Abstract
Purpose: Monoclonal antibodies are potentially powerful tools used in biomedical research, diagnosis, and treatment of infectious diseases and cancers. The monoclonal antibody against Human IgA can be used as a diagnostic application to detect infectious diseases. The aim of this study was to improve an appropriate protocol for large-scale production of mAbs against IgA.
Methods: For large-scale production of the monoclonal antibody, hybridoma cells that produce monoclonal antibodies against Human IgA were injected intraperitoneally into Balb/c mice that were previously primed with 0.5 ml Pristane. After ten days, ascitic fluid was harvested from the peritoneum of each mouse. The ELISA method was carried out for evaluation of the titration of produced mAbs. The ascitic fluid was investigated in terms of class and subclass by a mouse mAb isotyping kit. MAb was purified from the ascitic fluid by ion exchange chromatography. The purity of the monoclonal antibody was confirmed by SDS-PAGE, and the purified monoclonal antibody was conjugated with HRP.
Results: Monoclonal antibodies with high specificity and sensitivity against Human IgA were prepared by hybridoma technology. The subclass of antibody was IgG1 and its light chain was the kappa type.
Conclusion: This conjugated monoclonal antibody could have applications in designing ELISA kits in order to diagnose different infectious diseases such as toxoplasmosis and H. Pylori.
Keywords: Large-scale, Generation, Monoclonal antibody, IgA
Introduction
In 1975, monoclonal antibodies (mAbs) were originally improved by Milstein and Kohler whenthey effectively fused an antibody-producing B-cell with an immortalized myeloma cell line,resulting in a hybridoma, or clone.1 Monoclonal antibodies are potentially powerful tools used in biomedical research, diagnosis and treatment of infectious diseases and cancers. They are also usefulfor investigating the function of cell surface markers and vaccine designs.1,2 Currently, monoclonal antibodies are molecules that possess a binding domain with an extreme affinity for a specific antigen; this characteristic of these antibodies has contributed to their routine usage in diagnostic kits, and reveals the function of such antigens that are involved in a number of physiological and pathological conditions.3,4 The detection of IgA may indicate recent infection or reactivation. In diagnostic kits designed to detect infectious diseases like acute and congenital toxoplasmosis, Hepatitis E and early detection HIV, monoclonal antibodies against human IgA conjugated with enzymes, fluorochromes or radioactive labels fulfill an important role.5-7 The diagnosis of viral infections (VZV, EBV and CMV) are made by detecting IgA antibodies.8 The IgA-specific mAbs have potential as immunochemical reagents to identify and quantify monomeric and polymeric IgA in human serum and secretions.9,10 Monoclonal antibodies are largely produced by these hybridomas,or cell lines of Balb/c mice hyperimmunized with the requested antigens. For large-scale generation of the monoclonal antibody, hybridoma cells must be grown by one of the following methods: in-vivo method; injection of requested clone into the abdominal cavity of a suitably prepared mouse (or in-vitro method); and culture of the cells in tissue culture flasks. Further processing of the mouse ascitic fluid and of the tissue culture supernatant are required to obtain mAb with the required purity and concentration. The mouse method is generally familiar, well understood, and widely available in many laboratories. The tissue culture methods have been expensive, time-consuming, and often fail to produce the required amount of antibodies without considerably skilled manipulation.11-13 Therefore, antibody production in ascitic fluid is an appropriate and economical method.14,15 The aim of this study was to produce large-scale amounts of monoclonal antibody against IgA in order tobe used in the diagnosis of infectious diseases.
Materials and Methods
Production of ascitic fluid in mice peritoneum
Four Balb/cfemale mice (4-6 weeks old) were obtained from the Pasteur Institute of Iran. 0.5 ml Pristane (2, 6, 10, 14 tetra methyl pentadecane, Sigma) was injected intraperitoneally into each mouse. Ten days after priming with Pristane, 1–2×106 cells per 0.5 ml PBS, from the suitable densities of a clone, were injected intraperitoneally into each mouse. Five days after the injection of hybridoma cells, the mice were investigated daily for production of ascitic fluid. About ten days later, the abdomens of the mice wereenlarged, and their skins were extended.Using 19 gauge needles, their ascitic fluid was harvested.After four days, the ascitic fluid of the mice was harvested again and centrifuged at 12,000 rpm for five min and the related supernatants were collected for purification and characterization.
Titration of antibody
The titer of the monoclonal antibodies was determined by the ELISA method. Wells of ELISA plates (Nunc,Germany) were coated with 100 µl of human IgA (5 μg/ml in PBS) and kept at 4°C overnight. The next day, the wells were washed three-times using the washing buffer PBS-T (PBS, pH 7.2+0.05% Tween-20) for 5 min. Non-specific sites of the wells were blocked with 2% BSA and incubated at 37°C for 1.5 hours. Wells were then washed three times, as mentioned, and then 100μl of the continuous dilution (two fold serial dilutions starting from 1:1000) of ascitic fluid were added to the wells. The plate was incubated at 37°C for 90 minutes and washed again with PBS-T. At the next step, 100 μl of a 1:4000 dilution of HRP-conjugated rabbit anti-mouse Ig (Sigma-Aldrich Co. Louis, USA) was added to the wells, and incubation continued for 90 minutes at 37°C. After washing, 100 μl of tetra methyl benzidine (TMB) substrate solution (Sigma-Aldrich Co.) was added to each well, and the plate was incubated at room temperature in a dark place. After 20 min, the reaction was stopped by adding 100μ1 of stopping solution (0.16 M H2SO4) per well and then the Optical Density (OD) of the reactions was read at 450 nmwith STAT FAX 303+ELISA reader.
Determination of mAbisotype
The class and subclass of the mAbs were determined with an ELISA mouse mAb isotyping kit (Thermo, USA). In this assay, we used ELISA strip-well plates with individual wells pre-coated with anti-mouse heavy-chain capture antibody (anti-IgG1, IgG2a, IgG2b, IgG3, IgA and IgM), or anti-mouse light-chain capture antibody (kappa or lambda). First, Tris buffer saline (TBS) was used for 1/100,000 dilution of the ascitic fluid and 50 µl of diluted antibody was added to each well of the 8-well strip. Then, 50 µl of the anti-mouse IgG1, IgG2a, IgG2b, IgG3, IgA, IgM HRP conjugated was added to each well of the 8-well strip and incubated for an hour at room temperature. After three washes, 75 µl of TMB substrate was added to each well and the plate was incubated at room temperature in a dark place for10 min. At the next step, the reaction was stopped by 75μl of a 5% solution of sulfuric acid. The absorbance of each well was read by ELISA Reader (STAT FAX 303+) at 450nm (Table 1).
Table 1. Determination of the isotype of mAb by ELISA.Classes and subclasses of monoclonal antibodies of 3-D5 monoclone .
| Class | IgG1 | IgG2a | IgG3 | IgG2b | IgA | IgM | kappa | Lambda |
| Clone 3-D5 | 1.053 | 0.139 | 0.137 | 0.101 | 0.114 | 0.119 | 1.724 | 0.124 |
Antibody purification
The ascitic fluids were diluted (at a ratio of 1:1) with PBS, and fractionated with a 40% saturated ammonium sulfate. After several rounds of washing with 40% ammonium sulfate, the fraction was centrifuged for 15 minutes in 5000g. The precipitated fraction was dialyzed against 0.05mM PBS, pH 7.4 and IgG was purified by ion exchange chromatography (DEAE-Sepharose 6B), which is a simple and economical method. The distinct antibody was eluted from the column through a washing buffer containing 50 mM NaCl, and the fractions were collected at 5 mL/20. Confirmation of the purified fractions was done by SDS-PAGE in reducing condition. Finally, the purified fractions were kept for conjugation with peroxidase.
Confirmation of the mAb purity by SDS-PAGE
Confirmation of the mAb purity was monitored by SDS-PAGE in non-reducing and reducing condition. 10 µl of purified mAb was mixed with 10 µl of sample buffer, then boiled for 2-5 min and cooled on ice. Electrophoresis was done in a 12% SDS-PAGE gel with a mini-PROTEAN electrophoresis instrument (Bio-Rad Laboratories, Hercules, CA, USA) 100 mA for 1 hr. The gel was stained with Coomassie Brilliant Blue R-250 (Sigma). (Figure 1)
Figure 1 .
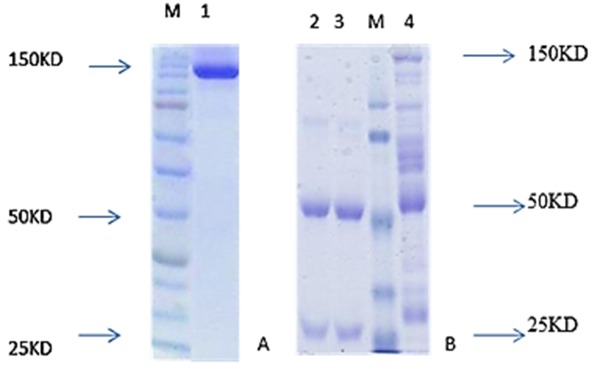
SDS-PAGE analysis non-reducing SDS-PAGE (A) and reducing SDS-PAGE (B) of produced monoclonal antibody. In reduced form (2 and 3), two bands were seen in 50 and 25 kDa but in non-reducing SDS-PAGE condition, only one band was seen in about 150 kDa. The SDS-PAGE gel lane 1 contains a band at 150 kDa is in non-reducing condition, in lane 2, 3 contain two band at 25 and 50 kDa in reducing condition and lane 4 produced antibody before purification.
Western Blotting
Western blotting was used to confirm the result of SDS-PAGE and to see pattern of specificity and cross-reactivity of anti-IgA monoclonal antibody. IgA, IgG and IgM was subjected to SDS-PAGE and then immunoblotting assay. Briefly, the nitrocellulose membrane and several thicknesses of whatman chromatography papers were soaked in the transfer buffer (25 mMTris, 192 mM glycine, 20% V/V methanol, pH 8.3). The wet nitrocellulose membrane was over laid on the wet Whatman sheets by taking precaution to avoid bubbles. Then, the SDS-PAGE gel was placed on the wet nitrocellulose membrane, where several wet Whatman papers were placed on it. Transfer of the proteins from the gel to the nitrocellulose membrane was done in 100V for three hours. Then, non-specific sites were blocked with a 2% BSA solution. After three rounds of washing, the membrane was cut into strips and incubated for two hours at 37 °C with the supernatants of suitable clones. Again, after five washes, the strips were incubated for two hours at 37 °C with Rabbit Anti-Mouse IgA conjugate (1/2000 dilution). The strips were washed and detected by ECL (Amersham Phamacia Biotech Inc, USA) hyperfilm after exposure for five min.
Conjugation of Monoclonal Antibody with Horseradish Peroxidase enzyme (HRP)
Two mg of HRP was dissolved in 1 mL of distilled water. We then added 0.2 ml of freshly prepared sodium Periodate solution (21mg/ml), and the solution was stirred for 20 min. at room temperature. The solution was dialyzed against an acetate buffer (pH 4.4) over night at 4°C. About 5 mg of the purified monoclonal antibody was dissolved in 0.625 mL sodium carbonate (10 mM, pH 9.5).The pH of the dialyzed enzyme was raised to 9, and immediately the solution containing monoclonal antibody was added and then shaken for two hours at room temperature. Then, 50µl of the freshly prepared sodium borohydride was added and incubated for 30 min. at room temperature. The final solution was precipitated with ammonium sulfate and then was dialyzed against the PBS buffer.
Results
After priming the peritoneum of the mice with Pristane, 1 million cells associated with the appropriate monoclonewere suspended in 0.5 mL of sterile PBS and injected into the each mouse. Ten days later, about 3 mL of ascitic fluids rich with an appropriate monoclonal antibody were harvested from each mouse. Then, after 4 days, a second round of about 2-3 mL ascetic fluid was harvested from the peritoneum of the mice. The titer of monoclonal antibody in ascitic fluid was measured by ELISA method. Absorbance (OD) of 1/100,000 dilution was above 1. With the same titer, the appropriate monoclonal antibody did not demonstrate any cross-reactivity in reaction with IgM and IgG. Immunoblotting was performed for confirming the results of the SDS-PAGE method. The result of immunoblotting demonstrated that the produced antibody was against the IgA heavy chain. This antibody did not demonstrate any cross-reactivity with the heavy chains of IgM and IgG, and a sharp band only existed in the IgA heavy chain position (Figure 2). The ascitic fluid was investigated for class and subclasses using amouse isotyping kit (ZYMED). The subclass of monoclonal antibody was IgG1 and its light chain was “kappa” type (Table 1). The diluted ascitic fluid was precipitated with saturated ammonium sulfate and dialyzed against PBS pH 7.4. The concentration of the dialyzed product in assay with UV at 280 nm was about 25 mg. Ascitic fluid was then purified with ion exchange chromatography and conjugated with HRP. There was 25 mg of protein in the ascitic fluid that reduced to 5 mg of antibody after the purification step. The results of purification were confirmed with SDS-PAGE in reducing and non-reducing condition. In SDS PAGE, two bands of 50 kDa and 25 kDa appeared that demonstrated heavy and light chains. In the non-reducing condition, only one 150 kDa band appeared that demonstrated the purified antibody. The specificity and cross-reactivity of the anti-IgA was also confirmed by an immunoblotting procedure. The titer of mAb in asciticfluid was examined using the ELISA method and the results showed that its 1/100,000 dilution had a high absorbance of IgA (Table 1), but had no absorbance with IgMand IgG (Table 2). Also, the results of conjugation indicated that a 1/10,000 dilution of conjugate had high absorbance with IgA (Table 1) and did not show any cross-reactivity with IgMand IgG.
Figure 2 .
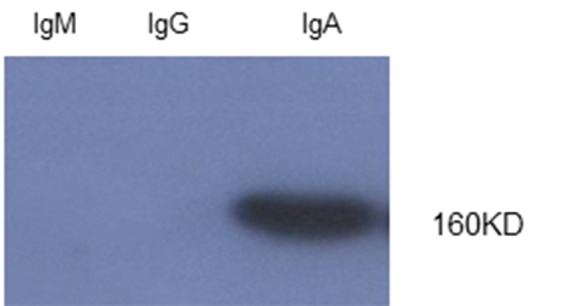
Immunoblotting of monoclonal antibodies from IgM, IgG, IgA. Only one specific band was seen in 160 kDa in IgA.
Table 2. Examining the cross-reactivity of the mouse mAbs with indirect ELISA. Mouse mAbswith 1/100,000 dilution .
| Class | IgA | IgG | IgM |
| Optical density | 1.81 | 0.21 | 0.33 |
Discussion
Antibodies are biologically valuable molecules for medical diagnostic purposes. Their highly specific ability to bind to an extensive variety of molecules and their capacity to identify specific antigenic determinants in these molecules make them an extremely useful accessory in biomedical research. Alternatively, mAbs also have many applications for medical treatment, diagnosis and purification.16 Forthe majority of infectious diseases, particularly those examined in acute condition, one of the important classes of the produced antibody against pathogens is IgA. Consequently, the monoclonal anti-human IgA conjugate is crucial for its recognition as a key reagent. Therefore, production of a monoclonal antibody with no cross-reactivity with homologous molecules (such as IgM and IgG) can be applied in diagnostic kits for infectious diseases. The production of monoclonal antibody in ascitic fluid is commercially valuable for large-scale production. Ascitic fluid production enriched with the monoclonal antibody in mice is a quick and profitable method. The amount of injected Pristane and the period between priming with monoclonal cells are both significant factors in ascitic fluid production.14,17,18 In addition, the number of hybridoma cells injected to the peritoneum of the mice is efficient with regards to the increased speed of ascitic fluid production. 3×106 cells injected to the peritoneum of mice could facilitate the harvest of about 3-4 mL ascitic fluid from the peritoneum of each mouse. Since several liters of RPMI medium and FCS (as well as several months of continuous struggle and attention) are required for the production of this amount of antibody by in-vitro method, it seems that the ascetic fluid production method is a very valuable and economic method.
The choice of procedure for antibody purification depends on the intended use of the antibodies, isotyping of antibodies and the available resources.19 The subclass of monoclonal antibody was IgG1 and its light chain was “kappa” type. Affinity chromatography is costly, can result in poor resolution for subclasses (or is only group-specific), and leads to possible leaching of contaminants into the purified products. Ion-exchange chromatography has shown great potential and is increasingly being used in the purification of immunoglobulins.20 Therefore, we utilized this technique for purification of the produced mAb. The method of choice for determining purity is SDS-PAGE. Purity was evaluated by SDS-PAGE in non-reducing and reducing conditions. SDS-PAGE analysis showed that we obtained a protein with suitable purity after the purification step. Currently, we know two ways for production of the desired antibody: the mouse ascites method, and the tissue-culture method that could be performed in vivo and in vitro.21 In vitro cell-culture method requires some expertise, requires special media, and can be expensive and time-consuming. Moreover, in this technique, unsuitable glycosylation may lead the antibody to be unusable for in vivo experiments resulting from changes in immunogenicity, binding affinity, biologic functions, or clearance in vivo.22 In the process of in vivo production method, at first, a primer such as Pristane or Freud’s incomplete adjuvant is injected to suppress the immune systems, and then the multiplied hybridoma cells form antibody-rich ascitic fluid in the peritoneal cavity. The ascites technique has some advantages; mainly, high levels of antibody production range from 1 to 20 mg/ml. In addition, this technique is not excessively labor- intensive.13 In summary, ascitic fluid production enriched with mAb in mice is both a rapid and economical method.
The amount of the injected Pristane and the intervals of priming with hybridoma cells are extremely important factors in ascetic fluid production. In addition, it is important to note thatthe side effects of tumor growth can become more severe due to incorrect i.p. injection of hybridoma cells because of insemination of hybridoma cells in abdominal organs, such as the urinary bladder or intestines.23 On the other hand, the number of cells injected to the mouseperitoneum is highly effective towards the acceleration of ascitic fluid production.24 During ascites development, animals should be observed at least three times per week for the first week and daily thereafter to monitor the degree of abdominal distention and signs of illness.25 Peterson et al. evaluated the effects on the well-being of animals injected with Pristane and ascites production using factors such as wheel-running activity, food and water consumption, clinical observation, and plasma corticoster one concentration. No significant evidence of distress was obtained in the animals studied.26 However, Mauch et al. reported that elevation of the diaphragm due to ascites is associated with dyspnea, or thopnea, or tachypnea. It therefore seems reasonable to assume that mice with large accumulations of ascitic fluid experience discomfort and distress.27 In a similar study conducted previously, Baradaran et al. used the in vivo method for mass production of a monoclonal antibody against EGFR in ascitic fluid efficiently and 10.4 mg of antibodies were purified with ion exchange chromatography.28 Inanother study, Brian Scott Hafley used in vivo method for the development of monoclonal antibodies.29 Moreover,Mittal et al. used the same in production of murinemonoclonal antibodies against Haemophilus parasuis.30 Furthermore, Galen et al. used the in vivo method for the mass production of monoclonal antibodies against human rennin in ascitic fluid, then mAb with high purity was obtained by affinity chromatography.31 In all of these studies, the mouse ascites methods were preferred for their economical, efficient and high concentrations of produced mAbs. On the other hand, Shu-Fen Chou et al. utilized the tissue culture in flasks method for the scaling-up of anti-AFP mAbs. Then, monoclonal antibodies were purified by affinity chromatography on protein A Sepharose.32
Based on documented evidence, an analysis of mAb produced in tissue culture reveals that a desired function of the antibody is often diminished or lost. Furthermore, tissue culture might be maintained for long periods, and some mAb were denatured during concentration or purification. Nevertheless, due to several parameters, the in-vivo method has fallen into disfavor. The most important reasons relate to the following: significant pain and distress in mice; high-quality in vitro production systems are progressing; and there is a high risk of contaminating mAb with infectious agents, such asviruses and other microorganisms.24 Although, based on various reports, there is now consensus that ascites production should be the exception, requiring rigorous and well-documented justification. Special circumstances that might justify the use of ascites production include the following: emerging therapeutic applications; downstream concentration of mAb from in-vitro; denaturation and decreased antibody activity in the tissue culture; and poor growth of hybridoma cells in the in-vitro system.13,22 Based on the reasons described above, it is reasonable to conclude that new developments in in-vitro mAb production gradually will limit the use of animals for this purpose. In general, the ascitic fluid production method seems to be very useful, inexpensive and economic. Finally, we recommend that mAb production by the mouse ascites method be permitted if scientifically justified and approved by the relevant Institutional Animal Care and Use Committee (IACUC).
Conclusion
Taking all information together, the ascetic fluid production method seems to be a reasonable and economical approach for mAb production with suitable purity and concentration.
Acknowledgments
This work was financially supported by the Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran, and the manuscript was written based on a dataset of a Master’s thesis registered in Tabriz University of Medical Sciences.
Ethical Issues
Not applicable.
Conflict of Interest
The authors report no conflicts of interest in this work.
References
- 1.Waldmann TA. Monoclonal antibodies in diagnosis and therapy. Science . 1991;252(5013):1657–62. doi: 10.1126/science.2047874. [DOI] [PubMed] [Google Scholar]
- 2.Sapra P, Shor B. Monoclonal antibody-based therapies in cancer: advances and challenges. Pharmacol Ther . 2013;138(3):452–69. doi: 10.1016/j.pharmthera.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Xu H, Di B, Pan YX, Qiu LW, Wang YD, Hao W. et al. Serotype 1-specific monoclonal antibody-based antigen capture immunoassay for detection of circulating nonstructural protein NS1: Implications for early diagnosis and serotyping of dengue virus infections. J Clin Microbiol . 2006;44(8):2872–8. doi: 10.1128/JCM.00777-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Troiano LD, Thomaz-Soccol V, Agottani JV, Brodzinski J, Penha TR, Ozaki SC. Production, Characterization, and Use of Monoclonal Antibodies Against gp51 Protein to Diagnose Bovine Leukemia Virus Infection. BioRes Open Access . 2013;2(1):55–60. doi: 10.1089/biores.2012.0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montoya JG. Laboratory diagnosis of Toxoplasma gondii infection and toxoplasmosis. J Infect Dis . 2002;185 Suppl 1:S73–82. doi: 10.1086/338827. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi M, Kusakai S, Mizuo H, Suzuki K, Fujimura K, Masuko K. et al. Simultaneous detection of immunoglobulin A (IgA) and IgM antibodies against hepatitis E virus (HEV) Is highly specific for diagnosis of acute HEV infection. J Clin Microbiol . 2005;43(1):49–56. doi: 10.1128/JCM.43.1.49-56.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazzoli S, Lopalco L, Salvi A, Trabattoni D, Lo Caputo S, Semplici F. et al. Human immunodeficiency virus (HIV)-specific IgA and HIV neutralizing activity in the serum of exposed seronegative partners of HIV-seropositive persons. J Infect Dis . 1999;180(3):871–5. doi: 10.1086/314934. [DOI] [PubMed] [Google Scholar]
- 8.Doerr HW, Rentschler M, Scheifler G. Serologic detection of active infections with human herpes viruses (CMV, EBV, HSV, VZV): diagnostic potential of IgA class and IgG subclass-specific antibodies. Infection . 1987;15(2):93–8. doi: 10.1007/BF01650204. [DOI] [PubMed] [Google Scholar]
- 9.Farris MA, Hardie D, De Lange G, Jefferis R. Immunogenic and antigenic epitopes of immunoglobulins. X: Monoclonal antibodies specific for human IgA, the IgA1 and IgA2 subclasses and an nA2m(2) iso-allotypic epitope. Vox Sang. 1985;48(2):116–21. doi: 10.1111/j.1423-0410.1985.tb00155.x. [DOI] [PubMed] [Google Scholar]
- 10.Mestecky J, Hamilton RG, Magnusson CG, Jefferis R, Vaerman JP, Goodall M. et al. Evaluation of monoclonal antibodies with specificity for human IgA, IgA subclasses and allotypes and secretory component. Results of an IUIS/WHO collaborative study. J Immunol Methods. 1996;193(2):103–48. doi: 10.1016/0022-1759(95)00289-8. [DOI] [PubMed] [Google Scholar]
- 11.Aghebati Maleki L, Majidi J, Baradaran B, Abdolalizadeh J, Kazemi T, Aghebati Maleki A. et al. Large Scale Generation and Characterization of Anti-Human CD34 Monoclonal Antibody in Ascetic Fluid of Balb/c Mice. Adv Pharm Bull . 2013;3(1):211–6. doi: 10.5681/apb.2013.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baradaran B, Majidi J, Hassan ZM, Abdolalizadeh J. Large scale production and characterization of anti-human IgG monoclonal antibody in peritoneum of Balb/c mice. Am J Biochem Biotechnol . 2005;1(4):189–92. [Google Scholar]
- 13.Jackson LR, Trudel LJ, Fox JG, Lipman NS. Monoclonal antibody production in murine ascitesI. Clinical and pathologic features. Lab Anim Sci. 1999;49(1):70–80. [PubMed] [Google Scholar]
- 14.Mc Ardle J. Alternatives to ascites production of monoclonal antibodies. Ani Welf Inform Cent Newslett . 1998;8:3–4. [Google Scholar]
- 15.Lang AB, Schuerch U, Cryz SJ, Jr. Optimization of growth and secretion of human monoclonal antibodies by hybridomas cultured in serum-free media. Hybridoma . 1991;10(3):401–9. doi: 10.1089/hyb.1991.10.401. [DOI] [PubMed] [Google Scholar]
- 16.Yucel F, Manav A, Basalp A. Production and characterization of monoclonal antibodies against hepatitis B viruses and application of a quick sandwich ELISA. Hybrid Hybridomics . 2003;22(3):173–7. doi: 10.1089/153685903322286593. [DOI] [PubMed] [Google Scholar]
- 17.Brijesh Kachariya, Vihar Gadhvi, Gupta A, Roopchandani K, Patel N. A Review: Production of Monoclonal Antibody. Research Journal of Pharmacy and Technology . 2013;7:701–5. [Google Scholar]
- 18.Goncalves AL, Rocha CA, Gonzaga HT, Goncalves-Pires Mdo R, Ueta MT, Costa-Cruz JM. Specific IgG and IgA to larvae, parthenogenetic females, and eggs of Strongyloides venezuelensis in the immunodiagnosis of human strongyloidiasis. Diagn Microbiol Infect Dis . 2012;72(1):79–84. doi: 10.1016/j.diagmicrobio.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Fitzgerald J, Leonard P, Darcy E, O'kennedy R. Immunoaffinity chromatography. Methods Mol Biol . 2011;681:35–59. doi: 10.1007/978-1-60761-913-0_3. [DOI] [PubMed] [Google Scholar]
- 20.Yang YB, Harrison K. Influence of column type and chromatographic conditions on the ion-exchange chromatography of immunoglobulins. J Chromatogr A . 1996;743(1):171–80. doi: 10.1016/0021-9673(96)00255-5. [DOI] [PubMed] [Google Scholar]
- 21.Peterson NC, Peavey JE. Comparison of in vitro monoclonal antibody production methods with an in vivo ascites production technique. Contemp Top Lab Anim Sci . 1998;37(5):61–6. [PubMed] [Google Scholar]
- 22.Jackson LR, Trudel LJ, Fox JG, Lipman NS. Monoclonal antibody production in murine ascites. II. Production characteristics. Lab Anim Sci. 1999;49(1):81–6. [PubMed] [Google Scholar]
- 23.Walvoort NC. Assessment of distress through pathological examination. In: Hendriksen CFM, Köeter HBWM, editors. Replacement, Reduction and Refinement: Present Possibilities and Future Prospects. Amsterdam: Elsevier; 1991. P. 265-73.
- 24.Leenaars M, Hendriksen CF. Critical steps in the production of polyclonal and monoclonal antibodies: evaluation and recommendations. ILAR J . 2005;46(3):269–79. doi: 10.1093/ilar.46.3.269. [DOI] [PubMed] [Google Scholar]
- 25.Peterson NC. Advances in monoclonal antibody technology: genetic engineering of mice, cells, and immunoglobulins. ILAR J . 2005;46(3):314–9. doi: 10.1093/ilar.46.3.314. [DOI] [PubMed] [Google Scholar]
- 26.Peterson NC. Behavioral, clinical, and physiologic analysis of mice used for ascites monoclonal antibody production. Comp Med . 2000;50(5):516–26. [PubMed] [Google Scholar]
- 27.Mauch P,Ultmann. Treatment of malignant ascites. In: DeVita VT, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. Philadelphia: Lippincott; 1985. P. 2150-3.
- 28.Baradaran B, Hosseini AZ, Majidi J, Farajnia S, Barar J, Saraf ZH. et al. Development and characterization of monoclonal antibodies against human epidermal growth factor receptor in Balb/c mice. Hum Antibodies . 2009;18(1-2):11–6. doi: 10.3233/HAB-2009-0195. [DOI] [PubMed] [Google Scholar]
- 29.Hafley BS. Development of Monoclonal Antibodies for a Multiple Antigen ELISA to Verify Safe Cooking End-Point Temperatures in Beef and Pork [PhD Dissertation]. USA: Texas A&M University; 2005.
- 30.Tadjine M, Mittal KR, Bourdon S, Gottschalk M. Production and characterization of murine monoclonal antibodies against Haemophilus parasuis and study of their protective role in mice. Microbiology . 2004;150(Pt 12):3935–45. doi: 10.1099/mic.0.27443-0. [DOI] [PubMed] [Google Scholar]
- 31.Galen FX, Devaux C, Atlas S, Guyenne T, Menard J, Corvol P. et al. New monoclonal antibodies directed against human reninPowerful tools for the investigation of the renin system. J Clin Invest. 1984;74(3):723–35. doi: 10.1172/JCI111488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chou SF, Hsu WL, Hwang JM, Chen CY. Production of monoclonal and polyclonal antibodies against human alphafetoprotein, a hepatocellular tumor marker. Hybrid Hybridomics . 2002;21(4):301–5. doi: 10.1089/153685902760213921. [DOI] [PubMed] [Google Scholar]


