Summary
Background
Treatment of breast cancer with aromatase inhibitors is associated with damage to bones. NCIC CTG MA.27 was an open-label, phase 3, randomised controlled trial in which women with breast cancer were assigned to one of two adjuvant oral aromatase inhibitors—exemestane or anastrozole. We postulated that exemestane—a mildly androgenic steroid—might have a less detrimental effect on bone than non-steroidal anastrozole. In this companion study to MA.27, we compared changes in bone mineral density (BMD) in the lumbar spine and total hip between patients treated with exemestane and patients treated with anastrozole.
Methods
In MA.27, postmenopausal women with early stage hormone (oestrogen) receptor-positive invasive breast cancer were randomly assigned to exemestane 25 mg versus anastrozole 1 mg, daily. MA.27B recruited two groups of women from MA.27: those with BMD T-scores of –2·0 or more (up to 2 SDs below sex-matched, young adult mean) and those with at least one T-score (hip or spine) less than –2·0. Both groups received vitamin D and calcium; those with baseline T-scores of less than –2·0 also received bisphosphonates. The primary endpoints were percent change of BMD at 2 years in lumbar spine and total hip for both groups. We analysed patients according to which aromatase inhibitor and T-score groups they were allocated to but BMD assessments ceased if patients deviated from protocol. This study is registered with ClinicalTrials.gov, NCT00354302.
Findings
Between April 24, 2006, and May 30, 2008, 300 patients with baseline T-scores of –2·0 or more were accrued (147 allocated exemestane, 153 anastrozole); and 197 patients with baseline T-scores of less than –2·0 (101 exemestane, 96 anastrozole). For patients with T-scores greater than –2·0 at baseline, mean change of bone mineral density in the spine at 2 years did not differ significantly between patients taking exemestane and patients taking anastrozole (−0·92%, 95% CI −2·35 to 0·50 vs −2·39%, 95% CI −3·77 to –1·01; p=0·08). Respective mean loss in the hip was −1·93% (95% CI −2·93 to –0·93) versus −2·71% (95% CI −4·32 to –1·11; p=0·10). Likewise for those who started with T-scores of less than –2·0, mean change of spine bone mineral density at 2 years did not differ significantly between the exemestane and anastrozole treatment groups (2·11%, 95% CI −0·84 to 5·06 vs 3·72%, 95% CI 1·54 to 5·89; p=0·26), nor did hip bone mineral density (2·09%, 95% CI −1·45 to 5·63 vs 0·0%, 95% CI −3·67 to 3·66; p=0·28). Patients with baseline T-score of –2·0 or more taking exemestane had two fragility fractures and two other fractures, those taking anastrozole had three fragility fractures and five other fractures. For patients who had baseline T-scores of less than –2·0 taking exemestane, one had a fragility fracture and four had other fractures, whereas those taking anastrozole had five fragility fractures and one other fracture.
Interpretation
Our results demonstrate that adjuvant treatment with aromatase inhibitors can be considered for breast cancer patients who have T-scores less than –2·0.
Funding
Canadian Cancer Society Research Institute, Pfizer, Canadian Institutes of Health Research.
Introduction
Aromatase inhibitors have largely replaced tamoxifen as adjuvant endocrine treatment for postmenopausal women with hormone-receptor-positive breast cancer.1–3 Two classes of aromatase inhibitors are prescribed at present: non-steroidal (anastrozole and letrozole), and steroidal (exemestane). Aromatase inhibitors decrease circulating oestrogen concentrations in postmenopausal women,4,5 resulting in accelerated bone loss, decreased bone mineral density, and increased risk of clinical fractures.6 Exemestane—with its unique androgenic structure—results in less bone loss according to studies of animals and man.7,8
Bone loss can be treated or prevented with bisphosphonates, which inhibit osteoclast-mediated bone resorption, and thereby increase bone mineral density, reducing the risk of fracture.9,10 Bisphosphonates11–14 and denosumab15 can counteract bone loss of women treated with aromatase inhibitors; however, most breast cancer trials excluded women with osteoporosis at baseline.11
NCIC CTG MA.27 was a randomised control trial16 of 7576 postmenopausal women assigned to adjuvant exemestane or anastrozole, which showed no significant difference for event-free survival between treatments. Patients in the exemestane group reported fewer new cases of osteopenia or osteoporosis than in the anastrozole group (1171 vs 1304; p<0·001). We did a companion study to answer two questions about bone health in these patients: first, did changes of bone mineral density differ between adjuvant anastrozole and adjuvant exemestane groups? And second, for women with osteopenia or osteoporosis, did bisphosphonate treatment restore bone mineral density equally in each treatment group?
Methods
Study design
The present study (MA.27B) is a companion study to NCIC CTG MA.27, an open-label phase 3 randomised controlled trial of the oral drugs exemestane 25 mg versus anastrozole 1 mg, done at 40 centres in Canada, 363 in the USA, and 43 worldwide through the International Breast Cancer Study Group. Treatment was given daily for 5 years as adjuvant treatment for postmenopausal patients with early, hormone receptor-positive breast cancer.16 Patients were randomised at the NCIC CTG central office with dynamic minimisation.17 Stratification was by lymph node status, adjuvant chemotherapy, and use of celecoxib, aspirin 81 mg, and trastuzumab. The primary endpoint was event-free survival. Provision of MA.27 trial treatment stopped on Dec 31, 2010.
Enrolment to MA.27B took place after patients discontinued celecoxib (due to reports of cardiotoxicity) in MA.27.16 MA.27B was done at nine centres in Canada and 89 in the USA. The study included patients who had a dual-energy x-ray absorptiometry scan of acceptable quality, done within 25 weeks of randomisation in MA.27. MA.27B included two distinct groups of patients from MA.27. The first included those with baseline T-scores for bone mineral density of –2·0 or greater (up to two SDs below sex-matched patients younger than age 30 years) at the spine and hip (no osteopenia or osteoporosis). These patients could not have received any drugs—including bisphosphonates—in the 6 months before registration for MA.27B. The second group consisted of patients with at least one T-score less than –2·0, who may have started bisphosphonates up to 25 weeks before registration. They were permitted to continue the same bisphosphonate treatment. If not started previously, bisphosphonate treatment began when these patients were enrolled into MA.27B. The bisphosphonate treatment was chosen by the patient and their doctor; we did not analyse differences in outcome by type of bisphosphonate. Patients with T-score less than –2·0 had to have a creatinine clearance of more than 35 mL/min according to the Cockcroft-Gault formula. The cutoff of –2·0 was chosen because it conforms with several clinical guidelines recommending bisphosphonate treatment.18
The full protocol is available online. MA.27B was approved by health regulatory authorities and participating centres’ institutional review boards. Patients provided written consent.
Procedures
We used dual-energy x-ray absorptiometry densitometers manufactured by Hologic (Bedford, MA, USA) or GE Lunar (Madison, WI, USA) to assess bone mineral density of the lumbar spine (L1–L4) and hip. Participants in MA.27B were prescribed calcium 1000 mg and vitamin D 800 IU daily.
All patients had to have had their 2-year assessments done by Jan 22, 2011. Patients had their bone mineral density assessed yearly for 5 years. The study was truncated when the core MA.27 trial was closed, so the original intention to assess change of bone mineral density at 3 years and 5 years was not possible.
When possible, bone scans were analysed centrally by technologists certified by the International Society of Clinical Densitometry. Areal bone mineral densities assessed by Lunar intruments were expressed as Hologic equivalents.19 We assessed changes by eye when density assessments were presented graphically, which was very rare, although we do not have a precise number. We calculated T-scores at baseline, 1 year, and 2 years centrally for all patients.
We measured changes in serum markers of bone turnover and bone formation (N-terminal of procollagen type 1 propeptide [PINP] and serum N-telopeptide [NTX]) at 6 months and 12 months compared with baseline. We measured PINP and NTX concentrations from non-fasting serum samples collected in yellow-top separator tubes, left to sit for 30 min after collection, then centrifuged, aliquoted, and frozen at −70°C (appendix p 23). Samples were batched and shipped on dry ice to a central laboratory (appendix).
Outcomes
The primary endpoints were percent change of spine and hip bone mineral density from baseline to 2 years. Our primary objectives were to assess whether there was a 5% difference between T-score groups for the primary endpoint. This analysis included only patients assessed at 2 years. Assessments of bone mineral density were stopped with discontinuation of MA.27 treatment, or for those patients with baseline T-score of –2·0 or more, with prescription of bisphosphonate.
Secondary objectives at 2 years were: percent change by patient of bone mineral density at 1 year; absolute change by patient of T-score between baseline and 1 year and 2 years; treatment group mean percent change of bone mineral density and T-score, between baseline and 1 year and 2 years; and clinically relevant changes at 2 years. For patients with baseline T-scores of –2·0 or more, clinically relevant changes were the development of T-score of less than –2·0 at the spine or hip, or clinical fracture, and for patients with baseline T-scores of less than –2·0, they were improvements of 5% or more in bone mineral density from baseline, or development of clinical fracture. Fractures were classified as fragility (eg, fractures resulting from low or no trauma) or other type.20
Statistical analysis
We estimated the SD of percent change of bone mineral density to be 0·10.21 Four comparisons of exemestane and anastrozole treatment assessed whether there was a 5% difference for change in bone mineral density: for each group of patients with different baseline T-scores, change was compared in spine and in hip. Each primary objective would be significant using a Bonferroni adjustment if two-sided p values were 0·0125 or less (0·05/4), with power of 0·80. Each T-score group within each treatment group required 89 patients. We expected 10% of patients to withdraw (missing assessments or loss to follow-up) and 5% to drop out by 2 years, for which we needed 102 patients per group; 408 patients overall.
Attrition at 2 years was greater than expected so we assessed whether baseline bone mineral density was different between those with and those without data for bone mineral density at 2 years; we also compared bone mineral density at baseline between patients with versus those without data at 1 year with a two-sided Student's t tests. We assessed patients who started bisphosphonates in the high baseline T-score group and discontinued treatment in the low baseline T-score group by baseline and 2-year T-scores from graphs.
We assessed the primary objective with two-sided Student's t tests of individual patient change of bone mineral density (g/cm2); patients included in these analyses had bone mineral density assessed at both timepoints, although not always at both hip and spine. We also compared treatment effects by T-score group, including patients with bone mineral density at baseline compared with those with data at 2 years.
We did sensitivity analyses of change by 1 year and 2 years with centrally calculated T-scores (differences between baseline and one timepoint). We did exploratory generalised linear model longitudinal investigations to assess whether hip and spine bone mineral density differed over time, by treatment, and whether there was an interaction of time and treatment. We used only patients with hip (or spine) bone mineral density assessments at all timepoints (baseline, 1 year, and 2 years). We also did an exploratory longitudinal assessment with SAS Proc Mixed of all available hip (or spine) bone mineral density assessments at all timepoints, adding baseline bone mineral density as an explanatory variable.
We analysed patients according to which aromatase inhibitor they were allocated and T-score at baseline groups but bone mineral density assessments after baseline ceased if patients deviated from their protocol-assigned treatment. Such patients were then excluded from the subsequent analyses.
We used exact Fisher tests to assess differences in fracture rates at 2 years and clinically relevant changes in each T-score group. We used t tests to assess both individual and group changes of PINP and NTX. We used SAS (version 9.2) for these analyses.
This study is registered with ClinicalTrials.gov, NCT00354302.
Role of the funding source
MA.27B data were collected, managed, and analysed by NCIC CTG. The trial committee decided to publish the results. The sponsor had no role in designing the study or writing the report. LES, CRE, and J-AWC had full access to all the data.
Results
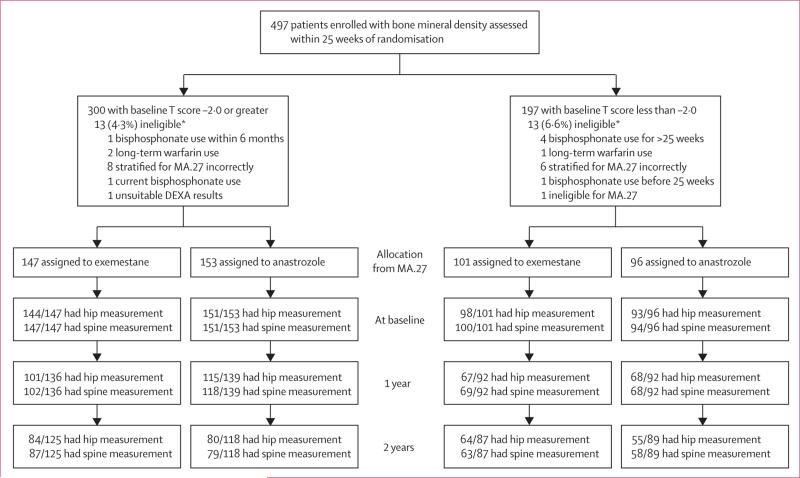
Between April 24, 2006, and May 30, 2008, 497 patients were accrued to MA.27B. 300 patients had baseline T-scores of –2·0 or more, 197 had scores of less than –2·0. 13 patients in each T-score group were ineligible (figure 1). All enrolled patients were included in the analyses; ineligible patients were not excluded if they had bone mineral density assessed at the protocol-specified times. Baseline patient characteristics (table 1) were similar to those of patients in MA.27 (data not shown).16 Characteristics were well balanced between treatment groups, within each T-score group. Calcium and vitamin D supplementation was maintained by 297 of 300 (99·0%) patients with baseline T-scores of –2·0 or more, and 194 of 197 (98·5%) with baseline T-scores of less than –2·0. Histograms of spine and hip bone mineral density at baseline and 2 years show a fairly symmetrical distribution (appendix pp 1–8).
Figure 1. Patient recruitment and analysis.
n/N=number of patients who had assessment/number of patients still on study. DEXA=dual-energy x-ray absorptiometry. *Did not meet eligibility criteria, but were still included in analyses.
Table 1.
Baseline characteristics
| Baseline T-score-2-0 or greater |
Baseline T-score less than-2-0 |
|||
|---|---|---|---|---|
| Exemestane (n=147) | Anastrozole (n=153) | Exemestane (n=101) | Anastrozole (n=96) | |
| Age at allocation (years) | ||||
| 40-49 | 4 (3%) | 4 (3%) | 1 (1%) | 0 (0%) |
| 50-59 | 50 (34%) | 59 (39%) | 21 (21%) | 15 (16%) |
| 60-69 | 52 (35%) | 63 (41%) | 37 (37%) | 28 (29%) |
| 70-79 | 34 (23%) | 17 (11%) | 31 (31%) | 42 (44%) |
| ≥80 | 7 (5%) | 10 (7%) | 11 (11%) | 11 (11%) |
| Median age (IQR; years) | 63.2 (58.1-70.9) | 62.0 (57.1-68.2) | 68.3 (61.1-73.7) | 71.0 (61.9-77.0) |
| Ethnic origin | ||||
| Data missing | 0 (0%) | 2 (1%) | 3 (3%) | 3 (3%) |
| White | 141 (96%) | 141 (92%) | 90 (89%) | 86 (90%) |
| Black | 4 (3%) | 7 (5%) | 4 (4%) | 3 (3%) |
| Asian | 1 (1%) | 1 (1%) | 4 (4%) | 3 (3%) |
| Unknown | 1 (1%) | 2 (1%) | 0 (0%) | 1 (1%) |
| ECOG performance status score | ||||
| 0 (fully) | 130 (88%) | 136 (89%) | 75 (74%) | 76 (79%) |
| 1 (restricted) | 15 (10%) | 17 (11%) | 23 (23%) | 20 (21%) |
| ≥2 (ambulatory) | 2 (1%) | 0 (0%) | 3 (3%) | 0 (0%) |
| Postmenopausal status | ||||
| Bilateral oophorectomy | 11 (7%) | 14 (9%) | 2 (2%) | 7 (7%) |
| Age ≥60 years | 89 (61%) | 88 (58%) | 79 (78%) | 77 (80%) |
| Age 45-59 years (<12 months since last menstruation; hysterectomy) | 5 (3%) | 9 (6%) | 2 (2%) | 2 (2%) |
| 45-59 years (>12 months) | 41 (28%) | 40 (26%) | 17 (17%) | 10 (10%) |
| 45-59 (<12 months; HRT) | 1 (1%) | 2 (1%) | 1 (1%) | 0 (0%) |
| Type of most extensive primary surgery | ||||
| Partial mastectomy | 111 (76%) | 113 (74%) | 61 (60%) | 55 (57%) |
| Mastectomy (not otherwise specified) | 36 (24%) | 40 (26%) | 40 (40%) | 41 (43%) |
| Axillary dissection | ||||
| No | 95 (65%) | 89 (58%) | 57 (56%) | 55 (57%) |
| Yes | 52 (35%) | 64 (42%) | 44 (44%) | 41 (43%) |
| Tumour laterality | ||||
| Bilateral | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Left | 77 (52%) | 83 (54%) | 59 (58%) | 52 (54%) |
| Right | 69 (47%) | 70 (46%) | 42 (42%) | 44 (46%) |
| Oestrogen-receptor status | ||||
| Negative | 1 (1%) | 1 (1%) | 0 (0%) | 1 (1%) |
| Positive | 146 (99%) | 152 (99%) | 101 (100%) | 95 (99%) |
| Progesterone-receptor status | ||||
| Negative | 18 (12%) | 25 (16%) | 19 (19%) | 22 (23%) |
| Positive | 129 (88%) | 128 (84%) | 82 (81%) | 74 (77%) |
| T stage | ||||
| T1 | 113 (77%) | 115 (75%) | 67 (66%) | 72 (75%) |
| T2 | 29 (20%) | 33 (22%) | 30 (30%) | 22 (23%) |
| T3 | 5 (3%) | 5 (3%) | 3 (3%) | 2 (2%) |
| TX | 0 (0%) | 0 (0%) | 1 (1%) | 0 (0%) |
| N stage | ||||
| N0 | 117 (80%) | 112 (73%) | 71 (70%) | 67 (70%) |
| N1 | 21 (14%) | 30 (20%) | 26 (26%) | 22 (23%) |
| N2 | 7 (5%) | 7 (5%) | 2 (2%) | 3 (3%) |
| N3 | 0 (0%) | 1 (1%) | 0 (0%) | 2 (2%) |
| NX | 2 (1%) | 3 (2%) | 2 (2%) | 2 (2%) |
| Previous adjuvant chemotherapy | ||||
| No | 101 (69%) | 97 (63%) | 73 (72%) | 73 (76%) |
| Yes | 46 (31%) | 56 (37%) | 28 (28%) | 23 (24%) |
| Previous raloxifene treatment | ||||
| No | 143 (97%) | 152 (99%) | 99 (98%) | 94 (98%) |
| Yes | 2 (1%) | 1 (1%) | 2 (2%) | 2 (2%) |
| Unknown | 2 (1%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Radiotherapy (previous or concurrent) | ||||
| Unknown or missing data | 1 (1%) | 0 (0%) | 1 (1%) | 1 (1%) |
| Yes | 115 (78%) | 115 (75%) | 63 (62%) | 54 (56%) |
| No | 31 (21%) | 38 (25%) | 37 (37%) | 41 (43%) |
| Fractures within 10 years before allocation? | ||||
| No | 130 (88%) | 143 (93%) | 91 (90%) | 79 (82%) |
| Yes | 17 (12%) | 10 (7%) | 10 (10%) | 17 (18%) |
| Cardiovascular history | ||||
| None | 55 (37%) | 58 (38%) | 52 (51%) | 47 (49%) |
| Ischaemia | 92 (63%) | 95 (62%) | 49 (49%) | 49 (51%) |
| Median bone mineral density index T-score (IQR) | ||||
| Hip | −0.1 (−0.8 to 0.6) | −0.4 (−0.9 to 0.5) | −1.9 (−2.5 to −1.3) | −1.8 (−2.3 to −1.3) |
| Lumbar spine | −0.1 (−0.9 to 0.9) | −0.2 (−1.0 to 0.6) | −2.3 (−2.8 to −1.7) | −2.4 (−2.7 to −2.1) |
| Bisphosphonate use | ||||
| No | 145 (99%) | 148 (97%) | 5 (5%) | 5 (5%) |
| Yes | 2 (1%) | 5 (3%) | 96 (95%) | 91 (95%) |
Data are n (%), unless stated otherwise. ECOG=Eastern Cooperative Oncology Group. HRT=hormone replacement treatment.
Figure 1 shows the number of patients and those with assessable bone mineral density at each timepoint according to T-score group and treatment allocation. The post-hoc power for the primary analysis was 0·65–0·72. Baseline bone mineral density of patients who had data available for 1 year and 2 years was generally not significantly different from those who did not have data at 1 year and 2 years (data not shown); the only significant difference was that patients with baseline T-score of less than –2·0 taking anastrozole assessed at 2 years had significantly lower spine bone mineral density at baseline (p=0·01) than those not assessed at 2 years. Plots of matched baseline to 2-year T-scores indicate that only two patients with baseline T-scores of –2·0 or more had a reduction in T-score to less than –2·0 and started bisphosphonates (appendix pp 9–10). Likewise, only nine women with baseline T-scores of less than –2·0 withdrew with 2-year T-scores which persisted to be less than –2·0 (appendix pp 11–12).
For patients with baseline T-score of –2·0 or more, we detected no significant difference for mean loss of bone mineral density in the hip at 2 years between patients taking exemestane (−1·93%, 95% CI −2·93 to –0·93) versus anastrozole (−2·71%, 95% CI −4·32 to –1·11; p=0·10; table 2). We report similar results for bone mineral density in the spine (−0·92%, 95% CI −2·35 to 0·50 vs −2·39%, 95% CI −3·77 to –1·01; p=0·08). Absolute changes of individual T-scores were not significantly different between treatment groups (table 3). Unmatched group comparisons between timepoints are shown in the appendix (p 13); loss of spine bone mineral density was significant for patients taking anastrozole (p=0·05) but not those taking exemestane. At 1 year, mean loss of bone mineral density in the hip was significantly less for patients taking exemestane versus those taking anastrozole (table 2), although again, absolute T-scores did not differ significantly (table 3). Loss of bone mineral density in the spine at 1 year did not differ significantly between treatment groups (tables 2 and 3).
Table 2.
Relative change of patients' bone mineral density from baseline
| Exemestane |
Anastrozole |
p value* | |||
|---|---|---|---|---|---|
| N | Mean % change (95% CI) | N | Mean % change (95% CI) | ||
| At 2 years | |||||
| Hip | |||||
| Baseline T-score ≥−2.0 | 83 | −1.93% (−2.93 to −0.93) | 80 | −2.71% (−4.32 to −1.11) | 0.10 |
| Baseline T-score <−2.0 | 61 | 2.09% (−1.45 to 5.63) | 55 | 0.00% (−3.67 to 3.66) | 0.28 |
| Lumbar spine | |||||
| Baseline T-score ≥−2.0 | 87 | −0.92% (−2.35 to 0.50) | 77 | −2.39% (−3.77 to −1.01) | 0.08 |
| Baseline T-score <−2.0 | 63 | 2.11% (−0.84 to 5.06) | 57 | 3.72% (1.54 to 5.89) | 0.26 |
| At 1 year | |||||
| Hip | |||||
| Baseline T-score ≥−2.0 | 99 | −0.62% (−1.48 to 0.23) | 114 | −1.66% (−2.73 to −0.59) | 0.01 |
| Baseline T-score <−2.0 | 64 | 0.61% (−1.81 to 3.04) | 66 | 0.83% (−0.16 to 1.82) | 0.23 |
| Lumbar spine | |||||
| Baseline T-score ≥−2.0 | 102 | −0.59% (−1.73 to 0.54) | 116 | −1.88% (−3.06 to −0.70) | 0.32 |
| Baseline T-score <−2.0 | 69 | 3.75% (2.13 to 5.36) | 66 | 2.60% (1.24 to 3.96) | 0.67 |
Based on two-sided Student's t test of individual patient change.
Table 3.
Absolute change of patients' T-scores from baseline
| Exemestane |
Anastrozole |
p value* | |||
|---|---|---|---|---|---|
| N | Mean % change (95% CI) | N | Mean % change (95% CI) | ||
| At 2 years | |||||
| Hip | |||||
| Baseline T-score ≥−2.0 | 83 | −0.16 (−0.29 to −0.03) | 80 | −0.16 (−0.27 to −0.05) | 0.98 |
| Baseline T-score <−2.0 | 61 | 0.12 (−0.06 to 0.31) | 55 | 0.01 (−0.19 to 0.21) | 0.41 |
| Lumbar spine | |||||
| Baseline T-score ≥−2.0 | 87 | −0.11 (−0.24 to 0.03) | 77 | −0.20 (−0.34 to −0.06) | 0.35 |
| Baseline T-score <−2.0 | 63 | 0.17 (−0.02 to 0.36) | 57 | 0.24 (0.07 to 0.41) | 0.61 |
| At 1 year | |||||
| Hip | |||||
| Baseline T-score ≥−2.0 | 97 | −0.03 (−0.13 to 0.07) | 111 | −0.12 (−0.21 to −0.04) | 0.18 |
| Baseline T-score <−2.0 | 61 | 0.04 (−0.10 to 0.80) | 66 | 0.05 (0.00 to 0.11) | 0.89 |
| Lumbar spine | |||||
| Baseline T-score ≥−2.0 | 99 | −0.08 (−0.18 to 0.02) | 111 | −0.21 (−0.33 to −0.09) | 0.11 |
| Baseline T-score <−2.0 | 69 | 0.29 (0.17 to 0.40) | 66 | 0.18 (0.08 to 0.28) | 0.18 |
Based on two-sided Student's t test of individual patient change.
For patients with baseline T-score of less than –2·0, mean change of bone mineral density in the spine at 2 years did not differ significantly between exemestane and anastrozole groups (2·11%, 95% CI −0·84 to 5·06 vs 3·72%, 95% CI 1·54 to 5·89; p=0·26; table 2). Likewise, there was no significant difference between groups for mean bone mineral density in the hip (2·09%, 95% CI −1·45 to 5·63 vs 0·00%, 95% CI −3·67 to 3·66; p=0·28; table 2). Individual and group changes in spine and hip T-scores were not significantly different between treatment groups (table 3; appendix p 15).
In longitudinal assessments, for patients who had data at all timepoints (baseline, 1 year, and 2 years; appendix p 14) and patients who had data missing at some timepoints (appendix p 15), we recorded similar results.
In patients who had a baseline T-score of –2·0 or more, spine bone mineral density for each patient decreased at 2 years, with less decrease for patients allocated to exemestane (figure 2A, appendix p 14); we found no significant interaction between treatment and time (p=0·21 for patients with complete data; p=0·10 for those with incomplete data). Hip bone mineral density for these patients decreased significantly by 2 years (figure 2A; p<0·0001, for patients with both complete and incomplete data; appendix p 14), with no significant treatment difference (appendix p 14), and no significant interaction of treatment and time (p=0·58 for patients with complete data; p=0·26 for those with incomplete data). Baseline hip and spine bone mineral density significantly affected the results (p<0·0001).
Figure 2. Individual patient change of bone mineral density by treatment group for patients with assessments at baseline, 1 years, and 2 years.
For patients with T-scores –2·0 or greater at baseline (A) and patients with T-scores less than –2·0 at baseline (B).
For patients with baseline T-scores of less than –2·0, individual patient spine bone mineral density increased significantly by 2 years (figure 2B; p<0·0001, for both those with complete and those with incomplete data; appendix p 14); there was no significant treatment difference or interaction between treatment and time (appendix pp 9–12). Hip bone mineral density in these patients changed significantly by 2 years for patients with complete data (figure 2B), but not for those with incomplete data (appendix p 14). Treatment groups did not differ significantly and we detected no interaction of treatment and time (p=0·51 for patients with complete data; p=0·65 for patients with incomplete data; appendix p 14). As for those with high baseline T-scores, baseline hip and spine bone mineral density significantly affected the results for those with low baseline T-scores (p<0·0001; appendix p 14).
In patients with baseline T-scores of –2·0 or more, fewer women who took exemestane (20/138, 14·5%) than took anastrozole (29/141, 20·6%) started bisphosphonate treatment by 2 years (p=0·21). However, six women in each treatment group developed hip T-score of less than –2·0 (p=1·00; appendix p 16), while 13 women taking exemestane and seven taking anastrozole had a similar reduction in spine T-score (p=0·34; appendix p 16). For patients with baseline T-scores of less than –2·0, 16 women taking exemestane and nine taking anastrozole had clinically relevant hip changes (≥5% improvement of bone mineral density; p=0·26; appendix p 16), while 25 women taking exemestane and 27 taking anastrozole had similar improvements of spine bone mineral density (p=0·46; appendix p 16).
For patients with baseline T-scores of –2·0 or more, two fragility fractures occurred in the exemestane group versus three in the anastrozole group. For those with low baseline T-scores, one versus five fragility fractures occurred. The proportions of other fractures did not differ significantly between treatment groups in either T-score group (appendix p 17).
Markers of bone turnover were assessed for 401 patients at baseline, 328 at 6 months, and 290 at 1 year. For patients with both high T-score and low T-score, changes in markers of bone turnover in one treatment group did not differ significantly from changes in the other treatment group (appendix p 18), although both markers increased slightly in those patients with baseline T-scores of –2·0 or more (table 4, appendix p 18) and decreased in those with T-scores of less than –2·0 at baseline (table 4, appendix p 18).
Table 4.
Changes in biomarkers of bone turnover between baseline and 1 year
| Exemestane group | Anastrozole group | p value* | |
|---|---|---|---|
| NTX | |||
| T-score >−2.0 (n=117) | |||
| Baseline | 13.6% (12.9-14.3) | 13.7% (13.1-14.4) | .. |
| 1 year | 14.9% (13.9-15.9) | 14.9% (14.9-15.8) | 0.96 |
| T-score ≤−2.0 (n=81) | |||
| Baseline | 15.0% (14.1-16.0) | 15.1% (14.1-16.2) | .. |
| 1 year | 10.5% (9.6-11.4) | 10.5% (9.7-11.3) | 0.31 |
| PINP | |||
| T-score >−2.0 (n=117) | |||
| Baseline | 52.9% (48.9-57.3) | 52.3% (48.4-56.6) | .. |
| 1 year | 58.4% (53.5-63.7) | 54.5% (49.7-59.8) | 0.61 |
| T-score ≤−2.0 (n=81) | |||
| Baseline | 58.2% (53.2-63.7) | 57.3% (51.8-63.3) | .. |
| 1 year | 30.9% (26.3-36.3) | 28.7% (25.2-32.7) | 0.12 |
Data are mean percentage change (95% CI).
Based on two-sided Student's t test of patient change.
Discussion
The effects of aromatase inhibitors on bone mineral density at 2 years did not differ significantly between patients treated with exemestane and those treated with anastrozole. Although we noted less loss of bone mineral density in the hip for patients with baseline T-scores of –2·0 or more taking exemestane after 1 year, this benefit was not maintained. However, this finding accords with the fewer self-reported diagnoses of osteopenia and osteoporosis by patients in the exemestane group than in the anastrozole group in MA.27.16 Previous studies22,23 of exemestane showed that bone mineral density fell during the first year and stabilised thereafter (panel).
We noted less of a decrease in bone mineral density—for both spine and hip—in patients with baseline T-scores of –2·0 or more taking exemestane than that reported in other trials of exemestane.25,26 For example, in the Intergroup Exemestane Study,25 median change of bone mineral density at 2 years was −3·93% in the hip and −2·88% in the spine. Patients treated in the TEAM trial had mean change of bone mineral density of −3·5% at 2 years.26 Our favourable results could be partly attributed to mandatory vitamin D and calcium supplementation, which was maintained by almost all patients; this hypothesis is supported by a meta-analysis showing that calcium and vitamin D can prevent bone loss for women aged 50 years and older.27 We have reported results of a trial of exemestane for prevention of breast cancer,28 which showed changes in bone mineral density at 2 years of −1·8% for hip and −2·4% for spine. Exemestane caused decreases of cortical thickness at the distal radius and distal tibia despite mandatory vitamin D and calcium supplementation. The large ATAC bone substudy29 showed that bone mineral density increases after cessation of aromatase inhibitors and increased fracture risk does not persist in the long term, suggesting that bone changes induced by aromatase inhibitors are largely reversible with cessation of treatment and normalisation of menopausal oestrogen concentrations.
The different use of bisphosphonates in our study for the groups of women was based on their baseline bone mineral assessment.30 General clinical practice is to prescribe bisphosphonates for patients with T-scores of less than –2·0, but not for those with a T-score of –2·0 or more: our study conforms with this practice. The differences in effects of bone mineral density between treatment groups might have been attenuated by greater than expected attrition of patients assigned to take bisphosphonates, which reduced the power of the analysis, and the fewer patients with a baseline T-score of –2·0 or more taking exemestane who started bisphosphonates than those taking anastrozole.
The role of oral bisphosphonates in conjunction with aromatase inhibitors has been tested in several trials.12–14,24 Women who received oral bisphosphonates had either stabilised or increased bone mineral density at 2 years12,14 and 3 years.24 None of the trials of intravenous bisphosphonates included women with T-scores of less than –2·0 when starting aromatase inhibitor treatment.11 MA.27B is the largest prospective bone study to assess the role of oral bisphosphonate treatment for women with T-scores less than –2·0 with concomitant aromatase inhibitors. We showed that—despite the likelihood of strong oestrogen suppression during treatment with aromatase inhibitors—bisphosphonates prevented aromatase inhibitor-induced bone loss. This observation accords with other studies that have shown preservation or improvement of bone mineral density for patients receiving aromatase inhibitor treatment and concurrent bisphophonates or denosumab. This result underscores that osteoporotic patients with breast cancer should not be denied the opportunity offered by aromatase inhibitors for treatment of breast cancer.
For clinicians concerned about the effects of adjuvant aromatase inhibitors for osteoporotic patients with breast cancer, our results show that osteoporosis can be managed with concomitant initiation of oral bisphosphonates and supplementation with calcium and vitamin D. Clinical fractures were rare. We found no difference in bone turnover biomarkers between the two aromatase inhibitors. Similarly, a randomised trial of healthy volunteers showed that aromatase inhibitors have similar effects on bone turnover.31
Our study has some limitations. Patients were predominantly white; however, two studies have shown that the effects of aromatase inhibitors on bone loss for Japanese women are similar to those for white women.32,33 We did not record detailed information about patients’ exercise habits, smoking or family bone health history, or vitamin D and parathormone concentrations, which might affect changes in bone mineral density over time. The doses of vitamin D used in MA.27B were lower than would be used at present. Samples of markers of bone turnover were not collected while fasting, which might have affected variability of the results. We did not record morphometric data for the reported fractures.
The analyses were intention to treat for patients who remained on protocol treatment. Full intention-to-treat analyses were not possible because data for bone mineral density were not available after baseline for patients who went off protocol treatment. We addressed this shortcoming by testing for differences by treatment group at baseline for patients who did or did not have bone mineral density assessments at 2 years or 1 year. Only one of 16 tests indicated a significant difference. Furthermore, we did all tests with use of individual change (matched comparisons where patients had bone mineral density assessed at both timepoints) and group change (grouped patient data with any patient bone mineral density available at either timepoint). The latter analyses used intention-to-treat population data for bone mineral density.
Attrition was greater than expected, which led to fewer than the 89 patients planned for each treatment group of the primary objectives. For patients with baseline T-scores of –2·0 or more, the primary results were not significant with little possibility that they would have been significant had we enrolled as many patients as planned—those not assessed at 2 years had similar baseline bone mineral density to those who were assessed. For patients with less than –2·0 at baseline, with p values of 0·28 for hip and 0·26 for spine bone mineral density, it is also unlikely that differences would have been significant had enrolment targets been met. How the lower baseline spine bone mineral density for patients taking anastrozole than those taking exemestane might have affected spine outcome is unclear.
MA.27B was originally planned to have assessments of bone mineral density at 3 years and 5 years. The study was truncated when the core MA.27 trial was closed; however, MA.27B had uniform 2-year follow-up to meet the primary objectives. This follow-up does not enable us to assess long-term effects of aromatase inhibitors on clinical fracture risk or bone health.
Our study provides evidence related to several clinically relevant issues. In women with T-scores of –2·0 or greater, ensuring adequate vitamin D and calcium supplementation is advisable for optimal bone health management. Our findings for women with T-scores less than –2·0 are of particular clinical relevance, since low T-score is a common reason to withhold treatment with aromatase inhibitors, particularly for older women. Our data show that in this often osteopenic or osteoporotic group, bisphosphonates can preserve or improve bone mineral density. Bone health should be taken into account when planning adjuvant endocrine treatment, but our results show that at least short-term effects of aromatase inhibitors on bone can be successfully managed. Our findings support the present guidelines of monitoring bone mineral density of women receiving aromatase inhibitors and managing bone loss.
Supplementary Material
Panel: Research in context.
Systematic review
We searched PubMed and the Cochrane Database of Systematic Reviews with the terms “breast cancer”, “aromatase inhibitors”, “exemestane”, “anastrozole”, “letrozole”, “bone”, “bone mineral density”, “bone turnover markers”, “osteoporosis”, “fractures”, and “bisphosphonates”. We identified substudies of bone from all major trials of aromatase inhibitors for adjuvant breast cancer treatment, which showed that aromatase inhibitors are associated with increased risk of bone loss.1–3 We found two preclinical studies6 and a randomised trial of healthy postmenopausal women7 suggesting that exemestane (a steroidal aromatase inhibitor) could cause less bone loss than other aromatase inhibitors. MA.27 directly compared two aromatase inhibitors in postmenopausal patients with breast cancer, and reported fewer cases of osteopenia or osteoporosis for patients taking exemestane than those taking anastrozole.16 We identified four trials12–14,24 of oral bisphosphonates for women with baseline osteoporosis starting an aromatase inhibitor.
Interpretation
We designed a bone substudy to directly compare the effect of two aromatase inhibitors (exemestane and anastrozole) in postmenopausal women with breast cancer enrolled in the MA.27 trial. We also assessed the role of oral bisphosphonate treatment for women with T-scores less than –2·0 with concomitant administration of an aromatase inhibitor. Both aromatase inhibitors had a similar effect on hip and spine bone mineral density at 2 years for patients with both T-scores of –2·0 or greater and those with T-scores of less than –2·0. Our result underscores the idea that osteoporotic patients with breast cancer should have the opportunity offered by aromatase inhibitors for improvement of breast cancer survival.
Acknowledgments
We thank the 497 women who participated in the study, the trial committee, the investigators, and clinical research associates from the NCIC Clinical Trials Group, NCCTG, CALGB, ECOG, and SWOG. PEG, JSL, and TBC were supported by the Avon Foundation New York.
Footnotes
Contributors
All authors wrote the report, collected and analysed data, and approved the final version. PEG, DLH, JNI, KIP, AMC, and J-AWC designed the study.
Declaration of interests
VS has received funding from Novartis and Pfizer. The other authors declare that they have no competing interests.
References
- 1.Baum M, Buzdar A, Cuzick J, et al. ATAC (Arimidex, Tamoxifen Alone or in Combination) Trialists’ Group Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (Arimidex, Tamoxifen Alone or in Combination) trial efficacy and safety update analyses. Cancer. 2003;98:1802–10. doi: 10.1002/cncr.11745. [DOI] [PubMed] [Google Scholar]
- 2.Coombes RC, Hall E, Gibson LJ, et al. Intergroup Exemestane Study A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350:1081–92. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- 3.Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28:509–18. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 4.Geisler J, Haynes B, Anker G, Dowsett M, Lønning PE. Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized, cross-over study. J Clin Oncol. 2002;20:751–57. doi: 10.1200/JCO.2002.20.3.751. [DOI] [PubMed] [Google Scholar]
- 5.Geisler J, King N, Anker G, et al. In vivo inhibition of aromatization by exemestane, a novel irreversible aromatase inhibitor, in postmenopausal breast cancer patients. Clin Cancer Res. 1998;4:2089–93. [PubMed] [Google Scholar]
- 6.Chien AJ, Goss PE. Aromatase inhibitors and bone health in women with breast cancer. J Clin Oncol. 2006;24:5305–12. doi: 10.1200/JCO.2006.07.5382. [DOI] [PubMed] [Google Scholar]
- 7.Goss PE, Qi S, Josse RG, et al. The steroidal aromatase inhibitor exemestane prevents bone loss in ovariectomized rats. Bone. 2004;34:384–92. doi: 10.1016/j.bone.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Goss PE, Hadji P, Subar M, Abreu P, Thomsen T, Banke-Bochita J. Effects of steroidal and nonsteroidal aromatase inhibitors on markers of bone turnover in healthy postmenopausal women. Breast Cancer Res. 2007;9:R52. doi: 10.1186/bcr1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black DM, Cummings SR, Karpf DB, et al. Fracture Intervention Trial Research Group Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535–41. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 10.Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280:2077–82. doi: 10.1001/jama.280.24.2077. [DOI] [PubMed] [Google Scholar]
- 11.Brufsky A, Bundred N, Coleman R, et al. Z-FAST and ZO-FAST Study Groups Integrated analysis of zoledronic acid for prevention of aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole. Oncologist. 2008;13:503–14. doi: 10.1634/theoncologist.2007-0206. [DOI] [PubMed] [Google Scholar]
- 12.Van Poznak C, Hannon RA, Mackey JR, et al. Prevention of aromatase inhibitor-induced bone loss using risedronate: the SABRE trial. J Clin Oncol. 2010;28:967–75. doi: 10.1200/JCO.2009.24.5902. [DOI] [PubMed] [Google Scholar]
- 13.Markopoulos C, Tzoracoleftherakis E, Polychronis A, et al. Management of anastrozole-induced bone loss in breast cancer patients with oral risedronate: results from the ARBI prospective clinical trial. Breast Cancer Res. 2010;12:R24. doi: 10.1186/bcr2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lester JE, Dodwell D, Brown JE, et al. Prevention of anastrozole induced bone loss with monthly oral ibandronate: final 5 year results from the ARIBON trial. J Bone Oncol. 2012;1:57–62. doi: 10.1016/j.jbo.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellis GK, Bone HG, Chlebowski R, et al. Effect of denosumab on bone mineral density in women receiving adjuvant aromatase inhibitors for non-metastatic breast cancer: subgroup analyses of a phase 3 study. Breast Cancer Res Treat. 2009;118:81–87. doi: 10.1007/s10549-009-0352-y. [DOI] [PubMed] [Google Scholar]
- 16.Goss PE, Ingle JN, Pritchard KI, et al. Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27—a randomized controlled phase III trial. J Clin Oncol. 2013;31:1398–404. doi: 10.1200/JCO.2012.44.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tu D. Minimization procedure. In: Chow SC, editor. Encyclopedia of Biopharmaceutical Statistics. 3rd edn. Marcel Dekker; New York: 2010. pp. 795–98. [Google Scholar]
- 18.Hodgson SF, Watts NB, Bilezikian JP, et al. AACE Osteoporosis Task Force American Association of Clinical Endocrinologists medical guidelines for clinical practice for the prevention and treatment of postmenopausal osteoporosis: 2001 edition, with selected updates for 2003. Endocr Pract. 2003;9:544–64. doi: 10.4158/EP.9.6.544. [DOI] [PubMed] [Google Scholar]
- 19.Genant HK, Grampp S, Glüer CC, et al. Universal standardization for dual x-ray absorptiometry: patient and phantom cross-calibration results. J Bone Miner Res. 1994;9:1503–14. doi: 10.1002/jbmr.5650091002. [DOI] [PubMed] [Google Scholar]
- 20.Melton LJ, 3rd, Hartmann LC, Achenbach SJ, Atkinson EJ, Therneau TM, Khosla S. Fracture risk in women with breast cancer: a population-based study. J Bone Miner Res. 2012;27:1196–205. doi: 10.1002/jbmr.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez EA, Josse RG, Pritchard KI, et al. Effect of letrozole versus placebo on bone mineral density in women with primary breast cancer completing 5 or more years of adjuvant tamoxifen: a companion study to NCIC CTG MA.17. J Clin Oncol. 2006;24:3629–35. doi: 10.1200/JCO.2005.05.4882. [DOI] [PubMed] [Google Scholar]
- 22.Jones S, Stokoe C, Sborov M, et al. The effect of tamoxifen or exemestane on bone mineral density during the first 2 years of adjuvant treatment of postmenopausal women with early breast cancer. Clin Breast Cancer. 2008;8:527–32. doi: 10.3816/CBC.2008.n.065. [DOI] [PubMed] [Google Scholar]
- 23.Coleman RE, Banks LM, Girgis SI, et al. Intergroup Exemestane Study group Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): a randomised controlled study. Lancet Oncol. 2007;8:119–27. doi: 10.1016/S1470-2045(07)70003-7. [DOI] [PubMed] [Google Scholar]
- 24.Lomax AJ, Yap SY, White K, et al. Prevention of aromatase inhibitor-induced bone loss with alendronate in postmenopausal women: the BATMAN Trial. J Bone Oncol. 2013;2:145–153. doi: 10.1016/j.jbo.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coleman RE, Banks LM, Girgis SI, et al. Reversal of skeletal effects of endocrine treatments in the Intergroup Exemestane Study. Breast Cancer Res Treat. 2010;124:153–61. doi: 10.1007/s10549-010-1121-7. [DOI] [PubMed] [Google Scholar]
- 26.Hadji P, Asmar L, van Nes JG, et al. The effect of exemestane and tamoxifen on bone health within the Tamoxifen Exemestane Adjuvant Multinational (TEAM) trial: a meta-analysis of the US, German, Netherlands, and Belgium sub-studies. J Cancer Res Clin Oncol. 2011;137:1015–25. doi: 10.1007/s00432-010-0964-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet. 2007;370:657–66. doi: 10.1016/S0140-6736(07)61342-7. [DOI] [PubMed] [Google Scholar]
- 28.Cheung AM, Tile L, Cardew S, et al. Bone density and structure in healthy postmenopausal women treated with exemestane for the primary prevention of breast cancer: a nested substudy of the MAP.3 randomised controlled trial. Lancet Oncol. 2012;13:275–84. doi: 10.1016/S1470-2045(11)70389-8. [DOI] [PubMed] [Google Scholar]
- 29.Eastell R, Adams J, Clack G, et al. Long-term effects of anastrozole on bone mineral density: 7-year results from the ATAC trial. Ann Oncol. 2011;22:857–62. doi: 10.1093/annonc/mdq541. [DOI] [PubMed] [Google Scholar]
- 30.National Osteoporosis Foundation . Clinician's Guide to Prevention and Treatment of Osteoporosis. National Osteoporosis Foundation; Washington, DC: 2010. [Google Scholar]
- 31.McCloskey E, Hannon R, Lakner G, Clack G, Miyamoto A, Eastell R. The letrozole (L), exemestane (E), and anastrozole (A) pharmacodynamics (LEAP) trial: a direct comparison of bone biochemical measurements between aromatase inhibitors (AIs) in healthy postmenopausal women. Proc Am Soc Clin Oncol. 2006;24(suppl) abstr 555. [Google Scholar]
- 32.Aihara T, Suemasu K, Takei H, et al. Effects of exemestane, anastrozole and tamoxifen on bone mineral density and bone turnover markers in postmenopausal early breast cancer patients, results of N-SAS BC 04, the TEAM Japan sub-study. Oncology. 2010;79:376–81. doi: 10.1159/000323489. [DOI] [PubMed] [Google Scholar]
- 33.Akiyoshi T, Shimomura Y, Masuda S, et al. Effect of aromatase inhibitors on bone mineral density in a Japanese breast cancer population. Drug Metab Pharmacokinet. 2013;28:446–50. doi: 10.2133/dmpk.dmpk-12-nt-095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.