Summary
In 1976, a series of 12 cases describing a lesion that had previously not been well characterized was reported as “trophoblastic pseudotumor of the uterus.” Up until that time rare reports of the lesion had classified it most often as an unusual type of sarcoma associated with pregnancy. All patients in that series were alive and well except for one who died from complications of a uterine perforation occurring at the time of a diagnostic curettage. Thus, it appeared to be a benign neoplasm but subsequently it was found that some exhibited malignant behavior and the tumor was renamed “placental site trophoblastic tumor.” A variety of observations pointed to an origin in a distinctive cell of the placental site, designated “intermediate trophoblast,” which physiologically is seen in the normal implantation site. Subsequently, another subset of intermediate trophoblast cells originating from the chorion laeve have been shown to give rise to the placental site nodule/plaque, a well-circumscribed and usually microscopic incidental finding as well as the epithelioid trophoblastic tumor, its putative malignant counterpart. The initial description of “trophoblastic pseudotumor” opened a new area of research which brought to bear immunohistochemical and molecular genetic analyses that eventually has led to new insights in the diverse morphologic changes occurring in early placentation and also led to the development of a new classification of trophoblastic tumors and tumor-like lesions.
Keywords: Gestational trophoblastic disease, Exaggerated placental site, Placental site trophoblastic tumor, Placental site nodule/plaque, Epithelioid trophoblastic tumor
“There is only one person you (R.J.K.) want to work with if you are interested in gynecologic pathology and that is Bob Scully.” That was the first time I heard of Dr Scully and that advice was given to me by Dr William Ober, himself a highly accomplished gynecologic pathologist. He and Dr Scully had been residents together at the Boston Hospital for Women (now incorporated in the Brigham and Women's Hospital) and remained friends and colleagues ever since. I spent the summer working with Dr Ober between my third and fourth year of medical school and he sparked my interest in pathology and specifically gynecologic pathology. This “introduction” began a relationship with Bob Scully, my teacher, mentor, colleague, and close friend that lasted over 40 years.
The prodigious and seminal contributions that Dr Scully made to ovarian pathology are well known but what is not as well appreciated were his equally important contributions to every area of gynecologic pathology including gestational trophoblastic disease (GTD), the subject of this essay.
I began my fellowship training with Dr Scully in July 1971. One day he showed me slides from endometrial curettings performed on a young woman with an enlarged uterus who was presumed to have had a missed abortion. The slides had been previously reviewed by a number of consultants and various diagnoses rendered, including leiomyosarcoma, nonspecific uterine sarcoma, and atypical choriocarcinoma, but it was Dr Scully's opinion that the lesion was benign and represented an exaggerated form of so-called “syncytial endometritis.” He recalled having seen 2 or 3 similar cases (one of which was sent to him by Dr Ober who had reported the case as “myosarcoma with gonadotropin-secreting cells” (1) and 1 case illustrated in the First Series AFIP fascicle (2) by Drs Arthur Hertig and Hazel Gore). He also recalled having been shown a similar case on a visit to the Armed Forces Institute of Pathology (AFIP). In 1973, I was assigned to the Gynecologic and Breast Pathology Department of the AFIP which at that time was under the direction of Dr Henry J. Norris. A review of the files of tumors classified as leiomyosarcoma and choriocarcinoma led to the identification of additional cases and resulted in a series of 12. Because it was not clear that this was a trophoblastic lesion (only 4 of the women had a positive pregnancy test which at that time was not nearly as sensitive as current assays) we performed immunohistochemistry to attempt to localize hCG in the tissue sections. Parenthetically, immunohisto-chemistry in surgical pathology in the mid-1970s was in its infancy. With the assistance of Dr Robert Colvin (subsequently chief of Pathology at the MGH and Benjamin Castleman Professor of Pathology at Harvard Medical School) who was fulfilling his military obligation at the Walter Reed Army Institute of Research (across the street from the AFIP) we first attempted to perform immunofluorescence on paraffin-embedded fixed tissue using an antibody against the β-subunit of hCG provided by Dr Judy Vaitukaitus at the NIH because commercial antibodies against hCG were not available at that time. Unfortunately, we could not visualize anything due to the intense autofluorescence of the fixed tissue. We then decided to try a newly described technique (immunoperoxidase) and were able to localize hCG in individual tumor cells in 10 of the 12 cases. An analysis of the outcome on the 12 patients revealed that except for 1 woman who died following uterine perforation at the time of a curettage, the remaining 11 were alive and well. Because of what appeared to be an excellent outcome, the lesion was reported as “trophoblastic pseudotumor of the uterus” (3). This illustrates the problem of small sample size because subsequently it was found that on occasion the tumor behaved in an aggressive manner (4) and therefore the term “trophoblastic pseudotumor” was replaced by “placental site trophoblastic tumor (PSTT)” (5). This was a designation Dr Scully “came up with” seemingly out of nowhere on a weekend day in early 1981 as he turned, perhaps for inspiration, to the pleasant view of the Charles River from his office and then turned back and said “how about placental site trophoblastic tumor” (R. H. Young, personal communication, 2014).
The detection of hCG within these lesions led to a systematic immunohistochemical analysis of placental tissue throughout gestation and various forms of trophoblastic disease with antibodies against hCG and human placental lactogen (hPL). It was found in a study of early implantation sites that the cells at the implantation site bore a striking resemblance to the cells in PSTT and furthermore that hPL rather than hCG was present in the majority of the cells. A similar immunohistochemical staining pattern was found in PSTTs thereby linking the cells at the implantation site with PSTTs.
At that time it was thought that trophoblast was composed of 2 distinct populations of cells, cytotrophoblast (CT) and syncytiotrophoblast (ST). The cells that comprised the implantation site and PSTTs were morphologically quite different. They varied in appearance from decidual-like cells to spindle-shaped cells resembling smooth muscle and when lining vascular spaces they mimicked endothelium. Consequently, it was not surprising that the tumors were initially thought to be an unusual type of leiomyosarcoma; however, the immunohistochemical detection of hCG and hPL pointed to a trophoblastic origin. The tumor cells were mononucleate but larger than CT and demonstrated a different immunohistochemical profile from cyotrophoblast, the latter negative for both hCG and hPL. They also differ both in their morphologic and immunohistochemical phenotype from ST cells, which are strongly positive for hCG and weakly positive for hPL early in gestation and in choriocarcinoma. Thus, these cells had characteristics that were “intermediate” between CT and ST and were designated “intermediate trophoblast (IT)” (6). At the implantation site, IT cells represent the dominant population and extensively infiltrate the endomyometrium. Even though the process had been initially termed “syncytial endometritis,” as most of the cells are mononucleate and the process is not inflammatory, it was subsequently renamed “exaggerated placental (implantation) site (EPS)” (7).” A few years later, a review of Dr Scully's consultation files by Dr R. H. Young led to the discovery of another trophoblastic lesion termed “placental site nodule/plaque (PSN)” (8,9). It was thought to also be derived from IT but its morphologic and immunohistochemical features differed from those of EPS and PSTT.
In 1996 Dr Ie-Ming Shih joined me in the pathology department at Johns Hopkins and we undertook a comprehensive series of studies correlating morphology, immunohistochemical and molecular genetic features of implantation site in the early stages of gestation, trophoblastic tumors, and tumor-like lesions. The discussion that follows will briefly summarize the advances that have occurred since that time in elucidating our understanding of normal trophoblastic development and relate them to the pathogenesis and classification of trophoblastic tumors and tumor-like lesions with the focus on IT. This is not intended to be a comprehensive review of the literature but rather a description of the work that Dr Shih and I have done as continuing the line of research initiated by Dr Scully.
SUBPOPULATIONS OF TROPHOBLAST. DIFFERENTIATION PATHWAYS
In the human placenta, the trophoblast growing on chorionic villi is referred to as “villous trophoblast,” whereas the trophoblast in all other locations is termed “extravillous trophoblast.” Villous tropho-blast is composed for the most part of CT and ST with small amounts of IT, whereas the extravillous trophoblast is almost exclusively composed of IT. The CT or Langhans cell is the germinative trophoblastic cell on the surface of chorionic villi. In early gestation, CT differentiates along 2 pathways— villous and extravillous. On the villous surface, CT fuses directly to form ST. The differentiation of CT into ST is accompanied by complete loss of proliferative activity (10,11). The second pathway of differentiation of CT occurs at the distal end of the villus that makes contact with the placental bed. These villi, are termed “anchoring villi” and they are composed of trophoblastic columns in which CT merges imperceptibly into IT and then ST. The IT cells in the trophoblastic columns are termed “villous IT.” At the base of trophoblastic column where it makes contact with the endometrium, IT infiltrates the decidua and myometrium and invades and replaces the distal ends of the spiral arteries of the implantation site to establish the maternal/fetal circulation. This differentiation pathway is accompanied by active gene transcription and translation that regulates the functional activity of IT. For example, CD146 (Mel-CAM) is gradually expressed by IT cells as they migrate from the trophoblastic column and infiltrate the placental site. In contrast, the proliferative activity of villous IT gradually decreases as the cells move away from the villi (11). This subpopulation of IT in the implantation site is designated “implantation site IT” (12,13). Although these IT cells extensively infiltrate the placental bed, they do not demonstrate proliferative activity. Some mononucleate implantation site IT cells fuse into multi-nucleated cells which are terminally differentiated. In contrast, IT away from implantation site (the chorion frondosum) differentiates into “chorionic-type IT.” At around 20 wk of gestation the expanding gestational sac obliterates the endometrial cavity and the chorion frondosum fuses with the decidua parietalis to form the chorion laeve. As the surface area of the chorion laeve increases towards term, the chorionic-type IT appears to proliferate at a low level throughout gestation. The trophoblastic cells in the trophoblastic shell, trophoblastic islands, and placental septae appear to be related to the implantation site IT and therefore all of these are considered subpopulations of IT. Besides the anatomic location and cell morphology, the different subpopulations of IT are characterized by distinctive gene expression profiles as revealed by immunohistochemistry (11,14). For example, villous IT exclusively expresses HNK-1 carbohydrate moiety, not expressed by other sub-types of IT (15). In contrast, almost all implantation site IT cells express CD146 (Mel-CAM) and hPL and most chorionic-type IT express p63 and placental alkaline phosphatase (13,14). These immunohisto-chemical markers not only shed light on the biology of the different types of IT but the antibodies that react with these markers provide useful reagents to dissect the lineage and molecular pathogenesis of the various types of GTD (12).
SUBPOPULATIONS OF TROPHOBLAST. FUNCTIONAL ASPECTS
CT is the trophoblastic stem cell and is located on the villous surface. CT expresses epidermal growth factor receptor (EGF-R) which binds to EGF secreted by the decidua (16). It has been postulated that paracrine-like mechanism EGF-R and its ligand may provide persistent growth stimulation for CT (17). As discussed previously, CT differentiates along 2 pathways. Along one CT proliferates and fuses to form the overlying ST. This process results in expansion of the surface area of chorionic villi in the developing placenta. In the second pathway, CT differentiates into villous IT in the trophoblastic columns and then into implantation site IT in the placental site or chorionic-type IT in the chorion laeve (12). The mechanisms underlying the differentiation of CT are largely unknown (16,18).
ST is composed of terminally differentiated cells which synthesize and secrete a variety of pregnancy-associated hormones including hPL, SP-1, and hCG, thought to be critical in the establishment and maintenance of pregnancy. In addition to its role as an endocrine organ, the ST is bathed in maternal blood and is responsible for the exchange of oxygen, nutrients, and a variety of metabolic products between the mother and fetus.
Villous IT cells proliferate in the proximal portion of trophoblastic columns and serve as source from which implantation site and chorionic-type IT are derived. In addition, villous IT cells may play an important role in maintaining the structural integrity of the villi that anchor the placenta to the decidua. It is speculated that HNK-1 carbohydrate moiety expressed on the surface of the villous IT may contribute to intercellular cohesion in the trophoblastic columns which counteract the mechanical-sheering forces resulting from fetal movements and the turbulence created by the pulsatile blood flow in the placental bed (15).
Implantation Site IT has as a major function to establish the maternal-fetal circulation by invading the spiral arteries in the endomyometrium during early pregnancy (19). It has been suggested that the mechanisms underlying trophoblastic invasion are similar to those involved in tumor cell invasion (20,21). For example, proteases are responsible for matrix degradation and tissue remodeling, a prerequisite for trophoblastic migration and invasion. Loss of E-cadherin expression is closely associated with the infiltrative phenotype of implantation site IT (I.M.S., unpublished data, 2007). Expression of growth factors and their receptors constitutes a unique molecular mechanism regulating trophoblastic behavior and cell-to-cell communication (autocrine or paracrine) including cellular migration, proliferation, and differentiation. Expression of cell adhesion molecules is important for trophoblastic migration in different extracellular substrates and for cross-talk between trophoblastic cells and their microenvironment. For example, it has been demonstrated that CD146 (Mel-CAM), expressed by implantation site IT cells, binds to its putative ligand on the surface of smooth muscle cells and the Mel-CAM-ligand interaction confers a stationary phenotype on trophoblastic cells, limiting their invasion into the superficial portion of myometrium (13).
Unlike malignant tumors, the invasion of implantation site IT is tightly regulated, confined spatially to the implantation site, and limited temporally to early pregnancy (17,19,22,23). While extensively infiltrating the endometrium of the basal plate, the implantation site IT invade only the inner third of the myometrium in the first trimester, decreasing to <10% of the myometrium by term. Although the molecular mechanisms underlying the control of trophoblastic invasion are unclear, the invasive process can be modulated by both the trophoblast and the local microenvironment (17,22,24). Fusion of mononucleate implantation site IT cells into multi-nucleated cells leads to the loss of their invasive and migratory phenotype.
Another feature that distinguishes non-neoplastic trophoblastic cells from tumor cells is their pattern of cellular proliferation. The differentiation of implantation site IT is accompanied by a decrease in cellular proliferation in contrast to the uncontrolled proliferation in malignant neoplasms. Indeed, implantation site IT cells are negative for Ki-67, a proliferation marker, and are positive for several proteins which are involved in the arrest of cell cycle progression including p21WAF1/CIP1 (25) and p57kip-2 (26).
Spiral arteries at the implantation site are the targets for invasion by implantation site IT. The mechanisms that are responsible for the tropism of implantation site IT to the spiral arteries and not to other structures are unclear; one postulate is that the oxygen gradient may be a cue. The trophoblastic invasion into the vascular wall is associated with abundant deposition of extra-cellular matrix which eventually replaces the entire smooth muscle layer of spiral arteries, resulting in the transformation of the arteries to large-caliber and low-resistant vascular channels. This unique feature of vascular invasion is not only observed in the normal placental site but also in the PSTT. Implantation site IT also replaces the lining endothelial cells of the distal ends of the spiral arteries (an epithelialendothelial transdifferentiation) characterized by the acquisition of several endothelial markers including VCAM-1, VE-cadherin, 4 integrin (27), and CD146 (Mel-CAM) (28) all of which are expressed by endothelial cells. Accordingly, the term “trophoblast pseudovasculogenesis” has been proposed to describe this unique differentiation pathway of implantation site IT (27,29). Some of the implantation site IT cells that invade the spiral arteries migrate along the vascular wall in a retrograde manner to reach the spiral arteries beyond the implantation site in the myometrium. The intravascular implantation site IT cells tend to form trophoblastic aggregates which act like valves or a sieve to control the blood flow in the trophoblast-modified spiral arteries. The process of aggregation is associated with the expression of NCAM and E-cadherin which may be responsible for the enhanced intercellular cohesion among cells in trophoblastic aggregates in the spiral arteries, a view supported by a study in which the E-cadherin gene was introduced into an E-cadherin-negative implantation site IT cell line, IST-1, resulting in a stationary and cohesive phenotype of IST-1 cells in culture (I.M.S., unpublished data).
Chorionic-type IT
The functional role of chorionic-type IT cells remains speculative. Unlike the implantation site IT, the chorionic-type IT proliferates throughout gestation as the total surface area of fetal membrane increases. Chorionic-type IT may contribute to the synthesis of extracelluar matrix which is required to maintain the tensile strength of the fetal membrane (30). It is also possible that chorionic-type IT acts as a biological and mechanical barrier to the maternal immune system and is important for fetal allograft survival.
CLASSIFICATION OF GTD
Before the publication of the trophoblastic pseudotumor, the classification of GTD consisted of hydatidiform mole (complete, partial, and invasive), choriocarcinoma and syncytial endometritis. Since the recognition of IT, the classification of GTD has been expanded to include PSTT, epithelioid trophoblastic tumor (ETT), and PSN. In addition, the term “syncytial endometritis” has been replaced by a more accurate one “exaggerated placental (implantation) site” (7).
PATHOLOGY OF TUMORS AND TUMOR-LIKE LESIONS OF TROPHOBLAST
In this discussion the focus will be on various trophoblastic lesions that were described based on the discovery of IT. Accordingly, hydatidiform moles and choriocarcinomas which were well-established entities before that discovery are not included.
PSTT
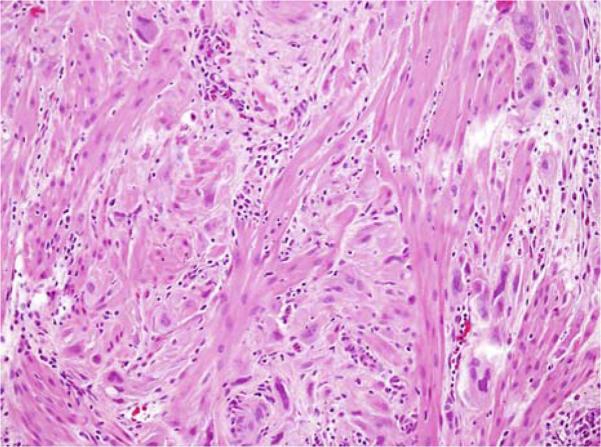
This trophoblastic tumor is a relatively uncommon form of GTD composed of neoplastic implantation site IT cells. Patients usually are in the reproductive age group and can present with either amenorrhea or abnormal bleeding, often accompanied by uterine enlargement (31,32). They frequently are thought to be pregnant. Microscopically, PSTT resembles the trophoblastic infiltration of the endometrium and myometrium of the placental site during early pregnancy (Fig. 1), but is generally more confluent. The predominant cell type in PSTT is implantation site IT based on the morphologic and immunohistochemical features (28,33). PSTT is generally associated with benign outcome except in approximately 15% of cases (4,32,34,35). With the use of chemo-therapy most patients with PSTT can be cured (36).
FIG. 1.
Placental site trophoblastic tumor. A confluent growth of neoplastic intermediate trophoblastic cells varying from rounded, simulating decidua, to spindle-shaped, simulating smooth muscle, infiltrates the myometrium.
On the basis of genotyping using fluorescent micro-satellite markers, Fisher and colleagues were able to confirm that PSTT is gestational in origin because paternal genomic contribution is present in all cases (37–39). Immunohistochemistry has also played an important role in delineating the pathogenesis of PSTT which is diffusely positive for hPL and CD146 (Mel-CAM), but rarely positive for hCG, p63, or HNK-1, an immunophenotype characteristic of implantation site IT. Accordingly, PSTT represents neoplastic transformation of implantation site IT (12,28). PSTT is associated with abnormal expression of cell cycle regulatory gene products including cyclins, cyclin-dependent kinases, and p53 (40).
ETT
This is an uncommon type of trophoblastic tumor that is distinct from PSTT and choriocarcinoma with features resembling a carcinoma. Patients are usually in their reproductive age and commonly present with vaginal bleeding (41). Microscopically, ETTs are nodular and generally well circumscribed although focal infiltrative features can be present at the periphery. The tumors are composed of a relatively uniform population of chorionic-type IT cells typically arranged in nests and cords and sheets of cells that are intimately associated with an eosinophillic, fibrillar, hyaline-like material, and necrotic debris (Fig. 2A). The extensive areas of necrosis that surround islands of viable tumor cells create a “geographic” pattern of necrosis (Fig. 2B). Typically, a small blood vessel is located in the center of the tumor nests. For the most part, ETTs behave in a benign manner but metastasis and death occurs in approximately 25% and 10% of patients, respectively (41–44).
FIG. 2.
Epithelioid trophoblastic tumor. Neoplastic chorionic-type intermediate trophoblastic cells surround blood vessels. Note geographic necrosis (A); Tumor cells are mononucleate, rounded and relatively uniform in size creating an “epithelioid” appearance (B).
The molecular features of ETTs are largely unknown as this tumor is relatively uncommon and has only been relatively recently recognized. Although the gestational origin of ETTs has not yet been demonstrated, the immunohistochemical studies suggest that they are related to chorionic-type IT. Besides cytokeratin, epithelial membrane antigen, E-cadherin, and EGF-R that are consistent with their epithelial origin, all the tumors are positive for placental alkaline phosphatase and p63 but only focally positive for hPL, hCG, and CD146 (Mel-CAM), an immunophenotype identical to that in chorionic-type IT (14,41) but different from PSTT which is diffusely positive for CD146 (Mel-CAM) and hPL. The mean Ki-67 labeling index in ETTs is 17.7 ± 4.5% (mean ± SD) with a range from 10% to 25%. Because PSNs are also composed of chorionic-type IT, it has been hypothesized that some PSNs may represent an intermediate stage in tumor progression to ETTs. This view is supported by our observations that PSNs have features intermediate between typical PSNs and ETTs and that in some cases this is an intimate association of some ETTs with PSNs (14,41).
EPS
The EPS is a benign non-neoplastic lesion characterized by an increased number of IT cells that extensively infiltrate the endometrium and underlying myometrium (Fig. 3). The EPS can occur in a normal pregnancy or an abortion from the first trimester (12). The incidence is approximately 1.6% of spontaneous and elective abortions from the first trimester. The trophoblastic cells display an identical morphologic and immunophenotypic profile to the implantation site IT cells in the normal placental site. The constituent cells contain abundant eosinophilic cytoplasm with hyperchromatic and irregular nuclei. In addition, they are strongly positive for CD146 (Mel-CAM) and hPL, moderately positive for EGF-R and E-cadherin, and negative for Ber-EP4, EMA, HNK-1, and NCAM. These findings indicate that the differentiation of IT is unaltered in an EPS, and supports the view that an EPS is a normal variation of an implantation site (12,28,31). Despite the profuse infiltration of IT in an EPS, the Ki-67 index is typically near zero, suggesting that the increased number of IT in EPS is probably not the result of de novo proliferation of IT in the implantation site (5). The precise mechanism underlying the exaggerated number of IT in EPS remains unclear, but may be due to rapid cell cycle progression of IT in the trophoblastic columns or the suppression of apoptosis of IT in the deep implantation site.
FIG. 3.
Exaggerated placental site. Placental site intermediate trophoblastic cells infiltrate the myometrium in a manner similar to the placental site trophoblastic tumor. In contrast to the latter, cells are arranged in small clusters and nests rather than in large confluent masses.
PSN
These lesions are composed of small, well-circumscribed nodular aggregates of chorionic-type IT cells that are embedded in a hyalinized stroma (Fig. 4). They are typically round but may be plaque-like; sometimes they exhibit necrosis. PSNs are benign non-neoplastic lesions and are typically incidental findings in uterine curettings, cervical biopsies, and occasionally in hysterectomy specimens (14,45,46). Because of their small size and circumscription, the lesions are usually removed completely by the surgical procedure that led to their discovery. Neither local recurrence nor progression to persistent GTD has been documented in PSNs (8,14). Accordingly, no treatment or follow-up is necessary.
FIG. 4.
Placental site nodule. A well-circumscribed nest of chorionic-type intermediate trophoblast cells are mononucleate and relatively uniform in size. Note the acellular hyaline-like material in the center of the nodule.
PSNs have been thought to represent a portion of uninvoluted placental site from remote gestations in the uterus. However, the constituent cells in PSNs are morphologically more closely related to the IT of chorion (chorionic-type IT) than to the IT of the placental site (implantation site IT cells) (14). In addition, the trophoblastic cells in the PSN exhibit an immunophenotype similar to that of trophoblastic cells in the chorion but distinct from the implantation site IT. They react with antibodies against cytokeratin, epithelial membrane antigen (EMA), pregnancy-specific SP-1, placental alkaline phosphatase, and inhibin-a (47–49). Most PSNs also express the “classic” IT markers including hPL, CD146 (Mel-CAM), and mucin-4 although only in a small number of cells (14). The above findings suggest that PSNs are derived from chorionic-type IT.
CONCLUDING REMARKS
Dr Scully's acute powers of observation played a critical role in the discovery of IT which opened a new area of investigation leading to a deeper understanding of placentation and to the development of a new classification of trophoblastic tumors and nonneoplastic trophoblastic proliferations. Given his interest in this area, it is appropriate that one of Dr. Scully's last papers was on the PSTT (32) and his historical forays included one pointing out that, although we have focused the story here on that since 1976, the old adage, “nothing new under the sun” comes to mind as when Dr Scully delved into the history of this topic he found evidence of the controversy concerning the nature of proliferations, likely of IT, almost a century ago. From a personal perspective one of us (R.J.K.) is warmed by the fact that one of Dr Scully's last essays was on his good friend and my other mentor Dr Ober (50). The field of gynecologic pathology has been forever changed by Dr Scully's contributions to this and numerous other areas.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Ober W. Excerpta Medica International Congress Series. Number 203. Excerpta Medica Foundation; Amsterdam and New York: 1970. Gestational choriocarcinoma. Immunologic aspects, diagnosis and treatment. In Gynecological Oncology-A Comprehensive Review and Evaluation (Proceedings of the Lenox Hill Hospital Symposium in New York, NY, 1969). pp. 304–315. [Google Scholar]
- 2.Hertig AT, Gore H, editors. Atlas of Tumor Pathology, Section IX Fascicle 33, Tumors of the Female Sex Organs, Part 2 (Supplement) Armed Forces Institute of Pathology; Washington, DC: 1960. pp. 329–34. [Google Scholar]
- 3.Kurman RJ, Scully RE, Norris HJ. Trophoblastic pseudotumor of the uterus. An exaggerated form of “syncytial endometritis” simulating a malignant tumor. Cancer. 1976;38:1214–26. doi: 10.1002/1097-0142(197609)38:3<1214::aid-cncr2820380323>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 4.Twiggs LB, Okagaki T, Phillips GL, et al. Trophoblastic pseudotumor—evidence of malignant disease potential. Gynecol Oncol. 1981;12(2 pt 1):238–48. doi: 10.1016/0090-8258(81)90153-0. [DOI] [PubMed] [Google Scholar]
- 5.Scully RE, Young RH. Trophoblastic pseudotumor: a reappraisal. Am J Surg Pathol. 1981;5:75. doi: 10.1097/00000478-198101000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Kurman RJ, Main CS, Chen HC. Intermediate trophoblast: a distinctive form of trophoblast with specific morphological, biochemical and functional features. Placenta. 1984;5:349–70. doi: 10.1016/s0143-4004(84)80015-6. [DOI] [PubMed] [Google Scholar]
- 7.Scully RE, Bonfiglio TA, Kurman RJ, et al., editors. Histological Typing of Female Genital Tract Tumours. 2nd ed. Springer-Verlag; Berlin: 1994. World Health Organization International Histological Classification of Tumors. [Google Scholar]
- 8.Young RH, Kurman RJ, Scully RE. Placental site nodules and plaques. A clinicopathologic analysis of 20 cases. Am J Surg Pathol. 1990;14:1001–9. doi: 10.1097/00000478-199011000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Young RH, Scully RE. Placental-site trophoblastic tumor: current status. Clin Obstet Gyncol. 1984;27:248–58. doi: 10.1097/00003081-198403000-00032. [DOI] [PubMed] [Google Scholar]
- 10.Loke YW, King A. Trophoblast interaction with extracellular matrix. In: Loke YW, King A, editors. Human Implantation-Cell Biology and Immunology. 1st ed. Cambridge University Press; Cambridge: 1995. pp. 151–79. [Google Scholar]
- 11.Shih IM, Kurman RJ. Ki-67 labeling index in the differential diagnosis of exaggerated placental site, placental site trophoblastic tumor, and choriocarcinoma: a double immunohisto-chemical staining technique using Ki-67 and Mel-CAM antibodies. Hum Pathol. 1998;29:27–33. doi: 10.1016/s0046-8177(98)90386-0. [DOI] [PubMed] [Google Scholar]
- 12.Shih I-M, Kurman RJ. The pathology of intermediate trophoblastic tumors and tumor-like lesions. Int J Gynecol Pathol. 2001;20:31–47. doi: 10.1097/00004347-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Shih I, et al. Expression of Mel-CAM in implantation site intermediate trophoblastic cell line, IST-1, limits its migration on uterine smooth muscle cells. J Cell Sci. 1998;111(pt 17):2655–64. doi: 10.1242/jcs.111.17.2655. [DOI] [PubMed] [Google Scholar]
- 14.Shih IM, Seidman JD, Kurman RJ. Placental site nodule and characterization of distinctive types of intermediate tropho-blast. Hum Pathol. 1999;30:687–94. doi: 10.1016/s0046-8177(99)90095-3. [DOI] [PubMed] [Google Scholar]
- 15.Shih IM, Schnaar RL, Gearhart JD, et al. Distribution of cells bearing the HNK-1 epitope in the human placenta. Placenta. 1997;18:667–74. doi: 10.1016/s0143-4004(97)90008-4. [DOI] [PubMed] [Google Scholar]
- 16.Mi S, Lee X, Li X, et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. 2000;403:785–9. doi: 10.1038/35001608. [DOI] [PubMed] [Google Scholar]
- 17.Bischof P, Meisser A, Campana A. Paracrine and autocrine regulators of trophoblast invasion-a review. Placenta. 2000;14(suppl A):S55–60. doi: 10.1053/plac.2000.0521. [DOI] [PubMed] [Google Scholar]
- 18.Janatpour MJ, McMaster MT, Genbacev O, et al. Id-2 regulates critical aspects of human cytotrophoblast differentiation, invasion and migration. Development. 2000;127:549–58. doi: 10.1242/dev.127.3.549. [DOI] [PubMed] [Google Scholar]
- 19.Fisher SJ, Damsky CH. Human cytotrophoblast invasion. Semin Cell Biol. 1993;4:183–8. doi: 10.1006/scel.1993.1022. [DOI] [PubMed] [Google Scholar]
- 20.Chassin D, Bénifla JL, Delattre C, et al. Identification of genes overexpressed in tumors through preferential expression screening in trophoblasts. Cancer Res. 1994;54:5217–23. [PubMed] [Google Scholar]
- 21.Yagel S, Parhar RS, Jeffrey JJ, et al. Normal nonmetastatic human trophoblast cells share in vitro invasive properties of malignant cells. J Cell Physiol. 1988;136:455–62. doi: 10.1002/jcp.1041360309. [DOI] [PubMed] [Google Scholar]
- 22.Graham CH, Connelly I, MacDougall JR, et al. Resistance of malignant trophoblast cells to both the anti-proliferative and anti-invasive effects of transforming growth factor-beta. Exp Cell Res. 1994;214:93–9. doi: 10.1006/excr.1994.1237. [DOI] [PubMed] [Google Scholar]
- 23.Graham CH. Effect of transforming growth factor-beta on the plasminogen activator system in cultured first trimester human cytotrophoblasts. Placenta. 1997;18:137–43. doi: 10.1016/s0143-4004(97)90085-0. [DOI] [PubMed] [Google Scholar]
- 24.Caniggia I, Grisaru-Gravnosky S, Kuliszewsky M, et al. Inhibition of TGF-beta 3 restores the invasive capability of extravillous trophoblasts in preeclamptic pregnancies. J Clin Invest. 1999;103:1641–50. doi: 10.1172/JCI6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheung AN, Shen DH, Khoo US, et al. p21WAF1/CIP1 expression in gestational trophoblastic disease: correlation with clinicopathological parameters, and Ki67 and p53 gene expression. J Clin Pathol. 1998;51:159–62. doi: 10.1136/jcp.51.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chilosi M, Piazzola E, Lestani M, et al. Differential expression of p57kip2, a maternally imprinted cdk inhibitor, in normal human placenta and gestational trophoblastic disease. Lab Invest. 1998;78:269–76. [PubMed] [Google Scholar]
- 27.Zhou Y, Fisher SJ, Janatpour M, et al. Human cytotropho-blasts adopt a vascular phenotype as they differentiate: a strategy for successful endovascular invasion? J Clin Invest. 1997;99:2139–51. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shih IM, Kurman RJ. Expression of melanoma cell adhesion molecule in intermediate trophoblast. Lab Invest. 1996;75:377–88. [PubMed] [Google Scholar]
- 29.Damsky CH, Fisher SJ. Trophoblast pseudo-vasculogenesis: faking it with endothelial adhesion receptors. Curr Opin Cell Biol. 1998;10:660–6. doi: 10.1016/s0955-0674(98)80043-4. [DOI] [PubMed] [Google Scholar]
- 30.Bryant-Greenwood GD. The extracellular matrix of the human fetal membranes: structure and function. Placenta. 1998;19:1–11. doi: 10.1016/s0143-4004(98)90092-3. [DOI] [PubMed] [Google Scholar]
- 31.Shih I-M, Mazur MT, Kurman RJ. Gestational trophoblastic disease. In: Sternberg SS, editor. Diagnostic Surgical Pathology. Lippincott Williams & Wilkins; New York: 1999. pp. 2067–86. [Google Scholar]
- 32.Baergen RN, Rutgers JL, Young RH, et al. Placental site trophoblastic tumor: a study of 55 cases and review of the literature emphasizing factors of prognostic significance. Gynecol Oncol. 2006;100:511–520. doi: 10.1016/j.ygyno.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 33.Shih IM. The role of CD146 (Mel-CAM) in biology and pathology. J Pathol. 1999;189:4–11. doi: 10.1002/(SICI)1096-9896(199909)189:1<4::AID-PATH332>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 34.Ekstein RP, Paradinas FJ, Bagshawe KD. Placental site trophoblastic tumour (trophoblastic pseudotumor): a study of four cases requiring hysterectomy including one fatal case. Histopathology. 1982;6:211–26. doi: 10.1111/j.1365-2559.1982.tb02716.x. [DOI] [PubMed] [Google Scholar]
- 35.Gloor E, Dialdas J, Hurlimann J, et al. Placental site trophoblastic tumor (trophoblastic pseudotumor) of the uterus with metastases and fatal outcome. Clinical and autopsy observations of a case. Am J Surg Pathol. 1983;7:483–6. doi: 10.1097/00000478-198307000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Twiggs LB, Hartenbach E, Saltzman AK, et al. Metastatic placental site trophoblastic tumor. Int J Gynaecol Obstet. 1998;60(suppl 1):S51–5. [PubMed] [Google Scholar]
- 37.Fisher RA, Khatoon R, Paradinas FJ, et al. Repetitive complete hydatidiform mole can be biparental in origin and either male or female. Hum Reprod. 2000;15:594–8. doi: 10.1093/humrep/15.3.594. [DOI] [PubMed] [Google Scholar]
- 38.Fisher RA, et al. Gestational and nongestational trophoblastic tumors distinguished by DNA analysis. Cancer. 1992;69:839–45. doi: 10.1002/1097-0142(19920201)69:3<839::aid-cncr2820690336>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 39.Arima T, Imamura T, Sakuragi N, et al. Malignant trophoblastic neoplasms with different modes of origin. Cancer Genet Cytogenet. 1995;85:5–15. doi: 10.1016/0165-4608(95)00109-3. [DOI] [PubMed] [Google Scholar]
- 40.Ichikawa N, Zhai YL, Shiozawa T, et al. Immunohistochemical analysis of cell cycle regulatory gene products in normal trophoblast and placental site trophoblastic tumor. Int J Gynecol Pathol. 1998;17:235–40. doi: 10.1097/00004347-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Shih I-M, Kurman RJ. Epithelioid trophoblastic tumor—a neoplasm distinct from choriocarcinoma and placental site trophoblastic tumor simulating carcinoma. Am J Surg Pathol. 1998;22:1393–403. doi: 10.1097/00000478-199811000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Hamazaki S, Nakamoto S, Okino T, et al. Epithelioid trophoblastic tumor: morphological and immunohistochemical study of three lung lesions. Hum Pathol. 1999;30:1321–7. doi: 10.1016/s0046-8177(99)90063-1. [DOI] [PubMed] [Google Scholar]
- 43.Silva EG, Tornos C, Lage J, et al. Multiple nodules of intermediate trophoblast following hydatidiform moles. Int J Gynecol Pathol. 1993;12:324–32. doi: 10.1097/00004347-199310000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Mazur MT. Metastatic gestational choriocarcinoma. Unusual pathologic variant following therapy. Cancer. 1989;63:1370–7. doi: 10.1002/1097-0142(19890401)63:7<1370::aid-cncr2820630723>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 45.Nayar R, Snell J, Silverberg SG, et al. Placental site nodule occurring in a fallopian tube. Hum Pathol. 1996;27:1243–5. doi: 10.1016/s0046-8177(96)90322-6. [DOI] [PubMed] [Google Scholar]
- 46.Campello TR, et al. Extrauterine (tubal) placental site nodule. Histopathology. 1998;32:562–5. [PubMed] [Google Scholar]
- 47.Huettner PC, Gersell DJ. Placental site nodule: a clinicopatho-logic study of 38 cases. Int J Gynecol Pathol. 1994;13:191–8. doi: 10.1097/00004347-199407000-00001. [DOI] [PubMed] [Google Scholar]
- 48.Shitabata PK, Rutgers JL. The placental site nodule: an immunohistochemical study. Hum Pathol. 1994;25:1295–301. doi: 10.1016/0046-8177(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 49.Shih IM, Kurman RJ. Immunohistochemical localization of inhibin-alpha in the placenta and gestational trophoblastic lesions. Int J Gynecol Pathol. 1999;18:144–50. doi: 10.1097/00004347-199904000-00008. [DOI] [PubMed] [Google Scholar]
- 50.Scully RE, Young RH, William B, Ober MD. humanist, humorist, historian, and histopathologist: recollections of his life and evaluation of his work. Semin Diagn Pathol. 1920-1993;2008;25:202–29. doi: 10.1053/j.semdp.2008.07.009. [DOI] [PubMed] [Google Scholar]