Abstract
Context
Eosinophilic renal neoplasms include a spectrum of solid and papillary tumors ranging from indolent benign oncocytoma to highly aggressive malignancies. Recognition of the correct nature of the tumor, especially in biopsy specimens, is paramount for patient management.
Objective
To review the diagnostic approach to eosinophilic renal neoplasms with light microscopy and ancillary techniques.
Data Sources
Review of the published literature and personal experience.
Conclusions
The following tumors are in the differential diagnosis of oncocytic renal cell neoplasm: oncocytoma, chromophobe renal cell carcinoma (RCC), hybrid tumor, tubulocystic carcinoma, papillary RCC, clear cell RCC with predominant eosinophilic cell morphology, follicular thyroid-like RCC, hereditary leiomyomatosis–associated RCC, acquired cystic disease–associated RCC, rhabdoid RCC, microphthalmia transcription factor translocation RCC, epithelioid angiomyolipoma, and unclassified RCC. In low-grade nonpapillary eosinophilic neoplasms, distinction between oncocytoma and low-grade RCC mostly rests on histomorphology; however, cytokeratin 7 immunostain may be helpful. In high-grade nonpapillary lesions, there is more of a role for ancillary techniques, including immunohistochemistry for cytokeratin 7, CA9, CD10, racemase, HMB45, and Melan-A. In papillary eosinophilic neoplasms, it is important to distinguish sporadic type 2 papillary RCC from microphthalmia transcription factor translocation and hereditary leiomyomatosis–associated RCC. Histologic and cytologic features along with immunohistochemistry and fluorescence in situ hybridization tests for TFE3 (Xp11.2) and TFEB [t(6;11)] are reliable confirmatory tests. Eosinophilic epithelial neoplasms with architecture, cytology, and/or immunoprofile not qualifying for either of the established types of RCC should be classified as unclassified eosinophilic RCC and arbitrarily assigned a grade (low or high).
Eosinophilic/oncocytic renal cell neoplasms constitute a major proportion of renal tumors. In low-grade non-papillary neoplasms, the major question is if the tumor meets criteria of oncocytoma, one of the few benign epithelial renal neoplasms. In high-grade tumors, one is expected to come up with an accurate diagnosis for prediction of clinical behavior and potential application of correct nonsurgical therapies. Herein we have organized a review describing first oncocytoma and tumors that may be considered in the differential diagnosis, and then high-grade nonpapillary and papillary tumors where a correct diagnosis may need more robust ancillary testing. Although there is extensive literature regarding each tumor type described in this review, we focus on the important morphologic aspects and controversial features prone to be missed or misinterpreted, and describe the ancillary tests recommended for clinical use. The Table summarizes the renal tumors that may display eosinophilic cytoplasm.
Table.
| Renal Tumors With Eosinophilic Cytoplasm |
|---|
| Oncocytoma |
| Chromophobe RCC |
| Hybrid tumor |
| Tubulocystic carcinoma |
| Papillary RCC |
| Clear cell (conventional) RCC |
| Follicular thyroid-like carcinoma |
| Hereditary leiomyomatosis–associated RCC |
| Acquired cystic kidney disease–associated RCC |
| Rhabdoid RCC |
| MiTF translocation carcinomas |
| Epithelioid angiomyolipoma |
| Unclassified RCC |
Abbreviations: MiTF, microphthalmia transcription factor; RCC, renal cell carcinoma.
NONPAPILLARY NEOPLASMS
Oncocytoma
Oncocytoma is a benign epithelial renal neoplasm representing less than 10% of renal tumors.1 Oncocytomas are usually seen in the sixth to seventh decade of life, with men affected 2 to 3 times more often than women.2 These tumors are incapable of metastases and, when large, may only pose a risk of intratumoral and retroperitoneal bleeding.1 The differential diagnosis of oncocytoma versus carcinoma is one of the most common reasons that low-grade nonpapillary oncocytic renal neoplasms are submitted for consultation.
To render the diagnosis of oncocytoma, the tumor needs to meet all diagnostic criteria and should not have features that are considered incompatible with this diagnosis. The classic macroscopic features of oncocytoma are a mahogany brown, well-demarcated lesion with central stellate scar. Presence of the stellate scar is one of the radiologic features of oncocytoma as well.3 However, a central scar is seen in only roughly 30% of the cases4 and consequently its absence does not impact the diagnosis. Moreover, the presence of the central scar is not specific, as it may be seen in other low-grade renal tumors.
One of the most important criteria for the diagnosis of oncocytoma is that at low-power magnification tumors are composed of small solid nests of cells within a myxoid or hyalinized stroma (Figure 1, A). Tubular or macrocystic structures with hemorrhage are seen in more than 70% of the cases (Figure 1, B).1 Extrarenal extension with fat involvement may be seen in 11% to 20% of cases and should not be considered a sign of malignancy.1,2,4 Even vascular invasion, including larger veins, may be seen in a minor subset of oncocytomas.1 Because of the likelihood of misinterpretation, cases showing especially the latter feature should be considered for expert consultation.5 By definition, renal oncocytomas lack significant areas of clear cells, papillary formation, and necrosis. An exception is that occasional simple papillary projections may be seen in dilated tubules. Clear cell changes are seen in up to 15% of cases and usually localized to areas of central scar.4 Necrosis should not be seen unless related to prior embolization or biopsy. Because oncocytomas can involve perinephric adipose tissue, when signing out resection specimens, it should be explained that this is not a feature of malignancy or increased likelihood of recurrence. Rather, the diagnosis should include the size (even though it is of no prognostic significance) and margin status.
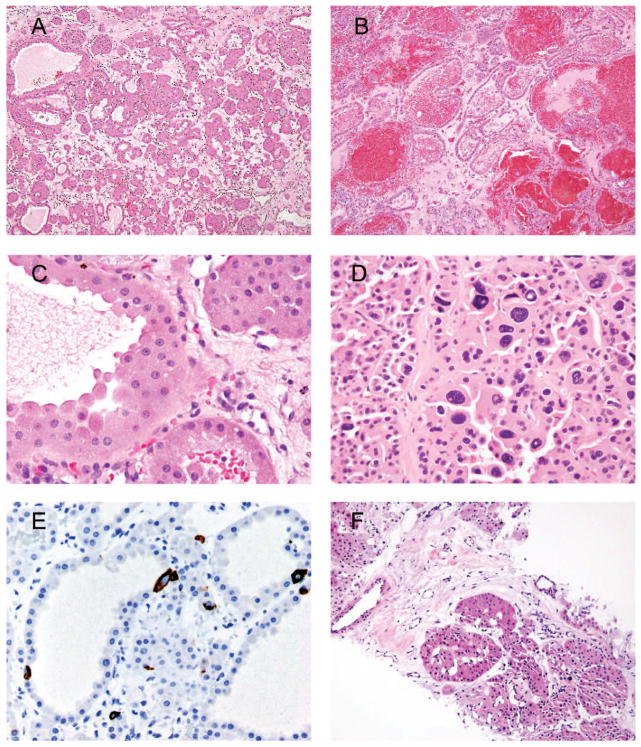
Figure 1.
A, Classic appearance of oncocytoma with small solid nests with myxoid or hyalinized background. B, Tubular architecture of oncocytoma with hemorrhage. C, Classic cytology of oncocytoma with abundant eosinophilic cytoplasm; indistinct cell borders; small, uniform nuclei; and prominent nucleoli. D, A cluster of degenerative-type atypia seen in oncocytoma. E, Scattered cytokeratin 7 immunoreactivity in oncocytoma. F, Biopsy of an oncocytic renal neoplasm consistent with oncocytoma (hematoxylin-eosin, original magnifications ×100 [A, B, and F], × 600 [C], and ×400 [D]; original magnification ×400 [E]).
Cytologic features are among the major defining criteria of renal oncocytoma. Cells of oncocytomas usually have voluminous, densely eosinophilic cytoplasm. Background nuclei are uniform and round, and may have prominent nucleoli, typically lacking binucleated forms (Figure 1, C). The only exception is that some oncocytomas have prominent degenerative-appearing nuclear atypia, which is usually clustered in small foci but less commonly can be more diffuse (Figure 1, D).6 In contrast to the notched raisinoid nuclei with detailed chromatin in chromophobe carcinoma, the degenerative atypia consists of large, often multinucleated cells with smudgy hyperchromatic nuclei and poorly preserved chromatin detail. Despite the atypia, mitoses are usually absent or very rare. Some cases may have clusters of small cells with scant cytoplasm and hyperchromatic nuclei, termed oncoblasts.1 These may be a source of diagnostic difficulty, especially in biopsy specimens. However, their admixture with more classical areas of oncocytoma, uniform small nuclei with smooth nuclear border, absence of mitoses and necrosis, and immunoprofile compatible with oncocytoma should assist with the correct identification. The presence of oncoblasts and cells with degenerative nuclear atypia has no clinical significance.
Many ancillary tests have been suggested for confirmation of oncocytoma.7 However, immunohistochemistry has limited applications in the differential diagnosis of oncocytoma and chromophobe renal cell carcinoma (chRCC). The only stain that may be useful is cytokeratin 7 (CK7), which typically shows isolated scattered cell staining in oncocytoma and diffuse strong staining in chRCC (Figure 1, E). Clear cells localized in the central scar of oncocytoma may be immunoreactive with CK7. However, the pitfall of this immunostain is that, according to some publications, up to 18% of chRCCs are negative for CK7.8,9 Hales colloidal iron stain in our experience has low reproducibility and is significantly operator dependent, and distinction of patterns of staining is prone to diagnostic errors.10
It is controversial whether a definitive diagnosis of oncocytoma can be rendered on needle biopsy. Although some experts make a definitive diagnosis on biopsy, for needle core biopsy specimens that have morphologic and immunophenotypic findings of oncocytoma (Figure 1, F), we interpret them as oncocytic renal cell neoplasm. We add the following comment: “If this biopsy is representative of the entire lesion, it would be consistent with an oncocytoma. However, renal cell carcinoma (RCC) and hybrid tumor may uncommonly show focal areas with oncocytic features.” Although in excision specimens with the classic morphology of oncocytoma the use of CK7 may be avoided, in core biopsy specimens CK7 immunostain is more widely accepted to avoid misclassification of low-grade RCC as oncocytoma.11,12 Frozen sections to assess status of resection margins in partial nephrectomies may show normal proximal tubules with their characteristic abundant eosinophilic cytoplasm, which could be misinterpreted as a low-grade oncocytic neoplasm. The presence of intermixed glomeruli is a reliable sign of benign histology, as none of the low-grade oncocytic renal tumors has an infiltrative growth pattern.
Chromophobe RCC
Chromophobe RCC is in general a low-grade renal malignancy with the capability to demonstrate aggressive clinical behavior, yet having a more favorable prognosis than clear cell RCC (ccRCC) and papillary RCC (pRCC).13 Chromophobe RCC affects almost equally men and women and typically develops in the sixth decade of life.14,15 According to different studies, its incidence varies from 3% to 10% of all RCC.14,16 Although many of these tumors qualify as Fuhrman nuclear grade 3, the Fuhrman nuclear grading system does not accurately reflect their prognosis. In general, patients with stage pT1 and pT2 chRCC are almost always cured by surgery, and presentation with pT3 or pT4 disease is rare. Consequently, some urologic pathologists do not recommended assigning Fuhrman nuclear grade to chRCC.17,18 Other grading systems have been suggested for chRCC, but none of them has found general acceptance amongst practitioners.19,20
Microscopically, chRCC usually has a solid growth pattern with thin fibrovascular septa (Figure 2, A). However, foci with less cellularity and a tubulocystic growth pattern may overlap with oncocytoma.21 Myxoid or hyalinized background is usually not a feature of chRCC. Similar to oncocytoma, recognition of cytologic features typical of chRCC is diagnostic. Chromophobe RCC has abundant cytoplasm with prominent cell borders (“vegetable cells”) and may not have classically described perinuclear halos. However, diagnostic of chRCC is the presence of nuclei with preserved chromatin and irregular, wrinkled nuclear membrane (“raisinoid” nuclei) (Figure 2, B).6 This atypia is different from the degenerative atypia seen in oncocytoma. Atypical cells in chRCC may be encountered in different proportions but are rather evenly disturbed and do not tend to cluster.
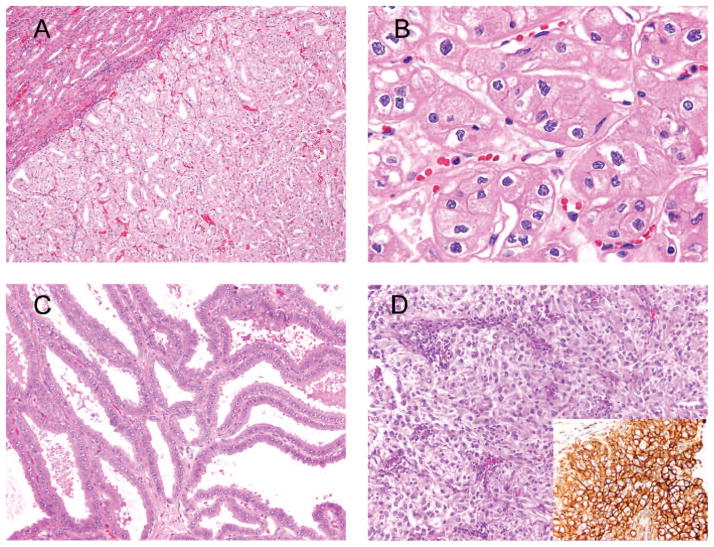
Figure 2.
A, Chromophobe renal cell carcinoma (RCC) with dense microtubular architecture. B, Enlarged pleomorphic nuclei with preserved chromatin and irregular, wrinkled nuclear membrane (“raisinoid” nuclei) diagnostic of chromophobe RCC. C, Tubulocystic carcinoma composed of tightly packed tubules and cysts. Prominent nucleoli are noted. D, Eosinophilic clear cell RCC with rich sinusoidal-like vasculature. Inset demonstrates CA-9 immunostain (hematoxylin-eosin, original magnifications ×100 [A], ×600 [B], and ×200 [C and D]; original magnification ×400 [D inset]).
CK7 is the best immunostain to distinguish chRCC from oncocytoma. Although CK7 is usually diffuse in chRCC, it is negative or only focal in oncocytoma. However, it should be recognized that there will be cases demonstrating classic cytologic features of chRCC yet are negative for CK7.8,9 In such cases, correct diagnosis of chRCC should be made by histomorphology alone. Some authors advocate using c-Kit (CD117) for diagnosis of oncocytoma.22 Although it is not expressed in most other RCCs,23 this stain is not specific and is also expressed in almost all chRCCs.24
Hybrid Tumor
This terminology refers to a specific tumor type that combines morphologic and immunohistochemical features of both oncocytoma and chRCC.25 Other tumors combining more than one pattern of neoplasm should be reported as unclassified. Its true incidence is not well established, which in part may be because of its rarity and subjectivity in its diagnosis. The existence of hybrid tumors is one of the examples precluding a definitive diagnosis of oncocytoma in needle core biopsy samples. Although these tumors may be sporadic, many are seen in patients with Birt-Hogg-Dubé syndrome.26,27 This syndrome can manifest with multifocal oncocytomas (oncocytosis) and chRCC and should be suggested by a pathologist when more than one lesion is present unilaterally or bilaterally. Fibrofolliculoma is a typical skin finding in patients with Birt-Hogg-Dubé syndrome.25 The clinical behavior of tumors in these settings is less aggressive than that of sporadic carcinomas, and active surveillance or kidney-sparing resection of the tumors should be considered by the surgeons.26,28,29
Tubulocystic Carcinoma
This is a rare variant of RCC with less than 100 cases reported.5,30,31 Its incidence is less than 1% of all renal carcinomas.32 Patients are usually in their sixth decade of life at presentation and demonstrate a striking male predominance (7:1).30 In older reports, tubulocystic RCC was proposed to be a low-grade carcinoma of collecting ducts of Bellini.33 However, subsequently it has been demonstrated that tubulocystic carcinoma and collecting duct carcinoma are distinct entities molecularly.34 Although the clinical behavior of tubulocystic carcinoma is usually favorable,30,32 we have seen 3 cases in which poorly differentiated foci were seen in otherwise classical tubulocystic carcinomas, imparting an adverse clinical behavior.35
Microscopically, tubulocystic carcinoma is composed of tightly packed tubules and cysts separated by thin fibrovascular septa (Figure 2, C). Papillary RCC architecture is seen in a minority of cases. The epithelial lining is represented by cuboidal or columnar cells with abundant eosinophilic cytoplasm containing large nuclei with prominent nucleoli.30,32 The latter is one of the major diagnostic pitfalls, as prominent nucleoli are one of the diagnostic features of tubulocystic carcinoma and should not be interpreted as evidence of a high-grade malignancy. Thus, Fuhrman nuclear grade is not assigned to tubulocystic carcinomas, recognizing their more favorable clinical behavior. The tubular pattern can mimic the tubular pattern seen in oncocytoma. The distinction is that typically in oncocytoma, in addition to the tubular pattern, there are other more classic areas with the fibromyxoid stroma. Also, although oncocytomas often have prominent nucleoli, the nuclei are perfectly round, whereas they are irregular in tubulocystic RCC.
Immunohistochemically and cytogenetically, there are overlapping findings with pRCC, both expressing racemase, CK7, CD10, and RCC antigen, and having gain of chromosomes 7 and 17 and loss of Y chromosome.31,32 However, in view of its distinct morphology and clinical behavior, tubulocystic carcinoma is best treated as a separate clinicopathologic entity.30,36 Distinguishing tubulocystic RCC and oncocytoma usually rests on morphology and is not aided by immunohistochemical stains.
ccRCC With Predominant Eosinophilic Cell Morphology
Clear cell RCC is the most common type of RCC, representing approximately 70% of the newly diagnosed cases, with peak incidence in the sixth to seventh decade. It affects men more often than women (2:1–3:1).37 Clear cell RCC has the worst prognosis among the most common types of RCC.38 Fuhrman nuclear grade was originally validated on ccRCC.39 Despite the name “clear cell,” a proportion of high-grade tumors acquire eosinophilic morphology, previously named granular cell RCC. However, designation of this tumor as clear cell (conventional) RCC is the current practice because these tumors with eosinophilic cytoplasm demonstrate the same molecular characteristics as those with classic clear cell morphology.37,40
Eosinophilic ccRCC grossly often has extensive hemorrhage and necrosis. Microscopically, it usually has a nested pattern of growth. One of the most pathognomonic morphologic features of ccRCC regardless of grade is a rich sinusoidal vasculature surrounding nests of neoplastic cells (Figure 2, D).37,38,41 This pattern of vasculature is preserved even in metastatic lesions, and it is a useful diagnostic clue in patients with metastatic disease of unknown primary.42 A wide spectrum of immunohistochemical stains is available to help discriminate this neoplasm from its mimickers. Clear cell RCC is usually positive for CD10 and CA-9 and negative for CK7 and high-molecular-weight keratin.11,43 Other useful positive markers are epithelial membrane antigen (EMA), vimentin, and RCC antigen. In contrast to microphthalmia transcription factor translocation RCC, ccRCC diffusely expresses epithelial markers (AE1/AE3 and EMA). Vimentin is positive in ccRCC, whereas it is negative in chRCC. Although having a limited role in differential diagnosis, PAX2 and PAX8 are reliable antibodies to establish renal origin in metastatic lesions. With the recognition of the morphology and immunoprofile, one may diagnose ccRCC even in the absence of clear cells.
Acquired Cystic Kidney Disease–Associated RCC
This is a recently recognized tumor that, as the name implies, develops only in the setting of acquired cystic disease.44 According to Tickoo et al, 45 acquired cystic kidney disease (ACKD)–associated RCC is the most common cancer subtype developing in end-stage kidney. It has a more favorable clinical behavior than carcinomas occurring in a sporadic setting.37,44 However, it is not clear if the tumor is indeed more indolent or simply detected at earlier stages because of regular screening of patients with end-stage kidney.12
Microscopically, the tumors are composed of large cells with abundant, deeply eosinophilic cytoplasm and large nuclei with prominent nucleoli (Figure 3, A). Different architectural patterns constituting different proportions of tumor may be observed. Solid and microcystic/macrocystic patterns are the most common. Intracytoplasmic vacuoles are a very common finding, creating a cribriform or sieve-like appearance of the neoplasm. Intratumoral oxalate crystals are consistently seen and are considered to be related to pathogenesis of ACKD-associated RCC.44,45 Fuhrman grade is not assigned to ACKD-associated RCC. Thorough examination of nephrectomy specimens with ACKD-associated RCC often reveals its precursor lesion—a cyst lined by atypical eosinophilic cells with hobnail appearance and containing intraluminal crystals (Figure 3, B). Papillary formation may be seen in a precursor lesion. Such cysts may be identified in otherwise nonneoplastic explants and should be reported as precursor lesions of ACKD-associated RCC. Although immunostains are rarely needed to diagnose these lesions, it is usually positive for racemase, CD10, and RCC and negative for CK7.
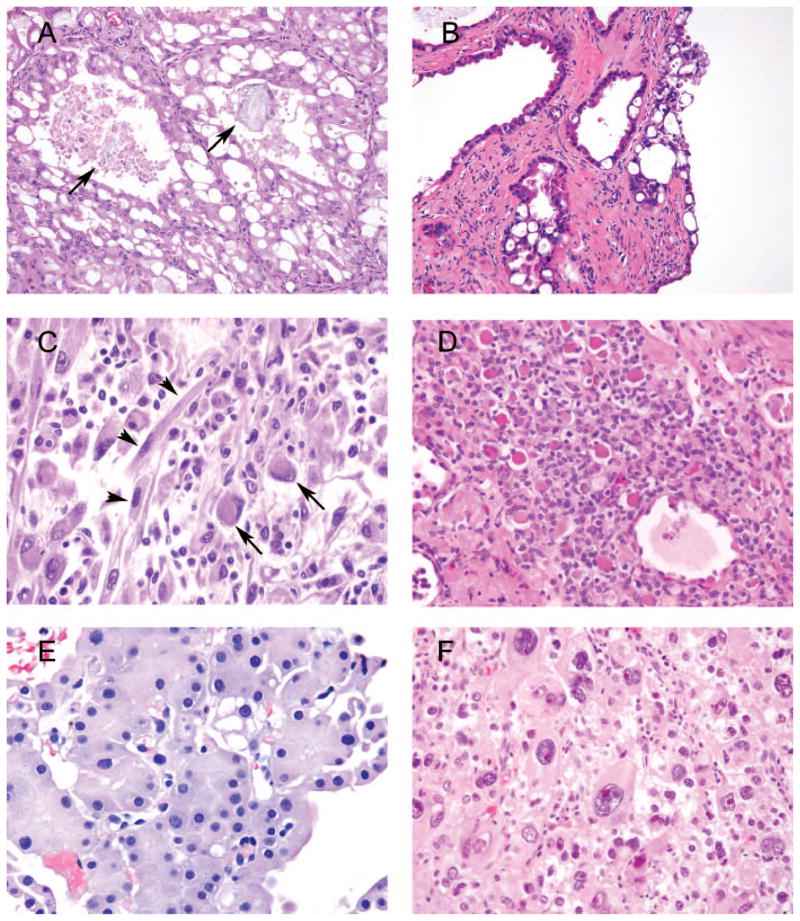
Figure 3.
A, Acquired cystic kidney disease (ACKD)–associated renal cell carcinoma (RCC) with intratumoral oxalate crystals (arrows). B, Atypical cysts in end-stage kidney disease with lining the same as ACKD-associated RCC, yet not forming solid mass. C, High-grade clear cell RCC (not shown) with rhabdoid (long arrows) and sarcomatoid (short arrows) differentiation. D, Microfollicular and macrofollicular architecture of follicular thyroid-like carcinoma with dense red and light pink secretions, correspondingly. E, Although cytologically this low-grade unclassified renal cell neoplasm is suggestive of oncocytoma, its dense growth pattern is not seen in the latter. F, Epithelioid angiomyolipoma composed of large bizarre cells with eosinophilic cytoplasm and smaller cells with pale cytoplasm and uniform small nucleoli (hematoxylin-eosin, original magnifications ×200 [A, B, and D], ×600 [C and E], and ×400 [F]).
RCC With Rhabdoid Features
This tumor is not a distinct type of carcinoma, but rather an extreme of dedifferentiation from any type of RCC. There is no specific immunoprofile of rhabdoid differentiation in RCC; rather, it should be identical to the immunoprofile of underlying RCC. The term rhabdoid designates a high-grade tumor cell with abundant eosinophilic cytoplasm and peripherally located nuclei, thus resembling rhabdomyoblasts (Figure 3, C).46,47 These highly aggressive morphologic patterns are more likely to be seen in high-grade tumors47 and, according to Gökden et al, 48 none of the 84 Fuhrman nuclear grade 1 tumors in their study had such changes.
In pediatric pathology a distinct neoplasm termed rhabdoid tumor of the kidney is recognized as a high-grade malignancy composed entirely of rhabdoid cells without true skeletal muscle differentiation.49,50 Rhabdoid differentiation seen in adult RCC is not related to pediatric rhabdoid tumor. Most rhabdoid tumors of kidney occur early in life (80% <2 years old; median age, 7–11 months).37,49,50 In the proper age range, loss of staining for INI-1 antibody is diagnostic of rhabdoid tumor.51 Expression of INI-1 is typically not lost in rhabdoid differentiation occurring in other defined types of high-grade RCC.
Follicular Thyroid-like Carcinoma
This is the least-frequent and least-studied variant of RCC. Eleven cases have been found in an unrestricted PubMed search.52–56 Two of these had metastasis—1 to hilar lymph node53 and 1 to lung54—proving their designation as a carcinoma. The nature of the tumor has no relationship to thyroid carcinoma, and the nomenclature stems purely from their light microscopic appearance.
Morphologically, these carcinomas have thick fibrous capsule and are composed of microfollicular and macro-follicular structures with light pink or dense red colloid-like secretions (Figure 3, D). The folliculi are lined by low cuboidal cells with lightly eosinophilic cytoplasm. Most of the tumors are reported as low Fuhrman nuclear grade. Immunohistochemical experience is limited, but thyroid transcription factor 1, thyroglobulin, RCC, and racemase appear to be negative. Some cases are reported to be immunoreactive with CK7, CD10, and PAX2. In addition to limited immunohistochemical experience, the light microscopy diagnosis may be problematic, as pRCC, clear cell pRCC, and some low-grade unclassified carcinomas may have areas that morphologically overlap with follicular thyroid-like carcinoma.
Eosinophilic Unclassified RCC
Unclassified RCC represents 1% to 5% of all RCCs.57–59 The definitions of unclassified RCC include (1) morphologic features that do not fit into any recognized RCC class, (2) combinations of 2 or more morphologic types by light microscopy, and (3) purely sarcomatoid carcinoma.12,37 Most unclassified RCC are more high-grade undifferentiated tumors and the Fuhrman grade has not been applied to these neoplasms.58,59 Consequently, if a tumor is diagnosed as unclassified RCC without any qualifiers, the assumption by clinicians is that it is high grade. However, it has been our experience that a subset of tumors that cannot be classified into well-known subtypes lack high cellularity, have minimal pleomorphism, do not exhibit necrosis, and have a low mitotic rate. We have termed these cases low-grade unclassified RCC, although there is no formal recognition of this dichotomization of unclassified RCC. In this review we restrict our description to only the less commonly seen low-grade unclassified RCC with cytoplasmic eosinophilia (oncocytoma-like).12
Low-grade eosinophilic unclassified RCC may architecturally mimic oncocytoma, but its nested arrangement is usually denser without the myxoid/hyalinized background (Figure 3, E). Cytologically, although these tumors do not have the nuclear features of chRCC (ie, raisinoid nuclei), binucleation and mitotic activity may be seen in excision specimens. In our experience, diffuse CK7 immunoreactivity is a helpful immunostain to rule out a definitive diagnosis of oncocytoma, keeping in mind that positive immunostaining is not equivalent to chRCC and pRCC. There are no prospective or retrospective studies describing these tumors, as this category of tumors represents an amalgamation of various distinct tumor types. Partial nephrectomy and ablation therapy may be acceptable treatment options for low-grade eosinophilic unclassified RCC.37
Epithelioid Angiomyolipoma
Epithelioid angiomyolipoma (EAML) may be misdiagnosed as unclassified high-grade carcinoma. Epithelioid angiomyolipoma represents approximately 8% of all angiomyolipomas.60,61 In contrast to triphasic angiomyolipomas, which are seen in the sixth decade of life, the mean age of the patients affected with EAML is 32 to 38 years according to different studies.60,61 Epithelioid angiomyolipoma is more commonly seen in tuberous sclerosis patients than its triphasic counterpart.60 Patients with tuberous sclerosis also have significantly larger angiomyolipomas.61
Histologically, there is no established cutoff of the percentage of epithelioid morphology required for a tumor to be designated as EAML, which in part could explain the diverse reports on the incidence of EAML in the literature. As such, it may be reasonable to report the percentage of tumor represented by EAML component. Epithelioid angiomyolipoma usually lacks a significant amount of intratumoral fat and malformed vessels. Architecturally, EAML grows in solid sheets or a large alveolar pattern. Cytologically, there may be 2 types of cells in EAML: (1) clear cells with finely granular cytoplasm and small monomorphic nuclei and (2) eosinophilic cells with abundant cytoplasm, epithelioid morphology, and large nuclei with prominent nucleoli, also known as amoeboid cells (Figure 3, F).12 The latter may demonstrate significant atypia and mitoses, leading to misdiagnosis of sarcoma or carcinoma.62 There are controversial data regarding clinical behavior of EAML.60,61,63–65 In our prior study we established criteria predicting clinical behavior of EAML with 78% sensitivity and 100% specificity.64 The presence of at least 3 of the following characteristics indicated a risk for malignant behavior: (1) at least 70% atypical epithelioid cells, (2) at least 2 mitoses per 10 high-power fields, (3) atypical mitoses, and (4) necrosis. Another group reported criteria for malignancy that partially overlap with ours, adding that extrarenal extension and renal vein involvement are also important prognostic factors.63 Although the 2 latter publications are based on consultation material and may be skewed towards more aggressive cases, a recent series by He et al66 analyzed 437 consecutive angiomyolipomas from 3 institutions and found only 20 cases of EAML, defined as showing more than 80% EAML morphology. Only one of these patients developed distant metastasis, and the authors concluded that overall the rate of aggressive behavior among EAMLs is very low. Epithelioid morphology without atypia has no prognostic significance per se. This entity can be confirmed using an immunohistochemical panel of pancytokeratin, EMA, PAX8, Melan-A, and HMB-45, with the latter 2 being immunoreactive in EAML. Diffuse reactivity with epithelial markers and PAX8 distinguishes sarcomatoid and high-grade unclassified RCC from EAML. The only other renal cell neoplasm positive for melanocytic markers, usually nonreactive with pancytokeratin and EMA, and variably reactive with PAX8 (~50%), is TFEB [t(6;11)] microphthalmia transcription factor translocation carcinoma. However, as described below, positive cathepsin K immunostain and specific molecular methods can reliably distinguish the latter.
PAPILLARY NEOPLASMS
Papillary RCC
Papillary RCC is the second most common renal cancer after ccRCC and comprises approximately 15% of renal cancers.67 The highest incidence of pRCC is in the sixth to seventh decade of life, with reported male predominance.68,69 Two types of pRCC are distinguished: type 1 is composed of linear arrays of small cells with scant amphophilic cytoplasm and low-grade nuclei, and type 2 has abundant eosinophilic cytoplasm with large crowded stratified nuclei with prominent nucleoli (Figure 4, A). A worse clinical behavior is attributed to type 2 pRCC.70 In addition to cytologic difference, presence of foamy macrophages and intracellular hemosiderin accumulation is uncommon in type 2 pRCC. Cytokeratin 7 immunoreactivity is seen in roughly 20% of type 2 pRCC cases, compared with around 80% in type 1 pRCC.68 Racemase is another useful marker expressed in pRCC.11 The oncocytic variant of pRCC is considered in the spectrum of type 2 carcinoma.28 Type 2 pRCC is usually higher grade (nucleolar grade 3), and this may account for the worse prognosis when compared with pRCC type 1, which is usually low-grade RCC. The identification of papillary architecture is usually not a challenge in most cases.
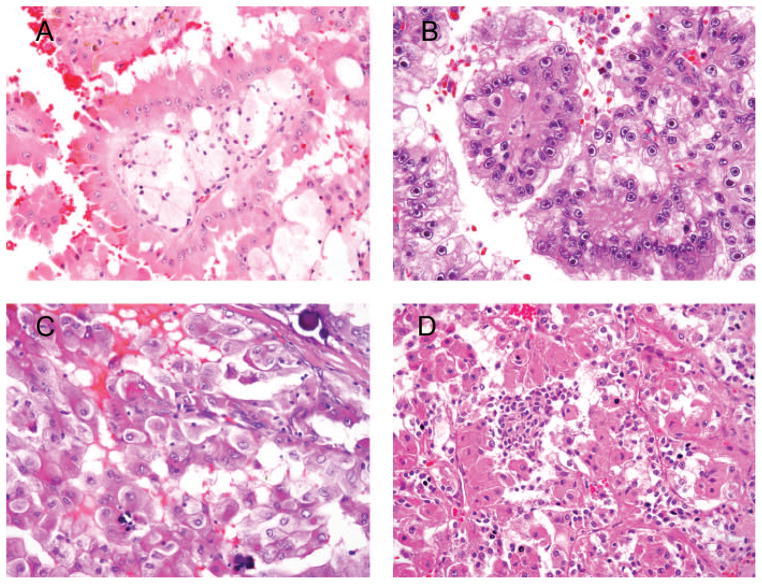
Figure 4.
A, Type 2 papillary renal cell carcinoma (RCC) with tall cell with eosinophilic cytoplasm, prominent nucleoli, intracytoplasmic pigment, and foamy macrophages (the latter 2 features are not common for type 2 papillary RCC). B, Hereditary leiomyomatosis–associated RCC composed of clear and eosinophilic cells. Large prominent nucleoli and perinucleolar halos are distinctive features. C, Xp11.2 (TFE3) translocation carcinoma with multiple psammoma bodies. D, Biphasic t(6;11) (TFEB) translocation carcinoma composed of large cells forming papillae and small cells clustered in between (hematoxylin-eosin, original magnifications ×400 [A, C, and D] and ×600 [B]).
Hereditary Leiomyomatosis and RCC
This rare syndrome is characterized by cutaneous leiomyomas and aggressive renal tumors developing in younger individuals (fourth decade of life) than sporadic tumors.71 Germline mutation in the fumarate hydratase gene is the underlying genetic alteration of hereditary leiomyomatosis and RCC.72 At time of presentation, lymph node metastases are not uncommon, and more than 50% of patients have advanced locoregional recurrent disease or succumb to renal cancer.71 Morphologically, by virtue of high-grade cytology, this type of RCC is reminiscent of type 2 pRCC, and some tumors may look akin to collecting-duct carcinoma. The distinctive morphologic feature of hereditary leiomyomatosis and RCC is prominent eosinophilic nucleoli with perinucleolar clearing (Figure 4, B).71 In isolation, however, these observations do not constitute sufficient ground for definitive diagnosis of hereditary leiomyomatosis and RCC. In our practice we call such tumors type 2 pRCC, assign them Fuhrman nuclear grade 3, and comment on peculiar morphology, highlighting that this may be seen in hereditary leiomyomatosis and RCC. Clinical information and genetic testing are essential for correct diagnosis.
Microphthalmia Transcription Factor Translocation Carcinoma
Two well-described entities with established diagnostic tools are included in this group: Xp11.2 and t(6;11) translocation RCC. A tendency toward young age at presentation is common to both neoplasms. There are specific immunostains for nuclear proteins produced by both translocations. However, the procedures are demanding and sensitive to preanalytical processing of the specimen, making it sometimes mandatory to use corresponding fluorescence in situ hybridization.
Xp11.2 Translocation RCC
TFE3 transcription factor gene maps to the short arm (p112) of the X chromosome. A number of translocations involving TFE3 resulting in fusion with other genes have been reported. Overall, although these carcinomas are relatively uncommon, comprising approximately 1% of adult RCC, they may represent up to 50% of RCC in children.28,73–76 Although behavior of these carcinomas in children is more favorable even in cases with lymph node metastases, adult disease is often lethal.77,78 Older age and advanced stage (distant metastases) are unfavorable prognostic factors.79
Histologically, the lesion is characterized by papillary architecture lined by clear and eosinophilic cells with abundant psammoma bodies (Figure 4, C). Xp11.2 RCC usually has clear to pale pink fluffy cytoplasm. Xp11.2 RCC often does not express epithelial markers (cytokeratin and EMA), but PAX2 and PAX8 are expressed in more than 50% of cases and useful for metastatic workup.80 The immunohistochemical marker for diagnosis of Xp11.2 RCC is the antibody against TFE3 protein.73 TFE3 immunohistochemistry may yield inaccurate results because of fixation issues. TFE3 break-apart fluorescence in situ hybridization may be performed on formalin-fixed, paraffin-embedded tissue and typically is more definitive.81 In a study by Martignoni et al, 82 cathepsin K immunoreactivity was demonstrated in 60% of Xp11.2 and 100% of t(6;11) carcinomas, and not seen in other types of RCC.
t(6;11) translocation RCC
There is limited experience with this renal cancer. Originally, it was considered to be a pediatric neoplasm, but later reports describe it in young adults.83,84 Most cases behave indolently, but rare tumors have metastasized and killed the patient. Molecularly, t(6;11) carcinoma is less heterogeneous than Xp11.2, and the underlying abnormality is α-TFEB gene fusion. Microscopically, t(6;11) carcinomas typically have a biphasic pattern (Figure 4, D). The majority of cells are large epithelioid eosinophilic and clear cells occasionally forming papillae. Clusters of small eosinophilic cells with small hyperchromatic nuclei are scattered throughout the tumor and often have rosettelike arrangement with accumulation of basement membrane–like material. TFEB carcinomas are uniformly positive for cathepsin K and melanocytic markers (HMB-45 and Melan-A), and only some may demonstrate focal staining with keratins.85 Antibodies against TFEB protein labeling nuclei and break-apart fluorescence in situ hybridization probes are available for clinical use.84,86
Summary
We have described common renal neoplasms with eosinophilic cytoplasm. Although many of those tumors have overlapping findings, recognition of key morphologic features and selection of correct ancillary tests (when needed) usually allow for a correct classification.
Footnotes
The authors have no relevant financial interest in the products or companies described in this article.
References
- 1.Perez-Ordonez B, Hamed G, Campbell S, et al. Renal oncocytoma: a clinicopathologic study of 70 cases. Am J Surg Pathol. 1997;21(8):871–883. doi: 10.1097/00000478-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Amin MB, Crotty TB, Tickoo SK, Farrow GM. Renal oncocytoma: a reappraisal of morphologic features with clinicopathologic findings in 80 cases. Am J Surg Pathol. 1997;21(1):1–12. doi: 10.1097/00000478-199701000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Choudhary S, Rajesh A, Mayer NJ, Mulcahy KA, Haroon A. Renal oncocytoma: CT features cannot reliably distinguish oncocytoma from other renal neoplasms. Clin Radiol. 2009;64(5):517–522. doi: 10.1016/j.crad.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Trpkov K, Yilmaz A, Uzer D, et al. Renal oncocytoma revisited: a clinicopathological study of 109 cases with emphasis on problematic diagnostic features. Histopathology. 2010;57(6):893–906. doi: 10.1111/j.1365-2559.2010.03726.x. [DOI] [PubMed] [Google Scholar]
- 5.Hes O, Michal M, Sima R, et al. Renal oncocytoma with and without intravascular extension into the branches of renal vein have the same morphological, immunohistochemical and genetic features. Virchows Arch. 2008;452(3):285–293. doi: 10.1007/s00428-007-0564-7. [DOI] [PubMed] [Google Scholar]
- 6.Tickoo SK, Amin MB. Discriminant nuclear features of renal oncocytoma and chromophobe renal cell carcinoma: analysis of their potential utility in the differential diagnosis. Am J Clin Pathol. 1998;110(6):782–787. doi: 10.1093/ajcp/110.6.782. [DOI] [PubMed] [Google Scholar]
- 7.Shen SS, Truong LD, Scarpelli M, Lopez-Beltran A. Role of immunohistochemistry in diagnosing renal neoplasms: when is it really useful? Arch Pathol Lab Med. 2012;136(4):410–417. doi: 10.5858/arpa.2011-0472-RA. [DOI] [PubMed] [Google Scholar]
- 8.Liu L, Qian J, Singh H, Meiers I, Zhou X, Bostwick DG. Immunohistochemical analysis of chromophobe renal cell carcinoma, renal oncocytoma, and clear cell carcinoma: an optimal and practical panel for differential diagnosis. Arch Pathol Lab Med. 2007;131(8):1290–1297. doi: 10.5858/2007-131-1290-IAOCRC. [DOI] [PubMed] [Google Scholar]
- 9.Wu SL, Kothari P, Wheeler TM, Reese T, Connelly JH. Cytokeratins 7 and 20 immunoreactivity in chromophobe renal cell carcinomas and renal oncocytomas. Mod Pathol. 2002;15(7):712–717. [Google Scholar]
- 10.Tickoo SK, Amin MB, Zarbo RJ. Colloidal iron staining in renal epithelial neoplasms, including chromophobe renal cell carcinoma: emphasis on technique and patterns of staining. Am J Surg Pathol. 1998;22(4):419–424. doi: 10.1097/00000478-199804000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Al-Ahmadie HA, Alden D, Fine SW, et al. Role of immunohistochemistry in the evaluation of needle core biopsies in adult renal cortical tumors: an ex vivo study. Am J Surg Pathol. 2011;35(7):949–961. doi: 10.1097/PAS.0b013e31821e25cd. [DOI] [PubMed] [Google Scholar]
- 12.Tickoo Satish K, Reuter Victor E. Kidney tumors and tumor-like conditions. In: Amin MB, editor. Genitourinary Diagnostic Pathology. Salt Lake City, UT: Amirsys; 2010. pp. 1.1–1.247. [Google Scholar]
- 13.Amin MB, Paner GP, Alvarado-Cabrero I, et al. Chromophobe renal cell carcinoma: histomorphologic characteristics and evaluation of conventional pathologic prognostic parameters in 145 cases. Am J Surg Pathol. 2008;32(12):1822–1834. doi: 10.1097/PAS.0b013e3181831e68. [DOI] [PubMed] [Google Scholar]
- 14.Cindolo L, de la Taille A, Schips L, et al. Chromophobe renal cell carcinoma: comprehensive analysis of 104 cases from multicenter European database. Urology. 2005;65(4):681–686. doi: 10.1016/j.urology.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Peyromaure M, Misrai V, Thiounn N, et al. Chromophobe renal cell carcinoma: analysis of 61 cases. Cancer. 2004;100(7):1406–1410. doi: 10.1002/cncr.20128. [DOI] [PubMed] [Google Scholar]
- 16.Beck SD, Patel MI, Snyder ME, et al. Effect of papillary and chromophobe cell type on disease-free survival after nephrectomy for renal cell carcinoma. Ann Surg Oncol. 2004;11(1):71–77. doi: 10.1007/BF02524349. [DOI] [PubMed] [Google Scholar]
- 17.Delahunt B, Sika-Paotonu D, Bethwaite PB, et al. Fuhrman grading is not appropriate for chromophobe renal cell carcinoma. Am J Surg Pathol. 2007;31(6):957–960. doi: 10.1097/01.pas.0000249446.28713.53. [DOI] [PubMed] [Google Scholar]
- 18.Cheville JC, Lohse CM, Sukov WR, Thompson RH, Leibovich BC. Chromophobe renal cell carcinoma: the impact of tumor grade on outcome. Am J Surg Pathol. 2012;36(6):851–856. doi: 10.1097/PAS.0b013e3182496895. [DOI] [PubMed] [Google Scholar]
- 19.Finley DS, Shuch B, Said JW, et al. The chromophobe tumor grading system is the preferred grading scheme for chromophobe renal cell carcinoma. J Urol. 2011;186(6):2168–2174. doi: 10.1016/j.juro.2011.07.068. [DOI] [PubMed] [Google Scholar]
- 20.Paner GP, Amin MB, Alvarado-Cabrero I, et al. A novel tumor grading scheme for chromophobe renal cell carcinoma: prognostic utility and comparison with Fuhrman nuclear grade. Am J Surg Pathol. 2010;34(9):1233–1240. doi: 10.1097/PAS.0b013e3181e96f2a. [DOI] [PubMed] [Google Scholar]
- 21.Thoenes W, Storkel S, Rumpelt HJ, Moll R, Baum HP, Werner S. Chromophobe cell renal carcinoma and its variants—a report on 32 cases. J Pathol. 1988;155(4):277–287. doi: 10.1002/path.1711550402. [DOI] [PubMed] [Google Scholar]
- 22.Carvalho JC, Wasco MJ, Kunju LP, Thomas DG, Shah RB. Cluster analysis of immunohistochemical profiles delineates CK7, vimentin, S100A1 and C-kit (CD117) as an optimal panel in the differential diagnosis of renal oncocytoma from its mimics. Histopathology. 2011;58(2):169–179. doi: 10.1111/j.1365-2559.2011.03753.x. [DOI] [PubMed] [Google Scholar]
- 23.Petit A, Castillo M, Santos M, Mellado B, Alcover JB, Mallofre C. KIT expression in chromophobe renal cell carcinoma: comparative immunohistochemical analysis of KIT expression in different renal cell neoplasms. Am J Surg Pathol. 2004;28(5):676–678. doi: 10.1097/00000478-200405000-00017. [DOI] [PubMed] [Google Scholar]
- 24.Pan CC, Chen PC, Chiang H. Overexpression of KIT (CD117) in chromophobe renal cell carcinoma and renal oncocytoma. Am J Clin Pathol. 2004;121(6):878–883. doi: 10.1309/A7M2-XTMJ-QK0K-PQER. [DOI] [PubMed] [Google Scholar]
- 25.Pavlovich CP, Walther MM, Eyler RA, et al. Renal tumors in the Birt-Hogg-Dubé syndrome. Am J Surg Pathol. 2002;26(12):1542–1552. doi: 10.1097/00000478-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Petersson F, Gatalica Z, Grossmann P, et al. Sporadic hybrid oncocytic/ chromophobe tumor of the kidney: a clinicopathologic, histomorphologic, immunohistochemical, ultrastructural, and molecular cytogenetic study of 14 cases. Virchows Arch. 2010;456(4):355–365. doi: 10.1007/s00428-010-0898-4. [DOI] [PubMed] [Google Scholar]
- 27.Delongchamps NB, Galmiche L, Eiss D, et al. Hybrid tumour “oncocytoma-chromophobe renal cell carcinoma” of the kidney: a report of seven sporadic cases. BJU Int. 2009;103(10):1381–1384. doi: 10.1111/j.1464-410X.2008.08263.x. [DOI] [PubMed] [Google Scholar]
- 28.Srigley JR, Delahunt B, Eble JN, et al. The International Society of Urological Pathology (ISUP) Vancouver classification of renal neoplasia. Am J Surg Pathol. 2013;37(10):1469–1489. doi: 10.1097/PAS.0b013e318299f2d1. [DOI] [PubMed] [Google Scholar]
- 29.Waldert M, Klatte T, Haitel A, et al. Hybrid renal cell carcinomas containing histopathologic features of chromophobe renal cell carcinomas and oncocytomas have excellent oncologic outcomes. Eur Urol. 2010;57(4):661–665. doi: 10.1016/j.eururo.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Amin MB, MacLennan GT, Gupta R, et al. Tubulocystic carcinoma of the kidney: clinicopathologic analysis of 31 cases of a distinctive rare subtype of renal cell carcinoma. Am J Surg Pathol. 2009;33(3):384–392. doi: 10.1097/PAS.0b013e3181872d3f. [DOI] [PubMed] [Google Scholar]
- 31.Zhou M, Yang XJ, Lopez JI, et al. Renal tubulocystic carcinoma is closely related to papillary renal cell carcinoma: implications for pathologic classification. Am J Surg Pathol. 2009;33(12):1840–1849. doi: 10.1097/PAS.0b013e3181be22d1. [DOI] [PubMed] [Google Scholar]
- 32.Alexiev BA, Drachenberg CB. Tubulocystic carcinoma of the kidney: a histologic, immunohistochemical, and ultrastructural study. Virchows Arch. 2013;462(5):575–581. doi: 10.1007/s00428-013-1398-0. [DOI] [PubMed] [Google Scholar]
- 33.MacLennan GT, Farrow GM, Bostwick DG. Low-grade collecting duct carcinoma of the kidney: report of 13 cases of low-grade mucinous tubulocystic renal carcinoma of possible collecting duct origin. Urology. 1997;50(5):679–684. doi: 10.1016/S0090-4295(97)00335-X. [DOI] [PubMed] [Google Scholar]
- 34.Osunkoya AO, Young AN, Wang W, Netto GJ, Epstein JI. Comparison of gene expression profiles in tubulocystic carcinoma and collecting duct carcinoma of the kidney. Am J Surg Pathol. 2009;33(7):1103–1106. doi: 10.1097/PAS.0b013e3181a13e7b. [DOI] [PubMed] [Google Scholar]
- 35.Al-Hussain TO, Cheng L, Zhang S, Epstein JI. Tubulocystic carcinoma of the kidney with poorly differentiated foci: a series of 3 cases with fluorescence in situ hybridization analysis. Hum Pathol. 2013;44(7):1406–1411. doi: 10.1016/j.humpath.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 36.Azoulay S, Vieillefond A, Paraf F, et al. Tubulocystic carcinoma of the kidney: a new entity among renal tumors. Virchows Arch. 2007;451(5):905–909. doi: 10.1007/s00428-007-0483-7. [DOI] [PubMed] [Google Scholar]
- 37.Zhou M, Netto GJ, Epstein JI. Neoplastic disease of the kidney. In: Zhou M, Netto GJ, Epstein JI, editors. Uropathology. Philadelphia, PA: Elsevier; 2012. pp. 266–338. [Google Scholar]
- 38.Grignon DJ, Che M. Clear cell renal cell carcinoma. Clin Lab Med. 2005;25(2):305–316. doi: 10.1016/j.cll.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6(7):655–663. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Yang XJ, Takahashi M, Schafernak KT, et al. Does “granular cell” renal cell carcinoma exist?: molecular and histological reclassification. Histopathology. 2007;50(5):678–680. doi: 10.1111/j.1365-2559.2007.02626.x. [DOI] [PubMed] [Google Scholar]
- 41.Kryvenko ON, Roquero L, Gupta NS, Lee MW, Epstein JI. Low-grade clear cell renal cell carcinoma mimicking hemangioma of the kidney: a series of 4 cases. Arch Pathol Lab Med. 2013;137(2):251–254. doi: 10.5858/arpa.2011-0615-OA. [DOI] [PubMed] [Google Scholar]
- 42.Sim SJ, Ro JY, Ordonez NG, Park YW, Kee KH, Ayala AG. Metastatic renal cell carcinoma to the bladder: a clinicopathologic and immunohistochemical study. Mod Pathol. 1999;12(4):351–355. [PubMed] [Google Scholar]
- 43.Yasir S, Herrera L, Gomez-Fernandez C, et al. CD10+ and CK7/RON– immunophenotype distinguishes renal cell carcinoma, conventional type with eosinophilic morphology from its mimickers. Appl Immunohistochem Mol Morphol. 2012;20(5):454–461. doi: 10.1097/PAI.0b013e31823fecd3. [DOI] [PubMed] [Google Scholar]
- 44.Sule N, Yakupoglu U, Shen SS, et al. Calcium oxalate deposition in renal cell carcinoma associated with acquired cystic kidney disease: a comprehensive study. Am J Surg Pathol. 2005;29(4):443–451. doi: 10.1097/01.pas.0000152131.58492.97. [DOI] [PubMed] [Google Scholar]
- 45.Tickoo SK, dePeralta-Venturina MN, Harik LR, et al. Spectrum of epithelial neoplasms in end-stage renal disease: an experience from 66 tumor-bearing kidneys with emphasis on histologic patterns distinct from those in sporadic adult renal neoplasia. Am J Surg Pathol. 2006;30(2):141–153. doi: 10.1097/01.pas.0000185382.80844.b1. [DOI] [PubMed] [Google Scholar]
- 46.Leroy X, Zini L, Buob D, Ballereau C, Villers A, Aubert S. Renal cell carcinoma with rhabdoid features: an aggressive neoplasm with overexpression of p53. Arch Pathol Lab Med. 2007;131(1):102–106. doi: 10.5858/2007-131-102-RCCWRF. [DOI] [PubMed] [Google Scholar]
- 47.Chapman-Fredricks JR, Herrera L, Bracho J, et al. Adult renal cell carcinoma with rhabdoid morphology represents a neoplastic dedifferentiation analogous to sarcomatoid carcinoma. Ann Diagn Pathol. 2011;15(5):333–337. doi: 10.1016/j.anndiagpath.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 48.Gökden N, Nappi O, Swanson PE, et al. Renal cell carcinoma with rhabdoid features. Am J Surg Pathol. 2000;24(10):1329–1338. doi: 10.1097/00000478-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 49.Vujanic GM, Sandstedt B, Harms D, Boccon-Gibod L, Delemarre JF. Rhabdoid tumour of the kidney: a clinicopathological study of 22 patients from the International Society of Paediatric Oncology (SIOP) nephroblastoma file. Histopathology. 1996;28(4):333–340. doi: 10.1046/j.1365-2559.1996.d01-436.x. [DOI] [PubMed] [Google Scholar]
- 50.Weeks DA, Beckwith JB, Mierau GW, Luckey DW. Rhabdoid tumor of kidney: a report of 111 cases from the National Wilms’ Tumor Study Pathology Center. Am J Surg Pathol. 1989;13(6):439–458. [PubMed] [Google Scholar]
- 51.Hoot AC, Russo P, Judkins AR, Perlman EJ, Biegel JA. Immunohistochemical analysis of hSNF5/INI1 distinguishes renal and extra-renal malignant rhabdoid tumors from other pediatric soft tissue tumors. Am J Surg Pathol. 2004;28(11):1485–1491. doi: 10.1097/01.pas.0000141390.14548.34. [DOI] [PubMed] [Google Scholar]
- 52.Jung SJ, Chung JI, Park SH, Ayala AG, Ro JY. Thyroid follicular carcinoma-like tumor of kidney: a case report with morphologic, immunohistochemical, and genetic analysis. Am J Surg Pathol. 2006;30(3):411–415. doi: 10.1097/01.pas.0000194745.10670.dd. [DOI] [PubMed] [Google Scholar]
- 53.Amin MB, Gupta R, Ondrej H, et al. Primary thyroid-like follicular carcinoma of the kidney: report of 6 cases of a histologically distinctive adult renal epithelial neoplasm. Am J Surg Pathol. 2009;33(3):393–400. doi: 10.1097/PAS.0b013e31818cb8f5. [DOI] [PubMed] [Google Scholar]
- 54.Dhillon J, Tannir NM, Matin SF, Tamboli P, Czerniak BA, Guo CC. Thyroid-like follicular carcinoma of the kidney with metastases to the lungs and retroperitoneal lymph nodes. Hum Pathol. 2011;42(1):146–150. doi: 10.1016/j.humpath.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sterlacci W, Verdorfer I, Gabriel M, Mikuz G. Thyroid follicular carcinoma-like renal tumor: a case report with morphologic, immunophenotypic, cytogenetic, and scintigraphic studies. Virchows Arch. 2008;452(1):91–95. doi: 10.1007/s00428-007-0486-4. [DOI] [PubMed] [Google Scholar]
- 56.Alessandrini L, Fassan M, Gardiman MP, Guttilla A, Zattoni F, Galletti TP. Thyroid-like follicular carcinoma of the kidney: report of two cases with detailed immunohistochemical profile and literature review. Virchows Arch. 2012;461(3):345–350. doi: 10.1007/s00428-012-1298-8. [DOI] [PubMed] [Google Scholar]
- 57.Lopez-Beltran A, Kirkali Z, Montironi R, et al. Unclassified renal cell carcinoma: a report of 56 cases. BJU Int. 2012;110(6):786–793. doi: 10.1111/j.1464-410X.2012.10934.x. [DOI] [PubMed] [Google Scholar]
- 58.Zisman A, Chao DH, Pantuck AJ, et al. Unclassified renal cell carcinoma: clinical features and prognostic impact of a new histological subtype. J Urol. 2002;168(3):950–955. doi: 10.1016/S0022-5347(05)64549-1. [DOI] [PubMed] [Google Scholar]
- 59.Karakiewicz PI, Hutterer GC, Trinh QD, et al. Unclassified renal cell carcinoma: an analysis of 85 cases. BJU Int. 2007;100(4):802–808. doi: 10.1111/j.1464-410X.2007.07148.x. [DOI] [PubMed] [Google Scholar]
- 60.Aydin H, Magi-Galluzzi C, Lane BR, et al. Renal angiomyolipoma: clinicopathologic study of 194 cases with emphasis on the epithelioid histology and tuberous sclerosis association. Am J Surg Pathol. 2009;33(2):289–297. doi: 10.1097/PAS.0b013e31817ed7a6. [DOI] [PubMed] [Google Scholar]
- 61.Lane BR, Aydin H, Danforth TL, et al. Clinical correlates of renal angiomyolipoma subtypes in 209 patients: classic, fat poor, tuberous sclerosis associated and epithelioid. J Urol. 2008;180(3):836–843. doi: 10.1016/j.juro.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 62.Delgado R, de Leon Bojorge B, Albores-Saavedra J. Atypical angiomyolipoma of the kidney: a distinct morphologic variant that is easily confused with a variety of malignant neoplasms. Cancer. 1998;83(8):1581–1592. [PubMed] [Google Scholar]
- 63.Nese N, Martignoni G, Fletcher CD, et al. Pure epithelioid PEComas (so-called epithelioid angiomyolipoma) of the kidney: a clinicopathologic study of 41 cases: detailed assessment of morphology and risk stratification. Am J Surg Pathol. 2011;35(2):161–176. doi: 10.1097/PAS.0b013e318206f2a9. [DOI] [PubMed] [Google Scholar]
- 64.Brimo F, Robinson B, Guo C, Zhou M, Latour M, Epstein JI. Renal epithelioid angiomyolipoma with atypia: a series of 40 cases with emphasis on clinicopathologic prognostic indicators of malignancy. Am J Surg Pathol. 2010;34(5):715–722. doi: 10.1097/PAS.0b013e3181d90370. [DOI] [PubMed] [Google Scholar]
- 65.L’Hostis H, Deminiere C, Ferriere JM, Coindre JM. Renal angiomyolipoma: a clinicopathologic, immunohistochemical, and follow-up study of 46 cases. Am J Surg Pathol. 1999;23(9):1011–1020. doi: 10.1097/00000478-199909000-00003. [DOI] [PubMed] [Google Scholar]
- 66.He W, Cheville JC, Sadow PM, et al. Epithelioid angiomyolipoma of the kidney: pathological features and clinical outcome in a series of consecutively resected tumors. Mod Pathol. 2013;26(10):1355–1364. doi: 10.1038/modpathol.2013.72. [DOI] [PubMed] [Google Scholar]
- 67.Tickoo SK, Reuter VE. Differential diagnosis of renal tumors with papillary architecture. Adv Anat Pathol. 2011;18(2):120–132. doi: 10.1097/PAP.0b013e31820cb3dd. [DOI] [PubMed] [Google Scholar]
- 68.Delahunt B, Eble JN. Papillary renal cell carcinoma: a clinicopathologic and immunohistochemical study of 105 tumors. Mod Pathol. 1997;10(6):537–544. [PubMed] [Google Scholar]
- 69.Amin MB, Corless CL, Renshaw AA, Tickoo SK, Kubus J, Schultz DS. Papillary (chromophil) renal cell carcinoma: histomorphologic characteristics and evaluation of conventional pathologic prognostic parameters in 62 cases. Am J Surg Pathol. 1997;21(6):621–635. doi: 10.1097/00000478-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 70.Delahunt B, Eble JN, McCredie MR, Bethwaite PB, Stewart JH, Bilous AM. Morphologic typing of papillary renal cell carcinoma: comparison of growth kinetics and patient survival in 66 cases. Hum Pathol. 2001;32(6):590–595. doi: 10.1053/hupa.2001.24984. [DOI] [PubMed] [Google Scholar]
- 71.Grubb RL, 3rd, Franks ME, Toro J, et al. Hereditary leiomyomatosis and renal cell cancer: a syndrome associated with an aggressive form of inherited renal cancer. J Urol. 2007;177(6):2074–2079. doi: 10.1016/j.juro.2007.01.155. discussion 2079–2080. [DOI] [PubMed] [Google Scholar]
- 72.Heinritz W, Paasch U, Sticherling M, et al. Evidence for a founder effect of the germline fumarate hydratase gene mutation R58P causing hereditary leiomyomatosis and renal cell cancer (HLRCC) Ann Hum Genet. 2008;72(pt 1):35–40. doi: 10.1111/j.1469-1809.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- 73.Argani P, Lal P, Hutchinson B, Lui MY, Reuter VE, Ladanyi M. Aberrant nuclear immunoreactivity for TFE3 in neoplasms with TFE3 gene fusions: a sensitive and specific immunohistochemical assay. Am J Surg Pathol. 2003;27(6):750–761. doi: 10.1097/00000478-200306000-00005. [DOI] [PubMed] [Google Scholar]
- 74.Argani P, Antonescu CR, Couturier J, et al. PRCC-TFE3 renal carcinomas: morphologic, immunohistochemical, ultrastructural, and molecular analysis of an entity associated with the t(X;1)(p11.2;q21) Am J Surg Pathol. 2002;26(12):1553–1566. doi: 10.1097/00000478-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 75.Argani P, Antonescu CR, Illei PB, et al. Primary renal neoplasms with the ASPL-TFE3 gene fusion of alveolar soft part sarcoma: a distinctive tumor entity previously included among renal cell carcinomas of children and adolescents. Am J Pathol. 2001;159(1):179–192. doi: 10.1016/S0002-9440(10)61684-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Argani P, Lui MY, Couturier J, Bouvier R, Fournet JC, Ladanyi M. A novel CLTC-TFE3 gene fusion in pediatric renal adenocarcinoma with t(X;17)(p11.2; q23) Oncogene. 2003;22(34):5374–5378. doi: 10.1038/sj.onc.1206686. [DOI] [PubMed] [Google Scholar]
- 77.Geller JI, Argani P, Adeniran A, et al. Translocation renal cell carcinoma: lack of negative impact due to lymph node spread. Cancer. 2008;112(7):1607–1616. doi: 10.1002/cncr.23331. [DOI] [PubMed] [Google Scholar]
- 78.Argani P, Olgac S, Tickoo SK, et al. Xp11 translocation renal cell carcinoma in adults: expanded clinical, pathologic, and genetic spectrum. Am J Surg Pathol. 2007;31(8):1149–1160. doi: 10.1097/PAS.0b013e318031ffff. [DOI] [PubMed] [Google Scholar]
- 79.Ellis CL, Eble JN, Subhawong AP, et al. Clinical heterogeneity of Xp11 translocation renal cell carcinoma: impact off fusion subtype, age and stage. Mod Pathol. 2014;27(6):875–886. doi: 10.1038/modpathol.2013.208. [DOI] [PubMed] [Google Scholar]
- 80.Argani P, Hicks J, De Marzo AM, et al. Xp11 translocation renal cell carcinoma (RCC): extended immunohistochemical profile emphasizing novel RCC markers. Am J Surg Pathol. 2010;34(9):1295–1303. doi: 10.1097/PAS.0b013e3181e8ce5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Green WM, Yonescu R, Morsberger L, et al. Utilization of a TFE3 break-apart FISH assay in a renal tumor consultation service. Am J Surg Pathol. 2013;37(8):1150–1163. doi: 10.1097/PAS.0b013e31828a69ae. [DOI] [PubMed] [Google Scholar]
- 82.Martignoni G, Pea M, Gobbo S, et al. Cathepsin-K immunoreactivity distinguishes MiTF/TFE family renal translocation carcinomas from other renal carcinomas. Mod Pathol. 2009;22(8):1016–1022. doi: 10.1038/modpathol.2009.58. [DOI] [PubMed] [Google Scholar]
- 83.Argani P, Hawkins A, Griffin CA, et al. A distinctive pediatric renal neoplasm characterized by epithelioid morphology, basement membrane production, focal HMB45 immunoreactivity, and t(6;11)(p21.1;q12) chromosome translocation. Am J Pathol. 2001;158(6):2089–2096. doi: 10.1016/S0002-9440(10)64680-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Argani P, Lae M, Hutchinson B, et al. Renal carcinomas with the t(6;11)(p21;q12): clinicopathologic features and demonstration of the specific alpha-TFEB gene fusion by immunohistochemistry, RT-PCR, and DNA PCR. Am J Surg Pathol. 2005;29(2):230–240. doi: 10.1097/01.pas.0000146007.54092.37. [DOI] [PubMed] [Google Scholar]
- 85.Camparo P, Vasiliu V, Molinie V, et al. Renal translocation carcinomas: clinicopathologic, immunohistochemical, and gene expression profiling analysis of 31 cases with a review of the literature. Am J Surg Pathol. 2008;32(5):656–670. doi: 10.1097/PAS.0b013e3181609914. [DOI] [PubMed] [Google Scholar]
- 86.Argani P, Yonescu R, Morsberger L, et al. Molecular confirmation of t(6;11)(p21;q12) renal cell carcinoma in archival paraffin-embedded material using a break-apart TFEB FISH assay expands its clinicopathologic spectrum. Am J Surg Pathol. 2012;36(10):1516–1526. doi: 10.1097/PAS.0b013e3182613d8f. [DOI] [PMC free article] [PubMed] [Google Scholar]