement. J Rehabil Res Dev . 2006;43(1):123–32. doi: 10.1682/jrrd.2004.08.0111. [DOI] [PubMed] [Google Scholar]
- 2.Kipp M, Amor S, Krauth R, Beyer C. Multiple sclerosis: neuroprotective alliance of estrogen-progesterone and gender. Front Neuroendocrinol . 2012;33(1):1–16. doi: 10.1016/j.yfrne.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Rahn K, Slusher B, Kaplin A. Cognitive impairment in multiple sclerosis: a forgotten disability remembered. Cerebrum . 2012;2012:14. [PMC free article] [PubMed] [Google Scholar]
- 4.Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N. et al. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell . 2002;110(4):429–41. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 5.Shin CM, Chung YH, Kim MJ, Lee EY, Kim EG, Cha CI. Age-related changes in the distribution of nitrotyrosine in the cerebral cortex and hippocampus of rats. Brain Res . 2002;931(2):194–9. doi: 10.1016/s0006-8993(01)03391-1. [DOI] [PubMed] [Google Scholar]
- 6.Becq H, Jorquera I, Ben-Ari Y, Weiss S, Represa A. Differential properties of dentate gyrus and CA1 neural precursors. J Neurobiol . 2005;62(2):243–61. doi: 10.1002/neu.20089. [DOI] [PubMed] [Google Scholar]
- 7.Moosmann B, Behl C. Antioxidants as treatment for neurodegenerative disorders. Expert Opin Investig Drugs . 2002;11(10):1407–35. doi: 10.1517/13543784.11.10.1407. [DOI] [PubMed] [Google Scholar]
- 8.Flora SJ, Tandon SK. Preventive and therapeutic effects of thiamine, ascorbic acid and their combination in lead intoxication. Acta Pharmacol Toxicol (Copenh) . 1986;58(5):374–8. doi: 10.1111/j.1600-0773.1986.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 9.Xavier SM, Barbosa CO, Barros DO, Silva RF, Oliveira AA, Freitas RM. Vitamin C antioxidant effects in hippocampus of adult Wistar rats after seizures and status epilepticus induced by pilocarpine. Neurosci Lett . 2007;420(1):76–9. doi: 10.1016/j.neulet.2007.04.056. [DOI] [PubMed] [Google Scholar]
- 10.Silva RH, Abilio VC, Takatsu AL, Kameda SR, Grassl C, Chehin AB. et al. Role of hippocampal oxidative stress in memory deficits induced by sleep deprivation in mice. Neuropharmacology . 2004;46(6):895–903. doi: 10.1016/j.neuropharm.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 11.Eldridge CF, Bunge MB, Bunge RP, Wood PM. Differentiation of axon-related Schwann cells in vitro. I. Ascorbic acid regulates basal lamina assembly and myelin formation. J Cell Biol. 1987;105(2):1023–34. doi: 10.1083/jcb.105.2.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrison FE, May JM. Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2. Free Radic Biol Med . 2009;46(6):719–30. doi: 10.1016/j.freeradbiomed.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson JX. Vitamin C transport in animals and plants. In: Asard H, May J, Smirnoff N, editors. Vitamin C—Functions and biochemistry in animals and plants. Oxford: BIOS Scientific Publisher Ltd; 2003. p. 97-117.
- 14.Hasanein P, Shahidi S. Effects of combined treatment with vitamins C and E on passive avoidance learning and memory in diabetic rats. Neurobiol Learn Mem . 2010;93(4):472–8. doi: 10.1016/j.nlm.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Shahidi S, Komaki A, Mahmoodi M, Atrvash N, Ghodrati M. Ascorbic acid supplementation could affect passive avoidance learning and memory in rat. Brain Res Bull . 2008;76(1-2):109–13. doi: 10.1016/j.brainresbull.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Haldane J. Sex Hormone Treatments for Multiple Sclerosis. J Orthomol Med . 2012;27(2):87–92. [Google Scholar]
- 17.Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE. et al. Progesterone receptors: form and function in brain. Front Neuroendocrinol . 2008;29(2):313–39. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bychowski ME, Auger CJ. Progesterone impairs social recognition in male rats. Horm Behav . 2012;61(4):598–604. doi: 10.1016/j.yhbeh.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orr PT, Lewis MC, Frick KM. Dorsal hippocampal progesterone infusions enhance object recognition in young female mice. Pharmacol Biochem Behav . 2009;93(2):177–82. doi: 10.1016/j.pbb.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kipp M, Beyer C. Impact of sex steroids on neuroinflammatory processes and experimental multiple sclerosis. Front Neuroendocrinol . 2009;30(2):188–200. doi: 10.1016/j.yfrne.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behav Neurosci . 2001;115(2):384–93. [PubMed] [Google Scholar]
- 22.Irwin RW, Yao J, Hamilton RT, Cadenas E, Brinton RD, Nilsen J. Progesterone and estrogen regulate oxidative metabolism in brain mitochondria. Endocrinology . 2008;149(6):3167–75. doi: 10.1210/en.2007-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gisou M, Soheila R, Nasser N. Evaluation of the effect of intrahippocampal injection of leptin on spatial memory. Afr J Pharm Pharmacol . 2009;3(9):443–8. [Google Scholar]
- 24.Goudarzvand M, Javan M, Mirnajafi-Zadeh J, Mozafari S, Tiraihi T. Vitamins E and D3 attenuate demyelination and potentiate remyelination processes of hippocampal formation of rats following local injection of ethidium bromide. Cell Mol Neurobiol . 2010;30(2):289–99. doi: 10.1007/s10571-009-9451-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paxinos G, Watson C. The mouse brain in stereotaxic coordinates. 5th ed. Academic press; 2004.
- 26.Miura S, Ishida A, Nakajima W, Ohmura A, Kawamura M, Takada G. Intraventricular ascorbic acid administration decreases hypoxic-ischemic brain injury in newborn rats. Brain Res . 2006;1095(1):159–66. doi: 10.1016/j.brainres.2006.04.045. [DOI] [PubMed] [Google Scholar]
- 27.Babri S, Badie HG, Khamenei S, Seyedlar MO. Intrahippocampal insulin improves memory in a passive-avoidance task in male wistar rats. Brain Cogn . 2007;64(1):86–91. doi: 10.1016/j.bandc.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Arzi A, Hemmati AA, Razian A. Effect of vitamins C and E on cognitive function in mouse. Pharmacol Res . 2004;49(3):249–52. doi: 10.1016/j.phrs.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Lee L, Kang SA, Lee HO, Lee BH, Jung IK, Lee JE. et al. Effect of supplementation of vitamin E and vitamin C on brain acetylcholinesterase activity and neurotransmitter levels in rats treated with scopolamine, an inducer of dementia. J Nutr Sci Vitaminol (Tokyo) . 2001;47(5):323–8. doi: 10.3177/jnsv.47.323. [DOI] [PubMed] [Google Scholar]
- 30.Reis EA, Zugno AI, Franzon R, Tagliari B, Matte C, Lammers ML. et al. Pretreatment with vitamins E and C prevent the impairment of memory caused by homocysteine administration in rats. Metab Brain Dis . 2002;17(3):211–7. doi: 10.1023/a:1019982223034. [DOI] [PubMed] [Google Scholar]
- 31.Ambali SF, Idris SB, Onukak C, Shittu M, Ayo JO. Ameliorative effects of vitamin C on short-term sensorimotor and cognitive changes induced by acute chlorpyrifos exposure in Wistar rats. Toxicol Ind Health . 2010;26(9):547–58. doi: 10.1177/0748233710373086. [DOI] [PubMed] [Google Scholar]
- 32.Naber PA, Witter MP, Lopes Silva FH. Networks of the hippocampal memory system of the rat. The pivotal role of the subiculum. Ann N Y Acad Sci. 2000;911:392–403. doi: 10.1111/j.1749-6632.2000.tb06739.x. [DOI] [PubMed] [Google Scholar]
- 33.Carlson NG, Rose JW. Antioxidants in multiple sclerosis: do they have a role in therapy? . CNS Drugs. 2006;20(6):433–41. doi: 10.2165/00023210-200620060-00001. [DOI] [PubMed] [Google Scholar]
- 34.Gilgun-Sherki Y, Melamed E, Offen D. The role of oxidative stress in the pathogenesis of multiple sclerosis: the need for effective antioxidant therapy. J Neurol . 2004;251(3):261–8. doi: 10.1007/s00415-004-0348-9. [DOI] [PubMed] [Google Scholar]
- 35.Harburger LL, Bennett JC, Frick KM. Effects of estrogen and progesterone on spatial memory consolidation in aged females. Neurobiol Aging . 2007;28(4):602–10. doi: 10.1016/j.neurobiolaging.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 36.Sun WL, Luine VN, Zhou L, Wu HB, Weierstall KM, Jenab S. et al. Acute progesterone treatment impairs spatial working memory in intact male and female rats. Ethn Dis . 2010;20(1 Suppl 1):S1–83. [PubMed] [Google Scholar]
- 37.El-Bakri NK, Islam A, Zhu S, Elhassan A, Mohammed A, Winblad B. et al. Effects of estrogen and progesterone treatment on rat hippocampal NMDA receptors: relationship to Morris water maze performance. J Cell Mol Med . 2004;8(4):537–44. doi: 10.1111/j.1582-4934.2004.tb00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bimonte-Nelson HA, Singleton RS, Williams BJ, Granholm AC. Ovarian hormones and cognition in the aged female rat:: II. progesterone supplementation reverses the cognitive enhancing effects of ovariectomy. Behav Neurosci. 2004;118(4):707–14. doi: 10.1037/0735-7044.118.4.707. [DOI] [PubMed] [Google Scholar]
- 39.Farr SA, Flood JF, Scherrer JF, Kaiser FE, Taylor GT, Morley JE. Effect of ovarian steroids on footshock avoidance learning and retention in female mice. Physiol Behav . 1995;58(4):715–23. doi: 10.1016/0031-9384(95)00124-2. [DOI] [PubMed] [Google Scholar]
- 40.Bian C, Zhang D, Guo Q, Cai W, Zhang J. Localization and sex-difference of steroid receptor coactivator-1 immunoreactivities in the brain of adult female and male mice. Steroids . 2011;76(3):269–79. doi: 10.1016/j.steroids.2010.11.009. [DOI] [PubMed] [Google Scholar]