Abstract
Objective
Neoadjuvant therapy increases rates of margin-negative resection of borderline resectable pancreatic ductal adenocarcinoma (BL-PDAC). Criteria for BL-PDAC resection following neoadjuvant chemotherapy and radiation therapy (NCRT) have not been clearly defined.
Methods
Fifty consecutive patients with BL-PDAC who received NCRT from 2007 to 2012 were identified. Computed tomography (CT) scans pre- and post-treatment were centrally reviewed.
Results
Twenty-nine patients (58 %) underwent resection following NCRT, while 21 (42 %) remained unresected. Patients selected for and successfully undergoing resection were more likely to have better performance status and absence of the following features on pre- and post-treatment CT: superior mesenteric vein/portal vein encasement, superior mesenteric artery involvement, tumor involvement of two or more vessels, and questionable/overt metastases (all p <0.05). Tumor volume and degree of tumor–vessel involvement did not significantly change in both groups after NCRT (all p > 0.05). The median overall survival was 22.9 months in resected versus 13.0 months in unresected patients (p < 0.001). Of patients undergoing resection, 93 % were margin-negative, 72 % were node-negative, and 54 % demonstrated moderate pathologic response to NCRT.
Conclusion
Apparent radiographic extent of vascular involvement does not change significantly after NCRT. Patients without metastatic disease should be chosen for surgical exploration based on adequate performance status and lack of disease progression.
Keywords: Pancreatic cancer, Borderline resectable, Neoadjuvant therapy, Chemoradiation, Computed tomography
Introduction
Each year, approximately 5,000 patients are diagnosed with borderline resectable pancreatic ductal adenocarcinoma (BL-PDAC) [1]. This unique stage encompasses localized primary pancreatic cancer with limited involvement of the superior mesenteric vein (SMV), portal vein (PV), superior mesenteric artery (SMA), common hepatic artery (HA), or celiac axis (CA). Though certain criteria exist for defining BL-PDAC [1–3], subjectivity in determining resectability creates some ambiguity in the diagnoses of BL-PDAC [1]. Regardless of the classification system used, BL-PDAC patients are more likely to require a highly challenging surgery leading to increased risk of complications, positive resection margins following a surgery-first approach, and early systemic metastases given the more advanced stage of primary tumor [4–6].
Downstaging with preoperative therapy has been well described for other malignancies, such as breast [7] and rectal [8] cancers; however, successful downstaging for BL-PDAC is rare (10–15 %) [8, 9]. Thus for patients with BL-PDAC, neoadjuvant therapy is administered at our institution with the following rationale: (1) to allow time to select patients for surgical resection that have more favorable tumor characteristics reflected by stable or responding disease given the potential morbidity of surgery, (2) to address micrometastatic disease earlier in the treatment sequence [10], (3) to take advantage of the superior performance status of patients in the preoperative setting to administer the adjuvant treatment regimen before the risk of delays in initiating postoperative treatment due to surgical recovery, and (4) to potentially enhance the chances of margin-negative (R0) tumor resection through radiation- and systemic chemotherapy-induced cell death [3, 5, 11]. A recently published consensus report supports this rational treatment approach to neoadjuvant therapy for BL-PDAC [11].
Based on previously published reports, over 40 % of patients with BL-PDAC who receive neoadjuvant therapy subsequently undergo successful resection. Resected BL-PDAC patients have a median overall survival of 23–40 months [3, 12–14] which is comparable to or better than the survival of patients presenting with resectable disease who undergo surgery and receive adjuvant therapy reported in large phase III trials (20–24 months) [15–18]. Moreover, most studies report R0 rates of at least 70 % for resected BL-PDAC [3, 9, 12, 19], which are comparable to rates observed for patients with resectable disease (65–81 %) [15–18]. This reflects the selection of BLPDAC patients with the most favorable clinical characteristics and tumor biology to benefit from successful resection following neoadjuvant therapy. However, criteria for attempting resection of BL-PDAC after neoadjuvant chemotherapy and radiation therapy (NCRT) are lacking [20]. As a result, patients without overt downstaging or improvement of vascular involvement may not be offered the opportunity for potentially curative surgical resection. The primary objective of the current study was to capture the treatment approach and subsequent response to therapy of patients who received NCRT at our institution and to identify which, if any, clinical and radiographic features could assist in selecting BL-PDAC patients for surgical resection following NCRT.
Methods
Patient selection
All BL-PDAC patients who received NCRT at our institution with curative intent between March 2007 and March 2012 were retrospectively reviewed. All patients had provided informed consent for treatment, and the study was approved by our internal institutional review board. Staging of BL-PDAC was determined by an institutional multidisciplinary pancreatic tumor board [21] in the presence of an attending surgical oncologist based on multidisciplinary discussion and guidance of the classification system criteria from the American Hepatopancreaticobiliary Association/Society of Surgical Oncology/Society for Surgery of the Alimentary Tract (AHPBA/SSO/SSAT) [1] on review of cross-sectional computed tomography (CT) scans. General features defining BL-PDAC status included tumor abutment (≤180°) of the CA and/or SMA, encasement of the gastroduodenal artery up to the HA with short-segment encasement/abutment not extending to the CA, or short-segment encasement/occlusion of the SMV/PV with suitable vessel above and below the region involved by the tumor for feasible reconstruction. Potential Appleby operation [22] candidates were also defined as BL-PDAC.
Neoadjuvant regimens
All patients received neoadjuvant chemotherapy and radiation therapy at our institution with the exception of four patients who received chemotherapy at an outside facility prior to radiation. Neoadjuvant therapy consisted of two strategies: (1) induction chemotherapy followed by chemoradiation or (2) upfront chemoradiation. Induction chemotherapy consisted of gemcitabine-based regimens or combination therapy of full or modified fluorouracil, leucovorin, and oxaliplatin with or without irinotecan (FOLFIRINOX and FOLFOX, respectively). Radiation therapy was delivered using three-dimensional conformal radiation therapy, intensity-modulated radiation therapy (IMRT), or volumetric modulated arc therapy (VMAT) techniques with concurrent chemotherapy consisting of gemcitabine, capecitabine, or combined gemcitabine and oxaliplatin. One patient received stereotactic body radiation therapy (SBRT) without concurrent chemotherapy.
Imaging acquisition and central review
Pancreas protocol CT imaging studies were performed within 1 month prior to the start of radiation therapy (pre-treatment CT) and 4–6 weeks after the last day of radiation therapy (post-treatment CT). All scans were performed with IV contrast and oral water using a thin-slice multidetector CT utilizing a dual-phase technique with acquisition of arterial phase and portal venous phase images 30 s and 1 min after intravenous injection of contrast, respectively. Pre- and post-treatment scans were centrally reviewed by an attending radiologist who was blinded to patient treatment and outcomes. Data collected during central review included three-dimensional tumor measurements and assessment for presence of ascites, metastases, possible metastases, tumor necrosis, and extent of tumor–vessel involvement. Classification of the extent of tumor–vessel involvement was performed as follows: (1) none; (2) abutment, <180° involvement; (3) partial encasement, >180° but <360° involvement; and (4) encasement including 360° vessel involvement or occlusion. Pre- and post-treatment scans were available for review at the time of the study for all patients except one; thus, this patient was not included in paired analyses of pre- and post-treatment scans. Response to NCRT was measured in three fashions: (1) by comparing tumor–vessel relationships in the pre-treatment scans to those in the post-treatment scans for the CA, HA, SMA, and SMV/PV; (2) by the percentage of change in tumor volume based on three-dimensional tumor measurements (formula for volume of an ellipsoid, length × width × height × π / 6); and (3) by the percentage change in the longest diameter of the primary tumor based on the revised Response Evaluation Criteria in Solid Tumors (RECIST) guidelines [23]. Preand post-treatment scans for all patients were centrally restaged using the AHPBA/SSO/SSAT criteria to compare our institutional interpretation of BL-PDAC with a standard definition.
Surgical and histopathological techniques
All diagnoses of pancreatic adenocarcinoma were pathologically confirmed by cytologic evaluation of fine-needle aspirate specimens prior to neoadjuvant therapy. After NCRT, patients were selected for surgical resection by an institutional multidisciplinary pancreatic tumor board (including an attending surgical oncologist) based on performance status, absence of metastatic disease, tumor characteristics on post-treatment CT scan, post-treatment carbohydrate antigen 19–9 (CA 19–9) level interpreted in context of the patient's baseline level, patient preferences, and feasibility of vessel reconstruction if applicable.
Following surgical resection, the pancreatectomy specimens were grossly examined for remaining tumor. Sections were submitted for diagnostic review according to standard institutional protocol, including 100 % of the pancreas in cases in which no tumor was grossly identified. Margin status of resection specimens was determined based on traditional methods [24]. Margins <1 mm from the cut edge were considered negative. At the time of this study, slides from sections grossly thought to contain tumor were reviewed by a single pathologist with expertise in pancreatic pathology to consistently assess treatment response and degree of differentiation. Treatment response was graded based on the College of American Pathology (CAP) criteria. This four-tier grading system classifies the extent of residual carcinoma in post-treatment pancreatectomy specimens as follows: grade 0, no viable residual tumor; grade 1, marked response; grade 2, moderate response; and grade 3, poor or no response [25].
Statistical analysis
Data regarding overall survival (OS), progression-free survival (PFS), and local progression-free survival (LPFS) were calculated from the date of pathologic diagnosis and analyzed using the Kaplan–Meier method and the log-rank test to assess for differences between subgroups. Means, medians, and proportions were compared between subgroups using the two-sided Student t test, Mann–Whitney U test, and two-sided Fisher's exact test, respectively. A p value of <0.05 was considered significant in this unadjusted exploratory analysis. Statistical analysis was performed using SPSS version 20.0 software (SPSS, Chicago, IL, USA) and S-Plus 8.0 Enterprise Developer.
Results
Patient characteristics
Of the 50 BL-PDAC patients who received NCRT at our institution between 2007and 2012, 31 (62 %) were offered surgical exploration; however, one patient refused surgery. At the time of surgery, liver metastases were identified in one patient. The remaining 29 of the 30 patients (97 %) proceeding to surgery underwent successful resection of the tumor. The two patients who did not undergo resection were analyzed with the unresected group. Clinicopathologic features of both resected and unresected groups are shown in Table 1. There were no statistically significant differences in features between groups with the exception of race (p =0.017) and proportion of patients with the Eastern Cooperative Oncology Group (ECOG) performance status of 0 and 2 at first follow-up post-NCRT (p =0.042 and p =0.041, respectively).
Table 1.
Baseline demographics, treatment, and clinical characteristics
| Factor | All (N = 50) | Unresected (N = 21) | Resected (N = 29) | p value |
|---|---|---|---|---|
| Age (years), median (IQR) | 63.5 (59–68) | 66 (61–74) | 62 (58–66) | 0.100 |
| Gender, n (%) | ||||
| Male | 32 (64) | 13 (62) | 19 (66) | |
| Female | 18 (36) | 8 (38) | 10 (35) | 0.999 |
| Race, n (%) | ||||
| White | 38 (76) | 12 (57) | 26 (90) | |
| Non-White | 12 (24) | 9 (43) | 3 (10) | 0.017 |
| Baseline ECOG status, n (%) | ||||
| 0 | 31 (62) | 11 (52) | 20 (69) | |
| 1 | 29 (38) | 10 (48) | 9 (31) | 0.110 |
| Post-NCRT ECOG status, n (%) | ||||
| 0 | 25 (50) | 7 (33) | 18 (62) | 0.042 |
| 1 | 19 (38) | 9 (43) | 10 (35) | <0.999 |
| 2 | 6 (12) | 5 (24) | 1 (3) | 0.041 |
| Tumor location, n (%) | ||||
| Head/neck | 40 (80) | 19 (91) | 21 (72) | |
| Body/tail | 10 (20) | 2 (10) | 8 (28) | 0.160 |
| Pre-radiation chemotherapy, n (%) | ||||
| No | 34 (68) | 13 (62) | 21 (72) | |
| Yes | 16 (32) | 8 (38) | 8 (28) | 0.543 |
| Gemcitabine-based | 10 (63) | 5 (50) | 5 (50) | |
| FOLFOX/FOLFIRINOX | 6 (37) | 3 (50) | 3 (50) | >0.999 |
| Concurrent chemotherapy during radiation, n (%) | ||||
| No | 3 (6) | 2 (10) | 1 (3) | |
| Yes | 47 (94) | 19 (91) | 28 (97) | 0.565 |
| Capecitabine | 29 (62) | 12 (63) | 17 (61) | >0.999 |
| Gemcitabine | 4 (9) | 3 (16) | 1 (4) | 0.292 |
| Radiation therapy modality, n (%) | ||||
| 3D-CRT | 13 (26) | 4 (19) | 9 (31) | 0.515 |
| IMRT/VMAT | 36 (72) | 16 (76) | 20 (69) | 0.752 |
| SBRT | 1 (2) | 1 (5) | 0 (0) | 0.420 |
| Neoadjuvant radiation dose (cGy), median (IQR) | 50.0 (40.0–50.0) | 50.0 (30.0–50.0) | 50.0 (50.0–50.0) | 0.719 |
| Baseline CA 19–9, N = 43 | ||||
| <90 U/mL, n (%) | 15 (35) | 7 (39) | 8 (32) | |
| ≥90 U/mL, n (%) | 28 (65) | 11 (61) | 17 (68) | 0.750 |
| Pre-radiation CA 19–9, N = 44 | ||||
| <90 U/mL, n (%) | 20 (46) | 9 (47) | 11 (44) | |
| ≥90 U/mL, n (%) | 24 (55) | 10 (53) | 14 (56) | 0.999 |
IQR interquartile range, ECOG Eastern Cooperative Oncology Group, 3D-CRT three-dimensional-conformal radiotherapy, IMRT intensity-modulated radiotherapy, SBRT stereotactic body radiotherapy, VMAT volumetric modulated arc therapy, Gy gray, CA 19–9 carbohydrate antigen 19–9
Neoadjuvant therapies received did not differ significantly between resected and unresected patients. Overall, 16 patients (32 %) received induction chemotherapy prior to radiation therapy, including 8 patients (28 %) in the resected group and 8 patients (38 %) in the unresected group (p =0.543). Similar proportions of resected and unresected patients received FOLFOX/FOLFIRINOX induction regimens (10 and 14 %, p =0.686). Of the 50 patients, 47 (94 %) received concurrent chemotherapy with radiation, including 28 (97 %) and 19 patients (90 %) in the resected and unresected groups, respectively (p =0.565). Similar proportions of resected and unresected patients treated with concurrent chemotherapy received capecitabine (61 % versus 63 %, p > 0.999), gemcitabine (4 % versus 16 %, p =0.292), and gemcitabine plus oxaliplatin (36 % versus 21 %, p =0.343) as the chemotherapeutic agent during radiation. The distribution of radiotherapy techniques did not differ significantly between resected and unresected patients (all p >0.05). Furthermore, there was no difference in the total dose of neoadjuvant radiation. Median radiation dose was 50 Gy overall (interquartile range (IQR), 40.0–50.0) and 50.0 Gy (IQR, 50.0–50.0) versus 50.0 Gy (IQR, 30.0–50.0) for the resected versus unresected groups (p =0.719). Median fraction size was 2.0 Gy (IQR, 2.0–2.0) for both groups.
Comparison of radiographic features between resected and unresected groups
Extent of tumor–vessel involvement
Table 2 presents the extent of tumor involvement with major vessels on centrally reviewed pre- and post-treatment CT scans. Resected and unresected patients were compared to determine which, if any, tumor–vessel relationships are associated with the decision to attempt surgical resection. On both pre- and post-treatment scans, resected patients were less likely than unresected patients to have SMV/PV encasement (45 % versus 81 % pre-treatment, p =0.018; and 45 % versus 85 % post-treatment, p =0.007) and SMA involvement (24 % versus 75 % pre-treatment, p =0.004; and 31 % versus 65 % post-treatment, p =0.023). Additionally, resected patients were less likely to have tumor involvement of two or more vessels compared to unresected patients on both pre- and post-treatment scans (55 % versus 90 % pre-treatment, p =0.011; and 62 % versus 90 % post-treatment, p =0.047).
Table 2.
Comparison of tumor–vessel interactions between unresected and resected patients on both pre- and post-treatment CT scans
| Radiographic features | Pre-treatment |
Post-treatment |
||||||
|---|---|---|---|---|---|---|---|---|
| All (N = 50) | Unresected (N = 21) | Resected (N = 29) | p value | All (N = 49) | Unresected (N = 20) | Resected (N = 29) | p value | |
| SMV/PV, n (%) | 0.066* | 0.135* | ||||||
| No involvement | 5 (10) | 0 (0) | 5 (17) | 4 (8) | 0 (0) | 4 (14) | ||
| Any involvement | 45 (90 %) | 21 (100 %) | 24 (83 %) | 45 (92 %) | 20 (100 %) | 25 (86 %) | ||
| SMV/PV, n (%) | 0.018 | 0.007 | ||||||
| No encasement | 20 (40) | 4 (19) | 16 (55) | 19 (39) | 3 (15) | 16 (55) | ||
| Encasement | 30 (60) | 17 (81) | 13 (45) | 30 (61) | 17 (85) | 13 (45) | ||
| SMA, n (%) | 0.004 | 0.023 | ||||||
| No involvement | 29 (58) | 7 (33) | 22 (76) | 27 (55) | 7 (35) | 20 (69) | ||
| Any involvement | 21 (42) | 14 (67) | 7 (24) | 22 (45) | 13 (65) | 9 (31) | ||
| SMA, n (%) | 0.223* | 0.133 | ||||||
| <180° | 44 (88) | 17 (81) | 27 (93) | 40 (82) | 14 (70) | 26 (90) | ||
| ≥180° | 6 (12) | 4 (19) | 2 (7) | 9 (18) | 6 (30) | 3 (10) | ||
| SMA, n (%) | 0.565* | >0.999* | ||||||
| No encasement | 47 (94) | 19 (91) | 28 (97) | 45 (92) | 18 (90) | 27 (93) | ||
| Encasement | 3 (6) | 2 (10) | 1 (3) | 4 (8) | 2 (10) | 2 (7) | ||
| HA, n (%) | 0.154 | 0.768 | ||||||
| No involvement | 30 (60) | 10 (48) | 20 (69) | 31 (63) | 12 (60) | 19 (66) | ||
| Any involvement | 20 (40) | 11 (52) | 9 (31) | 18 (37) | 8 (40) | 10 (35) | ||
| HA, n (%) | 0.716 | >0.999* | ||||||
| <180° | 41 (82) | 18 (86) | 23 (79) | 41 (84) | 17 (85) | 24 (83) | ||
| ≥180° | 9 (18) | 3 (14) | 6 (21) | 8 (16) | 3 (15) | 5 (17) | ||
| HA, n (%) | >0.999* | 0.387* | ||||||
| No encasement | 46 (92) | 19 (91) | 27 (93) | 44 (90) | 17 (85) | 27 (93) | ||
| Encasement | 4 (8) | 2 (10) | 2 (7) | 5 (10) | 3 (15) | 2 (7) | ||
| CA, n (%) | 0.271 | 0.496 | ||||||
| No involvement | 41 (82) | 19 (91) | 22 (76) | 39 (80) | 17 (85) | 22 (76) | ||
| Any involvement | 9 (18) | 2 (10) | 7 (24) | 10 (20) | 3 (15) | 7 (24) | ||
| CA, n (%) | 0.630* | >0.999* | ||||||
| <180° | 46 (92) | 20 (95) | 26 (90) | 43 (88) | 18 (90) | 25 (86) | ||
| ≥180° | 4 (8) | 1 (5) | 3 (10) | 6 (12) | 2 (10) | 4 (14) | ||
| CA, n (%) | >0.999* | 0.559* | ||||||
| No encasement | 48 (96) | 20 (95) | 28 (97) | 46 (94) | 18 (96) | 28 (97) | ||
| Encasement | 2 (4) | 1 (5) | 1 (3) | 3 (6) | 2 (4) | 1 (3) | ||
| Number of involved vessels, n (%) | 0.011 | 0.047 | ||||||
| One vessel | 15 (30) | 2 (10) | 13 (45) | 13 (27) | 2 (10) | 11 (38) | ||
| Two or more vessels | 35 (70) | 19 (90) | 16 (55) | 36 (74) | 18 (90) | 18 (62) | ||
| Ascites, n (%) | 0.171* | 0.003 | ||||||
| No | 48 (96) | 19 (91) | 29 (100) | 43 (88) | 14 (70) | 29 (100) | ||
| Yes | 2 (4) | 2 (10) | 0 (0) | 6 (12) | 6 (30) | 0 (0) | ||
| Questionable metastases, n (%) | 0.010 | 0.003 | ||||||
| No | 45 (90) | 16 (76) | 29 (100) | 43 (88) | 14 (70) | 29 (100) | ||
| Yes | 5 (10) | 5 (24) | 0 (0) | 6 (12) | 6 (30) | 0 (0) | ||
| Overt metastases, n (%) | >0.999* | <0.001 | ||||||
| No | 50 (100) | 21 (100) | 29 (100) | 41 (84) | 12 (60) | 29 (100) | ||
| Yes | 0 (0) | 0 (0) | 0 (0) | 8 (16) | 8 (40) | 0 (0) | ||
| Tumor volume (cm3), mean (SD) | 24.5 (34.8) | 41.6 (48.4) | 11.8 (7.9) | 0.002 | 23.9 (37.0) | 41.3 (52.3) | 11.8 (10.7) | 0.005 |
| Change in tumor volume (%), median (IQR) | – | – | – | – | –11.4 (–40.5–57.1) | –18.5 (–41.6–61.5) | –3.0 (–30.9–52.7) | 0.975 |
| RECIST category (primary tumor only), n (%) | ||||||||
| Partial response | – | – | – | – | 3 (6) | 2 (7) | 1 (5) | |
| Stable disease | – | – | – | – | 39 (80) | 23 (80) | 16 (80) | |
| Progressive disease | – | – | – | – | 7 (14) | 4 (14) | 3 (15) | |
SMV/PV superior mesenteric vein/portal vein, SMA superior mesenteric artery, HA hepatic artery, CA celiac artery, SD standard deviation, IQR interquartile range
p values from these comparisons need to be interpreted with caution due to the small sample size in some cells
We did not have a sufficient number of patients to test the difference between groups in the presence of involvement of the SMV/PV (including abutment and encasement) since all except five patients had some extent of SMV/PVinvolvement. We also could not determine the difference between groups in the presence of tumor abutment or encasement of both the SMA and HA due to the small number of patients who met the criteria; however, there was a higher proportion of resected patients with a lesser degree of involvement of both the SMA and HA on pre- and post-treatment scans.
Other imaging characteristics
Given the BL-PDAC stage, no patients had overt metastatic disease on pre-treatment CT scans. Questionable metastases were significantly more common on pre-treatment CT in unresected compared to resected patients (24 % versus 0 %, p =0.010). Due to our institutional policy to offer resection only in the absence of suspected metastatic disease or ascites, unresected patients were more likely to display evidence of overt metastases, questionable metastases, and ascites on post-treatment scan compared to unresected patients (40 % versus 0 %, p <0.001; 30 % versus 0 %, p =0.003; and 30 % versus 0 %, p =0.003, respectively). Five of the six unresected patients (83 %) with ascites on post-treatment scan had associated overt or questionable metastatic disease.
Changes in radiographic features over the course of neoadjuvant radiation
Tumor size
Patients undergoing resection had significantly smaller tumor volumes than those of unresected patients on both pre-treatment scans (mean 12.06 cm3 versus 41.6 cm3, p = 0.002) and post-treatment scans (11.8 cm3 versus 41.3 cm3, p =0.005) (Table 2). The tumor volume did not change significantly in either group following NCRT (median 11.0 cm3 pre-treatment versus 7.5 cm3 post-treatment for resected patients, p =0.549; median 30.5 cm3 pre-treatment versus 27.6 cm3 post-treatment for unresected patients, p =0.814), nor was there a significant difference in the percent change in tumor volume between pre- and post-treatment CT scans for the resected versus unresected groups (median −3.0 % versus −18.5 %, p =0.974). According to the RECIST, similar proportions of patients had partial response or stable disease following NCRT in the resected and unresected groups (86 % versus 85 %, p =0.609).
Extent of tumor–vessel involvement
Radiographic features on pre- and post-treatment scans for both resected and unresected patients were compared to determine whether any radiographic changes during neoadjuvant therapy were associated with future successful resection. The relationship of the tumor to the major vessels and proportion of patients with tumor involvement of two or more vessels did not change significantly over the course of NCRT in either the unresected or resected group based on paired analysis of the pre- and post-treatment CT scans for each patient (not shown).
Clinical management and outcomes
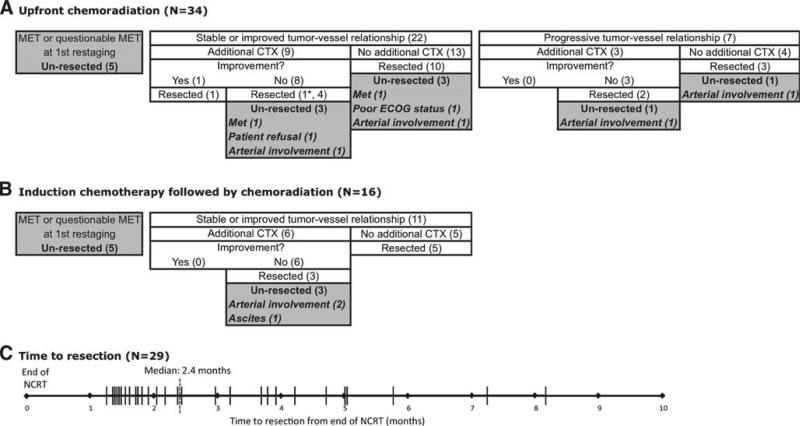
Figure 1 depicts the clinical treatment and response of all 50 patients with BL-PDAC based on NCRT approach consisting of upfront chemoradiation (Fig. 1a) versus induction chemo-therapy followed by chemoradiation (Fig. 1b). Response to treatment is divided between stable/improved versus progressive tumor–vessel relationships on post-treatment CT. Only four patients (8 %) demonstrated any degree of radiographic improvement in tumor–vessel relationships. Overall, ten patients (20 %) developed overt and/or questionable metastases on post-treatment CT and were not selected for surgical exploration. Thirty-three (66 %) patients had stable or improved tumor–vessel relationships on post-treatment CT, of which 15 patients received additional chemotherapy following this first restaging scan. Seven (14 %) patients had progressive tumor–vessel relationships on post-treatment scan, of which three received additional chemotherapy following the first restaging. In total, 18 (36 %) patients received post-radiation chemotherapy which consisted of gemcitabine in combination with either docetaxel and capecitabine (8 patients) or oxaliplatin/cisplatin (7 patients) with the exception of 1 patient who received FOLFIRINOX.
Fig. 1.
Treatment approach and response to neadjuvant therapy for BL-PDAC. Patients received either A upfront radiation or chemoradiation (N = 34) or B chemotherapy followed by chemoradiation (N = 16). Patients were grouped based on radiographic appearance of tumor–vessel relationships and appearance of overt or questionable metastatic disease on post-treatment CT scan. C Plot showing resection events from the date of the last day of radiation treatment (months). BL-PDAC borderline resectable pancreatic ductal adenocarcinoma, MET metastasis, CXT chemotherapy, RT radiation therapy, ECOG Eastern Cooperative Oncology Group
Twenty-nine patients (58 %) eventually underwent successful resection. Median time to resection was 2.4 months (IQR, 1.6–3.9 months) from the last day of radiation therapy (Fig. 1c). Of the initial 50 BL-PDAC patients, 21 (42 %) remained unresected. Of these patients, reasons for not achieving successful resection included patient refusal (5 %), poor ECOG status (5 %), ascites (5 %), arterial involvement concerning for margin-positive resection and inability to perform vascular reconstruction (29 %), and metastases on first restaging (48 %) or subsequent restaging (10 %) (Fig. 1a, b).
Survival
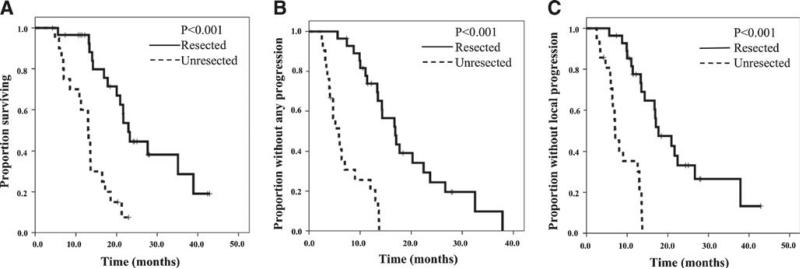
Survival data was calculated from the date of pathologic diagnosis. Median OS for all patients was 17.2 months (95 % CI, 13.4–22.2). Median PFS and LPFS were 13.4 months (95 % CI, 10.8–15.9) and 13.5 months (95 % CI, 12.8–14.2), respectively. Patients undergoing surgical resection had significantly longer OS (Fig. 2a), PFS (Fig. 2b), and LPFS (Fig. 2c) compared to unresected patients. Resected patients had a median OS of 22.9 months (95 % CI, 20.5–25.4) versus 13.0 months (95 % CI, 12.7–13.3 months) for unresected patients (p < 0.001). PFS and LPFS for resected patients were 16.7 months (95 % CI 13.4–20.0) and 17.0 months (95 % CI 13.2–20.9), respectively, compared to 5.9 months (95 % CI, 4.0–7.7 months) (p <0.001) and 7.1 months (95 % CI 5.4–8.7 months) (p <0.001) for unresected patients.
Fig. 2.
Survival data calculated from date of pathologic diagnosis. Patients undergoing surgical resection had significantly longer OS (A), PFS (B), and LPFS (C) compared to unresected patients
Of the 29 patients who underwent resection, 5 (17 %) patients developed radiographic appearance of apparent progression of tumor–vessel involvement over the course of NCRT (Fig. 1a). Still, all 5 patients achieved margin-negative resection and there were no statistically significant differences in OS, PFS, and LPFS when compared to the 24 patients who had stable or improved radiographic appearance of tumor–vessel interactions over the course of NCRT (all p <0.001).
Surgical and pathologic outcomes
Surgical and pathologic data from the resected group are shown in Table 3. Margin-negative resection was achieved in 27 patients (93 %). Eight patients (28 %) had loco-regional nodal involvement. Twenty-eight surgical specimens were available for central pathologic review. Of these specimens, three (11 %) demonstrated complete pathologic response (no viable tumor present). Fifteen patients (54 %) demonstrated CAP grade 0–2 (complete to moderate response). Of the 25 cases with viable tumor remaining, poor differentiation (hazard ratio (HR)=2.97, p =0.066), CAP grade 3 (HR=3.06, p =0.070), positive lymph node status (HR= 2.86, p =0.056), and perineural invasion (HR=2.97, p = 0.066) approached significance as prognostic factors for inferior survival on univariate analysis. Vascular resection and reconstruction was required in eight patients (27.6 %). Median estimated blood loss was 600 cm3 (IQR, 500–800 cm3) and median duration of postoperative hospital stay was 7.0 days (IQR, 7.0–12.25 days). This excludes one patient who died while still admitted 56 days after surgery after requesting for DNR/DNI status following multiple events of respiratory decompensation secondary to recurrent atrial fibrillation.
Table 3.
Surgical outcomes following resection and univariate analysis for prognostic significance
| Number | Events | Median OS (months) | HR | 95 % CI | p value | |
|---|---|---|---|---|---|---|
| Overall | 29 | 16 | 22.92 [20.46, 25.37] | |||
| Surgery type | ||||||
| Distal pancreatectomy | 7 | 2 | 38.89 [NA, NR] | 1.00 | – | |
| Whipple | 22 | 14 | 21.67 [18.90, 24.44] | 2.00 | 0.45–8.90 | 0.361 |
| Vascular reconstruction | ||||||
| No | 21 | 14 | 22.92 [20.62, 25.21] | 1.00 | – | |
| Yes | 8 | 2 | NA [NA, NR] | 0.71 | 0.16–3.23 | 0.667 |
| Operative blood loss | ||||||
| <700 cm3 | 18 | 12 | 22.92 [17.84, 27.99] | 1.00 | – | |
| ≥700 cm3 | 11 | 4 | NA [NA, NR] | 0.55 | 0.18–1.70 | 0.300 |
| CAP grade | ||||||
| Grade 0–2 | 15 | 7 | 35.11 [18.19, 52.04] | 1.00 | – | |
| Grade 3 | 13 | 8 | 21.67 [16.18, 27.16] | 3.06 | 0.91–10.25 | 0.070 |
| Pathologic grade | ||||||
| Moderate to well | 18 | 9 | 35.11 [18.50, 51.73] | 1.00 | – | |
| Poor | 10 | 6 | 17.82 [9.76, 25.89] | 2.97 | 0.93–9.50 | 0.066 |
| Tumor diameter | ||||||
| ≤3 cm | 19 | 12 | 21.67 [18.49, 24.84] | 1.00 | – | |
| >3 cm | 10 | 4 | 38.90 [NA, NR] | 0.58 | 0.19–1.81 | 0.350 |
| Margin status | ||||||
| Negative | 27 | 14 | 23.24 [15.62, 30.87] | 1.00 | – | |
| Positive | 2 | 2 | 13.22 [NA, NR] | 3.89 | 0.82–18.41 | 0.087 |
| Lymph node status | ||||||
| Negative | 21 | 9 | 35.11 [19.72, 50.51] | 1.00 | – | |
| Positive | 8 | 7 | 20.91 [15.62, 26.20] | 2.86 | 0.97–8.37 | 0.056 |
| Perineural invasion | ||||||
| No | 16 | 9 | 27.65 [12.7, 42.59] | 1.00 | – | |
| Yes | 13 | 7 | 20.9 11.88, 29.94] | 2.97 | 0.93–9.50 | 0.066 |
| Post-resection CA 19–9 | ||||||
| <90 U/mL (%) | 17 | 11 | 23.2 [15.23, 31.26] | 1.00 | – | |
| ≥90 U/mL (%) | 4 | 3 | 21.7 [18.8, 24.5] | 1.11 | 0.31–4.02 | 0.800 |
| Any adjuvant therapy | ||||||
| Yes | 21 | 13 | 23.1 [20.9, 25.4] | 1.00 | – | |
| No | 8 | 3 | 21.63 [8.8, 34.4] | 1.28 | 0.36–4.61 | 0.702 |
Overall survival for 29 patients who proceeded to successful resection. Median survival is shown in months with a 95 % CI. All hazard ratios (HR) and p values are from univariate models
CAP College of American Pathologists, CA 19–9 carbohydrate antigen 19–9, OS Overall survival, HR hazardratios, NA not analyzable, NR not reached
Comparison with standard staging
Pre- and post-treatment scans for all patients were centrally restaged using the AHPBA/SSO/SSAT criteria to compare our institutional interpretation of BL-PDAC to a standard definition. Forty patients (80 %) were defined as BL-PDAC stage and ten patients (20 %) as locally advanced (LA) according to the AHPBA/SSO/SSAT criteria due to the extent of tumor involvement with the CA and/or SMA. Of these LA patients, four (40 %) were ultimately resected. Two patients were Appleby candidates; each had a margin-negative resection. Tumors of the other two LA patients decreased in volume as a result of neoadjuvant therapy; however, there was no radiographic improvement in tumor–vessel interactions. Still, both patients underwent resection; however, one had microscopically positive margins. The six remaining LA patients were not explored due to development of metastatic disease (four patients), patient refusal (one patient), or fear of R1 resection due to the nature of arterial involvement (one patient).
Discussion
We report here the treatment, response, and outcomes of a series of consecutive patients with BL-PDAC disease, capturing the clinical decision making process of managing BL-PDAC at this institution. As suggested by Fig. 1, patients are treated with either upfront chemoradiation or chemotherapy followed by chemoradiation. The decision to pursue additional chemotherapy after the first restaging post-treatment versus attempting resection following radiation is based on the impression of vessel involvement determining resectability and preference of the attending surgical and medical oncologists. Few patients had improvement in the relationship of the tumor to abdominal vessels over the course of NCRT. Moreover, additional chemotherapy following NCRT rarely led to further improvements in the degree of tumor–vessel interactions.
This is the first study to report the frequencies of specific tumor–vessel interactions in BL-PDAC patients before and after NCRT. We observed a greater frequency of several tumor–vessel relationships on pre- and post-NCRT scans among patients who did not eventually proceed to surgery, including SMV/PV encasement and SMA involvement. Furthermore, unresected patients were more likely to have lower performance status and larger tumors (likely an indicator of our finding that unresected patients were more likely to have tumor involvement of two or more vessels on both pre- and post-treatment scans). Twenty-nine patients (58 %) successfully underwent resection. All except one resected patient had radiographically stable (80 %) or even progressed (17 %) tumor–vessel interactions. Thus, surgical exploration was not exclusively reserved for patients whose tumors demonstrated decreasing vessel involvement. Instead, patients without distant metastatic progression during the course of neoadjuvant therapy (we prefer 4 months or greater) who also exhibit stable or improved CA 19–9 following NCRT, good performance status, and willingness to undergo an aggressive surgical approach were considered candidates for resection. Although one surgery was aborted due to evidence of metastases using this general treatment approach, none were stopped due to inability to resect the tumor due to locally invasive disease. Subsequent margin-negative rates were still high.
Though resection is currently the only chance for cure for PDAC, continued careful selection of patients who proceed to surgery moving forward is crucial given the morbidity and mortality associated with pancreatectomy and the importance of an R0 resection [26]. Adequate performance status is crucial in selecting patients physically equipped to tolerate a more challenging surgery. The higher proportion of patients with ECOG 0 and lower proportion of ECOG 2 status in the resected compared to unresected patients in our study reflect this selection practice. Anecdotally, resection following neoadjuvant chemoradiation is a more technically challenging process when compared to a surgery-first resection as radiation creates scar tissue at the site of the primary tumor. In this study, several patients require vascular resection and reconstruction, reflecting either scarring or persistence of tumor–vessel involvement following NCRT. Nevertheless, R0 rates were high and the median duration of postoperative hospital stay and operative blood loss for patients undergoing resection following neoadjuvant therapy in our study were comparable to those for patients treated with a surgery-first approach at our institution [27].
A lack of tumor downstaging following successful neoadjuvant therapy was also suggested by a recently published study from the MD Anderson group in which tumor response to neoadjuvant therapy was evaluated in 122 patients with BL-PDAC using RECIST based on pre- and post-treatment CT images [9]. The majority of patients in their study received both chemotherapy and radiation as a combined-modality neoadjuvant therapy. Eighty-five patients (66 %) underwent pancreatectomy with 95 % achieving an R0 resection. Of the resected patients, only 1 had the disease downstaged to resectable stage and only 15 patients (12 %) had a partial response according to the RECIST classification. These authors conclude that radiographic downstaging following neoadjuvant therapy is rare and RECIST response is not an appropriate treatment goal. The results of our study support those of the MD Anderson group and others [9, 28]. None of the patients in this study were downstaged to resectable status and only two resected patients (7 %) demonstrated partial response to neoadjuvant therapy based on the RECIST criteria. Together, these data indicate that surgical exploration is warranted in patients who do not have evidence of radiographic downstaging or even improvement of tumor–vessel involvement as successful resection is common in these patients when attempted following NCRT.
Currently, computed tomography is the standard imaging modality for the diagnosis and staging of pancreatic cancer. Given that downstaging to resectable status and response measured by RECIST are not required for successful surgical outcomes following neoadjuvant therapy for BL-PDAC, there is increasing interest in determining which (if any) radiographic features are predictive for R0 resection. A previous study by Donahue and colleagues reported the low sensitivity and specificity of CT and magnetic resonance imaging (MRI) in predicting the presence of viable tumor at vessel interfaces following neoadjuvant therapy [29]. The frequency of apparent vessel involvement on CT/MRI in 34 patients with locally advanced pancreatic cancer was compared to the true incidence of tumor–vessel involvement at the time of resection. They report that CT/MRI was 71 % sensitive and 58 % specific in detecting viable tumor at vessel interfaces after neoadjuvant therapy. Rates of R0 and N0 were 83 and 81 %, respectively.
An important topic for future research is the capacity of functional imaging modalities such as positron emission tomography (PET) in providing reliable indicators for successful resection following NCRT. CT is restricted to evaluating the morphology of the pathology, and radiation-induced fibrosis and necrosis in the treated area create additional problems in assessing response with CT following NCRT [30]. PET, however, can more reliably assess the viability of the cancer cells following treatment. Choi and colleagues [31] performed a small study of 16 locally advanced patients who received pre- and post-treatment 18F-fluorodeoxyglucose (FDG) PET scans. They observed that patients exhibiting a ≥50 % decrease in SUV following neoadjuvant chemoradiation underwent successful resection while resection was uncommon in non-responders; however, only three patients in the entire cohort proceeded to resection. While these preliminary studies show promising results, much more research is warranted before FDG-PET parameter cutoffs are applied in the surgical candidate selection process for BL-PDAC.
In addition to studies improving clinical assessment of treatment response, more research is necessary to establish even a standard approach to treatment of BL-PDAC patients. Laurence and colleagues [32] published a meta-analysis of 19 cohorts from 1985 to 2007 on the utilization of neoadjuvant therapy for pancreatic cancer. They showed improved survival of patients with initially unresectable disease with the addition of neoadjuvant therapy; however, the survival of BL-PDAC patients was not separated in this analysis [32]. One prior multi-institutional trial for BL-PDAC was attempted, but it closed prematurely due to poor accrual and lack of a standardized therapeutic approach and study population. Fortunately, the recently approved Intergroup pilot study (Alliance A021101) dramatically improves on the shortcomings of prior studies on BL-PDAC. Data from this trial will not only contribute greatly to our literature on this unique population but also serve as a paradigm for future studies [33].
The role of radiation in the neoadjuvant approach to BL-PDAC is currently dependent on institutional preference. The rationale for a combined neoadjuvant approach at this institution are (1) the greater likelihood patients will receive both chemotherapy and radiation therapy when administered up-front without risk of delays due to postoperative recovery [34], (2) radiation can be targeted to the vessel locations where cell death is necessary for increased chance of R0 resection, and (3) the tumor, and thus radiation target, is better oxygenated with an intact vascular supply, increasing efficacy of radiation. Those who do not advocate for radiation in the preoperative management of BL-PDAC argue that it may interrupt full-dose chemotherapy or delay surgery, creating a window for metastatic disease, eliminating the chance of potentially curative resection. Research like the abovementioned study is critical in elucidating the standard treatment approach to this unique subset of patients.
The current study had several limitations. Given the relative rarity of BL-PDAC, this single-institution study was limited to only 50 patients. Furthermore, only 29 patients underwent resection, further limiting the study to its more anecdotal nature. While the neoadjuvant radiation doses were similar, radiation techniques were heterogeneous and patients received different chemotherapy regimens either before or during their neoadjuvant radiation therapy, though there were no significant differences in either neoadjuvant radiotherapy technique or chemotherapy regimens between resected and unresected patients. These variances were partly due to enrollment in different clinical trials during this period and/or preference of a specific regimen by the treating oncologists. Institutional bias may also be present especially with regard to experience with NCRT, operative techniques required for successful resection, and patient selection for surgical resection. Bias for surgical selection limits the interpretability of data regarding the unresected population who did not undergo surgery due to concern of arterial involvement. We are able to draw conclusions from the resected patient population, however, indicating that improved tumor–vessel involvement was not required for successful resection of the patients who were resected.
Conclusions
Apparent radiographic extent of vascular involvement does not change significantly after NCRT. Even in the absence of improvement in the radiographic tumor–vessel interactions on post-treatment CT scan, patients proceeding to surgery have a high rate of margin-negative resection. Instead of only offering surgical resection to BL-PDAC patients who demonstrate improved radiographic tumor–vessel relationships following NCRT, patients should be chosen for surgical exploration based on adequate performance status and lack of distant disease progression on imaging following neoadjuvant therapy.
Acknowledgments
Research support for study The following provided research support for the study: Claudio X. Gonzalez Family Foundation, Flannery Family Foundation, Alexander Family Foundation, Keeling Family Foundation, DeSanti Family Foundation, and National Cancer Institutes, P30 CA006973.
Footnotes
Presented at the American Society of Clinical Oncology Gastrointestinal Cancers Symposium, San Francisco, California, January 14–16, 2013 and the American Society of Clinical Oncology Annual Meeting, Chicago, Illinois, May 31–June 4, 2013
Conflict of interest
Avani Dholakia, Amy Hacker-Prietz, Aaron Wild, Siva Raman, Laura Wood, Peng Huang, Daniel Laheru, Lei Zheng, Ana De Jesus-Acosta, Dung Le, Richard Schulick, Barish Edil, Susannah Ellsworth, Timothy Pawlik, Christine Iacobuzio-Donahue, Ralph Hruban, John Cameron, Elliot Fishman, Christopher Wolfgang, and Joseph Herman all declare no conflicts of interest.
Ethical standards
Eleven patients were treated on one of two prospective trials and were required to provide written informed consent prior to study enrollment. The remaining 39 patients were treated per institutional standard of care and provided standard consent for treatment. These patients were included in an institutional review board approved retrospective study evaluating the outcomes of pancreatic cancer patients receiving radiation therapy at our institution.
Contributor Information
Avani S. Dholakia, Department of Radiation Oncology & Molecular Radiation Sciences, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins Hospital, Johns Hopkins University School of Medicine, 401 N. Broadway, Weinberg Suite 1440, Baltimore, MD 21231, USA
Amy Hacker-Prietz, Department of Radiation Oncology & Molecular Radiation Sciences, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins Hospital, Johns Hopkins University School of Medicine, 401 N. Broadway, Weinberg Suite 1440, Baltimore, MD 21231, USA.
Aaron T. Wild, Department of Radiation Oncology & Molecular Radiation Sciences, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins Hospital, Johns Hopkins University School of Medicine, 401 N. Broadway, Weinberg Suite 1440, Baltimore, MD 21231, USA
Siva P. Raman, Department of Radiology, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, 601 N. Broadway, Baltimore, MD 21231, USA
Laura D. Wood, Department of Pathology, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, 401 N. Broadway, Weinberg Suite 2242, Baltimore, MD 21231, USA
Peng Huang, Department of Oncology Biostatistics, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, 550 N. Broadway, Suite 1103, Baltimore, MD 21205, USA.
Daniel A. Laheru, Department of Oncology, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, 1650 Orleans St., Baltimore, MD 21287, USA
Lei Zheng, Department of Oncology, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, 1650 Orleans St., Baltimore, MD 21287, USA.
Ana De Jesus-Acosta, Department of Oncology, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, 1650 Orleans St., Baltimore, MD 21287, USA.
Dung T. Le, Department of Oncology, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, 1650 Orleans St., Baltimore, MD 21287, USA
Richard Schulick, Department of Surgery, University of Colorado, 12631 E. 17th Avenue, Suite 6117, Aurora, CO 80045, USA.
Barish Edil, Department of Surgery, University of Colorado, 12631 E. 17th Avenue, Suite 6117, Aurora, CO 80045, USA.
Susannah Ellsworth, Department of Radiation Oncology & Molecular Radiation Sciences, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins Hospital, Johns Hopkins University School of Medicine, 401 N. Broadway, Weinberg Suite 1440, Baltimore, MD 21231, USA.
Timothy M. Pawlik, Department of Surgery, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, 600 N. Wolfe St., Baltimore, MD 21287, USA
Christine A. Iacobuzio-Donahue, Department of Pathology, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, 401 N. Broadway, Weinberg Suite 2242, Baltimore, MD 21231, USA Department of Oncology, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, 1650 Orleans St., Baltimore, MD 21287, USA; Department of Surgery, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, 600 N. Wolfe St., Baltimore, MD 21287, USA.
Ralph H. Hruban, Department of Pathology, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, 401 N. Broadway, Weinberg Suite 2242, Baltimore, MD 21231, USA
John L. Cameron, Department of Surgery, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, 600 N. Wolfe St., Baltimore, MD 21287, USA
Elliot K. Fishman, Department of Radiology, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, 601 N. Broadway, Baltimore, MD 21231, USA
Christopher L. Wolfgang, Department of Surgery, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, 600 N. Wolfe St., Baltimore, MD 21287, USA
Joseph M. Herman, Department of Radiation Oncology & Molecular Radiation Sciences, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins Hospital, Johns Hopkins University School of Medicine, 401 N. Broadway, Weinberg Suite 1440, Baltimore, MD 21231, USA
References
- 1.Callery MP, Chang KJ, Fishman EK, Talamonti MS, William Traverso L, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1727–1733. doi: 10.1245/s10434-009-0408-6. doi:10.1245/s10434-009-0408-6. [DOI] [PubMed] [Google Scholar]
- 2.Tempero MA, Arnoletti JP, Behrman SW, Ben-Josef E, Benson AB, Casper ES, Cohen SJ, Czito B, Ellenhorn JD, Hawkins WG, Herman J, Hoffman JP, Ko A, Komanduri S, Koong A, Ma WW, Malafa MP, Merchant NB, Mulvihill SJ, Muscarella P, Nakakura EK, Obando J, Pitman MB, Sasson AR, Tally A, Thayer SP, Whiting S, Wolff RA, Wolpin BM, Freedman-Cass DA, Shead DA. Pancreatic adenocarcinoma, version 2.2012: featured updates to the NCCN Guidelines. J Natl Compr Cancer Netw. 2012;10:703–713. doi: 10.6004/jnccn.2012.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katz MH, Pisters PW, Evans DB, Sun CC, Lee JE, Fleming JB, Vauthey JN, Abdalla EK, Crane CH, Wolff RA, Varadhachary GR, Hwang RF. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833–846. doi: 10.1016/j.jamcollsurg.2007.12.020. doi:10.1016/j.jamcollsurg.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Geenen RC, ten Kate FJ, de Wit LT, van Gulik TM, Obertop H, Gouma DJ. Segmental resection and wedge excision of the portal or superior mesenteric vein during pancreatoduodenectomy. Surgery. 2001;129:158–163. doi: 10.1067/msy.2001.110221. doi:10.1067/msy.2001.110221. [DOI] [PubMed] [Google Scholar]
- 5.Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, Lee JE, Pisters PW, Evans DB, Wolff RA. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035–1046. doi: 10.1245/ASO.2006.08.011. doi:10.1245/ ASO.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Lu DS, Reber HA, Krasny RM, Kadell BM, Sayre J. Local staging of pancreatic cancer: criteria for unresectability of major vessels as revealed by pancreatic-phase, thin-section helical CT. Am J Roentgenol. 1997;168:1439–1443. doi: 10.2214/ajr.168.6.9168704. doi:10.2214/ajr.168.6.9168704. [DOI] [PubMed] [Google Scholar]
- 7.Straver ME, Loo CE, Rutgers EJ, Oldenburg HS, Wesseling J, Vrancken Peeters MJ, Gilhuijs KG. MRI-model to guide the surgical treatment in breast cancer patients after neoadjuvant chemotherapy. Ann Surg. 2010;251:701–707. doi: 10.1097/SLA.0b013e3181c5dda3. doi:10.1097/SLA.0b013e3181c5dda3. [DOI] [PubMed] [Google Scholar]
- 8.Gerard JP, Rostom Y, Gal J, Benchimol D, Ortholan C, Aschele C, Levi JM. Can we increase the chance of sphincter saving surgery in rectal cancer with neoadjuvant treatments: lessons from a systematic review of recent randomized trials. Crit Rev Oncol Hematol. 2012;81:21–28. doi: 10.1016/j.critrevonc.2011.02.001. doi:10.1016/j.critrevonc.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Katz MH, Fleming JB, Bhosale P, Varadhachary G, Lee JE, Wolff R, Wang H, Abbruzzese J, Pisters PW, Vauthey JN, Charnsangavej C, Tamm E, Crane CH, Balachandran A. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer. 2012;18:5749–5756. doi: 10.1002/cncr.27636. doi:10.1002/cncr.27636. [DOI] [PubMed] [Google Scholar]
- 10.Haeno H, Gonen M, Davis MB, Herman JM, Iacobuzio-Donahue CA, Michor F. Computational modeling of pancreatic cancer reveals kinetics of metastasis suggesting optimum treatment strategies. Cell. 2012;148:362–375. doi: 10.1016/j.cell.2011.11.060. doi:10.1016/j.cell.2011.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abrams RA, Lowy AM, O'Reilly EM, Wolff RA, Picozzi VJ, Pisters PW. Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1751–1756. doi: 10.1245/s10434-009-0413-9. doi:10.1245/s10434-009-0413-9. [DOI] [PubMed] [Google Scholar]
- 12.Stokes JB, Nolan NJ, Stelow EB, Walters DM, Weiss GR, de Lange EE, Rich TA, Adams RB, Bauer TW. Preoperative capecitabine and concurrent radiation for borderline resectable pancreatic cancer. Ann Surg Oncol. 2011;18:619–627. doi: 10.1245/s10434-010-1456-7. doi:10.1245/s10434-010-1456-7. [DOI] [PubMed] [Google Scholar]
- 13.McClaine RJ, Lowy AM, Sussman JJ, Schmulewitz N, Grisell DL, Ahmad SA. Neoadjuvant therapy may lead to successful surgical resection and improved survival in patients with borderline resectable pancreatic cancer. HPB (Oxford) 2010;12:73–79. doi: 10.1111/j.1477-2574.2009.00136.x. doi:10.1111/j.1477-2574.2009.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chun YS, Milestone BN, Watson JC, Cohen SJ, Burtness B, Engstrom PF, Haluszka O, Tokar JL, Hall MJ, Denlinger CS, Astsaturov I, Hoffman JP. Defining venous involvement in borderline resectable pancreatic cancer. Ann Surg Oncol. 2010;17:2832–2838. doi: 10.1245/s10434-010-1284-9. doi:10.1245/s10434-010-1284-9. [DOI] [PubMed] [Google Scholar]
- 15.Neoptolemos J, Buchler M, Stocken D, Ghaneh P, Smith D, Bassi C, Moore M, Cunningham D, Dervenis C, Goldstein D. ESPAC-3(v2): A multicenter, international, open-label, randomized, controlled phase III trial of adjuvant 5-fluorouracil/folinic acid (5-FU/FA) versus gemcitabine (GEM) in patients with resected pancreatic ductal adenocarcinoma [abstract]. J Clin Oncol. 2009;27:s18. [Google Scholar]
- 16.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, Gutberlet K, Kettner E, Schmalenberg H, Weigang-Koehler K, Bechstein WO, Niedergethmann M, Schmidt-Wolf I, Roll L, Doerken B, Riess H. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. doi:10.1001/jama. 297.3.267. [DOI] [PubMed] [Google Scholar]
- 17.Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, Bassi C, Falconi M, Pederzoli P, Dervenis C, Fernandez-Cruz L, Lacaine F, Pap A, Spooner D, Kerr DJ, Friess H, Buchler MW. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576–1585. doi: 10.1016/s0140-6736(01)06651-x. doi:10.1016/S0140-6736(01)06651-X. [DOI] [PubMed] [Google Scholar]
- 18.Smeenk HG, van Eijck CH, Hop WC, Erdmann J, Tran KC, Debois M, van Cutsem E, van Dekken H, Klinkenbijl JH, Jeekel J. Long-term survival and metastatic pattern of pancreatic and periampullary cancer after adjuvant chemoradiation or observation: long-term results of EORTC trial 40891. Ann Surg. 2007;246:734–740. doi: 10.1097/SLA.0b013e318156eef3. doi:10.1097/SLA.0b013e318156eef3. [DOI] [PubMed] [Google Scholar]
- 19.Chuong MD, Hayman TJ, Patel MR, Russell MS, Malafa MP, Hodul PJ, Springett GM, Choi J, Shridhar R, Hoffe SE. Comparison of 1-, 2-, and 3-dimensional tumor response assessment after neoadjuvant GTX-RT in borderline-resectable pancreatic cancer. Gastrointest Cancer Res. 2011;4:128–134. [PMC free article] [PubMed] [Google Scholar]
- 20.Kim EJ, Ben-Josef E, Herman JM, Bekaii-Saab T, Dawson LA, Griffith KA, Francis IR, Greenson JK, Simeone DM, Lawrence TS, Laheru D, Wolfgang CL, Williams T, Bloomston M, Moore MJ, Wei A, Zalupski MM. A multi-institutional phase 2 study of neoadjuvant gemcitabine and oxaliplatin with radiation therapy in patients with pancreatic cancer. Cancer. 2013 doi: 10.1002/cncr.28117. doi:10.1002/cncr.28117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pawlik TM, Laheru D, Hruban RH, Coleman J, Wolfgang CL, Campbell K, Ali S, Fishman EK, Schulick RD, Herman JM. Evaluating the impact of a single-day multidisciplinary clinic on the management of pancreatic cancer. Ann Surg Oncol. 2008;15:2081–2088. doi: 10.1245/s10434-008-9929-7. doi:10.1245/s10434-008-9929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimura W, Han I, Furukawa Y, Sunami E, Futakawa N, Inoue T, Shinkai H, Zhao B, Muto T, Makuuchi M, Komatsu H. Appleby operation for carcinoma of the body and tail of the pancreas. Hepatogastroenterology. 1997;44:387–393. [PubMed] [Google Scholar]
- 23.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. doi:10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 24.Hruban RHPM, Klimstra DS. Tumors of the pancreas. American Registry of Pathology; Washington, DC: 2007. [Google Scholar]
- 25.Washington K, Berlin J, Branton P, Burgart LJ, Carter DK, Fitzgibbons P, Frankel WL, Jessup J, Kakar S, Minsky B, Nakhleh R, Compton C. Protocol for the examination of specimens from patients with carcinoma of the exocrine pancreas, version 3.2.0.0. College of American Pathologists; Northfield, IL: 2010. Protocol for the examination of specimens from patients with carcinoma of the exocrine pancreas. [Google Scholar]
- 26.Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Buchler MW. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91:586–594. doi: 10.1002/bjs.4484. doi:10.1002/bjs.4484. [DOI] [PubMed] [Google Scholar]
- 27.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, Sauter PK, Coleman J, Hruban RH, Lillemoe KD. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. doi:10.1016/S1091-255X(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 28.Kim HJ, Czischke K, Brennan MF, Conlon KC. Does neoadjuvant chemoradiation downstage locally advanced pancreatic cancer? J Gastrointest Surg. 2002;6:763–769. doi: 10.1016/s1091-255x(02)00017-3. doi:10.1016/S1091-255X(02)00017-3. [DOI] [PubMed] [Google Scholar]
- 29.Donahue TR, Isacoff WH, Hines OJ, Tomlinson JS, Farrell JJ, Bhat YM, Garon E, Clerkin B, Reber HA. Downstaging chemo-therapy and alteration in the classic computed tomography/magnetic resonance imaging signs of vascular involvement in patients with pancreaticobiliary malignant tumors: influence on patient selection for surgery. Arch Surg. 2011;146:836–843. doi: 10.1001/archsurg.2011.152. doi:10.1001/archsurg.2011.152. [DOI] [PubMed] [Google Scholar]
- 30.Raman SP, Horton KM, Fishman EK. Multimodality imaging of pancreatic cancer-computed tomography, magnetic resonance imaging, and positron emission tomography. Cancer J. 2012;18:511–522. doi: 10.1097/PPO.0b013e318274a461. doi: 10.1097/PPO.0b013e318274a461. [DOI] [PubMed] [Google Scholar]
- 31.Choi M, Heilbrun LK, Venkatramanamoorthy R, Lawhorn-Crews JM, Zalupski MM, Shields AF. Using 18F-fluorodeoxyglucose positron emission tomography to monitor clinical outcomes in patients treated with neoadjuvant chemo-radiotherapy for locally advanced pancreatic cancer. Am J Clin Oncol. 2010;33:257–261. doi: 10.1097/COC.0b013e3181a76a0b. doi:10. 1097/COC.0b013e3181a76a0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laurence JM, Tran PD, Morarji K, Eslick GD, Lam VW, Sandroussi C. A systematic review and meta-analysis of survival and surgical outcomes following neoadjuvant chemoradiotherapy for pancreatic cancer. J Gastrointest Surg. 2011;15:2059–2069. doi: 10.1007/s11605-011-1659-7. doi:10.1007/ s11605-011-1659-7. [DOI] [PubMed] [Google Scholar]
- 33.Katz MH, Marsh R, Herman JM, Shi Q, Collison E, Venook AP, Kindler HL, Alberts SR, Philip P, Lowy AM, Pisters PW, Posner MC, Berlin JD, Ahmad SA. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg Oncol. 2013 doi: 10.1245/s10434-013-2886-9. doi:10.1245/s10434-013-2886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spitz FR, Abbruzzese JL, Lee JE, Pisters PW, Lowy AM, Fenoglio CJ, Cleary KR, Janjan NA, Goswitz MS, Rich TA, Evans DB. Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J Clin Oncol. 1997;15:928–937. doi: 10.1200/JCO.1997.15.3.928. [DOI] [PubMed] [Google Scholar]