Abstract
Constitutional SMARCB1 mutations at 22q11.23 have been found in ~50% of familial and <10% of sporadic schwannomatosis cases1. We sequenced highly conserved regions along 22q from eight individuals with schwannomatosis whose schwannomas involved somatic loss of one copy of 22q, encompassing SMARCB1 and NF2, with a different somatic mutation of the other NF2 allele in every schwannoma but no mutation of the remaining SMARCB1 allele in blood and tumor samples. LZTR1 germline mutations were identified in seven of the eight cases. LZTR1 sequencing in 12 further cases with the same molecular signature identified 9 additional germline mutations. Loss of heterozygosity with retention of an LZTR1 mutation was present in all 25 schwannomas studied. Mutations segregated with disease in all available affected first-degree relatives, although four asymptomatic parents also carried an LZTR1 mutation. Our findings identify LZTR1 as a gene predisposing to an autosomal dominant inherited disorder of multiple schwannomas in ~80% of 22q-related schwannomatosis cases lacking mutation in SMARCB1.
Schwannomatosis (MIM 162091), the third major form of neurofibromatosis, is a late-onset tumor predisposition disorder that is clinically and genetically distinct from neurofibromatosis types 1 (MIM 162200) and 2 (MIM 101000). Although isolated schwannomas are common benign tumors, schwannomatosis—characterized by the development of multiple schwannomas without the bilateral vestibular schwannomas, congenital cataracts or ependymomas typically associated with neurofibromatosis type 2—is rare; however, its exact incidence is unknown2,3. While constitutional NF2 mutations are not found, independent somatic mutations affecting both NF2 alleles are typically present in every schwannoma of individuals with schwannomatosis4. Multipoint linkage analysis in families with schwannomatosis pointed to an ~8.48-Mb region centromeric to the NF2 locus, between markers D22S420 and D22S1148, as the linked region5. Germline mutations in SMARCB1, located in this region and previously known to cause rhabdoid tumor predisposition syndrome (RTPS), have since been found in schwannomatosis cases2,6–10. Genetic analysis of schwannomas in cases with a SMARCB1 germline mutation (first event, E1) shows loss of a region at 22q (second event, E2), with retention of the SMARCB1 mutation in the schwannomas, followed by mutation of the remaining wild-type NF2 gene (third event, E3) in cis with the SMARCB1 germline mutation2,7. These three events result in biallelic loss of both the SMARCB1 and NF2 tumor suppressor genes in the schwannomas.
As germline SMARCB1 mutations account for only ~50% of familial and <10% of sporadic cases11, additional schwannomatosis-predisposing loci probably exist. A subset of cases had no constitutional first-hit SMARCB1 mutation but had deletion of part of 22q encompassing both NF2 and SMARCB1 and somatic mutation of the remaining NF2 allele in the schwannomas (Online Methods and Supplementary Fig. 1). We hypothesized that either functionally important sequences outside of the SMARCB1 regions previously analyzed through clinical testing (for example, introns, 5′ or 3′ UTRs or intergenic regions) or an alternative evolutionarily conserved locus on chromosome 22 might carry a first hit predisposing to schwannomatosis in these cases. Here we report studies of germline DNA in 20 unrelated probands (6 familial cases, 11 sporadic cases and 3 cases with unknown family history of schwannomatosis; Supplementary Table 1) with an unknown first-hit mutation in blood and schwannomas (E1?), loss of 22q (E2+) and a different NF2 mutation in every schwannoma (E3+) (Supplementary Fig. 1). We selectively enriched for 3.72 Mb of highly conserved sequence along chromosome 22 and initially performed deep parallel sequencing in eight cases (NGS1–NGS8) (Table 1 and Online Methods).
Table 1.
LZTR1 mutations identified in 16 unrelated schwannomatosis cases
| Subject | Genomic positiona | Genomic mutation | Exon or intron |
cDNA mutation (blood) |
Protein alteration | Type of mutation |
Predicted effect of missense mutation | ESP alleleb | ||
|---|---|---|---|---|---|---|---|---|---|---|
| PolyPhen | SIFT | MutationTaster | ||||||||
| S3 | Chr. 22: 21336687 | c.27delGc | E1 | – | p.Gln10Argfs*15 | Truncating | NA | NA | NA | G = 12,702 |
| S2 | Chr. 22: 21337353 | c.238dupA | E2 | r.238dupa | p.Ile80Asnfs*15 | Truncating | NA | NA | NA | A = 13,006 |
| NGS2 | Chr. 22: 21340117 | c.264–13G>Ac | IVS2 | r.263_264ins264– 11_264–1 |
p.Lys89Cysfs*16 | Truncating, out-of-frame splice site |
NA | NA | NA | G = 13,006 |
| NGS3 | Chr. 22: 21341837 | c.365C>Tc | E4 | – | p.Ser122Leu | Missense | Probably damaging (1.00) |
Deleterious (0.00) |
Disease causing (probability = 1.0) |
C = 13,006 |
| S7 | Chr. 22: 21343911 | c.594–3C>Gc | IVS6 | r.594_651del (skip E7) |
p.Leu199Trpfs*34 | Truncating/ out-of-frame splice |
NA | NA | NA | C = 12,998, T = 8f |
| S5 | Chr. 22: 21347143 | c.1210G>A | E11 | – | p.Gly404Arg | Missense | Probably damaging (0.999) |
Deleterious (0.01) |
Disease causing (probability = 1.0) |
G = 13,000 |
| NGS8 | Chr. 22: 21348226 | c.1367T>G | E13 | r.1367u>g | p.Val456Gly | Missense | Probably damaging (0.97) |
Deleterious (0.00) |
Disease causing (probability = 1.0) |
T = 13,006 |
| S9 | Chr. 22: 21348256 | c.1397G>Ac | E13 | – | p.Arg466Gln | Missense | Probably damaging (1.00) |
Deleterious (0.00) |
Disease causing (probability = 1.0) |
G = 13,002 |
| NGS6 | Chr. 22: 21348309 | c.1449+1G>A | IVS13 | r.1354_1449del (skip E13) |
p.Glu453_Lys484del | 32-residue deletion, in-frame splice site |
NA | NA | NA | G = 12,984 |
| NGS5 | Chr. 22: 21348502 | c.1559C>Tc | E14 | – | p.Pro520Leu | Missense | Probably damaging (0.98) |
Deleterious (0.02) |
Disease causing (probability = 1.0) |
C = 12,978 |
| S8 | Chr. 22: 21348982 | c.1751dupA | E15 | – | p.Ser585Glufs*84 | Truncating | NA | NA | NA | A = 13,004 |
| NGS7 | Chr. 22: 21350154 | c.2062C>Tc,d | E17 | r.2062c>u | p.Arg688Cys | Missense | Probably damaging (1.00) |
Deleterious (0.00) |
Disease causing (probability = 1.0) |
C = 13,002 |
| S4 | Chr. 22: 21350154 | c.2062C>Td | E17 | – | p.Arg688Cys | Missense | Probably damaging (1.00) |
Deleterious (0.00) |
Disease causing (probability = 1.0) |
C = 13,002 |
| NGS1 | Chr. 22: 21350969 | c.2220–16_2220– 14delCTT |
IVS18 | r. = | p. = ?e | Splicing? | NA | NA | NA | C,T,T = 13,006 |
| S6 | Chr. 22: 21351197 | c.2348_2351delCGCAc | E20 | – | p.Thr783Argfs*5 | Truncating | NA | NA | NA | C = 13,005; T = 1; G,C,A = 13,006g |
| S1 | Chr. 22: 21351552 | c.2438G>Tc | E21 | r.2438g>u | p.Ser813Ile | Missense | Probably damaging (0.996) |
Deleterious (0.01) |
Disease causing (probability = 1.0) |
G = 13,006 |
None of the mutations was present in dbSNP137, 1000 Genomes Project Phase 1 integrated release variations or the National Heart, Lung, and Blood Institute (NHLBI) GO Exome Sequencing Project (ESP) Exome Variant Server v.0.0.21 (August 2013). A prefix of “NGS” indicates samples included in the next-generation sequencing cohort, and a prefix of “S” indicates, samples included in the Sanger sequencing cohort.
Chr., chromosome; –, not tested; IVS, intron; E, exon, NA, not applicable.
Genomic positions are given according to the hg19 reference assembly. Reference transcript (hg19) is available under accession NM_006767.3.
Allele counts are according to the NHLBI GO ESP Exome Variant Server (v.0.0.18; accessed 8 February 2013).
Mutations were also found in relatives available for targeted LZTR1 mutation analysis (see Fig. 3).
A recurrent mutation found in two unrelated cases.
Likely pathogenic mutation affecting the highly evolutionarily conserved CTT nucleotide motif within the splice acceptor of exon 19: phastCons score of 1.00 and phyloP score for the consecutive nucleotides of 2.87, 2.95 and 2.14.
The c.594–3C>T transition is annotated in ESP but is predicted not to affect splicing, whereas the c.594–3C>G transversion has not previously been reported. c.594–3C>G is predicted to create a novel splice acceptor sequence and to decrease the strength of the wild-type splice acceptor site, and it was proven in this study to cause aberrant splicing (skipping of exon 7) at the mRNA (cDNA) level.
c.2348C>T is a SNP (rs143507674) at chr. 22: 21,351,197 in dbSNP137; however, no pathogenic c.2348_2351delCGCA is present in dbSNP137 or ESP.
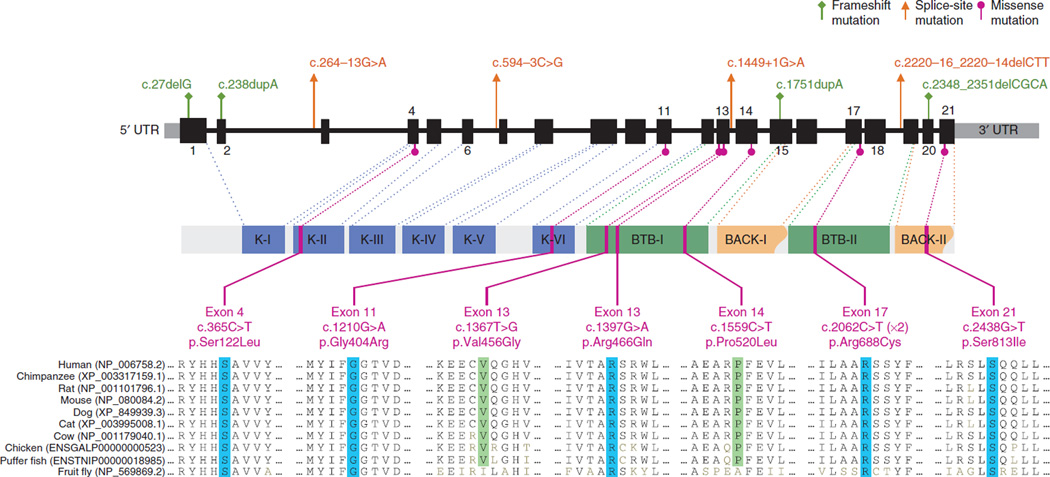
Variants were called with Platypus and SVDetect12, which, in addition to identifying germline mutations, includes a search for mosaic and structural variants. Initial filtering identified a single gene, LZTR1, carrying previously unreported exonic nonsynonymous variants in four of eight cases (Supplementary Table 2); these variants encoded p.Ser122Leu, p.Val456Gly, p.Pro520Leu and p.Arg688Cys alterations, all of which are missense changes affecting highly conserved amino acids and are predicted in silico to be damaging (Figs. 1 and 2, Table 1, Supplementary Fig. 2 and Supplementary Tables 3 and 4a). Manual examination of intronic LZTR1 sequences identified mutations affecting conserved splice sites in three additional probands of this initial cohort; these mutations included c.264–13G>A, c.1449+1G>A and c.2220–16_2220–14delCTT (Supplementary Fig. 3). All mutations were confirmed by Sanger sequencing. Analysis of discrepancies in insert size and anomalies in mapping information did not identify likely pathogenic intrachromosomal changes (Online Methods and Supplementary Table 5).
Figure 1.
Distribution of mutations identified in the LZTR1 gene in individuals with schwannomatosis. Top, locations of frameshift, splice-site and missense mutations. Exons, introns and 5′ and 3′ UTRs are indicated by thick, thin and gray segments, respectively. Middle, LZTR1 protein domains and the locations of the genomic sequences encoding them (dotted lines): K-I–K-VI, Kelch motifs of the Kelch domain; BTB-I and BTB-II, BACK-I and BACK-II (partial BACK) domains. Bottom, missense mutations and the evolutionary conservation of the affected amino acids across ten different species up to the fruit fly. Blue, amino acids conserved up to the fruit fly; green, amino acids conserved up to the puffer fish. Recurrent p.Arg688Cys alterations were found in two unrelated individuals. An accession code for the GenBank protein record is given in parentheses for each species. See also Table 1.
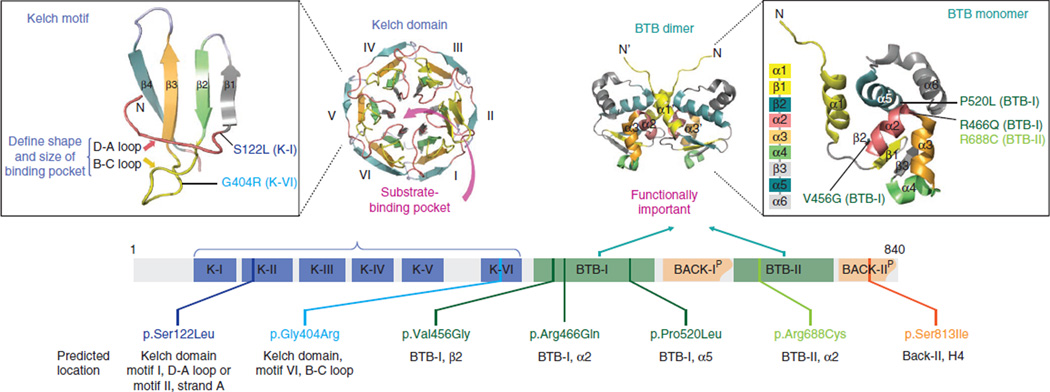
Figure 2.
Structural domains of LZTR1 and spatial predictions for missense alterations. Top left, structural modeling of a single Kelch motif and the predicted locations of missense alterations, as well as the entire Kelch domain consisting of six Kelch motifs (K-I to K-VI). Top right, structural modeling of dimeric and monomeric BTB domains (along with sequential distribution of α helices and β sheets) and the predicted locations of missense alterations in the BTB-I and BTB-II domains. Bottom, distribution and predicted locations of missense alterations.
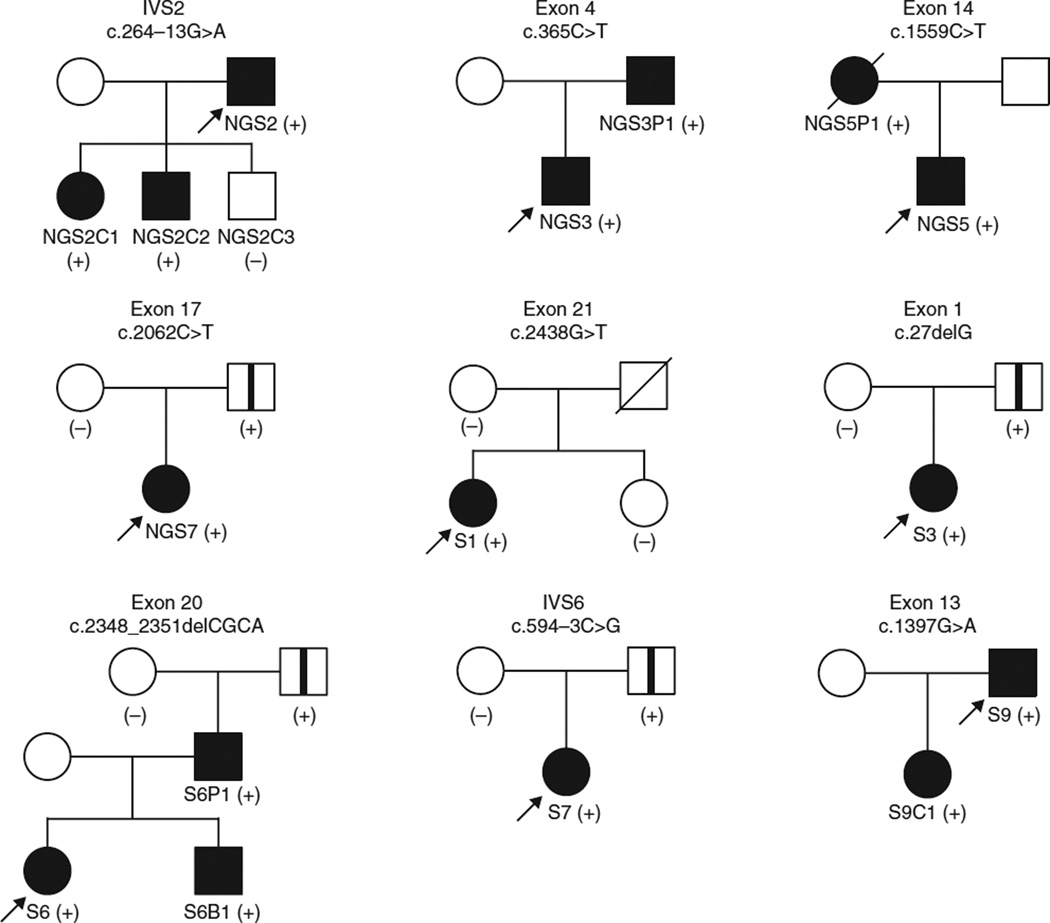
Sanger sequencing of LZTR1 in lymphocyte DNA from 12 further unrelated E1?E2+E3+ probands (S1–S12) identified additional mutations in 9 cases (Fig. 1 and Table 1). In total, 15 different previously unreported germline mutations in LZTR1 were found in 16 of 20 unrelated schwannomatosis probands negative for SMARCB1 mutation (E1?E2+E3+) but in 0 of 8 schwannomatosis probands positive for SMARCB1 mutation (E1+E2+E3+) (P = 0.0002, two-tailed Fisher’s exact test; Supplementary Table 6), including 6 truncating mutations (4 frameshift and 2 out-of-frame splice-site mutations), 1 in-frame splice-site mutation, a 3-nt deletion affecting a highly evolutionarily conserved splice acceptor sequence and 7 different missense mutations predicted to be damaging, all of which were absent in dbSNP137, the 1000 Genomes Project and ESP6500 (Fig. 1, Table 1, Supplementary Figs. 2–4 and Supplementary Table 4). The spectrum of mutations suggests that loss of function is the main mechanism. LZTR1, located at 22q11.21, contains 21 exons and generates multiple alternatively spliced transcripts, with the longest ORF encoding an 840-residue protein13. LZTR1 is expressed ubiquitously and abundantly in human tissues13,14. LZTR1 resides centromeric to SMARCB1, also within the previously identified schwannomatosis-associated linkage interval5. Loss of heterozygosity with retention of the case-specific LZTR1 mutation was found in all 25 schwannomas studied, strongly supporting the hypothesis that the LZTR1 mutations are pathogenic (the likelihood of such genetic changes occurring by chance in all 25 tumors in 16 unrelated cases is conservatively ~1.5 × 10−5) and consistent with a tumor suppressor mode of action for LZTR1 (Supplementary Figs. 1 and 5). The LZTR1 mutations segregated with the presence of multiple schwannomas in all seven affected first-degree relatives from five families, in line with autosomal dominant inheritance (Fig. 3). A germline LZTR1 mutation was identified in all familial cases studied and in 8 of 11 reportedly sporadic cases. No first-hit LZTR1 mutations were detected in the schwannomas of the remaining three sporadic cases; therefore, LZTR1 mosaicism is unlikely to explain the phenotype of these individuals (Supplementary Table 6).
Figure 3.
Pedigrees of families positive for LZTR1 mutation with information from relatives available for testing. Filled symbols represent individuals clinically affected by schwannomatosis. Open symbols with a vertical line represent clinically asymptomatic, (likely) non-penetrant individuals carrying the familial LZTR1 mutation. Plus and minus signs indicate individuals positive or negative for the family-specific mutation, respectively. LZTR1 mutation in probands (arrows) was initially identified by next-generation sequencing of evolutionarily conserved sequences at 22q (NGS2, NGS3, NGS5, NGS7) or by sequencing the entire coding sequence of the LZTR1 gene and flanking intronic sequences (S1, S3, S6, S7, S9). Relatives were subjected to targeted analysis of the family-specific LZTR1 mutation identified in the proband.
The clinically unaffected fathers of four reportedly sporadic cases carried the familial LZTR1 mutation (Fig. 3), probably demonstrating non-penetrance, which has previously been observed in familial schwannomatosis pedigrees9. As these individuals did not undergo full-body magnetic resonance imaging (MRI), they may carry unrecognized tumors. It is possible that LZTR1 mutations predispose to a phenotype at the mild end of the spectrum (even resulting in a single schwannoma, which is a common finding in the general population) with incomplete penetrance; larger studies including more affected individuals and their unaffected relatives will help resolve this question.
LZTR1 was recently characterized as a tumor suppressor gene and driver in glioblastoma multiforme (GBM) on the basis of the presence of biallelic mutations in 4 of 139 GBM samples, with mutations driving self-renewal and growth of glioma spheres15. Moreover, somatic LZTR1 mutations have been identified in several cancers (Catalogue of Somatic Mutations in Cancer (COSMIC) database) (Supplementary Table 4b). However, some loss-of-function mutations in LZTR1 have also been found in control populations (ESP2500 and the 1000 Genomes Project), a feature shared with some other tumor suppressor genes involved in hereditary predisposition to late-onset disorders, including MSH6, PMS2, BRCA1 and BRCA2. All these genes have a complex spectrum of mutations associated with variable expressivity and penetrance16–18, and, for BRCA2, MSH6 and PMS2, biallelic mutations have been found in rare individuals with distinct phenotypes19. Penetrance for associated tumors may be different for a given gene and even may be different depending on the specific mutation.
We performed a detailed analysis of the spectrum and frequency of LZTR1 mutations in tumor databases (schwannomatosis, GBM and the confirmed somatic cohort in COSMIC) versus control databases (ESP2500 and the 1000 Genomes Project) (Supplementary Figs. 6 and 7 and Supplementary Tables 3 and 4). The frequencies of predicted pathogenic mutations in LZTR1 in cases from the present study (16/20) and controls (27/3,292) were extremely statistically different (P < 0.0001; 2-tailed Fisher’s exact test). In addition, the difference in the predicted pathogenicity of observed missense mutations in the tumor databases (schwannomatosis, GBM and the confirmed somatic cohort of COSMIC) and control databases was also very statistically significant. The frequency of different/non-recurrent predicted damaging missense mutations in the tumor-associated cohorts (25/35) versus control populations (12/34) was also very statistically significant (P = 0.0037, 2-tailed Fisher’s exact test; Supplementary Fig. 6). None of the mutations in the tumor databases were present in the control databases, except for the mutation encoding a p.Phe447Leu alteration from COSMIC, which was predicted to be probably benign. Of the 35 different missense mutations reported in the tumor-associated data sets, 10 were found in >1 unrelated tumor sample or were affecting a critical amino acid (Arg68) observed to be targeted by different codon-changing mutations, implying functional significance. Nevertheless, the available data from control cohorts suggest that pathogenic mutations in the LZTR1 tumor suppressor gene are observed in presumably asymptomatic cases, and further studies in individuals heterozygous or compound heterozygous for such mutations will allow a better understanding of the spectrum of phenotypes associated with mutations in this gene.
The LZTR1 protein belongs to a functionally diverse superfamily of BTB/POZ (bric-a-brac, tramtrack and broad complex/pox virus and zinc-finger) proteins20. The LZTR1 domain arrangement is unique compared to all other known BBK proteins (N-BTB-BACK (BTB and C-terminal Kelch)-Kelch-C)21 and contains an N-terminal Kelch domain with six Kelch motifs followed by two BTB domains (Fig. 2). Following each BTB domain, a partial BACK domain (N-Kelch-BTB-BACK(p)-BTB-BACK(p)-C) is predicted (Supplementary Fig. 8)22, which may be important to position the Kelch domains for substrate recognition23. All seven missense mutations and the in-frame splice-site mutation c.1449+1G>A (encoding p.Glu453_Lys484del) affect highly evolutionarily conserved residues within functional domains of importance and are therefore predicted to be pathogenic (Figs. 1 and 2 and Supplementary Figs. 2 and 8–11).
BTB-containing proteins control fundamental cellular processes, ranging from the regulation of chromatin conformation to the cell cycle. Alterations in their activities have been linked to many inherited diseases and cancers (Supplementary Fig. 9)24,25. They share a role as substrate adaptors for cullin-3 (Cul3) RING ligase (CRL3), which recruits substrate-specific adaptors to catalyze protein ubiquitination20. Kelch domains are the most common substrate recognition domains for Cul3 (ref. 26). A mass spectroscopy study inferred an association of LZTR1 and Cul3 (ref. 27), which was recently proven by immunoprecipitation to specifically involve the BTB domain, as expected15. LZTR1 localizes to the Golgi network in endothelial, smooth muscle and HeLa cell lines, with this localization mediated by LZTR1–BTB-II (ref. 21), and it may stabilize the Golgi complex via interaction with other proteins21,28. Other roles for both BTB domains need further study.
LZTR1 contains a bipartite nuclear localization signal, which may facilitate its transport to the nucleus, as shown for another BBK family member localized at the Golgi in non-dividing cells and translocated to the spindle apparatus during mitosis29 (Online Methods and Supplementary Fig. 12).
Several proteins containing BTB/POZ domains interact with the N-CoR (nuclear receptor corepressors) and SMRT (silencing mediator for retinoid and thyroid receptors) nuclear receptor corepressors30,31. The N-CoR complex also contains components of the SWI/SNF chromatin-remodeling complex, and SMARCB1 was previously proven to interact with N-CoR, indicating a potential functional link between LZTR1 and SMARCB1 or other members of the SWI/SNF complex32. Moreover, studies in the evolutionarily distant organism Toxoplasma gondii have shown that the Toxoplasma homolog of LZTR1 interacts with SRCAP33, another member of the SWI/SNF complex.
SMARCB1 was previously shown to interact with HDAC4 (histone deacetylase 4)34, and mammalian two-hybrid analysis has recently shown that LZTR1 also interacts with HDAC4 (ref. 35). Furthermore, LZTR1 physically associates with STAT1 (signal transducer and activator of transcription 1) and PARP1 (poly (ADP-ribose) polymerase 1)35, both of which, through binding to the SMARCB1 promoter, are involved in the upstream regulation of SMARCB1 (refs. 36,37). Histone deacetylase inhibitors are emerging as a new class of antitumor drugs, and AR42, a novel compound with HDAC inhibitor activity, was recently shown to inhibit growth in schwannoma and meningioma cells, offering the prospect of its further evaluation as a potential treatment in schwannomatosis38.
Further in vivo and in vitro studies are needed to unravel the predicted tissue-specific functions of the different LZTR1 isoforms, their cellular localization and the proteins with which they interact in order to understand the mechanisms contributing to the pathogenesis of schwannomas and other tumors. In conclusion, we report the discovery of germline LZTR1 mutations whose frequency in individuals with schwannomatosis versus controls, retention in all studied schwannomas, segregation within affected families and predicted effects on protein function provide robust evidence that they are disease predisposing.
ONLINE METHODS
Cases and clinical data
All probands were diagnosed with either sporadic or familial schwannomatosis on the basis of diagnostic guidelines and were previously referred for genetic testing at the University of Alabama at Birmingham Medical Genomics Laboratory. The cohort of 20 probands studied here is a subpopulation of all schwannomatosis cases referred for genetic testing.
Through comprehensive mutational analyses of NF2, SMARCB1 and copy number changes at 22q in schwannomas and blood from schwannomatosis cases, we have identified five main groups of affected individuals:
E1+E2+E3+: cases carrying a SMARCB1 first-hit mutation in blood and schwannomas (positive (+) for mutational event 1, E1+), with loss of 22q (encompassing the region between and including LZTR1 and CABP7) in the schwannomas (E2+) and a different NF2 mutation in each schwannoma (E3+), resulting in biallelic loss of SMARCB1 and NF2.
E1?E2+E3+: cases with no SMARCB1 first-hit mutation detectable in blood and schwannomas, although they still have loss of a region at 22q (encompassing the region between and including LZTR1 and CABP7) in the schwannomas and a different NF2 mutation in each schwannoma. In the schwannomas both positive and negative for SMARCB1 mutation with loss of 22q and a different NF2 mutation in each schwannoma, the eventual (ultimate) target was inactivation of both NF2 copies. It is possible that the undetected first-hit mutations affect functionally important sequences outside the SMARCB1 coding region, such as the 5′ or 3′ UTR or a conserved intronic or intergenic region (that may not typically be part of the clinical testing that focuses on sequencing of the exons and their flanking intron sequence as well as on copy number analysis) or that an alternative gene on chromosome 22 carries the first predisposing hit (first event, first hit) in these cases.
E1?E2+E3?: cases with no SMARCB1 first-hit mutation detectable in blood and schwannomas, although they still have loss of a region at 22q (encompassing SMARCB1 and NF2) in the schwannomas but no mutations identified in the NF2 gene.
E1?E2−E3?: cases with no SMARCB1 first-hit mutation detectable in blood and schwannomas, no loss at 22q and no identified NF2 mutations. This group of cases may be a heterogeneous population, where the underlying genetic cause may be diverse and is more likely to be unrelated to chromosome 22.
Some cases were additionally identified as being mosaic NF2 mutation carriers, with the presence of a common first-hit NF2 mutation in their schwannomas (see the cautionary note on diagnostic criteria in Plotkin et al.2). In addition, for some cases, only blood was available for testing, and no germline SMARCB1 or NF2 mutation was detected; therefore, genetic analysis did not allow us to molecularly confirm suspected diagnosis.
All 20 probands in the current study had previously undergone clinical genetic testing for SMARCB1 and NF2 mutations at the University of Alabama at Birmingham Medical Genomics Laboratory on blood and tumor samples and belonged to group 2 (E1?E2+E3+). The study was approved by the institutional review board at the University of Alabama at Birmingham, and informed consent was obtained from all subjects. Clinical data are summarized in Supplementary Table 1. Mutational data are summarized in Figure 1, Table 1, Supplementary Figures 1 and 13, and Supplementary Table 7.
Targeted resequencing of chromosome 22
A custom SureSelect target enrichment library (design ID 0371891) was designed using the SureDesign online tool (Agilent Technologies). Briefly, the custom enrichment library targeted exonic and noncoding evolutionarily conserved elements (conserved in vertebrates with PhastCons scores of >0.85) within previously defined linkage intervals as well as other conserved regions along 22q. The library also included the entire repeat-masked genomic sequence of the SMARCB1, NF2 and CABIN1 genes, previously implied (SMARCB1 and NF2) or suggested (CABIN1) to be important in schwannomatosis39–42. In total, the library covered 3.72 Mb of genomic reference sequence along 22q. Target capture was carried out according to the manufacturer’s protocol (Agilent Technologies). Samples were subjected to paired-end sequencing on an Illumina HiSeq 2000 instrument. Corresponding data files have been deposited in ArrayExpress (accession E-MTAB-1574). Sequencing statistics are provided in Supplementary Table 2. Sequencing reads were aligned to the human reference genome (hg19) using the Burrows-Wheeler transform Aligner (BWA)43. Unmapped reads and reads with mapping quality of <30 (Phred scaled) were removed using SAMtools44.
Detection and annotation of sequence variants
Variant detection was carried out with Platypus (accessed March 2012) in variant-calling mode. Variants supported by high-quality bases (≥30) in fewer than five reads were filtered out. Variants called by Platypus were further annotated with SeattleSeq 134 (accessed August 2012), including allele counts from dbSNP134 and the ESP Exome Variant Server (v.0.0.14; released June 2012), and were filtered according to predicted effect using the in-house tool VCF File Comparator (P.M., L.M.M. and A.P., unpublished data).
Structural variant analysis
Sorted and indexed BAM files were preprocessed with the BAM_preprocessingPairs.pl script from SVDetect r.0.8 (ref. 12). This script filters out correctly mapped reads and outputs anomalously mapped reads for downstream analysis. SVDetect was run on the output files with the following parameters: sliding window size for partitioning the genome for intrachromosomal rearrangements, μ + 2σ (where μ is the mean of the insert size distribution and σ is the standard error of the insert size distribution); length of the sliding window step, one-fourth of the window size; minimum number of pairs in a cluster, one-fourth of the median depth of coverage for the experiment; minimum number of σ-fold for insert size filtering and to call insertions, deletions or tandem duplications, 3; minimal final filtering score for calling structural variants, 0.8.
cDNA-based mutation analysis
cDNA-based analysis of the SMARCB1, NF2 and LZTR1 genes began with RNA extraction from a phytohemagglutinin-stimulated short-term lymphocyte culture (STLC) and RT-PCR using SuperScript II Reverse Transcriptase (Invitrogen by Life Technologies, 18064014).
cDNA regions were amplified using TakaRa Ex Taq (TAKARA BIO, RR001A) for SMARCB1 and NF2 and the Expand Long-Template PCR system (Roche Applied Science, 11681842001) for LZTR1. Direct shotgun Sanger sequencing of the entire coding regions was subsequently performed on an ABI PRISM 3730 Genetic Analyzer, and sequences were analyzed using SeqScape software v 2.5 (Applied Biosystem by Life Technologies) and mutation interpretation software Alamut v.2.3 (Interactive Biosoftware). Primer sequences are available upon request.
To compensate for the location of the forward RT-PCR primer in exons 1 of SMARCB1 and LZTR1 and the alternative transcripts affecting SMARCB1 exon 2 and LZTR1 exon 15, genomic DNA analysis of exons 1–3 of SMARCB1 and of exons 1 and 15 of LZTR1 was performed for each sample in parallel to RT-PCR. All mutations found at the cDNA level were confirmed by analysis at the genomic DNA level.
Genomic DNA–based mutation analysis
Genomic DNA was amplified for all exons of the NF2, SMARCB1 and LZTR1 genes and for part of the 3′ UTR of the SMARCB1 and LZTR1 genes from peripheral blood leukocyte samples and fresh or formalin-fixed paraffin-embedded tumor tissues. SMARCB1 and LZTR1 amplicons were generated using LightScanner Master Mix (Idaho Technology, HRLS-ASY-0003), and NF2 amplicons were generated using the Platinum Taq DNA Polymerase kit (Invitrogen by Life Technologies, 10966-034).
PCR runs for all LZTR1 exons and for exons 1 of SMARCB1 and NF2 contained DMSO to a final concentration of 5%. All primers were tagged with M13 to facilitate downstream sequencing (primer sequences available upon request). PCR products were sequenced bidirectionally on an ABI PRISM 3130xl or 3730 Genetic Analyzer, and sequences were analyzed using SeqScape software v2.5 and the mutation interpretation software Alamut.
Comprehensive analysis of schwannomas included sequencing of all SMARCB1 and NF2 exons and flanking intronic sequences (at least from −20 to +15 bp relative to the exon boundaries). LZTR1 mutations identified in blood were confirmed in tumors by targeted sequencing of the corresponding exonic or intronic region. Family studies were conducted on genomic DNA extracted from the saliva or blood (if available) of relatives by targeted analysis of the family-specific LZTR1 mutation identified in the proband.
Multiplex ligation-dependent probe amplification
To detect copy number changes (deletions or duplications) of the NF2 and SMARCB1 loci and flanking genes, genomic DNA extracted from blood leukocytes and schwannoma tumors was analyzed by multiplex ligation-dependent probe amplification (MLPA) using the SALSA MLPA kit (MRC-Holland, P044_B1, P258_C1) according to the manufacturer’s suggestions. Notably, the SMARCB1 SALSA MLPA kit also contains a probe assessing LZTR1; therefore, a deletion or duplication encompassing this region would also be detected. Owing to the quality of the DNA extracted from formalin-fixated paraffin-embedded tissue, MLPA analysis was not of sufficient quality in some samples, and microsatellite marker analysis was therefore used to assess the loss of 22q in such cases.
Microsatellite marker analysis
Analysis of loss of heterozygosity for the region of chromosome 22 encompassing LZTR1, SMARCB1 and NF2 was analyzed using microsatellite markers as previously described42.
Predictions of the effects of missense mutations
Three software programs were used to predict the effects of missense mutations as previously described: PolyPhen45, SIFT46 and MutationTaster47.
In addition, predictions were generated on the basis of sequence alignment, secondary structure prediction, molecular modeling and residue permutation. All sequences were obtained from Ensembl. Conserved domains were searched against the CDDv3.08-43334 PSSMs (Position-Specific Score Matrices) and SMART v6.0-1013 PSSMs databases, through the NCBI website48–50. The Protein Homology/analogY Recognition Engine (Phyre) was used for secondary structure prediction51. SWISS-MODEL (automated mode) was applied for homology modeling52–54. Homology models used included the crystal structure of the Kelch domain of human Keap1 (Protein Data Bank (PDB) 1U6D), the crystal structure of the SPOP BTB domain complexed with the Cul3 N-terminal domain (PDB 4EOZ)55,56 and the solution structure of the BACK domain of Kelch repeat– and BTB domain–containing protein 4 (PDB 2EQX). Residue permutation and illustrations were generated with PyMOL (DeLano Scientific, PyMOL Molecular Graphics System, Version 1.5.0.4 Schrödinger).
Predictions of nuclear localization signal and subcellular localization
LZTR1 nuclear localization signal was predicted with MyHits Motif Scan57 and through PSORT II prediction58. LZTR1 subcellular localization was predicted with WoLF PSORT59.
Analysis of variants from ESP6500, ESP2500, the 1000 Genomes Project, dbSNP and COSMIC
All LZTR1 variants were downloaded from the ESP6500 database. Although this database was very useful in establishing the absence of any given mutation found in our cohort, it was less useful in evaluating the significance of rare LZTR1 variants, mainly because of a lack of large-scale validation of the variants. In general, indel calls were less robust than SNP calls and had a higher false positive rate. After review of the data, 12 possible loss-of-function mutations (9 mutations with a single occurrence, 1 mutation occurring twice and 2 recurrent mutations) were identified, 6 of which were indels. Five of the six indels affected homopolymer runs, which are known to be prone to artifacts. We have specifically investigated the two recurrent indel mutations present in ESP6500 (c.21del1 (allele count A1 = 66, R = 11,900; genotype count: A1/A1 = 7, A1/R = 52, R/R = 5,924) and c.1506_1507insG (allele count A1 = 143, R = 11,227; genotype count: A1/A1 = 17, A1/R = 109, R/R = 5,559) (where R is the reference allele), which both had genotype counts deviating from Hardy-Weinberg equilibri)um. We screened a large set of anonymous control samples (previously submitted for Fragile X testing and found to be negative for the presence of FMR1 intermediate alleles, premutation or full mutation and for the presence of the two recurrent frameshift indels c.21delG and c.1506_1507insG). These indels, respectively, were present in none of the 981 and 572 control individuals (c.21del1 (allele count A1 = 0, R = 1,962; genotype count: A1/A1 = 0, A1/R = 0, R/R = 981; two-tailed P value = 0.0018 using χ2 with Yates’ correction) and c.1506_1507insG (allele count A1 = 0, R = 1,144; genotype count: A1/A1 = 0, A1/R = 0, R/R = 572; two-tailed P value = 0.0002 using χ2 with Yates’ correction). These results are extremely statistically significant.
After we had proven that indel calls were less robust and represented false discovery data points, we used NHLBI ESP2500 and 1000 Genomes Project data as a point of reference for the assessment of LZTR1 variation in controls, as ESP2500 and 1000 Genomes Project data were obtained after applying more stringent filter criteria.
For the ESP2500 data, variants have been deposited in dbSNP (local batch ESP2500) by NHLBI ESP. From dbSNP138, we downloaded all the ESP2500 data by batch query of ESP2500 (ref. 60). However, on the basis of dbSNP entries, none of the LZTR1 ESP2500 data have been confirmed independently using a different method (such as Sanger sequencing), but all calls were obtained after more stringent filtering criteria were applied.
For the 1000 Genomes Project database, Phase 1 variants were extracted with the UCSC Table Browser from the 1000G Ph1 Vars track. Only Phase 1 variants were used in subsequent in silico analysis, as variant calls for this subset are of higher quality than variants from the pilot phase61. Variants were further annotated with SeattleSeq Annotation 137 (version 8.07 as of 3 July 2013).
Overall data in COSMIC include both mutations that are confirmed to be somatic and are therefore tumor specific and mutations whose somatic status is unknown (and where it is therefore unknown whether these mutations might be present in the germline). The overall spectrum of COSMIC LZTR1 mutations consisted of 83 mutations: 1 nonsense, 62 missense (25 of 62 confirmed to be somatic), 16 synonymous, 3 frameshift and 3 splice-site mutations. We have specifically selected from the COSMIC database all mutations that were confirmed to be somatic for further analysis and comparison with variants from the other databases.
Supplementary Material
Acknowledgments
We thank the patients for their participation in this study. A.P. is a recipient of a Children’s Tumor Foundation Young Investigator Award (grant 2009-01-004). The study was supported in part by the Children’s Tumor Foundation and by internal funds from the University of Alabama at Birmingham Medical Genomics Laboratory.
Footnotes
URLs. Platypus, http://www.well.ox.ac.uk/platypus; NHLBI Exome Variant Server, http://evs.gs.washington.edu/EVS/; PolyPhen-2, http://genetics.bwh.harvard.edu/pph2/; SIFT, http://sift.jcvi.org/; MutationTaster, http://www.mutationtaster.org/; Conserved Domains and Protein Classification, http://www.ncbi.nlm.nih.gov/Structure/cdd/docs/cdd_search.html; Phyre, http://www.sbg.bio.ic.ac.uk/phyre2/html/page.cgi?id=index; SWISS-MODEL, http://swissmodel.expasy.org/; Ensembl, http://www.ensembl.org/index.html; Protein Data Bank, http://www.pdb.org/pdb/home/home.do; MyHits Motif Scan, http://myhits.isb-sib.ch/cgi-bin/motif_scan; PSORT II Prediction, http://psort.hgc.jp/form2.html; WoLF PSORT, http://wolfpsort.org/; SureDesign, https://earray.chem.agilent.com/suredesign/; PyMOL, http://www.pymol.org/; UCSC Table Browser, http://genome.ucsc.edu/cgi-bin/hgTables; SeattleSeq Annotation, http://snp.gs.washington.edu/SeattleSeqAnnotation137/.
Accession codes. Deep sequencing files have been deposited in ArrayExpress under accession E-MTAB-1574.
Note: Any Supplementary Information and Source Data files are available in the online version of the paper.
AUTHOR CONTRIBUTIONS
The study was conceived and coordinated by A.P. and L.M.M. Patient phenotyping was performed by L.A., D.B.-V., A.B., J.O.B., A.L.B., M.S.D., H.F., K.G., S.H., C.K., C.L., R.N., K.A.R., J.M.S., P.S., J.A.W., A.Z. and B.R.K. Clinical data were collected by A.R.G. Design of the target enrichment library was performed by A.P. Paired-end next-generation sequencing was performed by M.R.C. and D.K.C. Detection of variants, filtering and annotation were performed by A.P., P.M., D.K.C. and L.M.M. NF2 and SMARCB1 mutation analyses and loss of heterozygosity studies were performed by A.B.P. Multiplex ligation-dependent probe amplification analyses were performed by C.F. LZTR1 mutation analyses and confirmatory tests were performed by J.X. and A.B.P. Prediction of protein structure and effects of missense mutations was performed by Y.F.L. and L.M.M. Analysis of mutational databases and statistical analyses were performed by J.X., Y.F.L. and L.M.M. The manuscript was written by A.P., J.X., A.B.P., Y.F.L. and L.M.M. All authors contributed to the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Smith MJ, et al. Frequency of SMARCB1 mutations in familial and sporadic schwannomatosis. Neurogenetics. 2012;13:141–145. doi: 10.1007/s10048-012-0319-8. [DOI] [PubMed] [Google Scholar]
- 2.Plotkin SR, et al. Update from the 2011 International Schwannomatosis Workshop: from genetics to diagnostic criteria. Am. J. Med. Genet. A. 2013;161A:405–416. doi: 10.1002/ajmg.a.35760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith MJ, et al. Vestibular schwannomas occur in schwannomatosis and should not be considered an exclusion criterion for clinical diagnosis. Am. J. Med. Genet. A. 2012;158A:215–219. doi: 10.1002/ajmg.a.34376. [DOI] [PubMed] [Google Scholar]
- 4.Jacoby LB, et al. Molecular analysis of the NF2 tumor-suppressor gene in schwannomatosis. Am. J. Hum. Genet. 1997;61:1293–1302. doi: 10.1086/301633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacCollin M, et al. Familial schwannomatosis: exclusion of the NF2 locus as the germline event. Neurology. 2003;60:1968–1974. doi: 10.1212/01.wnl.0000070184.08740.e0. [DOI] [PubMed] [Google Scholar]
- 6.Hulsebos TJ, et al. Germline mutation of INI1/SMARCB1 in familial schwannomatosis. Am. J. Hum. Genet. 2007;80:805–810. doi: 10.1086/513207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sestini R, Bacci C, Provenzano A, Genuardi M, Papi L. Evidence of a four-hit mechanism involving SMARCB1 and NF2 in schwannomatosis-associated schwannomas. Hum. Mutat. 2008;29:227–231. doi: 10.1002/humu.20679. [DOI] [PubMed] [Google Scholar]
- 8.Hadfield KD, et al. Molecular characterisation of SMARCB1 and NF2 in familial and sporadic schwannomatosis. J. Med. Genet. 2008;45:332–339. doi: 10.1136/jmg.2007.056499. [DOI] [PubMed] [Google Scholar]
- 9.Boyd C, et al. Alterations in the SMARCB1 (INI1) tumor suppressor gene in familial schwannomatosis. Clin. Genet. 2008;74:358–366. doi: 10.1111/j.1399-0004.2008.01060.x. [DOI] [PubMed] [Google Scholar]
- 10.Rousseau G, Noguchi T, Bourdon V, Sobol H, Olschwang S. SMARCB1/INI1 germline mutations contribute to 10% of sporadic schwannomatosis. BMC Neurol. 2011;11:9. doi: 10.1186/1471-2377-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith MJ, et al. Frequency of SMARCB1 mutations in familial and sporadic schwannomatosis. Neurogenetics. 2012;13:141–145. doi: 10.1007/s10048-012-0319-8. [DOI] [PubMed] [Google Scholar]
- 12.Zeitouni B, et al. SVDetect:a tool to identify genomic structural variations from paired-end and mate-pair sequencing data. Bioinformatics. 2010;26:1895–1896. doi: 10.1093/bioinformatics/btq293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thierry-Mieg D, Thierry-Mieg J. AceView: a comprehensive cDNA-supported gene and transcripts annotation. Genome Biol. 2006;7(suppl. 1):S12.1–S12.14. doi: 10.1186/gb-2006-7-s1-s12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu C, et al. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol. 2009;10:R130. doi: 10.1186/gb-2009-10-11-r130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frattini V, et al. The integrated landscape of driver genomic alterations in glioblastoma. Nat. Genet. 2013;45:1141–1149. doi: 10.1038/ng.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Senter L, et al. The clinical phenotype of Lynch syndrome due to germ-line PMS2 mutations. Gastroenterology. 2008;135:419–428. doi: 10.1053/j.gastro.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sjursen W, et al. Current clinical criteria for Lynch syndrome are not sensitive enough to identify MSH6 mutation carriers. J. Med. Genet. 2010;47:579–585. doi: 10.1136/jmg.2010.077677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lalloo F, Evans DG. Familial breast cancer. Clin. Genet. 2012;82:105–114. doi: 10.1111/j.1399-0004.2012.01859.x. [DOI] [PubMed] [Google Scholar]
- 19.Rahman N, Scott RH. Cancer genes associated with phenotypes in monoallelic and biallelic mutation carriers: new lessons from old players. Hum. Mol. Genet. 2007;16(1):R60. doi: 10.1093/hmg/ddm026. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Torrado R, Yamada D, Defossez PA. Born to bind: the BTB protein-protein interaction domain. Bioessays. 2006;28:1194–1202. doi: 10.1002/bies.20500. [DOI] [PubMed] [Google Scholar]
- 21.Nacak TG, Leptien K, Fellner D, Augustin HG, Kroll J. The BTB-kelch protein LZTR-1 is a novel Golgi protein that is degraded upon induction of apoptosis. J. Biol. Chem. 2006;281:5065–5071. doi: 10.1074/jbc.M509073200. [DOI] [PubMed] [Google Scholar]
- 22.Stogios PJ, Prive GG. The BACK domain in BTB-kelch proteins. Trends Biochem. Sci. 2004;29:634–637. doi: 10.1016/j.tibs.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Stogios PJ, Downs GS, Jauhal JJ, Nandra SK, Prive GG. Sequence and structural analysis of BTB domain proteins. Genome Biol. 2005;6:R82. doi: 10.1186/gb-2005-6-10-r82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly KE, Daniel JM. POZ for effect—POZ-ZF transcription factors in cancer and development. Trends Cell Biol. 2006;16:578–587. doi: 10.1016/j.tcb.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Dhanoa BS, Cogliati T, Satish AG, Bruford EA, Friedman JS. Update on the Kelch-like (KLHL) gene family. Hum. Genomics. 2013;7:13. doi: 10.1186/1479-7364-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canning P, et al. Structural basis for Cul3 protein assembly with the BTB-Kelch family of E3 ubiquitin ligases. J. Biol. Chem. 2013;288:7803–7814. doi: 10.1074/jbc.M112.437996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett EJ, Rush J, Gygi SP, Harper JW. Dynamics of cullin-RING ubiquitin ligase network revealed by systematic quantitative proteomics. Cell. 2010;143:951–965. doi: 10.1016/j.cell.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adams J, Kelso R, Cooley L. The kelch repeat superfamily of proteins: propellers of cell function. Trends Cell Biol. 2000;10:17–24. doi: 10.1016/s0962-8924(99)01673-6. [DOI] [PubMed] [Google Scholar]
- 29.Lührig S, Kolb S, Mellies N, Nolte J. The novel BTB-kelch protein, KBTBD8, is located in the Golgi apparatus and translocates to the spindle apparatus during mitosis. Cell Div. 2013;8:3. doi: 10.1186/1747-1028-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huynh KD, Bardwell VJ. The BCL-6 POZ domain and other POZ domains interact with the co-repressors N-CoR and SMRT. Oncogene. 1998;17:2473–2484. doi: 10.1038/sj.onc.1202197. [DOI] [PubMed] [Google Scholar]
- 31.Wong CW, Privalsky ML. Components of the SMRT corepressor complex exhibit distinctive interactions with the POZ domain oncoproteins PLZF, PLZF-RARα, and BCL-6. J. Biol. Chem. 1998;273:27695–27702. doi: 10.1074/jbc.273.42.27695. [DOI] [PubMed] [Google Scholar]
- 32.Underhill C, Qutob MS, Yee SP, Torchia J. A novel nuclear receptor corepressor complex, N-CoR, contains components of the mammalian SWI/SNF complex and the corepressor KAP-1. J. Biol. Chem. 2000;275:40463–40470. doi: 10.1074/jbc.M007864200. [DOI] [PubMed] [Google Scholar]
- 33.Nallani KC, Sullivan WJ., Jr Identification of proteins interacting with Toxoplasma SRCAP by yeast two-hybrid screening. Parasitol. Res. 2005;95:236–242. doi: 10.1007/s00436-004-1291-5. [DOI] [PubMed] [Google Scholar]
- 34.Pan X, Zhai L, Sun R, Li X, Zeng X. INI1/hSNF5/BAF47 represses c-fos transcription via a histone deacetylase–dependent manner. Biochem. Biophys. Res. Commun. 2005;337:1052–1058. doi: 10.1016/j.bbrc.2005.09.155. [DOI] [PubMed] [Google Scholar]
- 35.Ravasi T, et al. An atlas of combinatorial transcriptional regulation in mouse and man. Cell. 2010;140:744–752. doi: 10.1016/j.cell.2010.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell. 2008;132:363–374. doi: 10.1016/j.cell.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pottier N, et al. Expression of SMARCB1 modulates steroid sensitivity in human lymphoblastoid cells: identification of a promoter SNP that alters PARP1 binding and SMARCB1 expression. Hum. Mol. Genet. 2007;16:2261–2271. doi: 10.1093/hmg/ddm178. [DOI] [PubMed] [Google Scholar]
- 38.Bush ML, et al. AR42, a novel histone deacetylase inhibitor, as a potential therapy for vestibular schwannomas and meningiomas. Neuro-oncol. 2011;13:983–999. doi: 10.1093/neuonc/nor072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buckley PG, et al. Identification of genetic aberrations on chromosome 22 outside the NF2 locus in schwannomatosis and neurofibromatosis type 2. Hum. Mutat. 2005;26:540–549. doi: 10.1002/humu.20255. [DOI] [PubMed] [Google Scholar]
- 40.Hulsebos TJ, et al. Germline mutation of INI1/SMARCB1 in familial schwannomatosis. Am. J. Hum. Genet. 2007;80:805–810. doi: 10.1086/513207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacCollin M, et al. Familial schwannomatosis: exclusion of the NF2 locus as the germline event. Neurology. 2003;60:1968–1974. doi: 10.1212/01.wnl.0000070184.08740.e0. [DOI] [PubMed] [Google Scholar]
- 42.Sestini R, Bacci C, Provenzano A, Genuardi M, Papi L. Evidence of a four-hit mechanism involving SMARCB1 and NF2 in schwannomatosis-associated schwannomas. Hum. Mutat. 2008;29:227–231. doi: 10.1002/humu.20679. [DOI] [PubMed] [Google Scholar]
- 43.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li H, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adzhubei IA, et al. A method and server for predicting damaging missense mutations. Nat. Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ng PC, Henikoff S. Predicting deleterious amino acid substitutions. Genome Res. 2001;11:863–874. doi: 10.1101/gr.176601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwarz JM, Rödelsperger C, Schuelke M, Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat. Methods. 2010;7:575–576. doi: 10.1038/nmeth0810-575. [DOI] [PubMed] [Google Scholar]
- 48.Marchler-Bauer A, et al. CDD: specific functional annotation with the Conserved Domain Database. Nucleic Acids Res. 2009;37:D205. doi: 10.1093/nar/gkn845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marchler-Bauer A, Bryant SH. CD-Search: protein domain annotations on the fly. Nucleic Acids Res. 2004;32:W327–W331. doi: 10.1093/nar/gkh454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marchler-Bauer A, et al. CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 2011;39:D225. doi: 10.1093/nar/gkq1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kelley LA, Sternberg MJ. Protein structure prediction on the Web: a case study using the Phyre server. Nat. Protoc. 2009;4:363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- 52.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 53.Kiefer F, Arnold K, Künzli M, Bordoli L, Schwede T. The SWISS-MODEL Repository and associated resources. Nucleic Acids Res. 2009;37:D387. doi: 10.1093/nar/gkn750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manuel CP. Protein modeling by e-mail. Nat. Biotechnol. 1995;13:658–660. [Google Scholar]
- 55.Errington W, et al. Adaptor protein self-assembly drives the control of a cullin-RING ubiquitin ligase. Structure. 2012;20:1141–1153. doi: 10.1016/j.str.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 56.Li X, Zhang D, Hannink M, Beamer LJ. Crystal structure of the Kelch domain of human Keap1. J. Biol. Chem. 2004;279:54750–54758. doi: 10.1074/jbc.M410073200. [DOI] [PubMed] [Google Scholar]
- 57.Pagni M, et al. MyHits: improvements to an interactive resource for analyzing protein sequences. Nucleic Acids Res. 2007;35:W433–W437. doi: 10.1093/nar/gkm352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakai K, Horton P. PSORT: a program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem. Sci. 1999;24:34–36. doi: 10.1016/s0968-0004(98)01336-x. [DOI] [PubMed] [Google Scholar]
- 59.Horton P, et al. WoLF PSORT: protein localization predictor. Nucleic Acids Res. 2007;35:W585–W587. doi: 10.1093/nar/gkm259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tennessen JA, et al. Evolution and functional impact of rare coding variation from deep sequencing of human exomes. Science. 2012;337:64–69. doi: 10.1126/science.1219240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.1000 Genomes Project Consortium. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.