Abstract
Introduction
Differential hypoxia is a pivotal problem in patients with femoral veno-arterial (VA) extracorporeal membrane oxygenation (ECMO) support. Despite recognition of differential hypoxia and attempts to deliver more oxygenated blood to the upper body, the mechanism of differential hypoxia as well as prevention strategies have not been well investigated.
Methods
We used a sheep model of acute respiratory failure that was supported with femoral VA ECMO from the inferior vena cava to the femoral artery (IVC-FA), ECMO from the superior vena cava to the FA (SVC-FA), ECMO from the IVC to the carotid artery (IVC-CA) and ECMO with an additional return cannula to the internal jugular vein based on the femoral VA ECMO (FA-IJV). Angiography and blood gas analyses were performed.
Results
With IVC-FA, blood oxygen saturation (SO2) of the IVC (83.6 ± 0.8%) was higher than that of the SVC (40.3 ± 1.0%). Oxygen-rich blood was drained back to the ECMO circuit and poorly oxygenated blood in the SVC entered the right atrium (RA). SVC-FA achieved oxygen-rich blood return from the IVC to the RA without shifting the arterial cannulation. Subsequently, SO2 of the SVC and the pulmonary artery increased (70.4 ± 1.0% and 73.4 ± 1.1%, respectively). Compared with IVC-FA, a lesser difference in venous oxygen return and attenuated differential hypoxia were observed with IVC-CA and FA-IJV.
Conclusions
Differential venous oxygen return is a key factor in the etiology of differential hypoxia in VA ECMO. With knowledge of this mechanism, we can apply better cannula configurations in clinical practice.
Electronic supplementary material
The online version of this article (doi:10.1186/s13054-015-0791-2) contains supplementary material, which is available to authorized users.
Introduction
Acute respiratory distress syndrome (ARDS) is a severe lung disease with a high mortality rate [1-4]. Extracorporeal membrane oxygenation (ECMO) can provide gas exchange independently of mechanical ventilation, either as a rescue intervention or to minimize ventilator-induced lung injury [5-7]. Because of encouraging outcomes from the CESAR trial [5] and success with ECMO in patients with influenza A (H1N1) and ARDS [8-10], standard veno-venous ECMO has been proposed as the modality of choice for severe acute respiratory failure (ARF) without cardiac dysfunction [11,12]. When hemodynamic support is needed, such as in ARF patients with cardiac failure, veno-arterial ECMO (VA ECMO) has been considered a substitute for veno-venous ECMO to provide substantial hemodynamic and respiratory support [13,14]. However, when the heart recovers a certain extent of force capacities, ARDS patients with femoral VA ECMO display lower partial pressure of oxygen (PO2) in the upper body than in the lower body, which has been termed differential hypoxia [15,16]. Differential hypoxia might also occur in patients with VA ECMO support under certain conditions, for instance, hemodynamic instability with severe pulmonary hypertension, and respiratory dysfunction caused by pulmonary edema or infection following cardiogenic shock [17].
It has been reported that differential hypoxia could cause insufficient oxygen supply to the vital organs, such as the brain and heart [17,18]. To avoid the risk of differential hypoxia, a number of critical care professionals recommend that the clinical application of VA ECMO is best avoided in ARDS patients [19]. Meanwhile, some specialists are trying to solve the problem by elucidating the underlying mechanisms of differential hypoxia. Because hypoxemia occurs in the upper body, dual circulation has been proposed as the major reason for differential hypoxia in patients with femoral VA ECMO (from the inferior vena cava to the femoral artery, IVC-FA) [14,15]. According to this theory, in VA ECMO, oxygenated blood from the ECMO circuit enters the descending aorta to perfuse the lower body, whereas the blood flow of the upper body is from the left ventricle [14]. To deliver more oxygenated blood to the upper body, some clinicians have suggested: (1) to modify FA cannulation to the axillary artery or carotid artery cannulation (IVC-CA) [20,21]; or (2) to use veno-arterio-venous ECMO by adding an additional venous reinfusion cannula in the internal jugular vein to IVC-FA (FA-IJV) [17].
Interestingly, Kitamura and colleagues [15] reported that differential hypoxia could be ameliorated when IVC-FA was modified with superior vena cava (SVC) drainage (SVC-FA). Unlike IVC-CA and FA-IJV, which directly deliver oxygenated blood to the upper body, SVC-FA does not alter the blood supply to systemic circulation. We hypothesized that oxygen saturation in the SVC and IVC might be different during IVC-FA, which then contributes to differential hypoxia. In the present study, we provided evidence that differential venous oxygen return is an important modulator of differential hypoxia in VA ECMO. Moving forward, we can account for the differential venous oxygen return and apply a more appropriate cannula configuration in clinical practice.
Methods
Animals
Twenty adult male crossbred sheep (2 years old, weight 40 ± 5 kg) were provided by the animal centre of Beijing Anzhen Hospital, Capital Medical University. The protocol for animal care was approved by the Ethics Committee on Animal Experimentation of Beijing Anzhen Hospital, Capital Medical University.
ECMO circuit
The ECMO system consisted of a Quadrox-D hollow-fiber oxygenator with BIOLINE coating, a Rotaflow centrifugal pump (Maquet, Rastatt, Germany) with heparin-coated circuit tubing, a Sechrist oxygen/air blender and a water heater/cooler (Sarns/3M Healthcare, Ann Arbor, MI, USA). Carmeda heparin-coated cannulas (Medtronic, Minneapolis, MN, USA) were used in all animals. Blood flow was monitored using a Doppler flow probe placed on the arterial side of the circuit (Transonic, Ithaca, NY, USA). An oximeter (Medtronic, Minneapolis, MN, USA) was used to monitor venous blood oxygen saturation and hematocrit.
ARF model supported with femoral VA ECMO
The ECMO model was established as described previously with minor modifications [22-24]. Briefly, before anesthesia, all sheep were premedicated with dexmedetomidine (Dexdomitor; Orion Pharma, Madrid, Spain; 4 μg/kg) and morphine (Morfina 2%; B. Braun, Melsungen, Germany; 0.2 mg/kg) intravenously. Anesthesia was then induced with propofol (1% Propofol Lipuro; Fresenius Kabi AB, Beijing, China; 4 mg/kg) and maintained with sufentanil (5%; Yichang Humanwell Pharmaceutical Co., Ltd., Yichang, China; 5 μg/kg/h) and atracurium (0.2% Cisatracurium Besilate; Shanghai Hengrui Pharmaceutical Co. Ltd., Shanghai, China; 0.2 mg/kg/h) intravenously. After sheep were anesthetized, they were intubated with an endotracheal tube and connected to a mechanical ventilator (Servos-S, Maquet, Solna, Sweden) at a respiratory rate of 16 to 18 beats/min and a tidal volume of 6 to 8 ml/kg. An FA line was established to measure the arterial blood pressure (BP). A bolus of heparin (125 U/kg) was administered, and IVC-FA was established with 19-Fr and 15-Fr Carmeda heparin-coated cannulas (Medtronic) using the open Seldinger method. The venous cannula was placed within the IVC through the femoral vein. Placement of the cannulas was confirmed via ultrasonography. To stabilize the contribution of cardiac output (CO) and the pump flow to the body, we utilized dopamine, anesthesia and fluid to maintain heart rate (HR) and BP at a normal range. The pump flow was maintained at 50 ml/kg/min and 100% oxygen was administered at a flow rate equal to the blood flow rate. The sheep model of ARF was established as described previously through discontinuing ventilation (Figure 1) [25].
Figure 1.

Study protocol. Heparin was infused to maintain an active clotting time of 180 to 220 sec after ECMO cannulation during the whole experiment. Of the 20 sheep, two were used for angiography. The other 18 sheep were randomly assigned to undertake one of three cannulation procedures. After 15 min of ECMO, ARF was initiated by removing the ventilator and discontinuing mechanical ventilation. The ARF animals were supported with IVC-FA for another 15 min and then were shifted to SVC-FA, IVC-CA or FA-IJV depending on the group assignment. The black arrow indicates the drainage cannula and the white arrow indicates the return cannula. Comparisons between IVC-FA and SVC-FA, IVC-FA and IVC-CA and IVC-FA and FA-IJV were made with paired t test. ARF: acute respiratory failure; ECMO: extracorporeal membrane oxygenation; FA-IJV: an additional return cannula was added into the internal jugular vein on the basis of femoral veno-arterial extracorporeal membrane oxygenation; IVC-CA: a drainage cannula was inserted into the inferior vena cava and a return cannula was inserted into the carotid artery; IVC-FA: a drainage cannula was placed into the inferior vena cava through the femoral vein and a return cannula was inserted into the femoral artery; SVC-FA: a drainage cannula was placed into the superior vena cava through the femoral vein and a return cannula was placed into the femoral artery.
Shifting femoral VA ECMO to other cannulation approaches
As shown in Figure 1, after 15 min of running, IVC-FA cannulation was shifted to (1) SVC-FA: the drainage cannula was moved to the SVC and the return cannula remained unchanged, (2) IVC-CA: the return cannula was moved to the CA with a 15-Fr Carmeda heparin-coated cannula (Medtronic) and (3) FA-IJV: an additional return cannula (12-Fr Carmeda heparin-coated cannula; Medtronic) was added in the IJV to IVC-FA. The total flow rate of ECMO was maintained at 50 mL/kg/min with 30% shunt flow.
Blood gas analysis
Blood samples were collected by trocars at indicated time points and analyzed with a portable clinical blood gas analyzer (Abbott i-STAT, Chicago, IL, USA) (Figure 1). The sites where blood samples were obtained were as follows: (1) SVC: two centimeters distal to the orifice of the SVC; (2) pulmonary artery (PA): in the main pulmonary artery; (3) aorta: at the root of the ascending aorta (oxygen saturation (SO2) of the left atrium (LA) was also measured in IVC-CA); and (4) IVC: distal to the tip of ECMO drainage cannula.
Angiography
Two sheep were used for SVC, IVC and aorta angiography (Figure 1) with a C-arm angiographic machine (Siemens, Munich, Germany). Contrast medium (Iodixanol; GE Healthcare, Pittsburgh, PA, USA) was injected using a high-pressure injector. For aorta angiography, the catheter was placed into the descending aorta near the return cannula in the FA. For vena cava angiography, the catheter was placed into the SVC or IVC.
Statistical analysis
SO2 values are shown as the mean ± standard deviation (SD). The improvement of SO2 values based on different cannulation approaches was calculated and expressed as the difference of oxygen saturation values (ΔSO2). Statistical analysis was performed using SPSS 14.0 software (SPSS Inc., Chicago, IL, USA). The differences before and after discontinuing ventilation or before and after cannula shifting were analyzed with a paired t test. Differences among groups were analyzed with a Student’s t test or ANOVA. A P value less than 0.05 was considered statistically significant.
Results
Upper body hypoxia in the ARF sheep model supported with IVC-FA
Hemodynamic parameters, including HR and mean arterial pressure (MAP), were stable in each group of animals throughout the experiment and no significant differences were present among groups (see Additional files 1, 2, 3 and 4). Fifteen minutes after ARF, we observed that the SO2 of the SVC, PA and aorta were dramatically decreased (SVC: 85.3 ± 1.0% to 40.3 ± 1.0%, P <0.01; PA: 84.2 ± 1.1% to 33.9 ± 0.9%, P <0.01; aorta, 99.5 ± 0.2% to 35.3 ± 1.0%, P <0.01), whereas the SO2 of the IVC remained stable (83.7 ± 1.2% to 83.6 ± 0.8%, P = 0.83). Thus, similar to the clinical cases, upper body hypoxia occurred in the sheep model with ARF supported with IVC-FA (Figure 2A).
Figure 2.
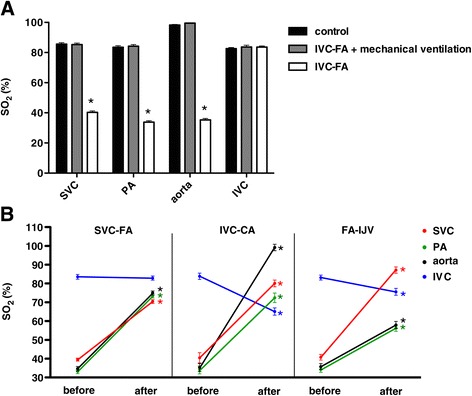
SO 2 in the ARF sheep model with different cannulations of VA ECMO. (A) The cannulation of IVC-FA in normal sheep did not affect the SO2 of the SVC, PA, aorta and IVC. After establishing ARF in these sheep, the SO2 of the SVC, PA and aorta decreased; the SO2 of the IVC remained high. (B) The SO2 in the ARF sheep model with SVC-FA, IVC-CA, and FA-IJV. ‘before’ indicates the SO2 value of IVC-FA. ‘after’ indicates the SO2 value after cannulation shifting. *Indicates P <0.01 between IVC-FA and mechanical ventilation or between IVC-FA and cannula-shifted sheep. ARF: acute respiratory failure; FA-IJV: an additional return cannula was added into the internal jugular vein on the basis of femoral veno-arterial extracorporeal membrane oxygenation; IVC-CA: a drainage cannula was inserted into the inferior vena cava and a return cannula was inserted into the carotid artery; IVC-FA: a drainage cannula was placed into the inferior vena cava through the femoral vein and a return cannula was inserted into the femoral artery; PA: pulmonary artery; SO2: oxygen saturation; SVC-FA: a drainage cannula was placed into the superior vena cava through the femoral vein and a return cannula was placed into the femoral artery; VA ECMO: veno-arterial extracorporeal membrane oxygenation.
Angiography in IVC-FA
To investigate vessels to which the blood from the return cannula in the FA flowed, we performed aorta angiography. We observed that fully oxygenated blood from the return cannula could reach the diaphragm level, but could not supply the upper body under such conditions (Figure 3). An additional movie file shows this in more detail (see Additional file 5).
Figure 3.
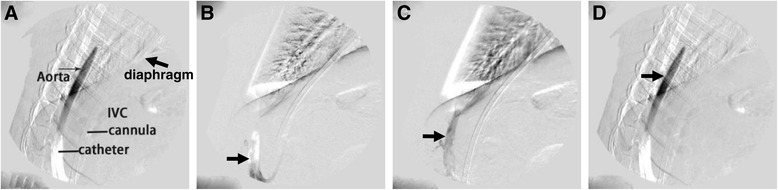
Aorta angiography in IVC-FA. (a) The diagram of aorta angiography. (b) Representative photos in the early stage of angiography. (c) Representative photos in the intermediate stage of angiography. (d) Representative photos in the late stage of angiography. The black arrow shows the contrast medium, which could only reach the diaphragm level. IVC-FA: inferior vena cava through the femoral vein and a return cannula was inserted into the femoral artery.
Vena cava angiography was performed in order to investigate whether the oxygenated blood entered the right atrium (RA). As shown in Figure 4 and the additional movie files (Additional files 6 and 7), under normal conditions without VA ECMO support, the contrast medium entered the RA from both the SVC and IVC. However, when the animals were supported with IVC-FA, the contrast medium failed to enter the RA from the IVC (Figure 4A; Additional file 8). In contrast, the SVC blood was conveyed to the RA (Figure 4B; Additional file 9). This observation indicates that high SO2 blood in the IVC was draining back into the drainage cannula instead of refluxing into the RA.
Figure 4.
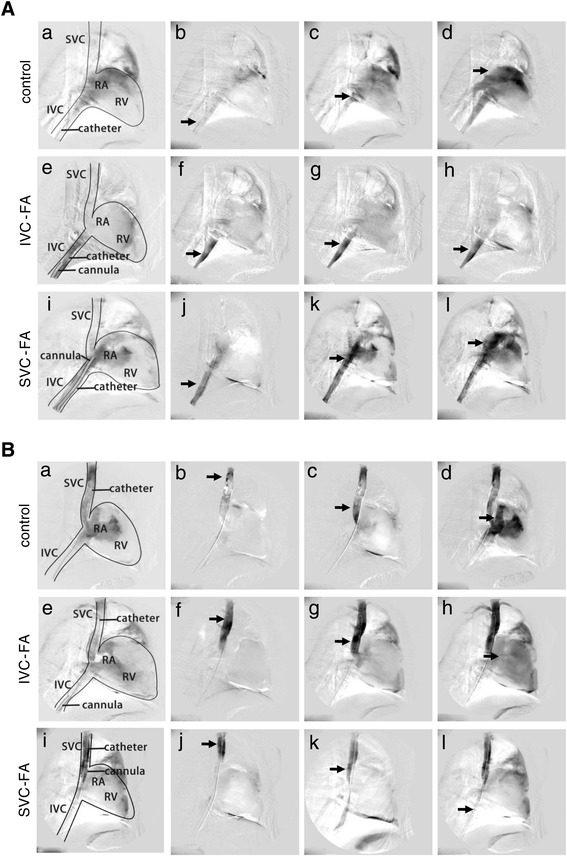
Vena cava angiography in IVC-FA and SVC-FA. The diagram (a, e and i) and representative photos in the early (b, f and j), intermediate (c, g and k) and late (d, h and l) stages of angiography are shown. (A) IVC angiography: contrast medium from the IVC. (a-d) IVC angiography in sheep without ECMO. (e-h) IVC angiography in sheep with IVC-FA. (i-l) IVC angiography in sheep with SVC-FA. Without ECMO, the contrast medium from the IVC entered the RA. In IVC-FA, the contrast medium from the IVC could not enter the RA. After shifting IVC-FA to SVC-FA, the contrast medium infused from the IVC could enter the RA again. (B) SVC angiography: contrast medium from the SVC. (a-d) SVC angiography in sheep without ECMO. (e-h) SVC angiography in sheep with IVC-FA. (i-l) SVC angiography in sheep with SVC-FA. Without ECMO or in IVC-FA, the contrast medium from the SVC entered the RA. After shifting IVC-FA to SVC-FA, the contrast medium infused from the SVC could barely enter the RA. The black arrow indicates the contrast medium. ECMO: extracorporeal membrane oxygenation; IVC-FA: a drainage cannula was placed into the inferior vena cava through the femoral vein and a return cannula was inserted into the femoral artery; RA: right atrium; RV: right ventricle; SVC-FA: a drainage cannula was placed into the superior vena cava through the femoral vein and a return cannula was placed into the femoral artery.
SVC-FA improved upper body oxygenation
Next, we modified IVC-FA to SVC-FA by placing the tip of the drainage cannula into the SVC. Vena cava angiography revealed that the contrast medium entered the RA from the IVC but not from the SVC (Figure 4; Additional files 10 and 11). Furthermore, 15 min after shifting to SVC-FA, the SO2 of the SVC, PA and aorta significantly increased (SVC: 39.5 ± 0.6% to 70.4 ± 1.0%, P <0.01; PA: 33.2 ± 1.1% to 73.4 ± 1.1%, P <0.01; aorta: 34.7 ± 1.2% to 75.0 ± 1.1%, P <0.01), which indicated improved upper body oxygen supply. In addition, the SO2 value at the IVC remained unchanged (83.6 ± 1.3% to 82.9 ± 1.1%, P = 0.31) (Figure 2B).
IVC-CA and FA-IJV improved upper body oxygenation
Next, we aimed to confirm the effects of IVC-CA on upper body oxygenation. The SO2 value of the aortic root was 99.2 ± 1.7%, whereas the SO2 value of the LA was 74.3 ± 2.3%. There was no significant SO2 difference between PA (72.4 ± 6.0%) and LA (74.3 ± 5.5%). The reason might be that oxygenated blood from the ECMO cannula in the CA caused a direct increase of the SO2 value at the aortic root. The SO2 value of SVC (80.1 ± 1.8%) and PA (72.4 ± 2.5%) were significantly higher than those in IVC-FA, which indicated that IVC-CA improved upper body oxygenation. Of note, the IVC SO2 value decreased under IVC-CA (83.9 ± 1.7% to 65.1 ± 1.9%, P <0.01) (Figure 2B).
Next, we achieved FA-IJV by shunting oxygenated blood from the returned cannula to the IJV. FA-IJV significantly increased the SO2 value at the SVC (40.8 ± 1.5% to 87.2 ± 1.7%, P <0.01) and improved upper body oxygen supply (PA: 34.3 ± 1.5% to 56.3 ± 1.7%, P <0.01; aorta: 35.8 ± 1.6% to 57.9 ± 1.9%, P <0.01). Of note, a decreased IVC SO2 value was observed in FA-IJV (83.2 ± 1.3% to 75.6 ± 1.8%, P <0.01) (Figure 2B). Moreover, as indicated by ΔSO2 values, the improvement in oxygen supply at the PA and the aorta was relatively lower in the animals supported with FA-IJV compared with those with SVC-FA and IVC-CA (P <0.01 for both). There was little change of ΔSO2 at IVC in SVC-FA sheep. In addition, there was a smaller decrease in SO2 at IVC in FA-IJV sheep than in IVC-CA sheep (Table 1).
Table 1.
The difference of oxygen saturation between IVC-FA and other approaches of cannulation
| SVC-FA | IVC-CA | FA-IJV | |
|---|---|---|---|
| SVC | 30.9 ± 0.5 | 39.6 ± 1.7* | 46.4 ± 1.4* |
| PA | 40.2 ± 1.4 | 38.3 ± 1.2 | 22.0 ± 0.4*# |
| Aorta | 40.3 ± 0.9 | 63.9 ± 1.3* | 22.1 ± 0.6*§ |
| IVC | −1.3 ± 0.9 | −18.8 ± 1.8* | −7.6 ± 0.7*# |
The SO2 values in IVC-FA were considered as basal level. The difference of oxygen saturation (ΔSO2) was obtained by subtracting the basal SO2 values from the SO2 values in SVC-FA, IVC-CA and FA-IJV, respectively. Basal levels were similar among sheep shifted to different cannulation. * P <0.01 vs. SVC-FA; # P <0.05 vs. IVC-CA; § P <0.01 vs. IVC-CA. FA-IJV: an additional return cannula was added into the internal jugular vein on the basis of femoral veno-arterial extracorporeal membrane oxygenation; IVC: inferior vena cava; IVC-CA: a drainage cannula was inserted into the inferior vena cava and a return cannula was inserted into the carotid artery; IVC-FA: a drainage cannula was placed into the inferior vena cava through the femoral vein and a return cannula was inserted into the femoral artery; PA: pulmonary artery; SO2: oxygen saturation; SVC: superior vena cava. SVC-FA: a drainage cannula was placed into the superior vena cava through the femoral vein and a return cannula was placed into the femoral artery.
Discussion
Although VA ECMO plays an important role in treating severe respiratory and circulatory failure [11,26-29], unfortunately, the phenomenon of differential hypoxia limits its clinical application [17]. In the present study, we utilized a sheep model to mimic differential hypoxia in VA ECMO as determined by SO2 values. Importantly, we found a significant difference in SO2 values between the IVC and the SVC. These data indicate the existence of differential oxygen return in venous system. Through angiography, we demonstrated that better oxygenated blood was drained back to ECMO instead of returning to the heart. Based on this observation, we can explain how differential hypoxia is attenuated by alternative modes of cannulation.
From angiography, we proved dual circulation by directly illustrating that blood flow to the lower body and the upper body was from the ECMO circuit and left ventricle, respectively. On one side, our results indicate that the femoral arterial reinfusion created a high level of lower body oxygenation when heart function is normal. This could lead to a high level of oxygen saturation in the returning venous blood from the lower body, as we saw from the high SO2 values in the IVC. This oxygen-rich blood was drained back into the ECMO circuit by the drainage cannula. On the other side, the oxygen-poor blood from the SVC entered the heart and perfused the upper body (Figure 5A). Thus, differential venous oxygen return between the IVC and the SVC was an important factor contributing to differential hypoxia. Draining the blood from the SVC where the returning blood is not as well saturated might be a key strategy for attenuating differential hypoxia (Figure 5B).
Figure 5.
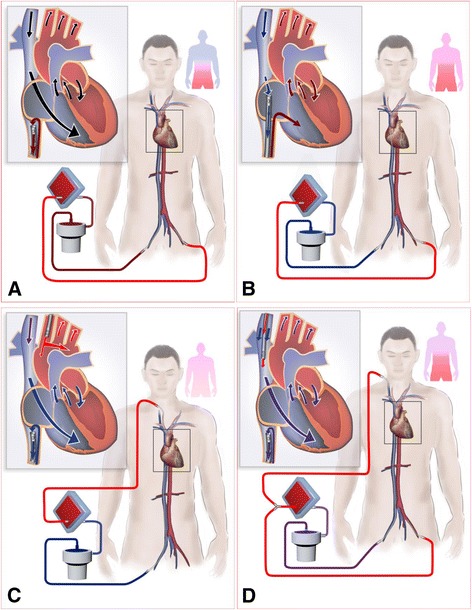
Paradigm depicting the mechanism of differential oxygen return. (A) Differential venous oxygen return between the IVC and the SVC exists in IVC-FA. Oxygen-rich blood is drained back to the ECMO circuit by the drainage cannula at the IVC, and the oxygen-poor blood from the SVC enters the heart and perfuses the upper body, which leads to differential hypoxia. (B) In SVC-FA, oxygen-poor blood in the SVC is drained to the ECMO circuit, whereas the oxygen-rich blood from the IVC enters the RA. (C) In IVC-CA, the oxygenated blood from the ECMO circuit is directly supplied to the whole body. (D) In FA-IJV, a certain amount of oxygenated blood is shunted into the SVC to improve upper body oxygenation. Differential venous oxygen return is attenuated in B, C, D. ECMO: extracorporeal membrane oxygenation; FA-IJV: an additional return cannula was added into the internal jugular vein on the basis of femoral veno-arterial extracorporeal membrane oxygenation; IVC-CA: a drainage cannula was inserted into the inferior vena cava and a return cannula was inserted into the carotid artery; IVC-FA: a drainage cannula was placed into the inferior vena cava through the femoral vein and a return cannula was inserted into the femoral artery; RA: right atrium; SO2: oxygen saturation; SVC-FA: a drainage cannula was placed into the superior vena cava through the femoral vein and a return cannula was placed into the femoral artery.
In IVC-CA (Figure 5C), through changing the arterial line of the VA ECMO circuit to the CA, the oxygenated blood from the ECMO circuit was directly supplied to the whole body. In addition, the oxygen supply to the heart increased indirectly because of the elevated SVC SO2 and/or LA SO2. Additionally, the venous oxygen return to the IVC was much lower than IVC oxygen of IVC-FA. In FA-IJV (Figure 5D), a certain amount of oxygenated blood, which originally perfused the lower body in IVC-FA, was shunted into the SVC to improve the upper body oxygenation. Additionally, the venous oxygen return at IVC (drainage) decreased, but not to the extent of SVC-FA and IVC-CA. As indicated by the ΔSO2, FA-IJV displayed lower efficacy in oxygenation improvement in cases where the pump flow remained at a constant rate compared with SVC-FA and IVC-CA. Although FA-IJV was less efficacious when compared with the other two cannulation approaches using the same pump flow, this deficiency can be abrogated in the clinic with additional flow to the SVC to meet the demands of the upper body.
Of note, we observed that SO2 in the aortic root was higher than in the PA in IVC-CA. However, because the PA and the LA displayed similar SO2 levels, potential confounding factors such as anesthesia levels could be excluded. An increase in aortic root SO2 might be a result from oxygenated blood from the ECMO cannula in the CA. This notion was supported by a recent finding [30] that determined that well-contrasted blood from the ECMO circuit (which was oxygenated) met low-contrasted blood from the left ventricle at the level of aortic root.
Although our present study explores how differential venous oxygen return results in differential hypoxia in IVC-FA, it is not limited to ECMO-supported patients. In minimal invasive cardiac surgery or in cardiac surgery requiring resternotomy (redo surgery), cardiopulmonary bypass is achieved through femoral cannulation. If the ventilation was stopped to facilitate operation before aortic cross-clamping or after declamping, differential hypoxia might occur when the heart ejects blood. This hypoxemia might lead to severe clinical consequences, such as brain death and ventricular fibrillation. Thus, ventilation should be maintained under such conditions.
Limitation
Differential venous oxygen return is strongly dependent on CO. If CO is very low, differential hypoxia is less a problem. We did not measure CO in this experiment. However, the hemodynamic parameters including BP and HR maintained stable during this study. Measurements of CO will be included in future studies.
Conclusions
In conclusion, our study indicates that differential venous oxygen return is a key factor in the etiology of differential hypoxia in VA ECMO. This observation provides new insight into the theory of dual circulation as well as clinical strategies of VA ECMO in pulmonary failure patients.
Key messages
Differential hypoxia is a pivotal problem in cardiopulmonary failure patients with veno-arterial extracorporeal membrane oxygenation (VA ECMO) support.
Dual circulation has been proposed as the major reason for differential hypoxia in femoral VA ECMO (a drainage cannula is placed within the inferior vena cava through the femoral vein and a return cannula is in the femoral artery (IVC-FA)).
The present study demonstrated that blood flow of lower body is from the ECMO circuit, whereas the blood flow of upper body is from the left ventricle.
Differential venous oxygen return between IVC and superior vena cava under IVC-FA support is an important contributor to low oxygen saturation in the upper body.
Draining the blood at the site where the returning blood was not as well saturated with oxygen may be a key strategy for attenuating differential hypoxia.
Acknowledgements
We thank Dr. Antonio Pesenti (Università di Milano-Bicocca, Milan, Italy), Dr. Nicolò Patroniti (Università di Milano-Bicocca, Milan, Italy), Dr. Daniel Brodie (Columbia University, College of Physicians and Surgeons, New York, USA) and Dr. Kenneth Palmér (Karolinska University, Stockholm, Sweden) for their helpful discussions. This study was supported by grants from the National Natural Science Foundation of China (Nos. 81270327 and 81470528 to Xiaotong Hou) and the Research Fund of Capital Medical Development (2014-1-1051 to Xiaotong Hou).
Abbreviations
- ARDS
acute respiratory distress syndrome
- ARF
acute respiratory failure
- BP
blood pressure
- CA
carotid artery
- CO
cardiac output
- ECMO
extracorporeal membrane oxygenation
- FA
femoral artery
- FA-IJV
an additional return cannula was added into the internal jugular vein on the basis of femoral veno-arterial extracorporeal membrane oxygenation
- HR
heart rate
- IJV
internal jugular vein
- IVC
inferior vena cava
- IVC-CA
drainage cannula is in the inferior vena cava and return cannula is in the carotid artery
- IVC-FA
drainage cannula is placed within the inferior vena cava through the femoral vein and return cannula is in the femoral artery
- LA
left atrium
- MAP
mean arterial blood pressure
- PA
pulmonary artery
- PO2
partial pressure of oxygen
- RA
right atrium
- SO2
oxygen saturation
- SVC
superior vena cava
- SVC-FA
drainage cannula is placed within the superior vena cava through the femoral vein and return cannula is in the femoral artery
- VA ECMO
veno-arterial extracorporeal membrane oxygenation
Additional files
Hemodynamic and blood gas changes from the baseline to IVC-FA.
Hemodynamic and blood gas parameters of SVC-FA.
Hemodynamic and blood gas parameters of IVC-CA.
Hemodynamic and blood gas parameters of FA-IJV.
Aorta angiography in IVC-FA. The catheter for contrast medium injection was placed into the descending aorta near the return cannula in the femoral artery. The blood from the return cannula could reach the diaphragm level, but could not supply the upper body. IVC-FA: drainage cannula is placed within the inferior vena cava through the femoral vein and return cannula is in the femoral artery.
Vena cava angiography under normal conditions. The contrast medium was from the inferior vena cava and entered the right atrium.
Vena cava angiography under normal conditions. The contrast medium was from the superior vena cava and entered the right atrium.
Vena cava angiography in IVC-FA. The contrast medium was from the inferior vena cava. When the animals were supported with IVC-FA, the contrast medium failed to enter the right atrium. IVC-FA: drainage cannula is in the inferior vena cava and return cannula is in the femoral artery.
Vena cava angiography in IVC-FA. The contrast medium was from the superior vena cava. When the animals were supported with IVC-FA, the contrast medium entered the right atrium. IVC-FA: drainage cannula is in the inferior vena cava and return cannula is in the femoral artery.
Vena cava angiography in SVC-FA. The contrast medium was from the inferior vena cava. When the animals were supported with SVC-FA, the contrast medium entered the right atrium. SVC-FA: drainage cannula is in the superior vena cava and return cannula is in the femoral artery.
Vena cava angiography in SVC-FA. The contrast medium was from the superior vena cava. When the animals were supported with SVC-FA, the contrast medium failed to enter the right atrium. SVC-FA: drainage cannula is in the superior vena cava and return cannula is in the femoral artery.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All authors read and approved the final manuscript. XH contributed to the design of the study, hypothesis delineation, and substantial revision of the manuscript. XY was involved in the conception, analysis of data, and drafting of the manuscript. ZD was involved in the conception, and analysis of data. JX was involved in the conception, and analysis of data. HL was involved in the conception, and design of the study. CJ contributed to acquisition of the data. JW contributed to acquisition of the data. ZX contributed to acquisition of the data. SL contributed to acquisition of the data. XL contributed to the design of the study, and substantial revision of the manuscript. FY contributed to acquisition of the data, and substantial revision of the manuscript. HW was involved in the conception, and substantial revision of the manuscript. HZ was involved in the conception, design of the study, and substantial revision of the manuscript.
Contributor Information
Xiaotong Hou, Email: xt.hou@ccmu.edu.cn.
Xiaofang Yang, Email: yangxiaofang_1985@163.com.
Zhongtao Du, Email: zhongtaodu@126.com.
Jialin Xing, Email: xingjl@163.com.
Hui Li, Email: 1737356020@qq.com.
Chunjing Jiang, Email: jcj525259@126.com.
Jinhong Wang, Email: wangjinhong002@163.com.
Zhichen Xing, Email: xingtc100@sohu.com.
Shuanglei Li, Email: 9300479@163.com.
Xiaokui Li, Email: xiaokui.li@ncich.com.cn.
Feng Yang, Email: 13581976632@163.com.
Hong Wang, Email: wanghong224@gmail.com.
Hui Zeng, Email: zenghui@ccmu.edu.cn.
References
- 1.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000; 342:1301–1308. [DOI] [PubMed]
- 3.Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:646–655. doi: 10.1001/jama.299.6.646. [DOI] [PubMed] [Google Scholar]
- 4.Stapleton RD, Wang BM, Hudson LD, Rubenfeld GD, Caldwell ES, Steinberg KP. Causes and timing of death in patients with ARDS. Chest. 2005;128:525–532. doi: 10.1378/chest.128.2.525. [DOI] [PubMed] [Google Scholar]
- 5.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt M, Zogheib E, Roze H, Repesse X, Lebreton G, Luyt CE, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med. 2013;39:1704–1713. doi: 10.1007/s00134-013-3037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brogan TV, Thiagarajan RR, Rycus PT, Bartlett RH, Bratton SL. Extracorporeal membrane oxygenation in adults with severe respiratory failure: a multi-center database. Intensive Care Med. 2009;35:2105–2114. doi: 10.1007/s00134-009-1661-7. [DOI] [PubMed] [Google Scholar]
- 8.Australia, New Zealand Extracorporeal Membrane Oxygenation Influenza I, Davies A, Jones D, Bailey M, Beca J, Bellomo R, et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009; 302:1888–95. [DOI] [PubMed]
- 9.Noah MA, Peek GJ, Finney SJ, Griffiths MJ, Harrison DA, Grieve R, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1) JAMA. 2011;306:1659–1668. doi: 10.1001/jama.2011.1471. [DOI] [PubMed] [Google Scholar]
- 10.Pham T, Combes A, Roze H, Chevret S, Mercat A, Roch A, et al. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2013;187:276–285. doi: 10.1164/rccm.201205-0815OC. [DOI] [PubMed] [Google Scholar]
- 11.Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. 2011;365:1905–1914. doi: 10.1056/NEJMct1103720. [DOI] [PubMed] [Google Scholar]
- 12.Linden V, Palmer K, Reinhard J, Westman R, Ehren H, Granholm T, et al. High survival in adult patients with acute respiratory distress syndrome treated by extracorporeal membrane oxygenation, minimal sedation, and pressure supported ventilation. Intensive Care Med. 2000;26:1630–1637. doi: 10.1007/s001340000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sidebotham D, McGeorge A, McGuinness S, Edwards M, Willcox T, Beca J. Extracorporeal membrane oxygenation for treating severe cardiac and respiratory disease in adults: part 1-overview of extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth. 2009;23:886–892. doi: 10.1053/j.jvca.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Sidebotham D, McGeorge A, McGuinness S, Edwards M, Willcox T, Beca J. Extracorporeal membrane oxygenation for treating severe cardiac and respiratory failure in adults: part 2-technical considerations. J Cardiothorac Vasc Anesth. 2010;24:164–172. doi: 10.1053/j.jvca.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Kitamura M, Shibuya M, Kurihara H, Akimoto T, Endo M, Koyanagi H. Effective cross-circulation technique of venoarterial bypass for differential hypoxia condition. Artif Organs. 1997;21:786–788. doi: 10.1111/j.1525-1594.1997.tb03743.x. [DOI] [PubMed] [Google Scholar]
- 16.Haft J, Firmin R. Adult cardiac support. In: Annich G, MacLaren G, editors. Extracorporeal cardiopulmonary support in critical care. Michigan: Extracorporeal Life Supporting Organization; 2012. pp. 327–328. [Google Scholar]
- 17.Choi JH, Kim SW, Kim YU, Kim SY, Kim KS, Joo SJ, et al. Application of veno-arterial-venous extracorporeal membrane oxygenation in differential hypoxia. Multidiscip Respir Med. 2014;9:55. doi: 10.1186/2049-6958-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wada H, Watari M, Sueda T, Kochi K, Sakai H, Shibamura H, et al. Cerebral tissue oxygen saturation during percutaneous cardiopulmonary support in a canine model of respiratory failure. Artif Organs. 2000;24:640–643. doi: 10.1046/j.1525-1594.2000.06601.x. [DOI] [PubMed] [Google Scholar]
- 19.Buscher H, Nair P. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. 2012;366:575. doi: 10.1056/NEJMc1114604. [DOI] [PubMed] [Google Scholar]
- 20.McGough EC, McGough S, Hawkins JA. Subclavian artery cannulation for infant extracorporeal membrane oxygenation. Ann Thorac Surg. 1993;55:787–788. doi: 10.1016/0003-4975(93)90301-W. [DOI] [PubMed] [Google Scholar]
- 21.Wickline SA, Soeter JR, McNamara JJ. Oxygenation of the cerebral and coronary circulation with right axillary artery perfusion during venoarterial bypass in primates. Ann Thorac Surg. 1977;24:560–565. doi: 10.1016/S0003-4975(10)63458-3. [DOI] [PubMed] [Google Scholar]
- 22.Stub D, Byrne M, Pellegrino V, Kaye DM. Extracorporeal membrane oxygenation to support cardiopulmonary resuscitation in a sheep model of refractory ischaemic cardiac arrest. Heart Lung Circ. 2013;22:421–427. doi: 10.1016/j.hlc.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 23.Wang D, Plunkett M, Lynch J, Zhou X, Ballard-Croft C, Zwischenberger JB. Wang-Zwische double-lumen cannula leads to total cavopulmonary support in a failing Fontan sheep model. Ann Thorac Surg. 2011;91:1956–1960. doi: 10.1016/j.athoracsur.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 24.Funes FJ, Granados MD, Morgaz J, Navarrete R, Fernandez-Sarmiento A, Gomez-Villamandos R, et al. Anaesthetic and cardiorespiratory effects of a constant rate infusion of fentanyl in isoflurane-anaesthetized sheep. Vet Anaesth Analg. 2015;42:157–164. doi: 10.1111/vaa.12216. [DOI] [PubMed] [Google Scholar]
- 25.Tamesue K, Ichiba S, Nawa S, Shimizu N. An experimental study on pumpless extracorporeal membrane oxygenation (ECMO) support in a canine model. Acta Med Okayama. 2006;60:167–172. doi: 10.18926/AMO/30748. [DOI] [PubMed] [Google Scholar]
- 26.Hemmila MR, Rowe SA, Boules TN, Miskulin J, McGillicuddy JW, Schuerer DJ, et al. Extracorporeal life support for severe acute respiratory distress syndrome in adults. Ann Surg. 2004;240:595–605. doi: 10.1097/01.sla.0000141159.90676.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakamoto S, Taniguchi N, Nakajima S, Takahashi A. Extracorporeal life support for cardiogenic shock or cardiac arrest due to acute coronary syndrome. Ann Thorac Surg. 2012;94:1–7. doi: 10.1016/j.athoracsur.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 28.Rastan AJ, Dege A, Mohr M, Doll N, Falk V, Walther T, et al. Early and late outcomes of 517 consecutive adult patients treated with extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. J Thorac Cardiovasc Surg. 2010; 139:302–11. 311 e301. [DOI] [PubMed]
- 29.Thiagarajan RR, Brogan TV, Scheurer MA, Laussen PC, Rycus PT, Bratton SL. Extracorporeal membrane oxygenation to support cardiopulmonary resuscitation in adults. Ann Thorac Surg. 2009;87:778–785. doi: 10.1016/j.athoracsur.2008.12.079. [DOI] [PubMed] [Google Scholar]
- 30.Hoeper MM, Tudorache I, Kuhn C, Marsch G, Hartung D, Wiesner O, et al. Extracorporeal membrane oxygenation watershed. Circulation. 2014;130:864–865. doi: 10.1161/CIRCULATIONAHA.114.011677. [DOI] [PubMed] [Google Scholar]


