Abstract
Purpose
To compare blood glucose levels in patients with or without “detectable” brown adipose tissue (BAT) using 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography/ computed tomography (FDG PET/CT).
Procedures
Nine hundred eight patients had PET/CT scans and were previously identified as having, or not having, FDG uptake in BAT. The original database was retrospectively reviewed for blood glucose level and body mass index (BMI) at the time of imaging. Blood glucose levels were compared between patients with or without FDG uptake in BAT, adjusting for age, sex, and BMI.
Results
Fifty-six patients (6.2%) had FDG uptake in BAT. In the univariate analysis, patients without FDG uptake in BAT had a higher risk of glucose ≥100 mg/dL (odds ratio 3.4, 95% CI= 1.6–7.3; P=0.0007). After adjustment for age, sex, BMI, and significant interaction of sex and BMI, patients without BAT tended to have a higher risk of glucose ≥100 mg/dL, although not statistically significant (odds ratio=1.6, 95% CI=0.7–3.6; P=0.268).
Conclusions
Although causal relationships are not specified, the data suggest that BAT uptake, glucose levels, BMI, sex, and age are inter-related and the possibility that presence of “detectable” BAT is protective against diabetes and obesity. FDG PET/CT may be a vital tool for further investigations of diabetes and obesity.
Keywords: Brown fat, BAT, Glucose, FDG, “USA”-Fat, PET, Brown adipose tissue
Introduction
Brown adipose tissue (BAT) in mammals primarily serves to generate heat [1]. By contrast, its counterpart white adipose tissue (WAT) is involved in caloric storage [1]. Uncoupling protein-1 is expressed in BAT and it is important in conferring the thermogenic ability of BAT by uncoupling adenosine-5′-triphosphate production from the movement of protons down their concentration gradient in the inner mitochondrial membrane [1]. The existence of BAT in hibernating rodents and human neonates is well established; however, its presence in adult humans remained controversial until its relatively recent description on 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography/computed tomography (FDG PET/CT) [2–7].
Cohade et al. [2] described foci of high metabolic activity localizing to fat density on CT scan in patients (mostly adults) undergoing FDG PET/CT imaging for oncologic evaluation, described as “USA-Fat” for its uptake mainly in the supraclavicular area of the body. The FDG uptake has since been described in other locations besides the supraclavicular area, for example the paraspinal region, mediastinum, neck, and upper abdomen [3, 8]. The high metabolic activity for FDG fuses to fat density on CT and is thus phenotypically due to active BAT. BAT has been shown to avidly accumulate FDG in animal studies [9–11].
In humans, FDG uptake in BAT is more common in females and younger patients and has been associated with cold exposure [3, 5–7, 12]. Studies have also suggested that patients with active BAT are thinner than those without active BAT [3, 5–7, 12]. In addition, pre-clinical and human studies have demonstrated that the use of beta-blockers is a negative predictor for the presence of active BAT detected on PET/CT [6, 11].
Mice without BAT are susceptible to insulin resistance and diet-induced diabetes and develop an obese phenotype [13–16]. BAT has been suggested to have a role in the regulation of insulin secretion and glucose homeostasis in animals [13–16]. Based on the animal data, we hypothesized that fasting glucose levels would be lower in adult humans with BAT uptake on FDG PET compared to those without BAT uptake. To test our hypothesis, we compared blood glucose levels in patients identified qualitatively as having active BAT on FDG PET/CT to patients with no “detectable” BAT uptake. We also considered other variables (age, sex, body mass index) that have also been shown to be associated with BAT uptake.
Materials and Methods
Patients
Nine hundred eight patients (445 females, 463 males, mean age 58 ± 15 years) underwent 1,016 consecutive FDG PET/CT scans between July 1, 2001 and June 30, 2002, all for the evaluation of known or suspected cancer. Seventy-five patients had more than one FDG PET/ CT scan during the time period of the study and only the first scan was considered for the purpose of this investigation.
Permission to conduct this retrospective study was obtained from our Institutional Review Board under an exempt review. The requirement for informed consent was waived.
FDG PET/CT Scans
Patients fasted for a minimum of 4 h and had blood glucose levels <200 mg/dL measured immediately prior to the intravenous injection of a weight-based amount of FDG (8.14 MBq/kg [0.22 mCi/kg]).
After an approximately 60-min tracer uptake phase, a combined PET/CT scan (Discovery LS; GE Healthcare, Waukesha, WI, USA) was obtained from the mid-skull level to the mid-femur level. The details of the image acquisition and reconstruction techniques were previously published [2, 12].
Data Analyses
Previously, all PET/CT scans were examined for the presence or absence of FDG uptake in BAT and the results reported [12]. For the current study, the original patient database and electronic medical record was reviewed to determine patients’ blood glucose level just prior to the intravenous administration of FDG, height and weight. Body mass index (BMI) was determined for each patient using the following equation: weight (kg)/height (m2). For the patients with BAT uptake and a sub-group without BAT uptake (2:1 for no BAT uptake to BAT uptake) matched for age, sex, and BMI, medical records were also reviewed for the use of beta-blockers and steroids.
Statistical Analyses
Results for continuous variables are expressed as mean±standard deviation and categorical variables as frequency and percentage. For the primary analysis, glucose level was considered as a binary outcome based on the current boundary between normoglycemia (glucose <100 mg/dL) and impaired fasting glucose level (glucose ≥100 mg/dL) [17]. The association between glucose group and BAT uptake, age, sex, and BMI was examined with the use of the logistic regression model. First, a univariate analysis was performed to assess the effects of individual variables on the risk of having glucose ≥100 mg/dL. Variables considered included BAT uptake (yes/no), age, sex (female/male), and BMI (underweight ≥18.5; normal=18.5–24.9; overweight=25–29.9; obesity ≥30) [18].
A multivariable logistic regression model was then applied to this cohort to assess the independent effects of risk factors with adjustment for potential confounders. A variable selection procedure was incorporated by stepwise selection while adhering to the hierarchical model building principal, that is, all effects contained in a significant interaction term must remain in the model, even if these effects are not conditionally significant. Potential interaction effects were considered between age and BAT, sex and BAT, BMI and BAT, age and BMI, and sex and BMI. As BAT uptake was of primary interest, it was included in the final model regardless of its outcome in the stepwise selection procedure. In this analysis, only two-way interaction was considered and data was modeled on probability of being at risk for diabetes (glucose level ≥100 mg/dL). Odds ratios and 95% confidence intervals are reported, which indicate the strength of the association between glucose group and BAT uptake, age, sex, and BMI.
We also explored the relationship between glucose level as a continuous variable and BAT uptake, age, sex, and BMI. For this analysis, a general linear model was employed and univariate and multivariable models were applied sequentially. The interaction effect (e.g., age and BAT, sex and BAT, BMI and BAT, age and BMI, sex and BMI) was assessed by including a cross-product term (two-way interaction) along with the associated main effects. Variables of significance based on the univariate models were retained in the multivariable models. As BAT uptake was of primary interest, it was considered in the final multivariable model regardless of its significance.
The analyses were performed with SAS (v. 9.2, SAS Institute, Cary, NC, USA). Throughout all analyses, statistical significance was considered at P value <0.05.
Results
Fifty-six of 908 patients (6.2%) had FDG uptake in BAT visualized on PET/CT (Fig. 1). Blood glucose level was not available for one patient and this patient was excluded from further analyses. The summary of results considering glucose level as a binary outcome variable is shown in Tables 1 and 2. Normal glucose levels (<100 mg/dL) were found in 590 patients and 317 patients had a glucose level ≥100 mg/dL. In the univariate analysis (Table 1), patients who had no BAT uptake, were older, males, or obese and were at higher risk for glucose levels ≥100 mg/dL.
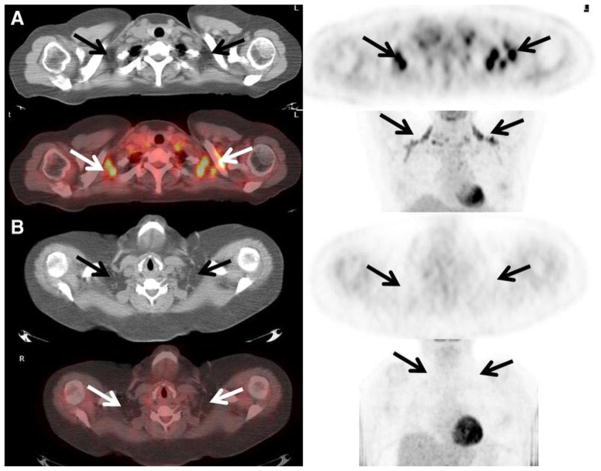
Fig. 1.
a Active brown adipose tissue detected on FDG PET/CT. A 48-year-old female with a history of endometrial carcinoma underwent FDG PET/CT to evaluate for recurrent disease. Foci of intense FDG uptake are seen fusing to fat in the supraclavicular regions consistent with the presence of active BAT (arrows). Her blood glucose level just prior to the administration of FDG was 72 mg/dL. Her body mass index was 26. b No active brown adipose tissue detected on FDG PET/ CT. A 50-year-old female with a history of non-Hodgkin’s lymphoma underwent FDG PET/CT for re-staging purposes. No increased FDG uptake was seen localizing to fat on the CT scan suggesting the absence of metabolically active BAT (arrows). Her blood glucose level just prior to the administration of FDG was 127 mg/dL. Her body mass index was 49.
Table 1.
Univariate analysis of association between predictors and risk of glucose level ≥100 mg/dL
| Variable | Glucose level ≥100 mg/dL (n=317) | Glucose level <100 mg/dL (n=590) | Odds ratio (95% CI) | P valuea |
|---|---|---|---|---|
| BAT uptake, n (%) | ||||
| Yes | 8 (2.5) | 48 (8.1) | 3.4 (1.6–7.3) | Reference |
| No | 309 (97.5) | 542 (91.9) | 0.0007 | |
| Age (years) | ||||
| Mean (±SD) | 63 (±12) | 55 (±17) | 1.4b (1.3–1.6) | <0.0001 |
| Median (range) | 63 (26–90) | 57 (1–93) | ||
| Sex, n (%) | ||||
| Female | 118 (37.2) | 327 (55.4) | 2.1 (1.6–2.8) | Reference |
| Male | 199 (62.8) | 263 (44.6) | <0.0001 | |
| BMI, n (%) | ||||
| Underweight | 7 (2.2) | 46 (7.8) | Reference | |
| Normal weight | 90 (28.4) | 259 (43.9) | 2.3 (1.0–5.2) | 0.051 |
| Overweight | 119 (37.5) | 164 (27.8) | 4.8 (2.1–10.9) | 0.0002 |
| Obesity | 101 (31.9) | 121 (20.5) | 5.5 (2.4–12.7) | <0.0001 |
Univariate logistic regression with exact procedure when appropriate
Unit of 10 years
Table 2.
Multivariable analysis of association between predictors and risk of glucose level ≥100 mg/dL
| Variable | Odds ratio (95% CI) | P value |
|---|---|---|
| BAT uptake | ||
| No vs. yes | 1.6 (0.7–3.6) | 0.268 |
| Age (unit of 10 years) | 1.5 (1.3–1.6) | <0.0001 |
| Sex×BMI | Overall: 0.027 | |
| Female | ||
| Obesity vs. normal weight | 5.6 (3.1–9.9) | |
| Overweight vs.normal weight | 2.8 (1.6–5.2) | |
| Underweight vs. normal weight | 1.7 (0.7–4.6) | |
| Obesity vs. overweight | 1.9 (1.1–3.4) | |
| Obesity vs. underweight | 3.2 (1.2–8.3) | |
| Overweight vs. underweight | 1.6 (0.6–4.3) | |
| Male | ||
| Obesity vs. normal weight | 1.7 (1.0–2.8) | |
| Overweight vs. normal weight | 1.7 (1.1–2.7) | |
| Underweight vs. normal weight | NA | |
| Obesity vs. overweight | 1.0 (0.6–1.6) | |
| Obesity vs. underweight | NA | |
| Overweight vs. underweight | NA | |
NA not estimable due to the zero count of underweight having diabetes
To further explore the independent effect of the predictors, a multivariable regression analysis was performed with a stepwise variable selection procedure, which included the main effects and all relevant two-way interactions. After controlling for age, sex, and BMI, BAT uptake did not remain an independent predictor of risk of glucose level ≥100 mg/dL. Age (P< 0.0001), sex (P=0.941), BMI (P< 0.0001) and interaction of sex and BMI (P=0.025) were retained in the model by stepwise selection.
A final model was fitted by adding BAT uptake to the above model as it was of primary interest to determine the strength of the association of BAT with risk of glucose level ≥100 mg/dL after controlling for confounders. In this final multivariable analysis (Table 2), age remained significant with older patients more associated with risk of glucose level ≥100 mg/dL (odds ratio for unit of 10 years 1.5, 95% CI 1.3–1.6, P<0.0001). There was a significant interaction between sex and BMI, where the likelihood of having a glucose level ≥100 mg/dL was significantly higher in obese females than males (P for interaction=0.027; Table 2). A trend was observed toward higher risk of glucose level ≥100 mg/dL in patients without BAT (odd ratio=1.6, 95% CI=0.7–3.6; P=0.268), although not statistically significant after adjustment for age, sex, BMI, and significant interaction of sex and BMI.
The relationships between glucose level considered as a continuous variable and BAT uptake, age, sex, and BMI are shown in Table 3, with similar findings to those with glucose as a binary outcome measure. In the univariate analysis, higher glucose was associated with no BAT uptake (P=0.002), older age (P<0.0001), male sex (P<0.0001), and higher BMI (P<0.0001). The only statistically significant interaction effect was between sex and BMI (P=0.024). After controlling for other co-variates in the multivariable analysis, there was no longer a significant interaction between sex and BMI (P=0.095). As such, a final model was fitted to the data by removing the interaction term and including only the main effects. Higher levels of glucose were correlated with higher BMI (P<0.0001) and older age (P<0.0001). The adjusted means of glucose levels tended to be higher in patients without BAT uptake (least square mean 98.4 mg/dL, 95% CI 96.6–100.3) compared to those with BAT uptake (least square mean 94.0 mg/dL, 95% CI 88.2–99.8); however, this was not statistically significant after adjustment for age and BMI (P=0.14).
Table 3.
Effects of independent predictors on glucose level as a continuou outcome variable
| Independent Variable |
P value
|
||
|---|---|---|---|
| Univariate analysis | Multivariable analysisa | Final modelb | |
| BAT uptake | 0.002 | 0.497 | 0.140 |
| Age | <0.0001 | <0.0001 | <0.0001 |
| Sex | <0.0001 | 0.064 | - |
| BMI | <0.0001 | <0.0001 | <0.0001 |
| Sex×BMI | 0.024 | 0.095 | - |
Multivariable model included BAT uptake, age, sex, BMI, and the interaction of sex and BMI
Final model included BAT uptake, age, and BMI
Medication histories were available for 35 patients with BAT uptake (median time from medication history to PET/ CT scan is 9 days, range 0–169 days). For the control group without BAT uptake that was matched for age, sex, and BMI, median time between available medication history and PET/CT scan is 7 days (range 0–78 days). No patient (0%) with BAT uptake was on beta-blocker therapy, but eight of 73 (11%) in the control group were receiving beta-blocker therapy (P=0.05, Fisher’s exact test). Seven patients were taking systemic steroids (n=6 glucocorticoids, n=2 sex hormones), seven were taking inhaled glucocorticoids, and two were taking systemic sex hormones and inhaled glucocorticoids. The steroid usage patterns between those with and without BAT uptake were not significantly different in regards to overall frequency of steroid usage (P=0.15, Fisher’s exact test) and inhaled versus systemic steroids (P=0.29, Fisher’s exact test).
Discussion
Most studies evaluating BAT visualization on FDG PET and PET/CT have focused on BAT as a confounding factor for image interpretation [2, 3, 8, 19], but the advent of PET/CT alleviates most, but not all of the challenges differentiating co-existing hypermetabolism in BAT and tumor. The ability to visualize hypermetabolism in BAT on FDG PET/CT has perhaps, even more significant implications because animal studies have suggested a role of BAT in the development of obesity, insulin resistance and diabetes [13–16, 20, 21]. FDG PET/CT may be used as non-invasive imaging tool to study the role of BAT in adult humans.
In univariate analyses, blood glucose levels were lower in patients with active BAT on FDG PET/CT versus those without active BAT. Patients without BAT tended to a higher risk of glucose level ≥100 mg/dL with an attenuating strength, but this was not statistically significant after adjustment for other factors which did remain associated with higher glucose levels (age, sex, and BMI). All patients were required to have glucose levels <200 mg/dL prior to FDG PET imaging and it is possible that the range of glucose levels evaluated was not large enough to show the correlation between glucose and BAT uptake in the multivariable analyses.
Possibly, a more important explanation, and what we speculate to be true, may be that glucose, age, sex, BMI, and BAT uptake are too closely inter-related to separate their interactions. In our model, the effects of BAT uptake on glucose level appear to have been attenuated by the effects of age, sex and BMI. From our model, it appears that obese females without BAT are probably at the highest risk for higher glucose levels. These results, while similar to those of Cypess et al. [6], are not directly comparable because the outcome variable was BAT uptake, and not glucose level, in that study.
Although the available data thus far does not conclusively demonstrate causal relationships between BAT uptake, glucose levels, BMI, sex, and age, one interpretation is that the presence of active BAT is protective against diabetes and obesity. This interpretation is supported by substantial animal data showing that BAT-deficient mice develop obesity, hyperphagia, and insulin resistance in the absence of hypothalamic and serum corticosterone level abnormalities [13–16]. The relationship between BAT activity and serum glucose levels could be tested in prospective human studies using PET. It is quite possible the sample size we examined is simply too small to clearly show such a relationship.
Cypess et al. [6] recently showed that beta-blocker therapy was significantly associated with a lack of “activated” BAT on PET in a larger patient population [6]. In our study, no patient with BAT was on beta-blocker therapy; however, generalizing the results of this sub-group analysis to the entire cohort is limited by patient numbers. Other animal models of obesity with and without a co-existing diabetic phenotype suggest impairment in the BAT adrenergic system [20–22]. Stimulation of brown adipocyte precursors to express uncoupling protein in WAT by a beta3 adrenergic agonist has been shown to be effective anti-obesity and anti-diabetic treatment in obese fa/fa rats [22]. We speculate that functional BAT may contribute to a lean, non-diabetic human phenotype.
Williams and Kolodny [23] recently reported a lower frequency of BAT visualization on FDG PET/CT and lower glucose levels (just before PET) in patients prepared with high-fat, very low-carbohydrate diets (compared to patients who fasted). They suggested this may be due to an inhibitory effect of fatty acid loading on glucose metabolism. In contrast, animal data suggest that BAT is increased in rats fed a high-fat “cafeteria” diet and is involved in diet-induced thermogenesis [24]. A major difference is that the animals were studied after 3 weeks of exposure to the high-fat diet whereas the patients’ exposure was less than 24 h. Our patients were fasting for at least 4 h prior to PET/CT, but we did not prescribe a specific preparatory diet.
The major limitations of our study are its retrospective nature and lack of control groups which did not allow compilation of complete clinical histories of documented cases of diabetes. The fasting serum glucose at the time of PET imaging was felt to serve as a surrogate and potentially found additional cases of undiagnosed diabetes. Other potential confounding factors that could not be controlled for were anxious states, cold weather or other cold conditions (i.e., air conditioning). In addition, the patients included in this study were referred for FDG PET/CT to evaluate for malignancy, and they could have metabolic states different from healthy adults.
Our study does not provide conclusive evidence that BAT is protective for the development of diabetes or obesity; however, our results along with other studies [5–7, 12] support this possibility. Further studies in humans are needed and FDG PET/CT may prove to be a vital tool for this purpose. Gaining a better understanding of the role of BAT in humans has potentially tremendous implications—potentially providing new treatment options for two major healthcare issues, diabetes and obesity.
Footnotes
Significance: This paper demonstrates that FDG PET/CT may be a vital tool for further investigations of diabetes and obesity and the possibility that the presence of “detectable” BAT is protective against diabetes and obesity.
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.Rosen ED, Spiegelman BM. Molecular regulation of adipo-genesis. Annu Rev Cell Dev Biol. 2000;16:145–171. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- 2.Cohade C, Osman M, Pannu HK, Wahl RL. Uptake in supraclavicular area fat (“USA-Fat”): description on 18F-FDG PET/ CT. J Nucl Med. 2003;44:170–176. [PubMed] [Google Scholar]
- 3.Hany TF, Gharehpapagh E, Kamel EM, Buck A, Himms-Hagen J, von Schulthess GK. Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur J Nucl Med Mol Imaging. 2002;29:1393–1398. doi: 10.1007/s00259-002-0902-6. [DOI] [PubMed] [Google Scholar]
- 4.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 5.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 6.Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Virtanen KA, Lidell ME, Orava J, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 8.Bar-Shalom R, Gaitini D, Keidar Z, Israel O. Non-malignant FDG uptake in infradiaphragmatic adipose tissue: a new site of physiological tracer biodistribution characterised by PET/CT. Eur J Nucl Med Mol Imaging. 2004;31:1105–1113. doi: 10.1007/s00259-004-1506-0. [DOI] [PubMed] [Google Scholar]
- 9.Baba S, Engles JM, Huso DL, Ishimori T, Wahl RL. Comparison of uptake of multiple clinical radiotracers into brown adipose tissue under cold-stimulated and nonstimulated conditions. J Nucl Med. 2007;48:1715–1723. doi: 10.2967/jnumed.107.041715. [DOI] [PubMed] [Google Scholar]
- 10.Baba S, Tatsumi M, Ishimori T, Lilien DL, Engles JM, Wahl RL. Effect of nicotine and ephedrine on the accumulation of 18F-FDG in brown adipose tissue. J Nucl Med. 2007;48:981–986. doi: 10.2967/jnumed.106.039065. [DOI] [PubMed] [Google Scholar]
- 11.Tatsumi M, Engles JM, Ishimori T, Nicely O, Cohade C, Wahl RL. Intense (18)F-FDG uptake in brown fat can be reduced pharmacologically. J Nucl Med. 2004;45:1189–1193. [PubMed] [Google Scholar]
- 12.Cohade C, Mourtzikos KA, Wahl RL. “USA-Fat”: prevalence is related to ambient outdoor temperature-evaluation with 18F-FDG PET/ CT. J Nucl Med. 2003;44:1267–1270. [PubMed] [Google Scholar]
- 13.Hamann A, Benecke H, Le Marchand-Brustel Y, Susulic VS, Lowell BB, Flier JS. Characterization of insulin resistance and NIDDM in transgenic mice with reduced brown fat. Diabetes. 1995;44:1266–1273. doi: 10.2337/diab.44.11.1266. [DOI] [PubMed] [Google Scholar]
- 14.Hamann A, Flier JS, Lowell BB. Decreased brown fat markedly enhances susceptibility to diet-induced obesity, diabetes, and hyper-lipidemia. Endocrinology. 1996;137:21–29. doi: 10.1210/endo.137.1.8536614. [DOI] [PubMed] [Google Scholar]
- 15.Lowell BB, Susulic V, Hamann A, et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366:740–742. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- 16.Ortmann S, Prinzler J, Klaus S. Self-selected macronutrient diet affects energy and glucose metabolism in brown fat-ablated mice. Obes Res. 2003;11:1536–1544. doi: 10.1038/oby.2003.205. [DOI] [PubMed] [Google Scholar]
- 17.Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diab Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 18.National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. Obes Res. 1998;6:51 S–209 S. [PubMed] [Google Scholar]
- 19.Yeung HW, Grewal RK, Gonen M, Schoder H, Larson SM. Patterns of (18)F-FDG uptake in adipose tissue and muscle: a potential source of false positives for PET. J Nucl Med. 2003;44:1789–1796. [PubMed] [Google Scholar]
- 20.Collins S, Daniel KW, Rohlfs EM, Ramkumar V, Taylor IL, Gettys TW. Impaired expression and functional activity of the beta 3- and beta 1-adrenergic receptors in adipose tissue of congenitally obese (C57BL/6 J ob/ob) mice. Mol Endocrinol. 1994;8:518–527. doi: 10.1210/mend.8.4.7914350. [DOI] [PubMed] [Google Scholar]
- 21.Marette A, Mauriege P, Despres JP, Tulp OL, Bukowiecki LJ. Norepinephrine- and insulin-resistant glucose transport in brown adipocytes from diabetic SHR/N-cp rats. Am J Physiol. 1993;265:R577–R583. doi: 10.1152/ajpregu.1993.265.3.R577. [DOI] [PubMed] [Google Scholar]
- 22.Ghorbani M, Himms-Hagen J. Appearance of brown adipocytes in white adipose tissue during CL 316, 243-induced reversal of obesity and diabetes in Zucker fa/fa rats. Int J Obes Relat Metab Disord. 1997;21:465–475. doi: 10.1038/sj.ijo.0800432. [DOI] [PubMed] [Google Scholar]
- 23.Williams G, Kolodny GM. Methods for decreasing uptake of 18F-FDG by hypermetabolic brown adipose tissue on PET. AJR Am J Roentgenol. 2008;190:1406–1409. doi: 10.2214/AJR.07.3205. [DOI] [PubMed] [Google Scholar]
- 24.Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979;281:31–35. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]