Abstract
Gold Standard allergen-specific immunotherapy is associated with low efficacy because it requires either many subcutaneous injections of allergen or even more numerous sublingual allergen administrations to achieve amelioration of symptoms. Intralymphatic vaccination can maximize immunogenicity and hence efficacy. We and others have demonstrated that as few as three low dose intralymphatic allergen administrations are sufficient to effectively alleviate symptoms. Results of recent prospective and controlled trials suggest that this strategy may be an effective form of allergen immunotherapy.
Keywords: Administration routes, Allergen immunotherapy, Intralympathic, Vaccination
Introduction
Specific immunotherapy (SIT) is the only disease modifying therapy for IgE-mediated allergic diseases. Subcutaneous immunotherapy is still considered the gold standard. One of the more recent developments is intralymphatic immunotherapy.
Frey and Wenk proved in 1957 [1] with a series of elegant skin flap experiments that antigens need to reach lymph nodes via afferent lymph vessels to induce a T-cell response. More recently experiments in spleenless (Hox11−/−) and alymphoplastic (aly/aly) mutant mice have confirmed the importance of secondary lymphoid organs, or neo-lymphoid aggregates [2], for elicting immune responses [3].
Early in lymphocyte development T- and B cell receptors are randomly rearranged resulting in T and B cells carrying a diverse repertoire of receptors. While this provides the ability of specific recognition of all possible antigens, it also requires antigens to be presented to approximately 107 T- and B cells before eliciting an immune response. Therefore, only antigens that are washed into secondary lymphoid organs, where exposure to high numbers of T and B cells can occur, will generate an immune response. Antigens, however, that bypass secondary lymphoid organs have a reduced likelihood to encounter specific T or B cells, and are thus largely ignored. The phenomenon is termed the “geographic concept of immunogenicity” [4-6]. This concept remains valid although it may appear rather simplistic in the light of current understanding of immune regulation by dendritic cells and T cells. Being aware of the complexity of immune regulation we should none the less remember that the key trigger and regulator of the immune response is the antigen.
The lymph vessels role has evolved to drain pathogens into lymph nodes, thus enabling the immune system to generate an immune response at the earliest. Small particles of 20–200 nm size, i.e. the size of viruses, are quite efficiently drained in a free form from peripheral injection sites into lymph nodes. Usually, however, only a few percent of the injected particles reach the lymph nodes [7]. Larger particles in the size range 500–2000 nm are mostly carried into lymph nodes by DCs [7]. Non-particulate antigens, however, are much less efficiently transported into lymph nodes. Only a very small fraction, i.e. between 10−3 and 10−6, of the injected doses arrive there. Many of today’s vaccines and immunotherapeutic agents are non-particulate, therefore the injection directly into a lymph node should boost antigen presentation in the lymph node and thence improve the immune response.
Review
As early as in 1977 a first review on intralymphatic vaccination was published [8]. In the early 1970s Juillard et al. used this method to enhance tumor cell based cancer vaccines in dogs. Ten years later, researchers were looking for the most efficient route of immunization for producing antibodies against purified proteins which were available in only very small amounts. In the 1980s reports were published of nanogram quantities of protein eliciting immune responses when injected into lymph nodes [9,10]. Thereafter in various fields where conventional routes of administration produced insufficient results or where maximizing the immune response was the goal, such as in cancer vaccines, intralymphatic vaccination was performed.
Intralympatic vaccination has been shown to improve the efficacy of various vaccines, e.g.
Immunostimulating complexes (ISCOMS) [19],
Protein based vaccines for immunization of macaques against SIV [28-34],
Protein based vaccines in cows [35],
Vaccines in cats against feline immunodeficiency virus using a protein based vaccine [41],
Moreover, lymph node targeting can also enhance the efficacy of adjuvants. Intralymphatic administration of the adjuvant CpG required 100 times lower doses of antigen compared with subcutaneous administration. Lower doses avoid undesired systemic adverse effects of the adjuvant [42]. This is in line with reports of enhanced efficacy of CpG and a better safety profile when targeting particles to lymph nodes [43,44].
Biodistribution studies in mice revealed that after direct lymph-node injection 100-fold higher antigen doses reached the lymph nodes than after subcutaneous injection in the drained area of a lymph node [45]. Intralymphatic and subcutaneous injections of radiotraced proteins in humans gave similar results. A 99mTc-labeled protein was injected directly into a superficial inguinal lymph node on the right abdominal side. On the left side, the same dose was injected subcutaneously 10 cm above the inguinal lymph nodes. Figure 1 shows that only a small fraction of the subcutaneously administered protein had reached the lymph nodes after 4 hours, and that this fraction had not increased after 25 hours. In contrast, after intralymphatic injection the protein had drained into the deep subcutaneous lymph nodes and already after 20 minutes it was detected in a pelvic lymph node. Intralymphatic injection could efficiently pulse five lymph nodes with the full amount of the protein.
Figure 1.
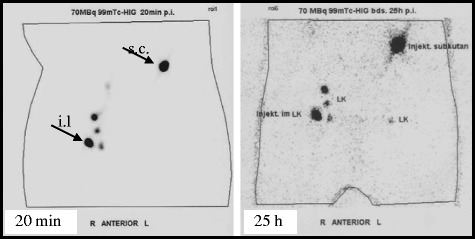
Biodistribution after intralymphatic administration. Biodistribution of 99mTc-labelled human IgG after intralymphatic (left abdominal side) and subcutaneous (right abdominal side) injections. Radio tracing was made by gamma-imaging 20 min (left panel) and 25 hours (right panel) after injection. Arrows indicate the site of injection (s.c., subcutaneous, i.l., intralymphatic).
Intralymphatic immunotherapy with allergen extracts
IgE-mediated allergies, such as allergic rhino-conjunctivitis and asthma today affect up to 35% of the population in westernized countries [46-49]. Subcutaneous allergen-specific immunotherapy (SIT) is the gold standard treatment, i.e. the administration of gradually increasing quantities of an allergen [50-52] over years. The immunotherapy confers long term symptom improvement [53-56], but the 30–80 visits of a physician over 3–5 years compromizes patient compliance. SIT is also associated with frequent allergic side effects and with a risk of anaphylaxis and death [57-59].
Allergen immunotherapy induces a phenotype shift in the T-cell response from Th2 to Th1 [60,61] and stimulates the generation of allergen-specific T-regulatory cells [60-62]. Serum titers of allergen-specific IgG antibodies, particularly IgG4, rise [63]. It is a matter of debate as to which of these immunological mediators is ultimately responsible for improving the allergic symptoms.
Intralymphatic administration of allergens to mice significantly enhanced the efficiency of immunization by inducing 10–20 times higher allergen-specific IgG2a antibody responses with as little as 0.1% of the allergen dose [45]. Intralymphatic injection of allergens also enhanced the secretion of IL-2, IL-4, IL-10 and IFN-γ compared to subcutaneous injection. This may indicate that intralymphatic administration does not polarize the response to the allergen, but overall generates a stronger Th1, Th2, and T-regulatory response [45].
Four separate clinical trials of the authors’ group have meanwhile demonstrated the feasibility, efficacy and safety of intralymphatic allergen immunotherapy. In the first clinical trial, eight patients allergic to bee-venom were given three low-dose injections of bee venom directly into their inguinal lymph nodes, whereas they would normally have received 70 subcutaneous injections. In this proof of concept trial seven of eight treated patients were protected against a subsequent bee sting challenge (Senti et al., manuscript in preparation). Similar results were achieved in a larger multi-center clinical trial with 66 bee venom-allergic patients (Senti et al., manuscript in preparation). In an other randomized controlled clinical trial, 165 patients with grass pollen-induced hay fever were administered either 54 subcutaneous injections with high dose pollen extract within three years or three low-dose intralymphatic injections over eight weeks. The three low-dose intralymphatic allergen injections reduced treatment time from three years to eight weeks and enhanced safety and efficacy of the treatment [64]. The results based on questionnaires and by combining patients treated with one of two allergens/seasons (grass and birch pollen) have been independently confirmed in a double-blind placebo-controlled trial using intralymphatic administration with the same dose, immunization regimen and grass pollen extract, and with tree pollen extract [65]. One trial with intralymphatic administration of grass pollen extract, however, only detected immunological alterations without clinical efficacy [66]. In that trial the time interval between injections was reduced to 2 weeks, whereas in the successful trials [64,65] the antigens were administered every 4 weeks. It is a well known fact of basic vaccine immunology that time intervals between injections of less than 4 weeks interferes with memory B cell formation and maturation of affinity [67,68]. Some authors, however, maintain that the time intervals argument is only valid for preventive vaccines, and that comparisons of low-power trials are strongly influenced by differences in endpoints and ways of assessment of clinical efficacy [69].
Targeting intralymphatic vaccines to the MHC class II pathway
As intralymphatic vaccination brings the antigen directly to the lymph node DCs, the CD4+ T cell response may be enhanced by intracellular translocation sequences and sequences further targeting the antigen to the MHC class II pathway. Such allergy vaccines can be targeted to MHC class II molecules located in the endoplasmatic reticulum by fusing allergens to a tat-translocation peptide derived from HIV and to a part of the invariant chain. Several experimental studies have shown that such targeting not only bypasses the inefficient pinocytosis process but also the enzymatic degradation in phagolysosomes. Both can significantly enhance immunogenicity [45,70,71,72]. A first clinical trial has already proved this concept in a double blinded placebo-controlled setup [73].
Intralymphatic immunotherapy is not painful
Subcutaneous lymph nodes are readily located by sonography since their paracortical area is hypoechoic (Figure 2). Injection into a superficial lymph node in the groin is usually performed in a few minutes and does not require great expertise in sonographic technique. What the patient feels during intralymphatic injection is solely the penetration of the skin, as lymph nodes carry few pain receptors. The pain of an intralymphatic injection thus is comparable with that of a subcutaneous injection. In the trials patients have rated intralymphatic injection less painful than venous puncture [64].
Figure 2.
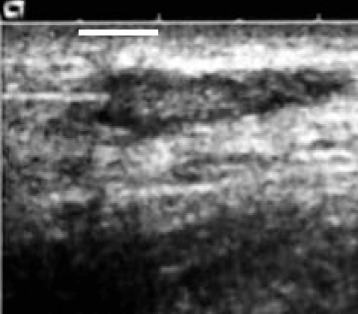
Intralymphatic injection. A sand blasted needle, being inserted into the lymph node from the right was used for better reflection and therefore visibility in the ultrasound. The dark, hypoechoic area represents the paracortex of the lymph node, which is approx. 15 mm long and 5 mm under the skin surface.
Conclusions
Clinical trials indicate intralymphatic immunotherapy to be not only efficient and safe, but also more convenient for the patient, as well as associated with a lower risk of systemic adverse effects, including anaphylaxis and lethal consequences. With as little as 3 injections within 12 weeks, a relief of symptoms can be achieved that is comparable to that obtained with standard subcutaneous immunotherapy necessitating up to 100 injections over 3 to 5 years. As clinical evidence so far is available for grass pollen and bee venom, more clinical trials are required to assess the clinical usefulness of intralymphatic immunotherapy for other common allergens.
Consent
Written informed consent was obtained from the patient for the publication of this report and any accompanying images.
Acknowledgements
Support for the dissemination of the WAO Immunotherapy and Biologics Online Monograph is provided by the following sponsors: Circassia, Boehringer-Ingleheim, and ORA Inc.
Abbreviations
- BCG
Bacillus calmette-guérin
- CD4+
Cluster of differentiation 4
- CpG
Dinucleotide cytosine-phosphate-guanine
- DC
Dendritic cell
- HIV
Human immunodeficiency virus
- IFNγ
Interferon gamma
- IL-
Interleukin-
- MHC
Major histocompatibility complex
- SIT
Specific immunotherapy
- SIV
Simian immunodeficiency virus
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
GS and TK contributed equally to the develoment of the manuscript. Both authors have reviewed and approved the final version.
Contributor Information
Gabriela Senti, Email: gabriela.senti@usz.ch.
Thomas M Kündig, Email: Thomas.Kuendig@usz.ch.
References
- 1.Frey JR, Wenk P. Experimental studies on the pathogenesis of contact eczema in the guinea-pig. Int Arch Allergy Appl Immunol. 1957;11:81–100. doi: 10.1159/000228405. [DOI] [PubMed] [Google Scholar]
- 2.Greter M, Hofmann J, Becher B. Neo-lymphoid aggregates in the adult liver can initiate potent cell-mediated immunity. PLoS Biol. 2009;7:e1000109. doi: 10.1371/journal.pbio.1000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karrer U, Althage A, Odermatt B, Roberts CW, Korsmeyer SJ, Miyawaki S, Hengartner H, Zinkernagel RM. On the key role of secondary lymphoid organs in antiviral immune responses studied in alymphoplastic (aly/aly) and spleenless (Hox11(−)/-) mutant mice. J Exp Med. 1997;185:2157–2170. doi: 10.1084/jem.185.12.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kundig TM, Bachmann MF, DiPaolo C, Simard JJ, Battegay M, Lother H, Gessner A, Kuhlcke K, Ohashi PS, Hengartner H, et al. Fibroblasts as efficient antigen-presenting cells in lymphoid organs. Science. 1995;268:1343–1347. doi: 10.1126/science.7761853. [DOI] [PubMed] [Google Scholar]
- 5.Zinkernagel RM, Ehl S, Aichele P, Oehen S, Kundig T, Hengartner H. Antigen localisation regulates immune responses in a dose- and time-dependent fashion: a geographical view of immune reactivity. Immunol Rev. 1997;156:199–209. doi: 10.1111/j.1600-065X.1997.tb00969.x. [DOI] [PubMed] [Google Scholar]
- 6.Zinkernagel RM. Localization dose and time of antigens determine immune reactivity. Semin Immunol. 2000;12:163–171. doi: 10.1006/smim.2000.0253. [DOI] [PubMed] [Google Scholar]
- 7.Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38:1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 8.Juillard GJ, Boyer PJ. Intralymphatic immunization: current status. Eur J Cancer. 1977;13:439–440. doi: 10.1016/0014-2964(77)90099-8. [DOI] [PubMed] [Google Scholar]
- 9.Sigel MB, Sinha YN, VanderLaan WP. Production of antibodies by inoculation into lymph nodes. Methods Enzymol. 1983;93:3–12. doi: 10.1016/S0076-6879(83)93031-8. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson BO, Svalander PC, Larsson A. Immunization of mice and rabbits by intrasplenic deposition of nanogram quantities of protein attached to Sepharose beads or nitrocellulose paper strips. J Immunol Methods. 1987;99:67–75. doi: 10.1016/0022-1759(87)90033-0. [DOI] [PubMed] [Google Scholar]
- 11.Waeckerle-Men Y, Bruffaerts N, Liang Y, Jurion F, Sander P, Kundig TM, Huygen K, Johansen P. Lymph node targeting of BCG vaccines amplifies CD4 and CD8 T-cell responses and protection against Mycobacterium tuberculosis. Vaccine. 2013;31:1057–1064. doi: 10.1016/j.vaccine.2012.12.034. [DOI] [PubMed] [Google Scholar]
- 12.Lesimple T, Neidhard EM, Vignard V, Lefeuvre C, Adamski H, Labarriere N, Carsin A, Monnier D, Collet B, Clapisson G, et al. Immunologic and clinical effects of injecting mature peptide-loaded dendritic cells by intralymphatic and intranodal routes in metastatic melanoma patients. Clin Cancer Res. 2006;12:7380–7388. doi: 10.1158/1078-0432.CCR-06-1879. [DOI] [PubMed] [Google Scholar]
- 13.Mackensen A, Krause T, Blum U, Uhrmeister P, Mertelsmann R, Lindemann A. Homing of intravenously and intralymphatically injected human dendritic cells generated in vitro from CD34+ hematopoietic progenitor cells. Cancer Immunol Immunother. 1999;48:118–122. doi: 10.1007/s002620050555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grover A, Kim GJ, Lizee G, Tschoi M, Wang G, Wunderlich JR, Rosenberg SA, Hwang ST, Hwu P. Intralymphatic dendritic cell vaccination induces tumor antigen-specific, skin-homing T lymphocytes. Clin Cancer Res. 2006;12:5801–5808. doi: 10.1158/1078-0432.CCR-05-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown K, Gao W, Alber S, Trichel A, Murphey-Corb M, Watkins SC, Gambotto A, Barratt-Boyes SM. Adenovirus-transduced dendritic cells injected into skin or lymph node prime potent simian immunodeficiency virus-specific T cell immunity in monkeys. J Immunol. 2003;171:6875–6882. doi: 10.4049/jimmunol.171.12.6875. [DOI] [PubMed] [Google Scholar]
- 16.de Vries IJ, Lesterhuis WJ, Barentsz JO, Verdijk P, van Krieken JH, Boerman OC, Oyen WJ, Bonenkamp JJ, Boezeman JB, Adema GJ, et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol. 2005;23:1407–1413. doi: 10.1038/nbt1154. [DOI] [PubMed] [Google Scholar]
- 17.De Vries IJ, Krooshoop DJ, Scharenborg NM, Lesterhuis WJ, Diepstra JH, Van Muijen GN, Strijk SP, Ruers TJ, Boerman OC, Oyen WJ, et al. Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Cancer Res. 2003;63:12–17. [PubMed] [Google Scholar]
- 18.Lesimple T, Moisan A, Carsin A, Ollivier I, Mousseau M, Meunier B, Leberre C, Collet B, Quillien V, Drenou B, et al. Injection by various routes of melanoma antigen-associated macrophages: biodistribution and clinical effects. Cancer Immunol Immunother. 2003;52:438–444. doi: 10.1007/s00262-003-0390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koopman G, Bogers WM, van Gils M, Koornstra W, Barnett S, Morein B, Lehner T, Heeney JL. Comparison of intranasal with targeted lymph node immunization using PR8-Flu ISCOM adjuvanted HIV antigens in macaques. J Med Virol. 2007;79:474–482. doi: 10.1002/jmv.20860. [DOI] [PubMed] [Google Scholar]
- 20.Johansen P, Haffner AC, Koch F, Zepter K, Erdmann I, Maloy K, Simard JJ, Storni T, Senti G, Bot A, et al. Direct intralymphatic injection of peptide vaccines enhances immunogenicity. Eur J Immunol. 2005;35:568–574. doi: 10.1002/eji.200425599. [DOI] [PubMed] [Google Scholar]
- 21.Smith KA, Tam VL, Wong RM, Pagarigan RR, Meisenburg BL, Joea DK, Liu X, Sanders C, Diamond D, Kundig TM, et al. Enhancing DNA vaccination by sequential injection of lymph nodes with plasmid vectors and peptides. Vaccine. 2009;27:2603–2615. doi: 10.1016/j.vaccine.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 22.Maloy KJ, Erdmann I, Basch V, Sierro S, Kramps TA, Zinkernagel RM, Oehen S, Kundig TM. Intralymphatic immunization enhances DNA vaccination. Proc Natl Acad Sci U S A. 2001;98:3299–3303. doi: 10.1073/pnas.051630798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heinzerling L, Basch V, Maloy K, Johansen P, Senti G, Wuthrich B, Storni T, Kundig TM. Critical role for DNA vaccination frequency in induction of antigen-specific cytotoxic responses. Vaccine. 2006;24:1389–1394. doi: 10.1016/j.vaccine.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Weber J, Boswell W, Smith J, Hersh E, Snively J, Diaz M, Miles S, Liu X, Obrocea M, Qiu Z, Bot A. Phase 1 trial of intranodal injection of a Melan-A/MART-1 DNA plasmid vaccine in patients with stage IV melanoma. J Immunother. 2008;31:215–223. doi: 10.1097/CJI.0b013e3181611420. [DOI] [PubMed] [Google Scholar]
- 25.Tagawa ST, Lee P, Snively J, Boswell W, Ounpraseuth S, Lee S, Hickingbottom B, Smith J, Johnson D, Weber JS. Phase I study of intranodal delivery of a plasmid DNA vaccine for patients with Stage IV melanoma. Cancer. 2003;98:144–154. doi: 10.1002/cncr.11462. [DOI] [PubMed] [Google Scholar]
- 26.Weber JS, Vogelzang NJ, Ernstoff MS, Goodman OB, Cranmer LD, Marshall JL, et al. A phase 1 study of a vaccine targeting preferentially expressed antigen in melanoma and prostate-specific membrane antigen in patients with advanced solid tumors. J Immunother. 2011;34:556–67. [DOI] [PMC free article] [PubMed]
- 27.Ribas A, Weber JS, Chmielowski B, Comin-Anduix B, Lu D, Douek M, et al. Intra-lymph node prime-boost vaccination against Melan A and tyrosinase for the treatment of metastatic melanoma: results of a phase 1 clinical trial. Clin Cancer Res. 2011;17:2987–96. [DOI] [PubMed]
- 28.Lehner T, Wang Y, Cranage M, Bergmeier LA, Mitchell E, Tao L, Hall G, Dennis M, Cook N, Brookes R, et al. Protective mucosal immunity elicited by targeted iliac lymph node immunization with a subunit SIV envelope and core vaccine in macaques. Nat Med. 1996;2:767–775. doi: 10.1038/nm0796-767. [DOI] [PubMed] [Google Scholar]
- 29.Kawabata S, Miller CJ, Lehner T, Fujihashi K, Kubota M, McGhee JR, Imaoka K, Hioi T, Kiyono H. Induction of Th2 cytokine expression for p27-specific IgA B-cell responses after targeted lymph node immunization with simian immunodeficiency virus in rhesus macaques. J Infect Dis. 1998;177:26–33. doi: 10.1086/513811. [DOI] [PubMed] [Google Scholar]
- 30.Lehner T, Mitchell E, Bergmeier L, Singh M, Spallek R, Cranage M, Hall G, Dennis M, Villinger F, Wang Y. The role of gammadelta T cells in generating antiviral factors and beta-chemokines in protection against mucosal simian immunodeficiency virus infection. Eur J Immunol. 2000;30:2245–2256. doi: 10.1002/1521-4141(2000)30:8<2245::AID-IMMU2245>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 31.Bogers WM, Bergmeier LA, Ma J, Oostermeijer H, Wang Y, Kelly CG, Ten Haaft P, Singh M, Heeney JL, Lehner T. A novel HIV-CCR5 receptor vaccine strategy in the control of mucosal SIV/HIV infection. Aids. 2004;18:25–36. doi: 10.1097/00002030-200401020-00003. [DOI] [PubMed] [Google Scholar]
- 32.Bogers WM, Bergmeier LA, Oostermeijer H, ten Haaft P, Wang Y, Kelly CG, Singh M, Heeney JL, Lehner T. CCR5 targeted SIV vaccination strategy preventing or inhibiting SIV infection. Vaccine. 2004;22:2974–2984. doi: 10.1016/j.vaccine.2004.02.050. [DOI] [PubMed] [Google Scholar]
- 33.Klavinskis LS, Bergmeier LA, Gao L, Mitchell E, Ward RG, Layton G, Brookes R, Meyers NJ, Lehner T. Mucosal or targeted lymph node immunization of macaques with a particulate SIVp27 protein elicits virus-specific CTL in the genito-rectal mucosa and draining lymph nodes. J Immunol. 1996;157:2521–2527. [PubMed] [Google Scholar]
- 34.Lehner T, Bergmeier LA, Tao L, Panagiotidi C, Klavinskis LS, Hussain L, Ward RG, Meyers N, Adams SE, Gearing AJ, et al. Targeted lymph node immunization with simian immunodeficiency virus p27 antigen to elicit genital, rectal, and urinary immune responses in nonhuman primates. J Immunol. 1994;153:1858–1868. [PubMed] [Google Scholar]
- 35.Guidry AJ, O’Brian CN, Oliver SP, Dowlen HH, Douglass LW. Effect of Whole Staphylococcus aureus and Mode of Immunization on Bovine Opsonizing Antibodies to Capsule. J Dairy Sci. 1994;77:2965–2974. doi: 10.3168/jds.S0022-0302(94)77238-6. [DOI] [PubMed] [Google Scholar]
- 36.Ochsenbein AF, Sierro S, Odermatt B, Pericin M, Karrer U, Hermans J, Hemmi S, Hengartner H, Zinkernagel RM. Roles of tumour localization, second signals and cross priming in cytotoxic T-cell induction. Nature. 2001;411:1058–1064. doi: 10.1038/35082583. [DOI] [PubMed] [Google Scholar]
- 37.Juillard GJ, Boyer PJ, Niewisch H, Hom M. Distribution and consequences of cell suspensions following intralymphatic infusion. Bull Cancer. 1979;66:217–228. [PubMed] [Google Scholar]
- 38.Juillard GJ, Boyer PJ, Snow HD. Intralymphatic infusion of autochthonous tumor cells in canine lymphoma. Int J Radiat Oncol Biol Phys. 1976;1:497–503. doi: 10.1016/0360-3016(76)90017-1. [DOI] [PubMed] [Google Scholar]
- 39.Juillard GJ, Boyer PJ, Yamashiro CH. A phase I study of active specific intralymphatic immunotherapy (ASILI) Cancer. 1978;41:2215–2225. doi: 10.1002/1097-0142(197806)41:6<2215::AID-CNCR2820410622>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 40.Juillard GJ, Boyer PJ, Yamashiro CH, Snow HD, Weisenburger TH, McCarthy T, Miller RJ. Regional intralymphatic infusion (ILI) of irradiated tumor cells with evidence of distant effects. Cancer. 1977;39:126–130. doi: 10.1002/1097-0142(197701)39:1<126::AID-CNCR2820390122>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 41.Finerty S, Stokes CR, Gruffydd-Jones TJ, Hillman TJ, Barr FJ, Harbour DA. Targeted lymph node immunization can protect cats from a mucosal challenge with feline immunodeficiency virus. Vaccine. 2001;20:49–58. doi: 10.1016/S0264-410X(01)00323-1. [DOI] [PubMed] [Google Scholar]
- 42.von Beust BR, Johansen P, Smith KA, Bot A, Storni T, Kundig TM. Improving the therapeutic index of CpG oligodeoxynucleotides by intralymphatic administration. Eur J Immunol. 2005;35:1869–1876. doi: 10.1002/eji.200526124. [DOI] [PubMed] [Google Scholar]
- 43.Storni T, Ruedl C, Schwarz K, Schwendener RA, Renner WA, Bachmann MF. Nonmethylated CG motifs packaged into virus-like particles induce protective cytotoxic T cell responses in the absence of systemic side effects. J Immunol. 2004;172:1777–1785. doi: 10.4049/jimmunol.172.3.1777. [DOI] [PubMed] [Google Scholar]
- 44.Bourquin C, Anz D, Zwiorek K, Lanz AL, Fuchs S, Weigel S, Wurzenberger C, von der Borch P, Golic M, Moder S, et al. Targeting CpG oligonucleotides to the lymph node by nanoparticles elicits efficient antitumoral immunity. J Immunol. 2008;181:2990–2998. doi: 10.4049/jimmunol.181.5.2990. [DOI] [PubMed] [Google Scholar]
- 45.Martinez-Gomez JM, Johansen P, Erdmann I, Senti G, Crameri R, Kundig TM. Intralymphatic Injections as a New Administration Route for Allergen-Specific Immunotherapy. Int Arch Allergy Immunol. 2009;150:59–65. doi: 10.1159/000210381. [DOI] [PubMed] [Google Scholar]
- 46.Arbes SJ, Jr, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116:377–383. doi: 10.1016/j.jaci.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 47.Verlato G, Corsico A, Villani S, Cerveri I, Migliore E, Accordini S, Carolei A, Piccioni P, Bugiani M, Lo Cascio V, et al. Is the prevalence of adult asthma and allergic rhinitis still increasing? Results of an Italian study. J Allergy Clin Immunol. 2003;111:1232–1238. doi: 10.1067/mai.2003.1484. [DOI] [PubMed] [Google Scholar]
- 48.Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Beasley R and the International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet. 1998;351:1225–1232. [PubMed]
- 49.Wuthrich B, Schindler C, Medici TC, Zellweger JP, Leuenberger P. IgE levels, atopy markers and hay fever in relation to age, sex and smoking status in a normal adult Swiss population. SAPALDIA (Swiss Study on Air Pollution and Lung Diseases in Adults) Team. Int Arch Allergy Immunol. 1996;111:396–402. doi: 10.1159/000237398. [DOI] [PubMed] [Google Scholar]
- 50.Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998;102:558–562. doi: 10.1016/S0091-6749(98)70271-4. [DOI] [PubMed] [Google Scholar]
- 51.Lockey RF. “ARIA”: global guidelines and new forms of allergen immunotherapy. J Allergy Clin Immunol. 2001;108:497–499. doi: 10.1067/mai.2001.118638. [DOI] [PubMed] [Google Scholar]
- 52.Varney VA, Gaga M, Frew AJ, Aber VR, Kay AB, Durham SR. Usefulness of immunotherapy in patients with severe summer hay fever uncontrolled by antiallergic drugs. BMJ. 1991;302:265–269. doi: 10.1136/bmj.302.6771.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Durham SR, Walker SM, Varga EM, Jacobson MR, O’Brien F, Noble W, Till SJ, Hamid QA, Nouri-Aria KT. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–475. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 54.Golden DB, Kwiterovich KA, Kagey-Sobotka A, Valentine MD, Lichtenstein LM. Discontinuing venom immunotherapy: outcome after five years. J Allergy Clin Immunol. 1996;97:579–587. doi: 10.1016/S0091-6749(96)70302-0. [DOI] [PubMed] [Google Scholar]
- 55.Pajno GB, Barberio G, De Luca F, Morabito L, Parmiani S. Prevention of new sensitizations in asthmatic children monosensitized to house dust mite by specific immunotherapy. A six-year follow-up study. Clin Exp Allergy. 2001;31:1392–1397. doi: 10.1046/j.1365-2222.2001.01161.x. [DOI] [PubMed] [Google Scholar]
- 56.Moller C, Dreborg S, Ferdousi HA, Halken S, Host A, Jacobsen L, Koivikko A, Koller DY, Niggemann B, Norberg LA, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-study) J Allergy Clin Immunol. 2002;109:251–256. doi: 10.1067/mai.2002.121317. [DOI] [PubMed] [Google Scholar]
- 57.Lockey RF, Benedict LM, Turkeltaub PC, Bukantz SC. Fatalities from immunotherapy (IT) and skin testing (ST) J Allergy Clin Immunol. 1987;79:660–677. doi: 10.1016/S0091-6749(87)80164-1. [DOI] [PubMed] [Google Scholar]
- 58.Lockey RF, Turkeltaub PC, Olive ES, Hubbard JM, Baird-Warren IA, Bukantz SC. The Hymenoptera venom study. III: Safety of venom immunotherapy. J Allergy Clin Immunol. 1990;86:775–780. doi: 10.1016/S0091-6749(05)80182-4. [DOI] [PubMed] [Google Scholar]
- 59.Stewart GE, 2nd, Lockey RF. Systemic reactions from allergen immunotherapy. J Allergy Clin Immunol. 1992;90:567–578. doi: 10.1016/0091-6749(92)90129-P. [DOI] [PubMed] [Google Scholar]
- 60.Till SJ, Francis JN, Nouri-Aria K, Durham SR. Mechanisms of immunotherapy. J Allergy Clin Immunol. 2004;113:1025–1034. doi: 10.1016/j.jaci.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 61.Norman PS. Immunotherapy: 1999–2004. J Allergy Clin Immunol. 2004;113:1013–1023. doi: 10.1016/j.jaci.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 62.Vissers JL, van Esch BC, Hofman GA, Kapsenberg ML, Weller FR, van Oosterhout AJ. Allergen immunotherapy induces a suppressive memory response mediated by IL-10 in a mouse asthma model. J Allergy Clin Immunol. 2004;113:1204–1210. doi: 10.1016/j.jaci.2004.02.041. [DOI] [PubMed] [Google Scholar]
- 63.Pierson-Mullany LK, Jackola D, Blumenthal M, Rosenberg A. Altered allergen binding capacities of Amb a 1-specific IgE and IgG4 from ragweed-sensitive patients receiving immunotherapy. Ann Allergy Asthma Immunol. 2000;84:241–243. doi: 10.1016/S1081-1206(10)62761-5. [DOI] [PubMed] [Google Scholar]
- 64.Senti G, Prinz Vavricka BM, Erdmann I, Diaz MI, Markus R, McCormack SJ, Simard JJ, Wuthrich B, Crameri R, Graf N, et al. Intralymphatic allergen administration renders specific immunotherapy faster and safer: a randomized controlled trial. Proc Natl Acad Sci U S A. 2008;105:17908–17912. doi: 10.1073/pnas.0803725105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hylander T, Latif L, Petersson-Westin U, Cardell LO. Intralymphatic allergen-specific immunotherapy: an effective and safe alternative treatment route for pollen-induced allergic rhinitis. J Allergy Clin Immunol. 2013;131:412–420. doi: 10.1016/j.jaci.2012.10.056. [DOI] [PubMed] [Google Scholar]
- 66.Witten M, Malling HJ, Blom L, Poulsen BC, Poulsen LK. Is intralymphatic immunotherapy ready for clinical use in patients with grass pollen allergy? J Allergy Clin Immunol. 2013;132:1248–1252. doi: 10.1016/j.jaci.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 67.Kundig TM, Johansen P, Bachmann MF, Cardell LO, Senti G. Intralymphatic immunotherapy: Time interval between injections is essential. J Allergy Clin Immunol. 2014;133:930–931. doi: 10.1016/j.jaci.2013.11.036. [DOI] [PubMed] [Google Scholar]
- 68.Siegrist CA. Vaccine Immunology. In Vaccines, 6th Edition. Edited by Plotkin SA: Elsevier; Saunders, Philadelphia 2013: 14–32
- 69.Malling HJ, Witten M, Poulsen LK. Intralymphatic immunotherapy: Time interval between injections is essential Reply. J Allergy Clin Immun. 2014;133:931–932. doi: 10.1016/j.jaci.2013.11.037. [DOI] [PubMed] [Google Scholar]
- 70.Martinez-Gomez JM, Johansen P, Rose H, Steiner M, Senti G, Rhyner C, Crameri R, Kundig TM. Targeting the MHC class II pathway of antigen presentation enhances immunogenicity and safety of allergen immunotherapy. Allergy. 2009;64:172–178. doi: 10.1111/j.1398-9995.2008.01812.x. [DOI] [PubMed] [Google Scholar]
- 71.Rhyner C, Kundig T, Akdis CA, Crameri R. Targeting the MHC II presentation pathway in allergy vaccine development. Biochem Soc Trans. 2007;35:833–834. doi: 10.1042/BST0350833. [DOI] [PubMed] [Google Scholar]
- 72.Crameri R, Fluckiger S, Daigle I, Kundig T, Rhyner C. Design, engineering and in vitro evaluation of MHC class-II targeting allergy vaccines. Allergy. 2007;62:197–206. doi: 10.1111/j.1398-9995.2006.01292.x. [DOI] [PubMed] [Google Scholar]
- 73.Senti G, Crameri R, Kuster D, Johansen P, Martinez-Gomez JM, Graf N, et al. Intralymphatic immunotherapy for cat allergy induces tolerance after only 3 injections. J Allergy Clin Immunol. 2012;129:1290–6. [DOI] [PubMed]


