Abstract
Use a French press to disrupt mammalian or Sf9 cells and generate a clarified lysate for subsequent use in protein purification.
1. THEORY
There are numerous methods to lyse cells including osmotic shock, freeze-thawing, and manual disruption such as Dounce homogenization. Lysis with a French Pressure Cell offers many advantages over these other techniques including speed, efficiency of cell disruption, and compatibility with numerous buffer conditions. Eukaryotic cells have higher amounts of nucleic acid than bacteria, and as a result a nuclease should be included to reduce the viscosity of the cell lysate.
2. EQUIPMENT
French Pressure Cell
Refrigerated centrifuge
Magnetic stir plate
Magnetic stir bars
Polycarbonate centrifuge tubes
3. MATERIALS
Tris base
Hydrochloric acid (HCl)
Sodium chloride (NaCl)
Glycerol
EDTA
Phenylmethylsulfonyl fluoride (PMSF)
Benzonase (Sigma)
3.1. Solutions & buffers
Step 1 Lysis Buffer
|
| |||
| Component | Final concentration | Stock | Amount |
|
| |||
| Tris–HCl, pH 8.0 | 25 mM | 1M | 2.5 ml |
|
| |||
| NaCl | 300 mM | 5M | 6 ml |
|
| |||
| Glycerol | 10% | 100% | 10 ml |
|
| |||
| EDTA | 1 mM | 500 mM | 0.2 ml |
|
| |||
| PMSF | 1 mM | 100 mM | 1 ml |
|
| |||
| Add water to 100 ml | |||
4. PROTOCOL
4.1. Duration
| Preparation | Variable |
|
| |
| Protocol | 1h |
|
| |
4.2. Preparation
Generate a pellet of cells expressing the protein of interest (Recombinant protein expression in baculovirus-infected insect cells or Single Cell Cloning of a Stable Mammalian Cell Line). The length of this step is variable, depending on the method chosen to express the protein.
Chill the French Pressure Cell and piston to 4 °C, about 2 h.
See Fig. 4.1 for the flowchart of the complete protocol.
Figure 4.1.
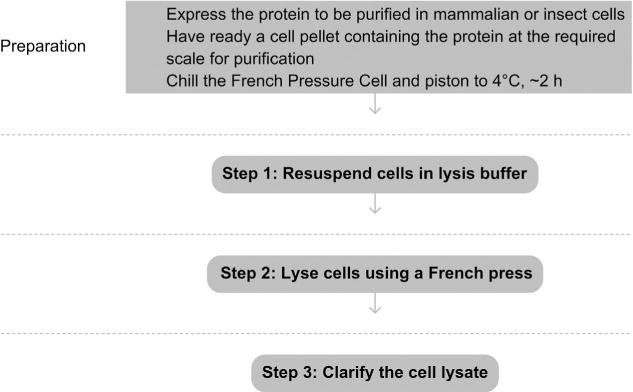
Flowchart of the complete protocol, including preparation.
5. STEP 1 RESUSPEND CELLS IN LYSIS BUFFER
5.1. Overview
Resuspend the cell pellet in a lysis buffer in which the protein of interest will be stable, soluble, and not aggregated.
5.2. Duration
30 min
1.1 Resuspend the cell pellet in 2–5 times its volume of Lysis Buffer (e.g., resuspend a 5-ml cell pellet in 10–25 ml of Lysis Buffer).
1.2 Add 1 μl of Benzonase per ml of cell pellet.
1.3 Stir cells on a magnetic stir plate at 4 °C until the solution is homogeneous and no cell clumps are visible.
5.3. Tip
The lysis buffer should be modified to ensure that the protein of interest is stable, soluble, and not aggregated (see Explanatory Chapter: Troubleshooting protein expression: what to do when the protein is not soluble).
5.4. Tip
Avoid vortexing the cell suspension or introducing bubbles throughout this protocol or the protein may become denatured.
5.5. Tip
DNase and RNase can be used in place of the Benzonase.
5.6. Tip
Protease Inhibitor cocktails can be used in place of the EDTA and PMSF.
5.7. Tip
EDTA should be omitted if the cell lysate is going to be used with metal affinity chromatography.
See Fig. 4.2 for the flowchart of Step 1.
Figure 4.2.
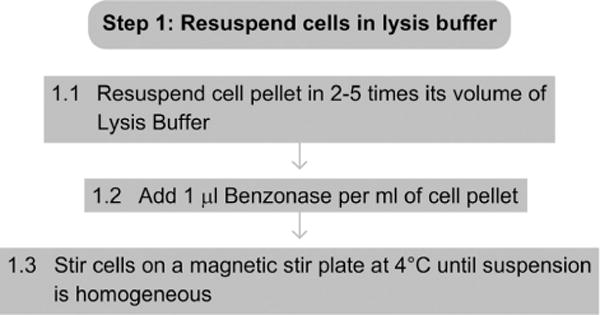
Flowchart of Step 1.
6. STEP 2 LYSE CELLS USING A FRENCH PRESS
6.1. Overview
Physical force disrupts the cells.
6.2. Duration
5 min
2.1 Load the resuspended cells into the prechilled French Pressure Cell.
2.2 Lyse the cells with one pass of the piston on the high setting, ~2500 psi.
2.3 Collect lysate and keep at 4 °C.
See Fig. 4.3 for the flowchart of Step 2.
Figure 4.3.
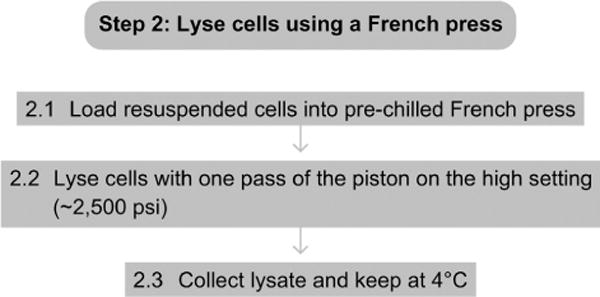
Flowchart of Step 2.
7. STEP 3 CLARIFY THE CELL LYSATE
7.1. Overview
In this step, you remove any insoluble and aggregated material from lysate.
7.2. Duration
30 min
3.1 Spin lysate in a centrifuge at 18 000×g at 4 °C for 30 min.
3.2 The supernatant should be used immediately in the next step of the purification protocol for the protein.
7.3. Tip
Storing or freezing the cell lysate can lead to protein aggregation and degradation.
7.4. Tip
Protein solubility can be analyzed by comparing the amount of protein in the lysate before centrifugation and the amount in the supernatant and pellet after centrifugation. If the amount of protein in the clarified lysate is low, the lysis buffer should be modified accordingly to increase the solubility of the protein.
See Fig. 4.4 for the flowchart of Step 3.
Figure 4.4.
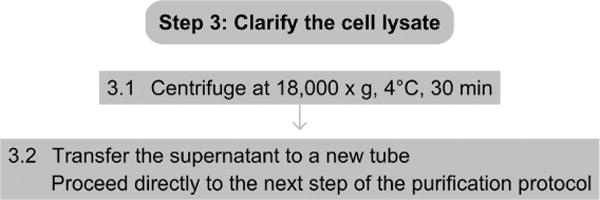
Flowchart of Step 3.
REFERENCES
Referenced Protocols in Methods Navigator
- Recombinant protein expression in baculovirus-infected insect cells.
- Single Cell Cloning of a Stable Mammalian Cell Line.
- Explanatory Chapter: Troubleshooting protein expression: what to do when the protein is not soluble.


