Abstract
We report the third case of a renal cell carcinoma bearing a fusion of the vinculin (VCL) and anaplastic lymphoma kinase (ALK) genes. Like the 2 other reported cases, this neoplasm occurred in a young patient (6 y old) with sickle-cell trait and demonstrated distinctive morphologic features including medullary epicenter, discohesive polygonal or spindle-shaped cells with prominent cytoplasmic vacuoles, and prominent lymphocytic infiltrate. The neoplastic cells demonstrated focal membranous labeling for ALK protein by immunohistochemistry, ALK gene rearrangement by fluorescence in situ hybridization, and a specific VCL-ALK gene fusion by reverse transcriptase polymerase chain reaction. VCL-ALK renal cell carcinoma may represent the eighth sickle-cell nephropathy.
Keywords: renal cell carcinoma, anaplastic lymphoma kinase, vinculin
Patients with sickle-cell trait, although largely spared of the devastating extrarenal vaso-occlusive pathology of sickle-cell disease, suffer from a variety of insults to the kidney.1 In 1974, Berman2 noted 6 nephropathies affecting patients with sickle-cell trait or disease including hematuria secondary to unilateral bleeding beneath the renal pelvic urothelium, papillary necrosis, nephrotic syndrome, renal infarction, the inability to concentrate urine, and pyelonephritis. In 1995, Davis et al3 described a distinctive subtype of renal cell carcinoma, renal medullary carcinoma (RMC), that occurs almost exclusively in these patients and termed it the seventh sickle-cell nephropathy. RMC is a highly aggressive carcinoma typically centered in the renal medulla which features an infiltrative growth pattern associated with prominent stromal desmoplasia and inflammation. The neoplastic cells may have solid, reticular, tubular, or cribriform architecture and typically demonstrate vesicular chromatin with prominent nucleoli. The morphology overlaps with that of collecting duct carcinomas in adults and, in some cases, pediatric rhabdoid tumor of the kidney.4–14 Interestingly, like rhabdoid tumors and a subset of collecting duct carcinomas, RMC typically demonstrates loss of the INI-1 protein by immunohistochemistry (IHC), a finding almost never seen in the more common renal cell carcinoma (RCC) subtypes.15–18
In the past 3 years, several examples of RCC demonstrating chromosome translocations resulting in rearrangements of the anaplastic lymphoma kinase (ALK) gene have been reported.19–22 ALK rearrangements are known to occur in several human cancers, including anaplastic large cell lymphoma (ALCL),23 inflammatory myofibroblastic tumor,24,25 and a subset of pulmonary adenocarcinoma.26,27 Two of the ALK-rearranged RCCs have occurred in young patients with sickle-cell trait, and on review these separately reported cases have many similarities. In both cases, the ALK gene was fused to the vinculin (VCL) gene, which functions as an adhesion protein that couples the extracellular matrix to the actinmyosin cytoskeleton.28,29 Both VCL-ALK RCC cases had solid architecture and featured polygonal to spindle-shaped cells with vesicular nuclei and abundant eosinophilic cytoplasm with prominent intracytoplasmic lumina. Both VCL-ALK RCC had a prominent lymphoplasmacytic infiltrate. Whereas the initial case reported was classified as RCC, unclassified, the second case was classified as an RMC given its association with sickle-cell trait. Subsequently, 4 cases of RCC associated with ALK rearrangements in which VCL was not the partner have been reported. All 4 of these neoplasms affected adults not afficted with sickle-cell trait, and their morphology has not been distinctive: 3 of the neoplasms have been classified as variants of papillary RCC, whereas the other was considered RCC unclassified. Given the small number of RCC with ALK rearrangements reported in the literature, the 2013 International Society of Urologic Pathology's 2013 Vancouver classification of renal neoplasia considers ALK-rearranged RCC to be a provisional new entity, with study of further cases required before a distinctive subtype can be accepted.30
We report herein the third case of an RCC with a VCL-ALK gene fusion. Like the other 2 cases reported in the literature, this RCC occurred in a young patient with sickle-cell trait and demonstrated similar distinctive morphologic and IHC features. We suggest that RCC with the VCL-ALK gene fusion may be a rare but distinctive complication of sickle-cell trait and thus potentially be considered the eighth sickle-cell nephropathy.
MATERIALS AND METHODS
All tissue samples were fixed in neutral-buffered formalin and embedded in formalin. Routine IHC labeling was performed on the Benchmark XT autostainer (Ventana Medical Systems Inc., Tucson, AZ) using the I-View detection kit. ALK IHC was performed using 2 methodologies. The standard methodology in our laboratory designed to detect ALK fusion proteins in ALCL and inflammatory myofibroblastic tumor uses the Dako Clone ALK1 antibody (catalog #M7195, 1:50 dilution). The more sensitive methodology designed to detect neoplasms with variant ALK gene fusions uses the prediluted Ventana ALK01 antibody (catalog #790-2918).
Fluorescence in situ hybridization studies were conducted using a dual-color, break-apart rearrangement probe (Vysis, Downers Grove, IL). A SpectrumOrange fluorophore-labeled 250 kbp DNA probe was hybridized to the telomeric side of the ALK breakpoint, and a SpectrumGreen fluorophore-labeled 300 kbp DNA probe was hybridized to the centromeric side of the ALK breakpoint. Unbound probe was removed by a series of washes, and the nuclei were counterstained with 4,6-diamidino-2-phenylindole. Hybridization of the ALK probes were viewed using a fluorescence microscope equipped with appropriate excitation and emission filters allowing visualization of the fluorescent signals. For 200 nuclei, the normal cutoff is <3.1% for split ALK signals.
Reverse transcriptase polymerase chain reaction (RT-PCR) and Sanger sequencing of PCR products were performed as previously described,20 on RNA extracted from formalin-fixed, paraffin-embedded tumor tissue. The following primers were designed to amplify a predicted 168-nucleotide-long fragment spanning the fusion site: VCL E16–68F (TGTGAAAGCTGCCTCTGATG) and ALK E20+60R (CGGAGCTTGCTCAGCTTGTA). RNA from a previously reported VCL-ALK-positive RCC was used as positive control.
CASE REPORT AND PATHOLOGIC FINDINGS
The patient is a 6-year-old African-American boy with sickle-cell trait who presented with a 3-week history of frank hematuria and blood clots in the urine. An ultrasound revealed a small mass in the upper pole of the right kidney that involved the collecting system and renal pelvis, for which the patient underwent a right radical nephrectomy. He was disease free by computed tomography scan and magnetic resonance imaging after 16 months and is disease free on clinical examination at 19 months.
The nephrectomy specimen revealed a localized 3 cm, well-circumscribed tumor involving the renal pelvis. Histologic examination revealed sheets of spindled to polygonal, dis-cohesive large cells with eosinophilic cytoplasm and indistinct cell borders but conspicuous intracytoplasmic vacuoles (Figs. 1A–D). Slit-like spaces separated aggregates of neoplastic cells. Only rare mitotic figures were identified (< 1/10 high-power fields). Nuclei were round to oval and vesicular with variably prominent nucleoli and occasional nuclear grooves. Mature lymphocytes were admixed throughout the tumor. Prominent sickled erythrocytes were noted within the vasculature and in thin slit-like spaces separating clusters of neoplastic cells. The resected perirenal lymph nodes contained rare displaced renal tubular epithelium associated with more abundant proteinaceous material consistent with Tamm-Horsfall protein (commonly seen in association with pediatric renal tumors that involve the medulla and obstruct lymphatic flow)31 but no evidence of metastatic disease.
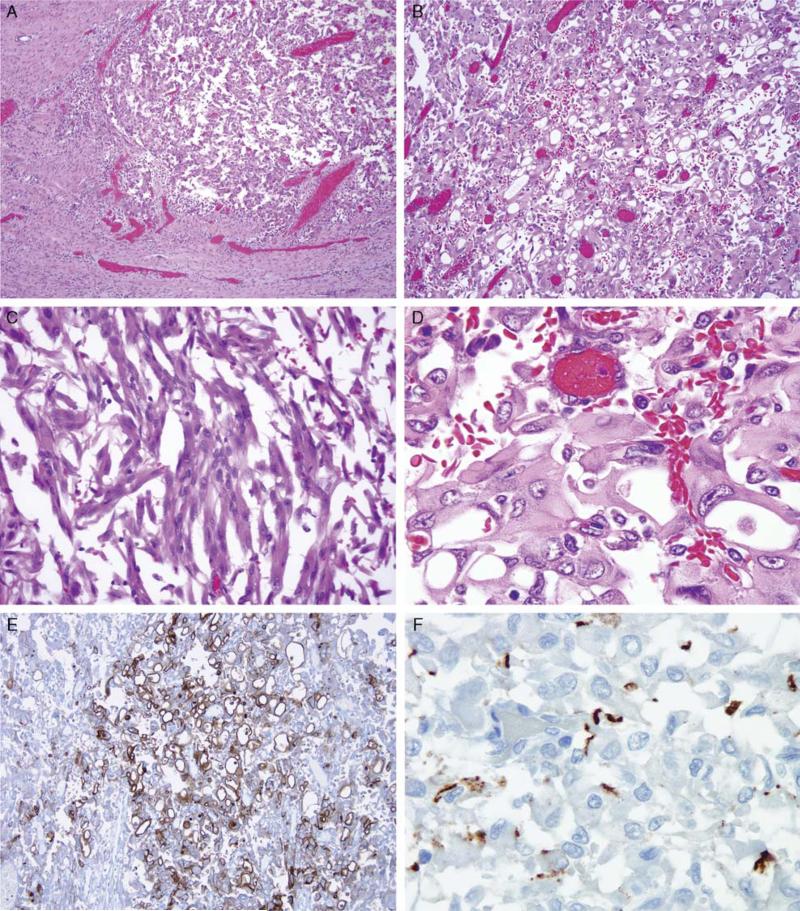
FIGURE 1.
Morphology of VCL-ALK RCC. The neoplasm is fairly well delineated and consists of a sheet-like proliferation of polygonal cells (A and B) with areas of spindling (C). Many of the cells have prominent intracytoplasmic vacuoles (D), which are highlighted on IHC for epithelial membrane antigen (E). F, Immunostaining for ALK showing discrete localization beneath the cell membrane.
By IHC, the neoplasm was positive for PAX8, focally positive for cytokeratins Cam5.2 and AE1/3, and negative for cathepsin K, Mart1, HMB45, p63, CD31, CD34, and desmin. p53 was not overexpressed. Nuclear labeling for INI-1 was intact. Epithelial membrane antigen accentuated the circumference of the prominent intracytoplasmic vacuoles (Fig. 1E). Consistent with the observed low mitotic rate, the neoplasm's Ki-67 index was relatively low at 5%. There was no labeling with the Dako ALK1 antibody, but Ventana ALK01 antibody demonstrated discrete, focal, noncircumferential ALK labeling at the cell membrane (Fig. 1F). Fluorescence in situ hybridization demonstrated that 95% of scored nuclei showed split ALK signals consistent with a rearrangement involving the ALK locus on chromosome 2p23 (Fig. 2).
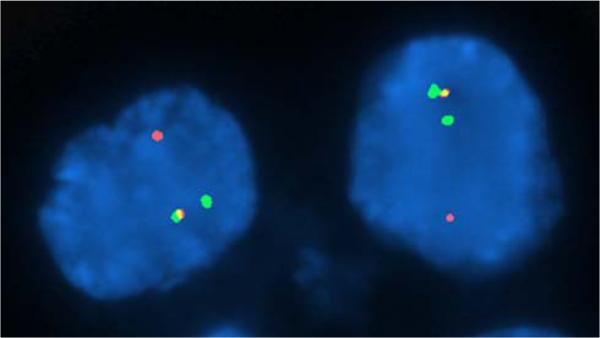
FIGURE 2.
FISH analysis of a representative tissue section of the neoplasm using an ALK dual-color break-apart probe set demonstrates split orange and green signals in the tumor nuclei indicative of a rearrangement of the ALK gene locus as well as juxtaposed or fused orange and green signals corresponding to a nonrearranged ALK locus.
RT-PCR using specific primers detected the presence of a 168-nucleotide band that corresponded to VCL-ALK fusion transcripts, as confirmed by direct sequencing (Fig. 3). The chromosomal breakpoints and the resulting fusion (VCL exon 16 to ALK exon 20) were the same as in the 2 previously reported cases.19,20
FIGURE 3.
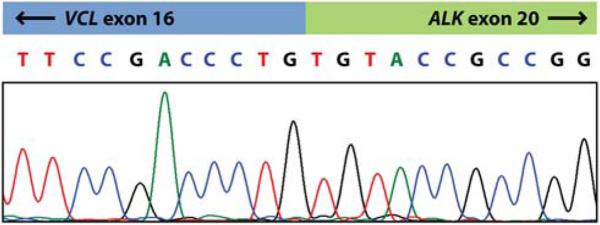
Sequencing trace of the VCL-ALK RT-PCR product, confirming an in-frame fusion of VCL exon 16 with ALK exon 20 (forward sequence is shown).
DISCUSSION
The ALK gene product is a receptor tyrosine kinase which is thought to mediate signal transduction in a regulated manner on the basis of ligand binding. In cancers, ALK fusion proteins generally consist of a portion of the ALK tyrosine kinase domain at the C-terminal end and a fusion partner at the N-terminal end. The N-terminal fusion partners generally are expressed at high levels and have the ability to self-oligomerize, thereby fostering close association and cross-activation of the ALK kinase domains. This process results in constitutive ligand-independent activation of the ALK tyrosine kinase. Constitutive activation of ALK in turn leads to unfettered activation of downstream signaling pathways that promote cell survival and replication, such as JAK3/STAT3, Ras/Mek/Erk, and PI3K/AKT.32,33 Neoplasms demonstrating ALK fusion proteins include ALCL,23 which typically harbors an NPM-ALK gene fusion, inflammatory myofibroblastic tumor,24,25 which most often harbors a TPM3-ALK or TPM4-ALK gene fusion, and a subset of pulmonary adenocarcinomas, which preferentially affect nonsmokers, have signet ring cell morphology,26,27 and most commonly harbor an EML4-ALK gene fusion.
Recently, VCL-ALK gene fusions were independently reported in 2 cases of RCCs occurring in young patients with sickle-cell trait.19,20 In 2010, Debelenko et al19 reported a 16-year-old African-American boy with sickle-cell trait who was found to have a 6.2 cm mass in the right renal collecting system causing hydro-nephrosis. Core biopsy was interpreted as consistent with RMC. Resection of the mass revealed a diffuse, sheet-like growth proliferation of discohesive, polygonal cells with round to oval vesicular nuclei, abundant eosinophilic cytoplasm, and prominent intracytoplasmic vacuoles. The resected neoplasm was diagnosed as RCC, indeterminate subtype on the basis of the unique constellation of histologic features. The pathologic stage was pT1N1MX. Cytogenetic studies revealed a complex karyotype including a t(2;10)(p23;q22) translocation with a resultant VCL-ALK fusion gene identified by RT-PCR and bidirectional cDNA sequencing. The patient was alive and well 4 months after surgery with no evidence of residual disease. Mariño-Eñquez et al20 shortly thereafter reported the case of a 6-year-old African-American boy with sickle-cell trait who was found to have a 4.6 cm mass centered in the renal medulla involving the upper pole of the left kidney. Histologically, the tumor was similarly characterized by sheets of polygonal to spindle-shaped cells with large vesicular nuclei, abundant eosinophilic cytoplasm, and frequent intracytoplasmic lumina. Ultra-structural examination showed that the intracytoplasmic lumina were lined by microvilli. The second case was classified as an RMC on the basis of the clinical association with sickle-cell trait. RT-PCR again identified a characteristic VCL-ALK gene fusion. The patient underwent a radical nephroureterectomy and was alive and disease free 21 months after surgery.
Subsequently, 2 groups have reported RCC associated with ALK translocations for which VCL was not the fusion partner. The histologic findings of these RCCs were different, however, from those seen in VCL-ALK RCC. Sugawara et al21 screened 355 adult RCCs for ALK IHC labeling using an intercalated antibody-enhanced polymer method and found that 2.3% of non–clear cell RCCs (2 of 88 tumors tested) were positive. Subsequent studies showed a TPM3-ALK fusion protein and an EML4-ALK fusion protein in these RCCs. Both adult patients (ages 36 and 53 y) with these tumors were alive and well 2 and 7 years after their initial diagnoses, respectively. The TPM3-ALK RCC displayed a papillary, tubular, and cribriform growth pattern, whereas the EML4-ALK RCC showed areas of both papillary and clear cell morphology. Sukov et al22 then reported 2 adult patients (ages 61 and 59 y) with papillary RCC associated with ALK gene fusions and later showed that VCL was not the fusion partner for either of these.34 Both of these RCCs behaved aggressively, and the patients died of disease at 1.4 and 4 years, respectively. Importantly, in none of the non-VCL-ALK–associated RCCs was a history of sickle-cell trait provided, and in no case were sickled red blood cells noted in the histologic sections.
Our case adds to the existing data regarding ALK-associated RCC and supports the possibility raised by Debelenko35 that VCL-ALK RCCs represent a distinctive entity. Distinctive features of the 3 reported VCL-ALK RCCs (including the current case) are listed in Table 1. Distinctive features of VCL-ALK RCC include their occurrence in young patients with sickle-cell trait, medullary epicenter, distinctive morphology featuring discohesive polygonal to spindle-shaped cells with prominent cytoplasmic vacuoles, prominent lymphoplasmacytic infiltrate, intact INI-1 protein, and low Ki-67 index. Debelenko et al19 have postulated that the distinctive intracytoplasmic vacuoles may be due to the structural effects of the VCL-ALK fusion protein. Vinculin is a protein crucial for the structural integrity of the cell and acts as a bridge between integrins on the cell surface and the actin cytoskeleton. The N-terminal head of vinculin binds to talin, which in turn links to integrins on the cell surface. The tail region of vinculin binds to α-actinin, thus acting as an intermediary between the cytoskeleton and talin.28,29 The VCL-ALK fusion protein created by the fusion of VCL exon 16 to ALK exon 20 lacks the tail region of vinculin and therefore lacks actin-binding ability, and thus the cells are functionally VCL haploinsufficient. The resulting disruption of cytoskeleton integrity could potentially promote the prominent intracytoplasmic lumina that these tumors show histologically.19 Integrins are also crucial for cellular cohesion with both the extracellular matrix and neighboring cells. Disruption of this cytoskeletal linkage may also explain the poor intercellular cohesion seen in the VCL-ALK RCC. These mechanisms provide a biological rationale for the concept that the distinctive histologic pattern found in VCL-ALK RCC is conferred by the specific biological properties of the vinculin component of the fusion oncoprotein. The normal multivalent binding of vinculin to talin also suggests the mechanism by which ALK may become constitutively active in these RCCs.28 The ability of the vinculin head domains of the VCLALK fusion protein to bind to talin in close association may promote phosphorylation of the associated ALK domain leading to activation of downstream proliferation and survival signals. This hypothesis is in keeping with the observation that ALK immunolabeling was restricted directly beneath the cellular membrane in all 3 VCL-ALK RCCs, including the current case. Furthermore, ALK and VCL were found to colocalize beneath the cellular membrane in the case reported by Debelenko et al.19
TABLE 1.
Distinctive Features of VCL-ALK RCC
| Occurrence in sickle-cell trait |
| Young age (mean 9.3 y) |
| Epicenter in renal medulla |
| Polygonal to spindle-shaped cells with cytoplasmic vacuoles |
| Prominent intratumoral lymphocytic infiltrate |
| Intact INI-1 |
| Low Ki-67 index |
Given the myriad morphologic patterns reported in RMC (including one with intracytoplasmic vacuoles) and the occurrence of RMC in young patients with sickle-cell trait, it has been proposed34 that VCL-ALK RCC might represent a subtype of RMC. However, several features distinguish VCL-ALK RCC from classic RMC. First, INI-1 protein is intact in VCL-ALK RCC and consistently lost in RMC. Second, RMCs are high-grade cancers characterized by an extremely high mitotic rate and Ki-67 proliferation index, whereas proliferative activity of the VCL-ALK RCC is relatively low. In a series of 14 RMCs, the median Ki-67 labeling was 87.5%.36 Conversely, the Ki-67 index was 5% in the case of VCL-ALK RCC reported by Debelenko and colleagues and 5% in our case. Two classic RMC comparison cases in the report by Debelenko et al19 showed 60% and 80% proliferation indices. Although follow-up is limited, none of the VCL-ALK RCCs have recurred, which contrasts with the 15-week mean survival from RMC.3 As Marinño-Eñríquez et al20 have indicated, it would be of interest to examine classic RMC for ALK rearrangements.
Identification of ALK-translocation RCC may have potential therapeutic implications. An ALK fusion protein inhibitor (crizotinib) was recently approved by the United States Food and Drug Administration for the subset of adenocarcinoma of the lung harboring an EML4-ALK fusion protein. Clinical trials showed a partial or complete tumor response in 57% of patients receiving the drug and stabilized disease in 33% of patients. This is compared with a 10% response rate in patients receiving conventional chemotherapy.37 It may be possible that ALK-translocation renal carcinomas may similarly benefit from targeted therapeutics, underscoring the importance of their recognition by surgical pathologists.
ACKNOWLEDGMENTS
The authors appreciate the outstanding technical assistance of Yuexiang Wang, PhD, Derrick Tao, and Dali Huang, MD.
Footnotes
Conflicts of Interest and Source of Funding: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
REFERENCES
- 1.Tsaras G, Owusu-Ansah A, Boateng FO, et al. Complications associated with sickle cell trait: a brief narrative overview. Am J Med. 2009;122:507–512. doi: 10.1016/j.amjmed.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 2.Berman LB. Sickle cell nephropathy. JAMA. 1974;228:1279. [PubMed] [Google Scholar]
- 3.Davis CJ, Jr, Mostofi FK, Sesterhenn IA. Renal medullary carcinoma. The seventh sickle cell nephropathy. Am J Surg Pathol. 1995;19:1–11. doi: 10.1097/00000478-199501000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Assad L, Resetkova E, Oliveira VL, et al. Cytologic features of renal medullary carcinoma. Cancer. 2005;105:28–34. doi: 10.1002/cncr.20764. [DOI] [PubMed] [Google Scholar]
- 5.Avery RA, Harris JE, Davis CJ, Jr, et al. Renal medullary carcinoma: clinical and therapeutic aspects of a newly described tumor. Cancer. 1996;78:128–132. doi: 10.1002/(SICI)1097-0142(19960701)78:1<128::AID-CNCR18>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Dimashkieh H, Choe J, Mutema G. Renal medullary carcinoma: a report of 2 cases and review of the literature. Arch Pathol Lab Med. 2003;127:e135–e138. doi: 10.5858/2003-127-e135-RMCARO. [DOI] [PubMed] [Google Scholar]
- 7.Gatalica Z, Lilleberg SL, Monzon FA, et al. Renal medullary carcinomas: histopathologic phenotype associated with diverse genotypes. Hum Pathol. 2011;42:1979–1988. doi: 10.1016/j.humpath.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 8.Hakimi AA, Koi PT, Milhoua PM, et al. Renal medullary carcinoma: the Bronx experience. Urology. 2007;70:878–882. doi: 10.1016/j.urology.2007.06.1124. [DOI] [PubMed] [Google Scholar]
- 9.Schaeffer EM, Guzzo TJ, Furge KA, et al. Renal medullary carcinoma: molecular, pathological and clinical evidence for treatment with topoisomerase-inhibiting therapy. BJU Int. 2010;106:62–65. doi: 10.1111/j.1464-410X.2009.09139.x. [DOI] [PubMed] [Google Scholar]
- 10.Simpson L, He X, Pins M, et al. Renal medullary carcinoma and ABL gene amplification. J Urol. 2005;173:1883–1888. doi: 10.1097/01.ju.0000158448.56888.09. [DOI] [PubMed] [Google Scholar]
- 11.Stahlschmidt J, Cullinane C, Roberts P, et al. Renal medullary carcinoma: prolonged remission with chemotherapy, immunohistochemical characterisation and evidence of bcr/abl rearrangement. Med Pediatr Oncol. 1999;33:551–557. doi: 10.1002/(sici)1096-911x(199912)33:6<551::aid-mpo5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe IC, Billis A, Guimaraes MS, et al. Renal medullary carcinoma: report of seven cases from Brazil. Mod Pathol. 2007;20:914–920. doi: 10.1038/modpathol.3800934. [DOI] [PubMed] [Google Scholar]
- 13.Wesche WA, Wilimas J, Khare V, et al. Renal medullary carcinoma: a potential sickle cell nephropathy of children and adolescents. Pediatr Pathol Lab Med. 1998;18:97–113. [PubMed] [Google Scholar]
- 14.Yang XJ, Sugimura J, Tretiakova MS, et al. Gene expression profiling of renal medullary carcinoma: potential clinical relevance. Cancer. 2004;100:976–985. doi: 10.1002/cncr.20049. [DOI] [PubMed] [Google Scholar]
- 15.Cheng JX, Tretiakova M, Gong C, et al. Renal medullary carcinoma: rhabdoid features and the absence of INI1 expression as markers of aggressive behavior. Mod Pathol. 2008;21:647–652. doi: 10.1038/modpathol.2008.44. [DOI] [PubMed] [Google Scholar]
- 16.Hollmann TJ, Hornick JL. INI1-deficient tumors: diagnostic features and molecular genetics. Am J Surg Pathol. 2011;35:e47–e63. doi: 10.1097/PAS.0b013e31822b325b. [DOI] [PubMed] [Google Scholar]
- 17.Elwood H, Chaux A, Schultz L, et al. Immunohistochemical analysis of SMARCB1/INI-1 expression in collecting duct carcinoma. Urology. 2011;78:e1–e5. doi: 10.1016/j.urology.2011.04.043. [DOI] [PubMed] [Google Scholar]
- 18.Judkins AR. Immunohistochemistry of INI1 expression: a new tool for old challenges in CNS and soft tissue pathology. Adv Anat Pathol. 2007;14:335–339. doi: 10.1097/PAP.0b013e3180ca8b08. [DOI] [PubMed] [Google Scholar]
- 19.Debelenko LV, Raimondi SC, Daw N, et al. Renal cell carcinoma with novel VCL-ALK fusion: new representative of ALK-associated tumor spectrum. Mod Pathol. 2011;24:430–442. doi: 10.1038/modpathol.2010.213. [DOI] [PubMed] [Google Scholar]
- 20.Mariño-Eñríquez A, Ou WB, Weldon CB, et al. ALK rearrangement in sickle cell trait-associated renal medullary carcinoma. Genes Chromosomes Cancer. 2011;50:146–153. doi: 10.1002/gcc.20839. [DOI] [PubMed] [Google Scholar]
- 21.Sugawara E, Togashi Y, Kuroda N, et al. Identification of anaplastic lymphoma kinase fusions in renal cancer. Cancer. 2012;118:4427–4436. doi: 10.1002/cncr.27391. [DOI] [PubMed] [Google Scholar]
- 22.Sukov WR, Hodge JC, Lohse CM, et al. ALK alterations in adult renal cell carcinoma: frequency, clinicopathologic features and outcome in a large series of consecutively treated patients. Mod Pathol. 2012;25:1516–1525. doi: 10.1038/modpathol.2012.107. [DOI] [PubMed] [Google Scholar]
- 23.Morris SW, Kirstein MN, Valentine MB, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science. 1994;263:1281–1284. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 24.Griffin CA, Hawkins AL, Dvorak C, et al. Recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res. 1999;59:2776–2780. [PubMed] [Google Scholar]
- 25.Lawrence B, Perez-Atayde A, Hibbard MK, et al. TPM3-ALK and TPM4-ALK oncogenes in inflammatory myofibroblastic tumors. Am J Pathol. 2000;157:377–384. doi: 10.1016/S0002-9440(10)64550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 27.Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 28.Johnson RP, Craig SW. F-actin binding site masked by the intramolecular association of vinculin head and tail domains. Nature. 1995;373:261–264. doi: 10.1038/373261a0. [DOI] [PubMed] [Google Scholar]
- 29.Hemler M. Integrins. Guidebook to the Extracellular Matrix, Anchor, and Adhesion Proteins. 2nd ed. Oxford University Press; Oxford: 1999. pp. 196–216. [Google Scholar]
- 30.Srigley JR, Delahunt B, Eble JN, et al. ISUP Renal Tumor Panel The International Society of Urological Pathology (ISUP) Vancouver Classification of Renal Neoplasia. Am J Surg Pathol. 2013;37:1469–1489. doi: 10.1097/PAS.0b013e318299f2d1. [DOI] [PubMed] [Google Scholar]
- 31.Weeks DA, Beckwith JB, Mierau GW. Benign nodal lesions mimicking metastases from pediatric renal neoplasms: a report of the National Wilms’ Tumor Study pathology center. Hum Pathol. 1990;21:1239–1244. doi: 10.1016/s0046-8177(06)80037-7. [DOI] [PubMed] [Google Scholar]
- 32.Mano H. ALKoma: a cancer subtype with a shared target. Cancer Discov. 2012;2:495–502. doi: 10.1158/2159-8290.CD-12-0009. [DOI] [PubMed] [Google Scholar]
- 33.Gunby RH, Sala E, Tartari CJ, et al. Oncogenic fusion tyrosine kinases as molecular targets for anti-cancer therapy. Anticancer Agents Med Chem. 2007;7:594–611. doi: 10.2174/187152007784111340. [DOI] [PubMed] [Google Scholar]
- 34.Hodge JC, Pearce KE, Sukov WR. Distinct ALK-rearranged and VCL-negative papillary renal cell carcinoma variant in two adults without sickle cell trait. Mod Pathol. 2013;26:604–605. doi: 10.1038/modpathol.2012.144. [DOI] [PubMed] [Google Scholar]
- 35.Debelenko LV. Reply to ‘Distinct ALK-rearranged and VCL-negative papillary renal cell carcinoma variants in two adults without sickle cell trait’. Mod Pathol. 2013;26:605–607. doi: 10.1038/modpathol.2013.37. [DOI] [PubMed] [Google Scholar]
- 36.Albadine R, Wang W, Brownlee NA, et al. Topoisomerase II alpha status in renal medullary carcinoma: immuno-expression and gene copy alterations of a potential target of therapy. J Urol. 2009;182:735–740. doi: 10.1016/j.juro.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwak EL, Bang Y, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non–small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]