Abstract
Objective
Prostate cancer is a disease of older men. Weekly docetaxel (DPq1w) is often favored over the standard three-weekly regimen (DPq3w) due to concerns about safety and tolerability in this population.
Materials and Methods
Two subgroup analyses of TAX 327 were conducted. Among patients receiving DPq3w, tolerability and efficacy were compared between three age groups: <65, 65–74 and ≥75 years. For men ≥75 years, these outcomes were compared between DPq3w, DPq1w, and mitoxantrone (MP) arms. Tolerability outcomes included dose delivery, grade 3/4 adverse events and quality of life. Efficacy outcomes included overall survival and tumor response.
Results
Of 1006 men with metastatic castrate-resistant prostate cancer (mCRPC) in the trial, 335 received DPq3w. Among these, 20% were age ≥75 years. For DPq3w, there were non-significant associations of worse tolerability and efficacy with advancing age. Twenty-eight percent of men age ≥75 years had an objective pain response, compared to 38% and 34% of patients 65–74 and <65 years, respectively. There were no significant differences in prostate-specific antigen (PSA) response (43–48%, p = 0.74) or measurable tumor response (7–17%, p = 0.30) according to age. Among men ≥75 years, DPq3w resulted in more dose reductions than DPq1w (22% versus 8%, p = 0.007), but tolerability was otherwise comparable. Both were associated with more favorable efficacy than mitoxantrone.
Conclusions
Tolerability and efficacy of DPq3w appear less favorable with advancing age. Compared to DPq1w, DPq3w is associated with better survival outcomes, but similar tolerability, and remains the standard first-line chemotherapy option in mCRPC. Toxicity is substantial, therefore careful patient selection, close monitoring and early management of toxicities is advised.
Keywords: Prostate cancer, Elderly, Docetaxel
1. Introduction
Prostate cancer affects older patients disproportionately, with a peak incidence at 70–74 years, and 25% aged ≥75 years at diagnosis.1 For men with metastatic castrate-resistant prostate cancer (mCRPC), docetaxel is a standard first-line treatment based on two pivotal phase III studies.2,3 The South West Oncology Group (SWOG 9916) and TAX 327 studies showed a 2–3 month improvement in overall survival (OS) for docetaxel-based chemotherapy as compared with mitoxantrone and prednisone (MP). Furthermore, both the 3-weekly and weekly docetaxel and prednisone regimens (DPq3w and DPq1w, respectively) evaluated in TAX 327 led to a greater improvement in quality of life as compared with MP.3 These improvements appeared to be independent of age, since an updated survival analysis of TAX 327 demonstrated a benefit in OS of similar magnitude in men age ≤68 and ≥69 years.4 Similar results were found using a cutoff of 75 years.4
The toxicity profile of DPq3w and DPq1w differed in TAX 327, with DPq1w being less myelosuppressive, but with no substantial difference in non-hematologic events in the overall population.3 Tolerability of docetaxel in older men was not reported independently. Observational data, however, suggest that older men have a high probability of substantial toxicity, including infection and diarrhea, when treated with DPq3w or DPq1w.5,6
Against this background, we report a retrospective analysis of tolerability and efficacy of chemotherapy in older patients in TAX 327. We hypothesized that MP and/or DPq1w might display better safety and tolerability than DPq3w for older patients in this retrospective analysis.
2. Materials and Methods
Full protocol details and results from TAX 327 have been reported previously.3 In TAX 327, 1006 men with mCRPC were randomly assigned to DPq3w (docetaxel 75 mg/m2 every 3 weeks; n = 315), DPq1w (docetaxel 35 mg/m2 weekly; n = 334) or MP (mitoxantrone 12 mg/m2 every 3 weeks; n = 337), each with prednisone 5 mg twice daily. Participating institutions received approval from their ethics review boards, and patients provided written informed consent. There was no upper age limit for inclusion, and patients with a Karnofsky performance status (PS) of ≥60 were eligible.
2.1. Age-Specific
Analyses Our analysis is based on three age groups: <65, 65–74 and ≥75 years, chosen based on commonly used age strata in published literature. Tolerability and efficacy outcomes with DPq3w were compared between these three groups. For men ≥75 years, the same outcomes were compared between the three randomized treatment arms (DPq3w, DPq1w, and MP).
2.2. Outcome Variables
Tolerability was determined by dose reductions, discontinuations due to adverse events and delays in treatment administration. Adverse events in TAX 327 were assessed using the National Cancer Institute Common Toxicity Criteria (version 2). Previous observational studies identified diarrhea, infection, fever, weight loss and dehydration as adverse events of potential concern.5 Therefore, these adverse events were a priori identified and statistically compared between the three age groups in men treated with DPq3w and in men ≥75 years treated with the different regimens.
Quality of life (QoL) in TAX 327 was assessed with the Functional Assessment of Cancer Therapy—Prostate (FACT-P) questionnaire.7 FACT-P consists of the 27-itemFACT-G (general) questionnaire and a 12-item prostate-specific concern subscale (PSC). The FACT-P Trial Outcome Index (TOI) is based on the physical and functional well-being subscales of the FACT-G and PSC. Clinically meaningful improvements in these QoL scales have been defined previously as an increase in score of: ≥6 points for the FACT-P total score, ≥5 points for FACT-P TOI, ≥2 points for FACT-P PSC, on two measurements obtained three weeks apart.8 These values were thus used in this age-specific analysis.
Efficacy outcomes included OS, prostate-specific antigen (PSA) response (a reduction of ≥50% maintained for ≥3 weeks), and objective tumor response.
2.3. Co-morbidities
Complete co-morbidity data were not available. In an exploratory analysis, using a comprehensive list of concomitant medications at randomization, categories of co-morbidity were generated and both mean number of medications and a weighted chronic disease score (CDS)9 were calculated. This was compared between age groups for DPq3w and between treatment groups for men ≥75 years.
2.4. Statistical Methods
Cox proportional hazards, χ2 and Kruskal–Wallis tests were used to test for statistical significance between age groups for DPq3w and between treatments for men ≥75 years. All analyses were performed in SAS v9.1 (SAS Institute, Cary, NC). All tests were two-sided and a p value of ≤0.05 was considered statistically significant. No adjustment for multiple analyses was performed.
3. Results
Between March 2000 and June 2002, 1006 men with mCRPC participated in TAX 327. Of the 335 patients assigned to DPq3w, 126 (38%) were <65 years, 141 (42%) were 65 to 74 years and 68 (20%) were ≥75 years. In the overall population, 207 (20%) patients were ≥75 years. Of these, 68 (33%) were treated with DPq3w, 71 (34%) with DPq1w and 68 (33%) with MP.
4. Outcomes by Age
ForDPq3w, baseline characteristics are summarized in Table 1A. Men ≥75 years were more likely to have poorer PS (<80), compared to those age 65–74 and <65 years (18% vs. 16% vs. 6%, respectively),more visceral disease (27%vs. 24%vs. 18%) and higher median PSA levels (162 vs. 129 vs. 98 ng/ml).
Table 1.
| A – Patient characteristics at baseline. Patients treated with DPq3w. | ||||||
|---|---|---|---|---|---|---|
| DPq3w | ||||||
| <65 y (n = 126) |
65–74 y (n = 141) |
=75 y (n = 68) |
||||
| n | % | N | % | n | % | |
| KPS | ||||||
| 60–70 | 7 | 6 | 23 | 16 | 12 | 18 |
| ≥80 | 119 | 94 | 118 | 84 | 56 | 82 |
| Gleason score | ||||||
| ≤7 | 51 | 41 | 63 | 45 | 28 | 41 |
| 8–10 | 48 | 38 | 31 | 22 | 26 | 38 |
| Not available | 27 | 21 | 47 | 33 | 14 | 21 |
| Stage at diagnosis | ||||||
| II + III | 30 | 24 | 52 | 37 | 32 | 47 |
| IV | 86 | 68 | 79 | 56 | 27 | 40 |
| NA | 10 | 8 | 10 | 7 | 9 | 12 |
| Prior treatment | ||||||
| Radiotherapy | 66 | 52 | 75 | 53 | 34 | 50 |
| Estramustine | 22 | 18 | 31 | 22 | 11 | 16 |
| Serum PSA | ||||||
| Median (ng/ml) | 98 | 129 | 162 | |||
| ≥20 ng/ml; (%) | 103 | 82 | 125 | 89 | 63 | 93 |
| Extent of disease | ||||||
| Bone metastases | 114 | 91 | 127 | 90 | 62 | 91 |
| Visceral disease | 23 | 18 | 34 | 24 | 18 | 27 |
| Evidence of progression at entry (%) | ||||||
| Bone scan | 87 | 69 | 97 | 69 | 54 | 79 |
| ↑(non) measurable lesion | 44 | 35 | 62 | 44 | 33 | 49 |
| ↑PSA | 90 | 71 | 98 | 70 | 53 | 78 |
| Treatment after discontinuation | ||||||
| Chemotherapy | 84 | 67 | 82 | 58 | 33 | 49 |
| Other | 33 | 26 | 40 | 28 | 25 | 37 |
| None | 9 | 7 | 19 | 14 | 10 | 15 |
| B – Patient characteristics at baseline. Patients ≥75 years. | ||||||
|---|---|---|---|---|---|---|
| ≥75 years | ||||||
| MP (n = 68) |
DPq1W (n = 71) |
DPq3W (n = 68) |
||||
| n | % | N | % | n | % | |
| KPSa | ||||||
| 60–70 | 9 | 13 | 9 | 13 | 12 | 18 |
| ≥80 | 59 | 87 | 62 | 87 | 56 | 82 |
| Gleason score | ||||||
| ≤7 | 35 | 52 | 34 | 48 | 28 | 41 |
| 8–10 | 10 | 15 | 20 | 28 | 26 | 38 |
| Not available | 23 | 34 | 17 | 24 | 14 | 21 |
| Stage at diagnosis | ||||||
| II + III | 27 | 40 | 28 | 39 | 32 | 47 |
| IV | 34 | 50 | 27 | 38 | 27 | 40 |
| NA | 7 | 10 | 16 | 23 | 9 | 12 |
| Prior treatment | ||||||
| Radiotherapy | 35 | 52 | 33 | 47 | 34 | 50 |
| Estramustine | 8 | 12 | 7 | 10 | 11 | 16 |
| Serum PSAb | ||||||
| Median (ng/ml) | 101 | 111 | 162 | |||
| ≥20 ng/ml; (%) | 62 | 91 | 62 | 87 | 63 | 93 |
| Extent of diseasec | ||||||
| Bone metastases | 61 | 90 | 67 | 94 | 62 | 91 |
| Visceral disease | 18 | 27 | 20 | 28 | 18 | 27 |
| Evidence of progression at entry (%) | ||||||
| Bone scan | 48 | 71 | 50 | 70 | 54 | 79 |
| ↑(non) measurable lesion | 31 | 46 | 38 | 53 | 33 | 49 |
| ↑PSA | 49 | 72 | 46 | 66 | 53 | 78 |
| Treatment after discontinuation | ||||||
| Chemotherapy | 36 | 53 | 40 | 56 | 33 | 49 |
| Other | 19 | 28 | 24 | 34 | 25 | 37 |
| None | 13 | 19 | 7 | 10 | 10 | 15 |
Abbreviations: y: years; DPq3w: three-weekly docetaxel/prednisone; MP: mitoxantrone/prednisone; DPq1w: weekly docetaxel/prednisone; KPS = Karnofsky Performance Status; NA = not applicable; PSA = Prostate Specific Antigen.
Baseline KPS ≥80 in the MP and DPq1W groups, 86% and 88%, respectively, in patients <75 years.
Baseline serum PSA ≥20 ng/ml in the MP and DPq1W groups, 84% and 88%, respectively in patients <75 years.
Visceral disease in the MP and DPq1W groups, 21% and 22%, respectively in patients <75 years.
4.1. Treatment Delivery
There were non-significant associations of lower drug exposure in patients ≥75 years compared to those age 65–74 and <65 years (Table 2). The median number of cycles of DPq3w was 7 cycles, 10 cycles, and 10 cycles, respectively (p = 0.22), and duration of treatment was 18.7, 26.7, and 27.0 weeks, respectively (p = 0.19). The proportion of patients with dose reductions increased with increasing age (Table 2), while dose delays were similar in the 65–74 and ≥75 age groups and lowest in those <65 years. Nineteen percent of patients ≥75 years stopped DPq3w early due to adverse events, compared with 3% of those <65 years (p = 0.23).
Table 2.
Efficacy outcomes and treatment tolerability by age group for DPq3w.
| <65 yrs | 65–74 yrs | ≥75 yrs | p | |
|---|---|---|---|---|
| Efficacy end point | ||||
| Pain | ||||
| No. evaluated (n) | 59 | 69 | 25 | |
| Response rate (n/%) | 20 (34) | 26 (38) | 7 (28) | 0.69 |
| Median duration (months) | 4.9 | 2.7 | 2.8 | 0.90 |
| ≥50% reduction in PSA | ||||
| No. evaluated (n) | 103 | 125 | 63 | |
| Response rate (n/%) | 45 (44) | 60 (48) | 27 (43) | 0.74 |
| Median duration (months) | 8.8 | 7.2 | 10.3 | 0.22 |
| Tumor response | ||||
| No. evaluated | 54 | 56 | 31 | |
| Response rate (n/%) | 9 (17) | 4 (7) | 4 (13) | 0.30 |
| Overall survival | ||||
| Median (months) | 20.4 | 18.6 | 18.9 | 0.53 |
| 1 year (%) | 76 | 74 | 68 | |
| Treatment intensity | ||||
| Drug exposure | ||||
| Median no. of cycles | 10 | 10 | 7 | 0.22 |
| Median duration of | 27 | 26.7 | 18.7 | 0.19 |
| Rx (wks) | ||||
| No. (%) with dose reductions | 9 (7) | 17 (12) | 15 (22) | 0.010 |
| >1 reductions | 0 | 2 (1) | 1 (2) | |
| No. (%) with cycle delays | 27 (21) | 36 (26) | 17 (25) | 0.72 |
| >1 cycles delayed | 3 (2) | 8 (6) | 4 (6) | |
| Dose discontinuation | ||||
| Completed Rx | 62 (49) | 67 (48) | 25 (37) | 0.23 |
| Disease progression | 58 (46) | 46 (33) | 23 (34) | |
| Adverse event | 4 (3) | 19 (14) | 13 (19) | |
| Death | 0 | 3 (2) | 1 (2) | |
| Other | 2 (2) | 6 (4) | 6 (9) |
P value of ≤ 0.05 was considered statistically significant.
Abbreviations: yrs: years; wks: weeks; PSA = Prostate Specific Antigen; Rx = treatment.
4.2. Quality of Life
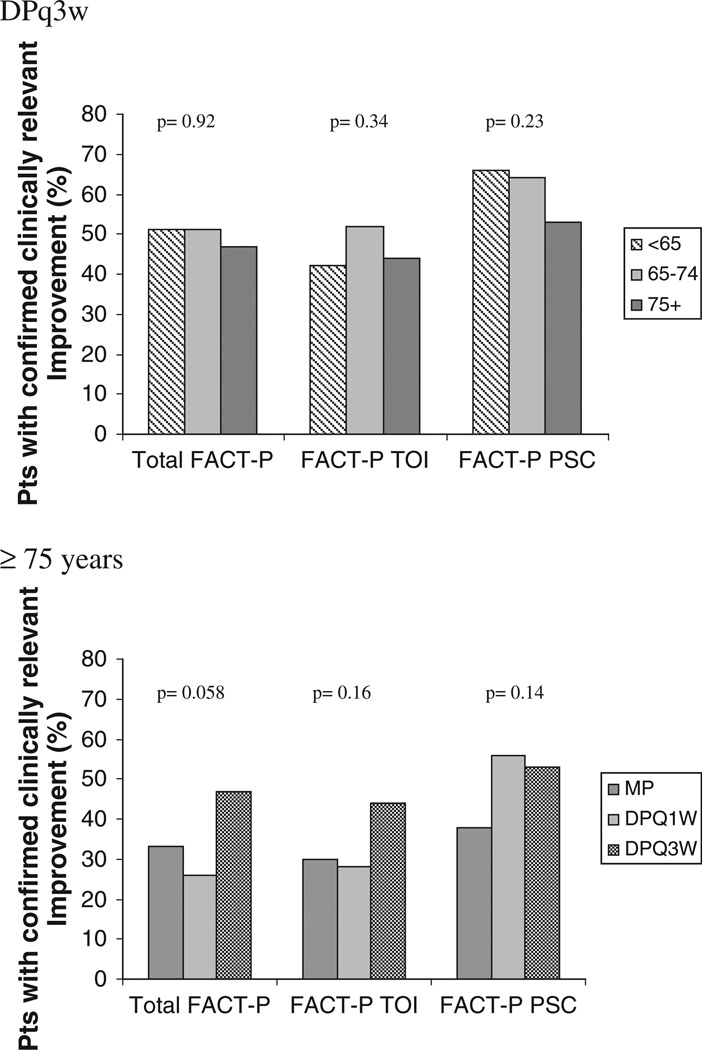
Similar proportions of older and younger patients had improvements in the overall FACT-P, and the FACT-P TOI (Fig. 1). Although the proportion reporting improvement in the prostate-specific concern (PSC) subscale was numerically smaller in patients ≥75 years compared to those 65–74 and <65 years, this was not statistically significant (53% vs 64% vs 66%, p = 0.23).
Fig. 1.
Improvement rates for quality of life and disease-related symptoms in the evaluable population.
4.3. Toxicity
Grade 3–4 events were uncommon, although there was a trend toward increasing frequency with increasing age (Table 3). Non-hematological toxicities were more common, with grade 3–4 fatigue in 10%, 4% and 2% of men aged ≥75, 65–74 and <65 years respectively. Diarrhea was common across all age categories, although the incidence of grade 3–4 diarrhea was <3% in all age groups. All grade weight loss and infection occurred with increasing frequency with increasing age. Five patients died secondary to drug-related toxicity, and all were ≥70 years, one ofwhomreceivedDPq3w, three received MP, and one received DPq1w.
Table 3.
Treatment-related adverse events for DPq3w (occurring in ≥5% of subjects in any treatment group).
| DPq3W | |||||||
|---|---|---|---|---|---|---|---|
| Toxicity | <65 (n = 126) | 65–74 (n = 141) | ≥75 (n = 68) | pa any grade |
|||
| All grades | Grades 3–4 | All grades | Grades 3–4 | All grades | Grades 3–4 | ||
| Diarrhea | 37 (30) | 2 (2) | 41(29) | 3 (2) | 27 (40) | 2 (3) | 0.25 |
| Infection | 30 (24) | 5 (4) | 49 (35) | 8 (6) | 28 (42) | 6 (9) | 0.030 |
| Fever | 10 (8) | 14 (10) | 12 (18) | 0.85 | |||
| Weight loss | 6 (5) | 18 (13) | 14 (21) | 0.003 | |||
| Dehydration | 3 (2) | 7 (5) | 6 (9) | 0.13 | |||
| Fatigue | 64 (52) | 2 (2) | 75 (54) | 6 (4) | 38 (57) | 7 (10) | |
| Neutrophils | 1 (1) | 1 (1) | 7 (5) | 7 (5) | 6 (9) | 5 (8) | |
| Bone pain | 42 (34) | 12 (10) | 43 (31) | 10 (7) | 16 (24) | 4 (6) | |
| Musculoskeletal | 10 (8) | 4 (3) | 13 (6) | 1 (1) | 7(10) | 1 (2) | |
| Nausea | 49 (40) | 60 (43) | 27 (40) | ||||
| Neuropathy | 40 (32) | 41(29) | 20 (30) | ||||
| Constipation | 30 (24) | 33 (24) | 21 (31) | ||||
| Vomiting | 21 (17) | 25 (18) | 10 (15) | ||||
| Edema | 21 (17) | 22 (16) | 21 (31) | ||||
| Anorexia | 13 (10) | 23 (16) | 20 (30) | ||||
| Hypotension | 2 (2) | 4 (3) | 7 (10) | ||||
| Stomatitis | 23 (19) | 25 (18) | 17 (25) | ||||
| Cardiac LVF | 11 (9) | 15 (11) | 6 (9) | ||||
P value of ≤ 0.05 was considered statistically significant.
Abbreviations: DPq3W: three-weekly docetaxel/prednisone; LVF: left ventricular function.
Percent of patients is indicated in parentheses.
Only adverse events identified a priori were statistically compared.
4.4. Response and Overall Survival
Twenty-eight percent of patients ≥75 years had an objective pain response, with a median duration of 2.8 months, compared to 38% and 34% of 65–74 and <65 years, respectively, and median durations of 2.7 and 4.9 months (Table 2). There were no significant differences in PSA response (43–48%, p = 0.74) or measurable tumor response (7–17%, p = 0.30) according to age. Median and 1-year OS were modestly lower with increased age, 18.9 vs 18.6 vs 20.4 months, and 68% vs 74% vs 76% for ≥75, 65–74 and <65 years, respectively (p = 0.53).
4.5. Co-morbidity
There was no difference (p = 0.13) in the number of medications for DPq3w, 4.7 (standard deviation (SD) 3.0), 4.6 (SD 2.7), and 4.2 (SD 3.2) respectively for ≥75, 65–74 and <65 years. However, older patients had a higher CDS score in the DPq3w group, largely driven by cardiac, hypertension, ulcer and cholesterol medications (p < 0.001) [Table 6].
Table 6.
Adapted chronic disease score.
| DPq3w | ≥75 years | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <65 (n = 126) |
65–74 (n = 141) |
≥75 (n = 68) |
MP (n = 68) |
DPq1W (n = 71) |
DPq3W (n = 68) |
|||||||
| Medication Category | n | % | N | % | n | % | n | % | n | % | n | % |
| Cardiac | 19 | 15.1 | 39 | 27.7 | 24 | 35.3 | 24 | 35.3 | 29 | 40.9 | 25 | 36.8 |
| Respiratory | 4 | 3.2 | 2 | 1.4 | 5 | 7.4 | 5 | 7.4 | 2 | 2.8 | 6 | 8.8 |
| Hypertension | 19 | 15.1 | 53 | 37.6 | 27 | 39.7 | 27 | 39.7 | 25 | 35.2 | 21 | 30.9 |
| Diabetes | 10 | 7.9 | 7 | 5.0 | 3 | 4.4 | 3 | 4.4 | 4 | 5.6 | 3 | 4.4 |
| Ulcers | 13 | 10.3 | 20 | 14.2 | 17 | 25.0 | 17 | 25.0 | 7 | 9.9 | 3 | 4.4 |
| Gout | 2 | 1.6 | 6 | 4.3 | 4 | 5.9 | 4 | 5.9 | 2 | 2.8 | 3 | 4.4 |
| Cholesterol | 9 | 7.1 | 14 | 9.9 | 8 | 11.8 | 8 | 11.8 | 7 | 9.9 | 8 | 11.8 |
| Mean (SD) CDSa | 1.3 | (2.1) | 2.1 | (2.3) | 2.7 | (2.5) | 2.7 | (2.5) | 2.6 | (2.4) | 2.3 | (2.5) |
Abbreviations: DPq3w: three weekly docetaxel/prednisone; DPq1w: weekly docetaxel/prednisone; MP: mitoxantrone/prednisone; CDS: chronic disease score.
CDS score was significantly different between age groups among men receiving DPq3W (p < 0.001). However, no significant difference was observed between treatment arms amongst men ≥75 (p = 0.54).
5. Outcomes by Treatment Group
For patients ≥75 years, baseline characteristics were well-balanced among the three treatment groups (Table 1B).
5.1. Treatment Delivery
There was little difference in the duration of therapy between patients treated with DPq3w and DPq1w (18.7 and 19.3 weeks, respectively), however this was significantly longer than for MP (12.1 weeks), p = 0.014. Less than 10% of patients in the MP and DPq1w groups required dose reductions compared to 22% of those treated with DPq3w (p = 0.007). Nineteen percent of patients required dose discontinuation due to toxicity in the DPq3w group, compared to 23% and 12% in the DPq1w and MP groups respectively, p = 0.017 (Table 4).
Table 4.
Efficacy Outcomes and Treatment Tolerability by Treatment Groups in ≥75 years.
| MP | DPq1w | DPq3w | p | |
|---|---|---|---|---|
| Efficacy end point | ||||
| Pain (n evaluable) | 27 | 32 | 25 | |
| Response rate (n/%) | 2 (7) | 14 (44) | 7 (28) | 0.007 |
| Median duration (months) | 4 | 6 | 3 | 0.94 |
| ≥50% reduction in PSA (n evaluable) | 62 | 62 | 63 | |
| Response rate (n/%) | 20 (32) | 24 (39) | 27 (43) | 0.48 |
| Median duration (months) | NR | 9 | 10 | 0.93 |
| Tumor response (n evaluable) | 24 | 34 | 31 | |
| Response rate (n/%) | 2 (8) | 2 (6) | 4 (13) | 0.66 |
| Overall survival | ||||
| Median (months) | 12.5 | 16.1 | 18.9 | 0.29 |
| 1 year (%) | 57 | 59 | 68 | |
| Treatment intensity | ||||
| Drug exposure | ||||
| Median no. of cycles | 5 | 4 | 7 | NC |
| Median duration of Rx (wks) | 12 | 19 | 19 | 0.014 |
| No. (%) with dose reductions | 4 (6) | 6 (8) | 15 (22) | 0.007 |
| No. (%) with cycle delays | 14 (21) | 15 (21) | 17 (25) | 0.83 |
| Dose discontinuation | ||||
| Completed Rx | 11 (16) | 16 (23) | 25 (37) | 0.017 |
| Disease progression | 39 (57) | 24 (34) | 23 (34) | |
| Adverse event | 8 (12) | 16 (23) | 13 (19) | |
| Death | 5 (7) | 2 (3) | 1 (2) | |
| Other | 1 (2) | 1 (1) | 1 (2) |
P value of ≤ 0.05 was considered statistically significant.
Abbreviations: MP: mitoxantrone/prednisone; DPq1w: weekly docetaxel/prednisone; DPq3w: three-weekly docetaxel/prednisone; wks: weeks; NR: not reached; NC: not comparable.
5.2. Quality of Life
Quality of life outcomes favored DPq3w, coming close to statistical significance for the Total FACT-P scale (p = 0.06, Fig. 1).
5.3. Toxicity
The toxicity profile was similar in the DPq1w and DPq3w arms, with diarrhea and fatigue being two of the most common adverse events seen (Table 5). While the incidence was significantly higher than with MP, there was little difference in the frequency of grade 3–4 events. All other toxicities were more common in the docetaxel arms compared with MP apart from impairment in left ventricular function.
Table 5.
Treatment-related adverse events per treatment group for patients ≥75 years (occurring in ≥5% of subjects in any treatment group).
| ≥75 years | |||||||
|---|---|---|---|---|---|---|---|
| MP (n = 68) | DPq1W (n = 71) | DPq3W (n = 68) | p a any grade | ||||
| Toxicity | All grades | Grades 3–4 | All grades | Grades 3–4 | All grades | Grades 3–4 | |
| Fever | 9 (13) | 2 (3) | 12 (18) | 0.015 | |||
| Weight loss | 11 (16) | 12 (17) | 14 (21) | 0.74 | |||
| Dehydration | 1 (2) | 5 (7) | 6 (9) | 0.15 | |||
| Diarrhea | 7 (10) | 0 (0) | 30 (42) | 4 (6) | 27 (40) | 2 (3) | <0.001 |
| Infection | 14 (21) | 7 (10) | 32 (45) | 5 (7) | 28 (42) | 6 (9) | 0.005 |
| Fatigue | 24 (35) | 5 (7) | 42 (59) | 8 (11) | 38 (57) | 7 (10) | |
| Neutrophils | 5 (7) | 1 (2) | 2 (3) | 2 (3) | 6 (9) | 5 (8) | |
| Bone pain | 26 (38) | 10 (15) | 15 (21) | 5 (7) | 16 (24) | 4 (6) | |
| Musculoskeletal | 3 (4) | 0 (0) | 9 (13) | 4 (6) | 7 (10) | 1 (2) | |
| Nausea | 20 (29) | 30 (42) | 27 (40) | ||||
| Neuropathy | 4 (6) | 17 (24) | 20 (30) | ||||
| Constipation | 8 (12) | 18 (25) | 21 (31) | ||||
| Vomiting | 10 (15) | 19 (27) | 10 (15) | ||||
| Edema | 0 (0) | 14 (20) | 21 (31) | ||||
| Anorexia | 11 (16) | 25 (35) | 20 (30) | ||||
| Hypotension | 2 (3) | 5 (7) | 7 (10) | ||||
| Stomatitis | 8 (12) | 16 (23) | 17 (25) | ||||
| Cardiac LVF | 17 (25) | 5 (7) | 6 (9) | ||||
P value of ≤ 0.05 was considered statistically significant.
Abbreviations: MP: mitoxantrone/prednisone; DPq1W: weekly docetaxel/prednisone; DPq3W: three-weekly docetaxel/prednisone; LVF: left ventricular function.
Percent of patients is indicated in parentheses.
Only adverse events identified a priori were statistically compared.
5.4. Response and Overall Survival
The rates of objective pain response were greatest with DPq1w (44%), compared with either MP (7.4%) or DPq3w (28%), p = 0.007. Although not statistically significant for any outcome, rates of tumor response, ≥50% PSA reduction and duration of reduction, and OS estimates were greatest for patients treated with DPq3w (Table 4). Median OS was 18.9, 16.1 and 12.5 months (p = 0.29) for DPq3w, DPq1w and MP respectively.
5.5. Co-morbidity
The number of medications per treatment group was well-balanced (p = 0.48), 4.6 (SD2.9), 5.3 (SD3.4) and 4.7 (SD3.0) for MP, DPq1w and DPq3w, respectively. Using the CDS as a surrogate for co-morbidity, no significant difference was observed between treatment groups among men ≥75 years (p = 0.54).
6. Discussion
As men with mCRPC often present at an older age, the impact of age on tolerability and efficacy of docetaxel-based treatment is important. In this retrospective analysis of TAX 327, DPq3w remains the preferable treatment option in fit elderly men with better survival outcomes compared to either DPq1w or MP. When treated with DPq3w older men should be closely monitored for toxicity.
Although older men treated with DPq3w had worse baseline prognostic factors and more often had dose reductions and discontinuation compared to younger men, efficacy was comparable in both groups. Despite higher toxicity of DPq3w, a similar proportion of older and younger men had improvements in QoL; this may be due to higher disease burden and more symptomatic disease in older men at baseline.
In daily practice DPq1w is often prescribed for older men, with the assumption that it is better tolerated than DPq3w. In TAX 327 there was little difference in tolerability in older men treated with DPq1w or DPq3w (Table 5). This is consistent with results of recent studies that have explored lower dosing of docetaxel (30 mg/m2 weekly).6,10 A review of patients treated with three-weekly (n = 95) or weekly (n = 80) docetaxel reported a greater percentage of treatment discontinuation with the weekly regimen (30 versus 8%),6 challenging the belief that weekly regimens are more suitable for frail patients. However, patients on the weekly regimen were older and in poorer general health than patients on the standard schedule. A pooled analysis of two phase II trials with weekly docetaxel in men less than and greater than age 70 with mCRPC (n = 86) confirmed equivalence.10 There were no significant differences in grade 3–4 toxicities, but grade 3+ non-hematologic toxicity was high at 46%. Shepard et al. reported their experience with three-weekly docetaxel in patients ≥74 years5. Dose reductions were required in 23%, 34% were hospitalized for toxicity and docetaxel was discontinued for toxicity in 27%. The pharmacokinetic profile of three-weekly docetaxel is also reported as similar for older and younger patients.11 The feasibility of docetaxel-based chemotherapy in frail men with mCRPC is being evaluated in GERICO-10, a phase II randomized trial comparing adjusted doses of DPq3w and DPq1w.1
Our data show that docetaxel carries a substantial risk of toxicity in older patients. Various methods have been studied to identify risk factors for toxicity in the elderly. Hurria et al. reported risk stratification incorporating age, tumor type, chemotherapy, laboratory and geriatric assessment variables.12 This study (n = 500) supports the view that PS is not representative of health status in the elderly.13 The variables used in the model are easily measurable, but the study included a heterogeneous population and results are yet to be validated. Other studies have assessed the potential of a comprehensive geriatric assessments (CGA) to identify predictive and prognostic factors14,15 and have proven to detect otherwise unknown problems.16,17 Variables not routinely measured in oncology practice have shown to be associated with increased toxicity (e.g. depression, functional dependency) and poorer prognosis (e.g. mental health, geriatric syndromes).18,19 The International Society of Geriatric Oncology (SIOG) Prostate Cancer Working Group advocates adapting treatment to PS, co-morbidities and nutritional status, again supporting the use of a CGA.20 However, these assessments are time-consuming, resource intensive and often difficult to interpret in directing treatment modifications.17 Most patients in TAX 327 had a good PS, and the additional value of CGA in a fit older population is not clear. Describing the population with traditional baseline measures including medication use (as a measure of co-morbidity) with PS provides a reasonable description of our population.
With the high rates of toxicity seen in older patients in TAX 327, prevention should be prioritized. The National Comprehensive Cancer Network (NCCN) guidelines regard patients >65 years as high risk for neutropenia and should be considered for primary prophylaxis with myeloid stimulating factors (G-CSF).21 In TAX 327, G-CSF was only used following prolonged- or febrile-neutropenia. In the overall population, 7%, 0% and 3% received G-CSF inDPq3w, DPq1w and MP groups, respectively. The high rates of infection seen in older patients in TAX 327 provide some support for primary prophylaxis, particularly ≥75 years. However, this should be individualized as DPq3W is generally associated with a low risk of febrile neutropenia and G-CSF is expensive and associated with toxicity.
This analysis has limitations. First, this is an unplanned, retrospective, subgroup analysis and therefore, conclusions should be interpreted cautiously. Older men enrolled into randomized trials usually have less co-morbidity than those in everyday practice. Findings from our analysis may thus be applicable only to fit older patients. Second, the analysis of efficacy and tolerability of DPq3w involved case matching of subgroups from a larger randomized trial and this could introduce bias. Thirdly, the number of patients aged ≥75 years was relatively small, thereby reducing the statistical power of our results. Finally, a comprehensive list of co-morbidities was not available. In an effort to quantify baseline health status, we reviewed the medications and generated a CDS score. This confirmed that older patients receiving DPq3w had greater co-morbidity than younger patients, which may contribute to the increased toxicity seen.
Given the negative results of phase 3 trials investigating docetaxel combinations over the past 8 years, DPq3w 75 mg/m2 with prednisone 10 mg/day has remained the standard treatment of mCRPC. In this analysis, 20% of patients are ≥75 years and thus these data provide meaningful information. DPq3w remains the preferable treatment option in older men, based on efficacy outcomes and comparable tolerability to DPq1w, and superior efficacy to MP. It is important to highlight, however, that there are increasing options available to patients for the treatment of mCRPC, with four new therapies approved since 2010, namely sipuleucel-T, cabazitaxel, abiraterone, and enzalutamide. These therapies have all been shown to improve overall survival in metastatic castration-resistant disease. As further data emerge, both in a general population and specifically for older patients, the sequencing of these agents will be clarified. It is anticipated that cytotoxic therapy will gradually be pushed down the treatment paradigm, replaced by agents with better tolerability. This will have a particular positive impact for older patients, and should simplify the current complex decision regarding initiating docetaxel.
While awaiting these data the development of studies that integrate clinical instruments that better determine which patient characteristics predict chemotherapy toxicity12 is important for optimizing the treatment of older men with mCRPC.
Acknowledgments
A. Horgan was supported by the Division of Medical Oncology, Princess Margaret Hospital/University Health Network, University of Toronto, Ontario, Canada. M. Eisenberger has acted in a consultant/advisory role for Sanofi Aventis, has received honoraria from Sanofi Aventis and has received research funding from Sanofi Aventis. R. De Wit has acted in a consultant/advisory role for Sanofi Aventis. I. F. Tannock has acted in an advisory role for Sanofi Aventis and has received research funding from Sanofi Aventis.
Footnotes
Prior presentations. The study was presented as a poster discussion at the ASCO Annual Meeting 2011, J Clin Oncol 29: 2011 (suppl; abstr 4530).
Disclosures and Conflict of Interest Statements
Remaining authors do not have any disclosures.
Author Contributions
Study concept and design: A. Horgan, B. Seruga, G.R. Pond, E. Amir, I.F. Tannock, S.M. Alibhai.
Data acquisition: A.M. Horgan, B. Seruga, G.R. Pond, S.M. Alibhai, E. Amir, R. De Wit, M.A. Eisenberger, I.F. Tannock.
Quality control of data and algorithms: A.M. Horgan, B. Seruga, G.R. Pond, S.M. Alibhai, E. Amir, R. De Wit, M.A. Eisenberger, I.F. Tannock.
Data analysis and interpretation: A.M. Horgan, B. Seruga, G.R. Pond, S.M. Alibhai, E. Amir, R. De Wit, M.A. Eisenberger, I.F. Tannock.
Statistical analysis: G.R. Pond, E. Amir, A.M. Horgan, B. Seruga, S.M. Alibhai.
Manuscript preparation: A.M. Horgan, E. Amir, B. Seruga, S.M. Alibhai, I.F. Tannock, G.R. Pond.
Manuscript editing and review: A.M. Horgan, B. Seruga, G.R. Pond, S.M. Alibhai, E. Amir, R. De Wit, M.A. Eisenberger, I.F. Tannock.
REFERENCES
- 1. http://clinicaltrials.gov/show/NCT01254513.
- 2.Petrylak DP, Tangen CM, Hussain MH, Jnr Lara PN, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 3.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 4.Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242–245. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 5.Shepard DR, Weil A, Garcia JA, Dreicer R, Raghavan D. Efficacy and toxicity of docetaxel in elderly men with castrate-resistant metastatic prostate cancer. J Clin Oncol. 2010;28(5) [(suppl; abstr 4687) 2010]. [Google Scholar]
- 6.Italiano A, Ortholan C, Oudard S, Pouessel D, Gravis G, Beuzeboc P, et al. Docetaxel-based chemotherapy in elderly patients (age 75 and older) with castration-resistant prostate cancer. Eur Urol. 2009;55:1368–1375. doi: 10.1016/j.eururo.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 7.Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology. 1997;50:920–928. doi: 10.1016/S0090-4295(97)00459-7. [DOI] [PubMed] [Google Scholar]
- 8.Cella D, Nichol MB, Eton D, Nelson JB, Mulani P. Estimating clinically meaningful changes for the Functional Assessment of Cancer Therapy—Prostate: results from a clinical trial of patients with metastatic hormone-refractory prostate cancer. Value Health. 2009;12:124–129. doi: 10.1111/j.1524-4733.2008.00409.x. [DOI] [PubMed] [Google Scholar]
- 9.Von Korff M, Wagner EH, Saunders K. A chronic disease score from automated pharmacy data. J Clin Epidemiol. 1992;45:197–203. doi: 10.1016/0895-4356(92)90016-g. [DOI] [PubMed] [Google Scholar]
- 10.Beer TM, Berry W, Wersinger EM, Bland LB. Weekly docetaxel in elderly patients with prostate cancer: efficacy and toxicity in patients at least 70 years of age compared with patients younger than 70 years. Clin Prostate Cancer. 2003;2:167–172. doi: 10.3816/cgc.2003.n.025. [DOI] [PubMed] [Google Scholar]
- 11.ten Tije AJ, Verweij J, Carducci MA, Graveland W, Rogers T, Pronk T, et al. Prospective evaluation of the pharmacokinetics and toxicity profile of docetaxel in the elderly. J Clin Oncol. 2005;23:1070–1077. doi: 10.1200/JCO.2005.03.082. [DOI] [PubMed] [Google Scholar]
- 12.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29:3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Repetto L, Fratino L, Audisio RA, Venturino A, Gianni W, Vercelli M, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. J Clin Oncol. 2002;20:494–502. doi: 10.1200/JCO.2002.20.2.494. [DOI] [PubMed] [Google Scholar]
- 14.Extermann M. Geriatric assessment with focus on instrument selectivity for outcomes. Cancer J. 2005;11:474–480. doi: 10.1097/00130404-200511000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Extermann M, Aapro M, Bernabei R, Cohen HJ, Droz JP, Lichtman S, et al. Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG) Crit Rev Oncol Hematol. 2005;55:241–252. doi: 10.1016/j.critrevonc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Extermann M, Meyer J, McGinnis M, Crocker TT, Corcoran MB, Yoder J, et al. A comprehensive geriatric intervention detects multiple problems in older breast cancer patients. Crit Rev Oncol Hematol. 2004;49:69–75. doi: 10.1016/s1040-8428(03)00099-4. [DOI] [PubMed] [Google Scholar]
- 17.Horgan AM, Leighl NB, Coate L, et al. Impact and Feasibility of a Comprehensive Geriatric Assessment in the Oncology Setting: A Pilot Study. Am J Clin Oncol. 2012 doi: 10.1097/COC.0b013e318210f9ce. [DOI] [PubMed] [Google Scholar]
- 18.Clough-Gorr KM, Stuck AE, Thwin SS, Silliman RA. Older breast cancer survivors: geriatric assessment domains are associated with poor tolerance of treatment adverse effects and predict mortality over 7 years of follow-up. J Clin Oncol. 2010;28:380–386. doi: 10.1200/JCO.2009.23.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freyer G, Geay JF, Touzet S, Provencal J, Weber B, Jacquin JP, et al. Comprehensive geriatric assessment predicts tolerance to chemotherapy and survival in elderly patients with advanced ovarian carcinoma: a GINECO study. Ann Oncol. 2005;16:1795–1800. doi: 10.1093/annonc/mdi368. [DOI] [PubMed] [Google Scholar]
- 20.Droz JP, Balducci L, Bolla M, Emberton M, Fitzpatrick JM, Joniau S, et al. Background for the proposal of SIOG guidelines for the management of prostate cancer in senior adults. Crit Rev Oncol Hematol. 2010;73:68–91. doi: 10.1016/j.critrevonc.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Myeloid growth factors. [Accessed April 2012]; http://www.nccn.org. [Google Scholar]