Abstract
The handicap principle predicts that sexual traits are more susceptible to inbreeding depression than nonsexual traits. However, this hypothesis has received little testing and results are inconsistent. We used 11 generations of full-sibling mating to test the effect of inbreeding on sexual and nonsexual traits in the stalk-eyed fly Diasemopsis meigenii. Consistent with the theoretical predictions, the male sexual trait (eyespan) decreased more than nonsexual traits (female eyespan and male wing length), even after controlling for body size variation. In addition, male eyespan was a reliable predictor of line extinction, unlike other nonsexual traits. After 11 generations, inbred lines were crossed to generate inbred and outbred families. All morphological traits were larger in outbred individuals than inbred individuals. This heterosis was greater in male eyespan than in male wing length, but not female eyespan. The elevated response in male eyespan to genetic stress mirrored the result found using environmental stress during larval development and suggests that common mechanisms underlie the patterns observed. Overall, these results support the hypothesis that male sexual traits suffer more from inbreeding depression than nonsexual traits and are in line with predictions based on the handicap principle.
Keywords: Condition dependence, extinction risk, heterosis, inbreeding depression, sexual ornament, stalk-eyed fly
The handicap principle predicts that exaggerated sexual ornaments can evolve when they act as signals of quality (Zahavi 1975; Enquist 1985; Pomiankowski 1987; Maynard Smith and Harper 2003). The association of the sexual trait with quality arises when only individuals in good condition are able to afford the cost of having costly sexual traits. Consequently, sexual trait expression is said to be condition dependent (Pomiankowski 1987; Johnstone et al. 2009). The application of the handicap principle to cases where males advertise their genetic quality was initially contentious, but is now supported by an abundance of theoretical work (Andersson 1986; Pomiankowski 1987; Grafen 1990; Iwasa et al. 1991; Rowe and Houle 1996; Cotton et al. 2004a; Johnstone et al. 2009) and by some empirical studies (reviewed in Johnstone 1995; Cotton et al. 2004a), although not by a recent meta-analysis (Prokop et al. 2012).
Most investigations into the condition dependence of male sexual ornaments have focused on manipulating environmental quality, for example altering diet (Kotiaho 2000; Hunt et al. 2004; Cotton et al. ,) or parasite load (Zuk et al. 1990; Thompson et al. 1997; Costa and Macedo 2005). This approach reflects the ease with which the environment can be manipulated under laboratory conditions. In contrast, it is much harder to manipulate genetic quality in a controlled manner and the genetic basis of the condition dependence of sexual traits remains relatively poorly investigated (Cotton et al. 2004a). A common approach has been to demonstrate that there is genetic variation in the condition-dependent response of traits to environmental stress (reviewed in Bussiere et al. 2008), although this does not directly show whether genetic stress causes a condition-dependent response. In this study, we bridge this gap in our understanding by using inbreeding to explicitly induce genetic stress and measure the condition-dependent response in a male sexual ornament.
Inbreeding has been advocated as a useful tool for studying the genetics of condition dependence (Rowe and Houle 1996; Tomkins et al. 2004) because an increase in homozygosity is associated with a reduction in fitness, called inbreeding depression (Roff 2002). Two separate mechanisms have been proposed to explain inbreeding depression. The dominance hypothesis predicts that fitness decreases because inbreeding exposes rare recessive deleterious mutations (Rowe and Houle 1996; Roff 2002; Tomkins et al. 2004). Alternatively, the overdominance hypothesis predicts that fitness decreases under inbreeding if heterozygotes have a fitness advantage compared with homozygotes (Wright 1977). Although the exact importance of each mechanism is still unclear, recessive deleterious mutations appear to account for a greater proportion of inbreeding depression (Charlesworth and Charlesworth 1999; Charlesworth and Willis 2009).
Generally, a trait will show greater inbreeding depression if it harbors higher standing genetic variation controlled by many loci with nonadditive effects (Charlesworth and Willis 2009). This has led to the prediction that condition-dependent sexual traits will suffer from elevated inbreeding depression in comparison with nonsexual traits (Rowe and Houle 1996; Tomkins et al. 2004). This prediction stems from the observations that condition-dependent sexual traits, like other fitness-related traits, have high standing genetic variation and in theory these traits are affected by allelic variation at multiple loci (Pomiankowski and Moller 1995; Rowe and Houle 1996). An extrapolation of this view is that where the male trait signals genetic quality, males with larger sexual ornaments are expected to be more resistant to the effects of inbreeding and so produce offspring with higher viability.
A small number of studies have compared the effect of inbreeding on sexual and nonsexual traits. The results have not produced a consistent pattern, with inbreeding depression in male sexual traits being demonstrated in some species including zebra finches (Bolund et al. 2010), guppies (Sheridan and Pomiankowski 1997; van Oosterhout et al. 2003; Mariette et al. 2006; Ala-Honkola et al. 2009; Zajitschek and Brooks 2010) and Drosophila (Sharp 1984; Aspi 2000). However, inbreeding was not associated with a reduction in male sexual traits in sticklebacks (Frommen et al. 2008), only in some finer-scale calling parameters in male crickets (Drayton et al. 2007) and not in the male sexual trait (eyespan) of an Asian stalk-eyed fly, Teleopsis dalmanni, after accounting for body scaling (Prokop et al. 2010).
Here, we investigate the effect of inbreeding in a different stalk-eyed fly species, Diasemopsis meigenii. As in many stalk-eyed fly species (Wilkinson 2001; Chapman et al. 2005), male D. meigenii have larger eyespan than females, even after controlling for body scaling (Baker and Wilkinson 2001) and females show strong mate preference for large eyespan males (Cotton et al. 2006). Here, a full-sibling mating design was used to generate inbred lines of D. meigenii. Unlike most previous studies investigating inbreeding in sexual traits, inbreeding was carried out over 11 successive generations, such that the lines were highly inbred (f ∼ 0.908; Falconer and Mackay 1996). The consequences of inbreeding were tracked for the male sexual trait (eyespan), other nonsexually selected traits in males and females and a life-history trait (female fecundity). To assess whether the male sexual trait indicated genetic quality as predicted by the handicap principle, we tested whether male eyespan decreased more than female eyespan and male wing length both before and after controlling for body size variation. We further tested whether male eyespan was a reliable predictor of line survival given repeated inbreeding (Radwan 2008). At the end of the period of inbreeding, the inbred lines were crossed to measure the degree of heterosis in sexual and nonsexual traits. This enabled a further test of the handicap principle. We asked whether male eyespan increased more than female eyespan and male wing length in outbred individuals when compared to inbred individuals. Finally, a more standard approach using environmental stress (larval diet) was used to study condition dependence in the male sexual trait of D. meigenii. This test was used to determine whether the effect of genetic stress was similar to that observed when applying environmental stress, and so indicative of common mechanisms underlying the responses of traits to different kinds of stress.
Methods
PRODUCTION OF STOCK FLIES
A stock population of D. meigenii was collected from Nelspruit, South Africa, in 2001 (by R. H. Baker) and maintained in cage culture (> 100 individuals per cage) in a 25°C temperature controlled room on a 12 h: 12 h light: dark cycle. Flies were fed twice a week on puréed sweetcorn.
PRODUCTION OF EXPERIMENTAL FLIES FOR THE INBREEDING REGIME
Inbred lines were founded from male–female virgin pairs (n = 105, defined as the F0 generation) taken from the stock population. Pairs were allowed to mate freely in 500 mL pots. The base of each pot was lined with moist cotton wool and blue tissue paper to visualize eggs, which were collected three times a week. At collection, eggs were transferred to Petri dishes lined with moist cotton wool with an excess of puréed sweetcorn to minimize larval competition. Pupae were transferred to a 500 mL pot and left to eclose. Male–female F0 pairs were kept until they produced approximately 10 male and 10 female F1 offspring. The F1 offspring were separated by sex within a week of eclosion and allowed to reach sexual maturity (approximately 7 weeks after eclosion).
Within each line up to five pairs of male and female F1 full-siblings were randomly paired, each pair being defined as a subline. These were maintained until one subline per line had produced 10 male and 10 female F2 adult offspring. This subline alone was chosen to maintain the line and the remaining sublines were culled. In cases where multiple sublines had produced more than 10 male and 10 female offspring, a single subline was randomly chosen to maintain the line. The procedure was repeated for 11 generations (f ∼ 0.908; Falconer and Mackay 1996) and took approximately 36 months.
EFFECT OF INBREEDING ON MORPHOLOGICAL TRAITS AND FEMALE FECUNDITY
All flies that eclosed were collected and stored at −20°C for future measurement. After generation 11, flies from the chosen subline for each generation were measured for sexual and nonsexual morphological traits. A maximum of 10 males and 10 females were measured from each line per generation. Flies were measured for eyespan (distance between the outmost tips of the eyes; Cotton et al. 2004b), thorax length (distance between the middle of the most anterior part of the head to the posterior edge of the thorax; Cotton et al. 2004b), and wing length (distance from the anterior cross-vein to the wing margin of the right wing; Baker and Wilkinson 2003). All measurements were made using a digital camera mounted on a monocular microscope using the image analysis program ImageJ (version 1.43).
The number of eggs laid (Cotton et al. 2006) by each female in each subline was recorded during the first four generations of inbreeding. Eggs were counted once a week over a 3-day period in each of the three successive weeks immediately post-sexual maturity and used to calculate the average fecundity per day for each female.
The effect of inbreeding was analyzed by comparing mean trait sizes (thorax length, eyespan, and wing length) for generations 1–11. The founding generation (F0) was excluded from all analyses because the flies were reared in a high-stress environment and measurements would be based on a sample of only two flies per line. General linear mixed models (GLMMs) were used to determine the effect of the inbreeding coefficient (f) on trait size separately for males and females. Each GLMM included line as a random effect, nested within generation. Models for eyespan and wing length were performed both with and without thorax length as a covariate, to control for allometric scaling (David et al. 1998). To distinguish between these tests, we refer to absolute eyespan and absolute wing length or relative eyespan and relative wing length (relative denoting that thorax length was included as a covariate).
The degree of inbreeding depression was quantified using 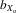 coefficients, that is, the trait value in inbred flies relative to the mean value in outbred flies (DeRose and Roff 1999). In our study, this is defined as
coefficients, that is, the trait value in inbred flies relative to the mean value in outbred flies (DeRose and Roff 1999). In our study, this is defined as 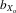 = (X1 − X11)/ fX1, where X1 is the mean trait size in F1, X11 is the mean trait size in F11, and f is the estimated inbreeding coefficient over the 11 generations of inbreeding (i.e., f = 0.908). The
= (X1 − X11)/ fX1, where X1 is the mean trait size in F1, X11 is the mean trait size in F11, and f is the estimated inbreeding coefficient over the 11 generations of inbreeding (i.e., f = 0.908). The 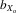 coefficients allow comparisons to be made with other studies (DeRose and Roff 1999; Prokop et al. 2010). We also calculated
coefficients allow comparisons to be made with other studies (DeRose and Roff 1999; Prokop et al. 2010). We also calculated 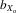 coefficients for crosses of the inbred lines. In this case,
coefficients for crosses of the inbred lines. In this case, 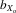 = (Xo − Xi)/fXo, where Xo is the mean trait size of outbred flies and Xi is the mean trait size of inbred flies (again f = 0.908).
= (Xo − Xi)/fXo, where Xo is the mean trait size of outbred flies and Xi is the mean trait size of inbred flies (again f = 0.908).
The effect of inbreeding on mean trait sizes is expected to decline with the number of rounds of inbreeding. The relationship of mean trait size with the inbreeding coefficient (f) is expected to be linear if the contribution of loci is additive (Falconer and Mackay 1996). If there are epistatic interactions between loci that contribute to the inbreeding depression of a trait then the mean trait size will be nonlinearly related to the inbreeding coefficient. If such interactions between loci are negative (diminishing epistasis), the rate of decline in the trait mean will be concave-up and this effect can be approximated by the frequency of double, or multiple, heterozygotes that decline in proportion to f2 (Crow and Kimura 1970). To test for this pattern, a regression model with both a linear and a quadratic term of the inbreeding coefficient (f and f2) was used for all absolute and relative morphological trait sizes. Traits were tested in males and females separately.
To test whether male eyespan responded more to inbreeding than the corresponding female eyespan trait and male wing length, we tested the interaction of inbreeding coefficient (f) × sex (male eyespan vs. female eyespan) and inbreeding coefficient (f) × trait (male eyespan vs. male wing length). This was done for both absolute and relative trait values.
The effect of inbreeding on female fecundity was tested over the first four generations. As female fecundity varies through time (Reguera et al. 2004), only females for whom we had obtained three fecundity measurements carried out over the full 3-week period were included. The analysis was performed for both absolute and relative measures (with thorax as a covariate) of fecundity.
To test whether line extinction and a loss of sample size was associated with trait changes, the above analysis was repeated using only the lines that were extant in generation 11 for all morphological analysis and generation 4 for fecundity analysis.
ASSOCIATION OF TRAIT VALUES WITH EXTINCTION
We assessed whether male and female F1 trait phenotypes were positively associated with the inbreeding coefficient at which the line went extinct. For each line, we calculated a mean F1 value for male and female thorax length, eyespan, wing length, and fecundity per day. The inbreeding coefficient (f) when the line went extinct was then regressed against the mean F1 trait value for that line. In cases where the line was extant in generation 11, we assigned a value of 1.0 for the inbreeding coefficient (f) of extinction. This was based on the assumption that all lines will eventually go extinct. This was done for both absolute trait values (thorax length, eyespan, wing length and fecundity per day) and relative trait values (eyespan, wing length, and fecundity per day).
CROSSING OF INBRED LINES
To test for the presence of inbreeding depression, the inbred lines were used to generate outbred and inbred crosses using a modified protocol developed by Prokop et al. (2010) shown in Figure1. Crosses were carried out using virgin flies collected from 17 inbred lines that had undergone at least 11 generations of full-sibling inbreeding. For each outbred cross (line i × line j), two reciprocal families were set up (one with male i × female j, the other with male j × female i). To balance this, each inbred cross (line i × line i) was also replicated (Fig.1). This design meant that the same number of inbred and outbred crosses were set up.
Figure 1.
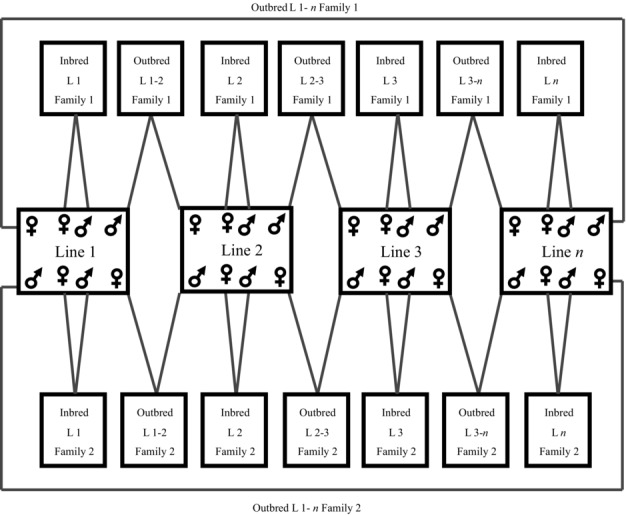
The mating design used to generate inbred and outbred families. Outbred families were generated by crossing each inbred line with two other inbred lines. To keep the number of inbred and outbred flies the same, two inbred families per line were generated.
Each family was generated by mating four males with four females (both outbred and inbred). Each was kept in a 1 L pot lined with moist blue tissue paper. Males and females were left to mate freely and eggs were collected twice a week. Eggs were transferred to Petri dishes with ad libitum puréed sweetcorn. A maximum of 10 eggs were transferred per Petri dish to minimize larval competition. Eggs were left to hatch and the resulting pupae were transferred to a 500 mL pot to eclose. All offspring were then killed and stored at −20°C for future measurement. Each family was maintained until they produced 10 male and 10 female offspring, which were measured for thorax length, eyespan, and wing length.
General linear mixed models were used to compare inbred versus outbred flies for thorax length, eyespan, and wing length in both males and females. As set-out earlier, two reciprocal families were set up for each outbred cross, with two inbred families set up to balance this (Fig.1). Cross was treated as a random effect. Family was also treated as a random effect (to avoid potential bias if one family produced more offspring than the other), nested within cross (Grafen and Hails 2002). The analysis was repeated for relative eyespan and wing length by including thorax length as a covariate. To test whether heterosis in male eyespan was exaggerated relative to nonsexual traits, we tested the interaction of inbreeding treatment (inbred or outbred) × sex (male eyespan vs. female eyespan) and inbreeding treatment × trait (male eyespan vs. male wing length). This was done both for absolute and relative trait values.
EFFECT OF ENVIRONMENTAL STRESS ON MORPHOLOGICAL TRAITS
We predicted that the response in morphological traits to genetic stress would mirror that observed to environmental stress. To test this hypothesis, we used a modified protocol from Cotton et al. (2004b) to manipulate larval condition by varying the amount of food available during development. Eggs were collected from a laboratory population of D. meigenii over a 24-h period and individually assigned to one of four food levels: 1.00, 0.30, 0.20, and 0.15 g of corn. Emerging adults were collected and then stored at −20°C for future measurement. All individuals were measured for thorax length, eyespan, and wing length.
General linear models (GLMs) were used to assess the effect of larval treatment on morphological traits, separately for males and females. The analysis was repeated for relative eyespan and wing length by including thorax length as a covariate. To consider whether the sexual trait was more sensitive to stress than nonsexual traits, we tested the interaction of treatment × sex (male eyespan vs. female eyespan) and treatment × trait (male eyespan vs. male wing length). This was done both for absolute and relative trait values.
All statistical analysis was performed using JMP Statistical Software (version 5.0.1). GLMM and GLM results tables and parameter estimates are provided in the Supporting Information.
Results
INBREEDING REGIME
There was a rapid and continual extinction of lines during the inbreeding procedure. Of the 105 male–female F0 pairs, the number of extant lines in successive generations was 58 in generation 1, 54 in generation 2, 48 in generation 3, 46 in generation 4, 41 in generation 5, 40 in generation 6, 33 in generation 7, 27 in generation 8, and 26 in generations 9–11. In total, 5167 flies (2663 males and 2504 females) from generations 1 to 11 were subject to analysis.
EFFECT OF INBREEDING ON MORPHOLOGICAL TRAITS AND FEMALE FECUNDITY
Male and female thorax length both suffered from inbreeding depression (males, 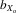 = 0.051, F1, 362.8 = 195.722, P < 0.001; females,
= 0.051, F1, 362.8 = 195.722, P < 0.001; females, 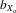 = 0.051, F1, 370.3 = 143.875, P < 0.001; Fig.2A). This pattern was also evident in absolute eyespan in both sexes (males,
= 0.051, F1, 370.3 = 143.875, P < 0.001; Fig.2A). This pattern was also evident in absolute eyespan in both sexes (males, 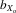 = 0.080, F1, 381.7 = 412.927, P < 0.001; females,
= 0.080, F1, 381.7 = 412.927, P < 0.001; females, 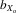 = 0.060, F1, 406.8 = 284.005, P < 0.001; Fig.2B) and persisted for relative eyespan after controlling for thorax length (males,
= 0.060, F1, 406.8 = 284.005, P < 0.001; Fig.2B) and persisted for relative eyespan after controlling for thorax length (males, 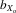 = 0.061, F1, 422.1 = 272.912, P < 0.001; females,
= 0.061, F1, 422.1 = 272.912, P < 0.001; females, 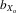 = 0.043, F1, 428.0 = 202.341, P < 0.001; Fig.2C). The pattern for wing length was much less pronounced compared to thorax and eyespan. There was a net decrease in male and female wing length over 11 generations, both for absolute wing length (males,
= 0.043, F1, 428.0 = 202.341, P < 0.001; Fig.2C). The pattern for wing length was much less pronounced compared to thorax and eyespan. There was a net decrease in male and female wing length over 11 generations, both for absolute wing length (males, 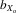 = 0.026, F1, 387.5 = 29.453, P < 0.001; females,
= 0.026, F1, 387.5 = 29.453, P < 0.001; females, 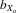 = 0.024, F1, 353.6 = 353.6, P < 0.001; Fig.2B) and relative wing length after controlling for thorax length (males,
= 0.024, F1, 353.6 = 353.6, P < 0.001; Fig.2B) and relative wing length after controlling for thorax length (males, 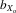 = 0.014, F1, 425.5 = 4.102, P = 0.044; females,
= 0.014, F1, 425.5 = 4.102, P = 0.044; females, 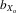 = 0.012, F1, 368.8 = 9.641, P = 0.002; Fig.2C).
= 0.012, F1, 368.8 = 9.641, P = 0.002; Fig.2C).
Figure 2.
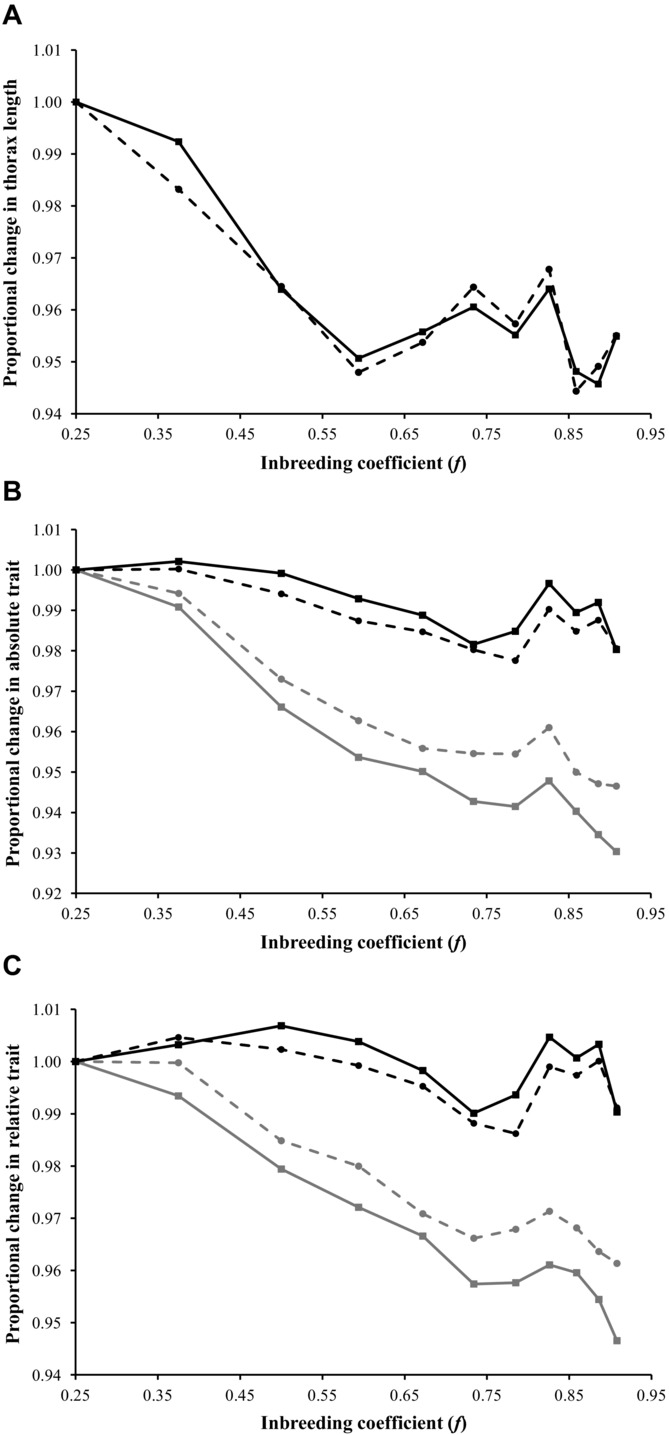
Proportional change in male (solid) and female (dashed) traits over 11 generations of inbreeding, standardized to one in F1, for (A) male and female thorax length, (B) absolute eyespan (gray) and absolute wing length (black), and (C) relative eyespan (gray) and relative wing length (black). Error bars were omitted for clarity.
It was hypothesized that the male sexual ornament (eyespan) would show greater inbreeding depression than less exaggerated nonsexual traits, namely the homologous female eyespan trait and male wing length. The response observed in absolute values of male eyespan was greater than in female eyespan (inbreeding coefficient, f × sex, F1, 367.2 = 105.128, P < 0.001) and male wing length (inbreeding coefficient, f × trait, F1, 361.3 = 419.609, P < 0.001). Similarly, there was a stronger response observed in relative values of male eyespan than in female eyespan (inbreeding coefficient, f × sex, F1, 375.4 = 108.493, P < 0.001) and male wing length (inbreeding coefficient, f × trait, F1, 361.9 = 429.345, P < 0.001). In contrast, there was no sex difference in absolute wing lengths (inbreeding coefficient, f × sex, F1, 313.8 = 0.366, P = 0.546) or relative wing lengths (inbreeding coefficient, f × sex, F1, 313.8 = 0.766, P = 0.382) responses to inbreeding.
There was evidence of a reduction in the rate of inbreeding depression because the inclusion of an additional quadratic term for the squared values of the inbreeding coefficient (f2) better explained the decline in male and female thorax length (males, F1,347.3 = 36.457, P < 0.001; females, F1,363.5 = 37.232, P < 0.001) and absolute eyespan (males, F1,373.0 = 10.572, P = 0.001; females, F1,404.4 = 9.355, P = 0.002), although not in absolute wing length (males, F1,372.8 = 0.029, P = 0.865; females, F1,345.8 = 1.594, P = 0.208). The latter result probably reflects the rather shallow inbreeding depression in this trait. After controlling for thorax length, the quadratic term was no longer significant for either male nor female relative eyespan (males, F1,383.7 = 1.218, P = 0.270; females, F1,404.7 = 1.026, P = 0.312).
Female fecundity was measured over the first four generations and was predicted to also show inbreeding depression. Both absolute and relative fecundity changed with inbreeding, but not in the predicted direction (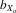 = −0.625 and −0.866 respectively, both comparisons, P < 0.001). In the light of these results, we checked whether the methods used to generate inbred lines inadvertently subjected them to artificial selection for female fecundity. To test for this possibility, a GLMM was used to test for differences in fecundity between flies from sublines chosen to maintain the line and sublines that were eliminated from further breeding. Unsurprisingly, sublines chosen to maintain the inbred line had higher female fecundities during the 3-week test period than those that were eliminated (F1,.454.6 = 16.239, P < 0.001). This relationship may explain why female fecundity increased over the first four generations.
= −0.625 and −0.866 respectively, both comparisons, P < 0.001). In the light of these results, we checked whether the methods used to generate inbred lines inadvertently subjected them to artificial selection for female fecundity. To test for this possibility, a GLMM was used to test for differences in fecundity between flies from sublines chosen to maintain the line and sublines that were eliminated from further breeding. Unsurprisingly, sublines chosen to maintain the inbred line had higher female fecundities during the 3-week test period than those that were eliminated (F1,.454.6 = 16.239, P < 0.001). This relationship may explain why female fecundity increased over the first four generations.
The prior analysis included all lines, those that went extinct and those that were still extant at generation 11. However, trait size may have been associated with the probability of line extinction and this might in part have accounted for the changes in trait sizes across generations. To control for this possibility, the morphology analysis was repeated using only the 26 lines that survived to generation 11 and the fecundity analysis using only the 46 lines surviving to generation 4. Overall, no change to the findings described earlier was identified. In the extant lines, all male and female absolute (thorax length, eyespan, and wing length) and relative traits (eyespan and wing length) decreased over 11 generations of inbreeding (all traits, P < 0.001). Once again the magnitude of the response in absolute and relative male eyespan (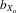 = 0.080 and 0.063, respectively) was greater than that in absolute and relative female eyespan (
= 0.080 and 0.063, respectively) was greater than that in absolute and relative female eyespan (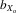 = 0.063 and 0.047, respectively) and absolute and relative male wing length (
= 0.063 and 0.047, respectively) and absolute and relative male wing length (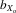 = 0.024 and 0.012, respectively; all comparisons, P < 0.001). Both absolute and relative fecundity still increased over the first four generations of inbreeding (
= 0.024 and 0.012, respectively; all comparisons, P < 0.001). Both absolute and relative fecundity still increased over the first four generations of inbreeding (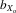 = −0.705 and −0.847, respectively, all comparisons P < 0.001).
= −0.705 and −0.847, respectively, all comparisons P < 0.001).
ASSOCIATION OF TRAIT VALUES WITH EXTINCTION
We tested whether particular values of male and female F1 absolute and relative trait phenotypes were associated with line extinction. When the analysis was performed on absolute morphological traits, only male absolute eyespan positively correlated with the inbreeding coefficient at which the line went extinct, that is, lines with larger F1 male absolute eyespan took more generations to go extinct (F1,56 = 4.125, P = 0.047; all other absolute morphological traits, F1,55–56 < 1.920, P > 0.131). After thorax was included as a covariate, only male relative eyespan was positively associated with the inbreeding coefficient at which the line went extinct (male relative eyespan, F1,55 = 7.179, P = 0.010; all other relative morphological traits, F1,54–55 < 1.971, P > 0.166). Surprisingly, neither absolute fecundity (F1,45 = 2.801, P = 0.101) nor relative fecundity (F1,44 = 3.058, P = 0.087) were significantly associated with extinction risk. We excluded the possibility that in the first generation, lines with larger male eyespan were associated with higher female fecundity as there were no correlation between these traits (both absolute and relative eyespan tests, P > 0.542).
CROSSING OF INBRED LINES
A total of 68 families were set up (34 inbred and 34 outbred). In total, 976 flies were measured: 481 females (237 inbreds and 244 outbreds) and 495 males (244 inbreds and 251 outbreds). Analysis was performed by pooling all flies and comparing inbred and outbred means. Evidence of heterosis was inferred if outbred trait means were significantly larger than inbred trait means. Male and female thorax length was higher in outbred flies than inbred flies (males, 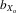 = 0.027, F1,25.5 = 15.280, P < 0.001; females,
= 0.027, F1,25.5 = 15.280, P < 0.001; females, 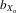 = 0.019, F1,28.1 = 8.495, P = 0.007; Fig.3A). The same was also true for absolute eyespan (males,
= 0.019, F1,28.1 = 8.495, P = 0.007; Fig.3A). The same was also true for absolute eyespan (males, 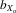 = 0.030, F1,30.8 = 11.964, P = 0.002; females,
= 0.030, F1,30.8 = 11.964, P = 0.002; females, 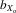 = 0.028, F1,29.1 = 10.084, P = 0.004) and relative eyespan (males,
= 0.028, F1,29.1 = 10.084, P = 0.004) and relative eyespan (males, 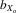 = 0.018, F1,31.1 = 5.256, P = 0.029; females,
= 0.018, F1,31.1 = 5.256, P = 0.029; females, 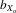 = 0.019, F1,28.0 = 7.794, P = 0.009; Fig.3B). Absolute wing length followed a similar pattern (males,
= 0.019, F1,28.0 = 7.794, P = 0.009; Fig.3B). Absolute wing length followed a similar pattern (males, 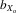 = 0.020, F1,29.9 = 7.749, P = 0.009; females,
= 0.020, F1,29.9 = 7.749, P = 0.009; females, 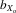 = 0.023, F1,31.4 = 11.986, P = 0.002). However, after controlling for thorax length, only female relative wing length still showed evidence of inbreeding depression (males,
= 0.023, F1,31.4 = 11.986, P = 0.002). However, after controlling for thorax length, only female relative wing length still showed evidence of inbreeding depression (males, 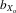 = 0.011, F1,31.5 = 3.047, P = 0.091; females,
= 0.011, F1,31.5 = 3.047, P = 0.091; females, 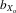 = 0.017, F1,31.4 = 9.967, P = 0.004; Fig.3C).
= 0.017, F1,31.4 = 9.967, P = 0.004; Fig.3C).
Figure 3.
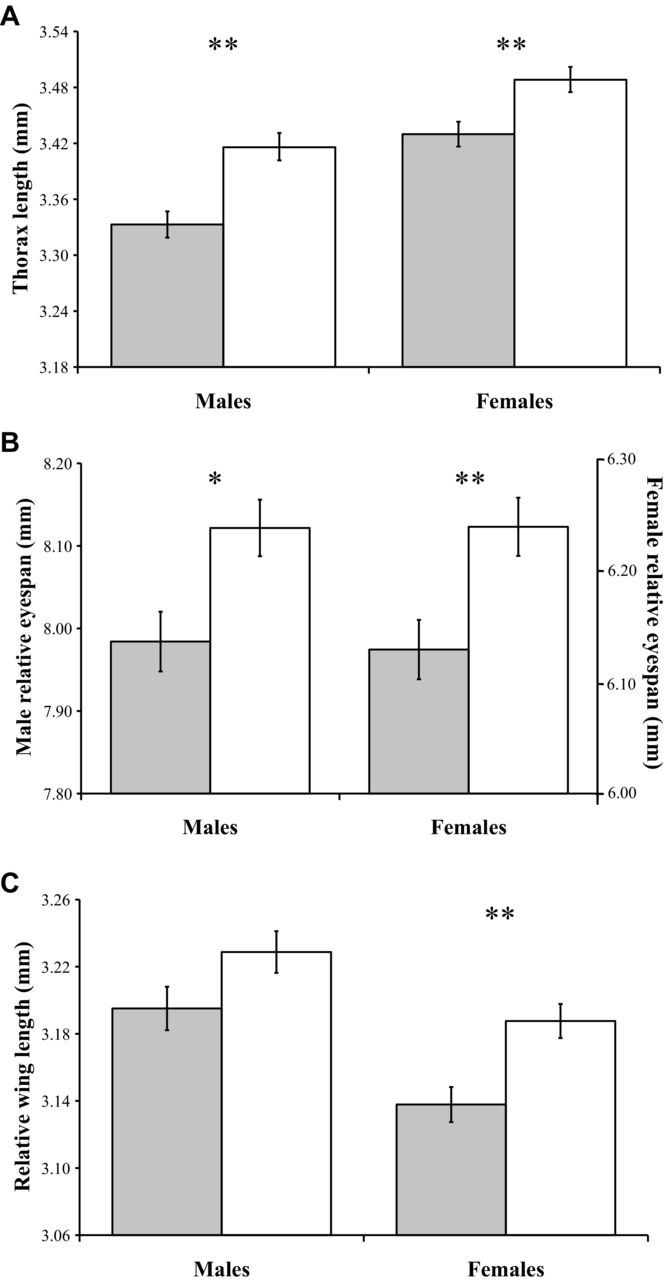
Mean (± standard error) values of inbred (gray bars) and outbred (white bars) individuals for (A) male and female thorax length, (B) male and female relative eyespan, and (C) male and female relative wing length. Asterisks denote significance level where *P < 0.050 and **P < 0.010.
Given that male eyespan is predicted to suffer greater inbreeding depression, it was predicted that it would also show greater heterosis than female eyespan and male wing length after a generation of outbreeding. However, the increase in male absolute eyespan was not significantly different from the increase in female absolute eyespan (inbreeding treatment × sex, F1,31.1 = 2.152, P = 0.152). Similarly, the observed increase in male relative eyespan did not significantly differ from the increase in female relative eyespan (inbreeding treatment × sex, F1,28.9 = 2.015, P = 0.167). However, the increase observed in absolute eyespan was significantly greater than the increase in absolute male wing length (inbreeding treatment × trait, F1,32.1 = 11.789, P = 0.002) and this pattern persisted after controlling for thorax length (inbreeding treatment × trait, F1,32.0 = 12.451, P = 0.001).
EFFECT OF ENVIRONMENTAL STRESS ON MORPHOLOGICAL TRAITS
In total, 547 flies were collected: 258 males and 289 females. All male and female absolute traits (thorax length, eyespan, and wing length) declined as the amount of food available decreased (all absolute traits, F1,249–286 > 403.743, P < 0.001). The response observed in male absolute eyespan (42.49% decrease over the four treatments) was greater than the response observed in female absolute eyespan (32.50% decrease; treatment × sex, F1,540 = 102.189, P < 0.001) and male absolute wing length (24.63% decrease; treatment × trait, F1,499 = 432.319, P < 0.001). The same comparisons were made using thorax length as a covariate. Again, all male and female relative traits (eyespan and wing length) declined as the amount of food available decreased (all relative traits, F1,243–279 > 44.573, P < 0.001; Fig.4). The response observed in male relative eyespan (21.71% decrease over the four treatments) was greater than the response observed in female relative eyespan (11.62% decrease; treatment × sex, F1,527 = 235.937, P < 0.001) and male relative wing length (10.26% decrease; treatment × trait, F1,491 = 732.948, P < 0.001).
Figure 4.
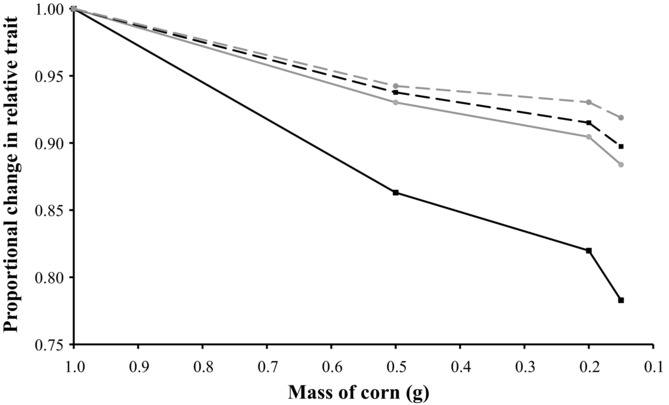
Proportional change in male (black) and female (gray) relative traits in response to environmental stress, standardized to one at 1.02 g of corn. Solid lines represent relative eyespan and dashed lines correspond to relative wing length. Error bars were omitted for clarity.
Discussion
In this article, we studied whether a sexual trait (male eyespan) in the stalk-eyed fly D. meigenii signals genetic stress induced by inbreeding. Unlike previous studies, male and female traits were measured over an extended period of full-sibling matings (11 generations, inbreeding coefficient f = 0.908). Male eyespan decreased as the inbreeding coefficient increased (Fig.2B), as did other nonsexually selected morphological traits like thorax length and wing length (in both males and females), and female eyespan (Fig.2A,B). Inbreeding depression was also evident after controlling for thorax length as relative morphological traits (eyespan and wing length) still declined over 11 generations of inbreeding in both sexes (Fig.2C).
The central prediction tested was that sexual traits should suffer more from inbreeding depression than nonsexual traits. In line with this, male absolute eyespan (the sexual trait) decreased more than female absolute eyespan and male absolute wing length (nonsexual traits). After controlling for body size, male relative eyespan still suffered more from inbreeding depression compared to female relative eyespan and male relative wing length. In contrast, there was no consistent sex difference in the response of male or female absolute or relative wing length (nonsexual traits) and in general the decline in wing length was much less marked than in eyespan.
A similar greater response in the sexual ornament was found when the inbred lines were crossed after generation 11. All traits showed heterosis, with higher absolute and relative values among outbred than inbred flies. The degree of heterosis was much greater for male relative eyespan compared to male relative wing length. However, the degree of heterosis observed in male absolute and relative eyespan did not significantly differ from that observed in female absolute and relative eyespan. Both tests approached significance (P ∼ 0.08) and a more intensive sampling of lines may have generated a significant response.
The extent of inbreeding depression is predicted to be dependent upon the genetic architecture of a trait (Rowe and Houle 1996; Tomkins et al. 2004). Generally, a locus is predicted to contribute a larger effect to inbreeding if the allelic variance at the locus is higher (as rare alleles are unlikely to be fixed) and if the dominance coefficient is higher (Falconer and Mackay 1996). The dominance effect depends on the degree to which the heterozygote differs from the mean of the homozygotes, and the mean effect of that locus on the trait. If there is no dominance there will be no inbreeding depression, as each allele in a heterozygote is equally likely to be fixed through inbreeding. In addition, a trait is predicted to suffer stronger inbreeding depression if it is affected by more loci, assuming additive interactions between loci and the same mean effect per locus (Falconer and Mackay 1996). This leads to a simple interpretation of our findings. Either the number of loci that affect variation or the mean per locus effect is greater in male eyespan compared to those attributes of nonsexual traits. Just such a pattern has been predicted and is explained by the idea of condition-dependent expression of sexual traits (Andersson 1986; Pomiankowski 1987; Iwasa et al. 1991; Pomiankowski and Moller 1995; Rowe and Houle 1996; Tomkins et al. 2004). More specifically, condition-dependent traits are likely to be affected by more loci (Pomiankowski and Moller 1995; Rowe and Houle 1996), have higher dominance coefficients typically of fitness related traits (Mousseau and Roff 1987; Roff and Mousseau 1987), and have higher allelic variation per locus as they have been subject to a history of directional rather than stabilizing selection (Pomiankowski and Moller 1995).
To distinguish the relative contribution of these causes of the patterns observed requires a more detailed specification of the genetic basis of traits in D. meigenii. Several quantitative trait loci (QTL) studies have assessed the number of genetic markers associated with sexual traits in other species (Gleason and Ritchie 2004; Huttunen et al. 2004; Moehring and Mackay 2004; Mundy 2005; Shaw and Lesnick 2009). However, few of these make direct comparisons of QTLs for sexual and nonsexual traits. One study that allows such a comparison of the number of QTLs is in a related stalk-eyed species, T. dalmanni (Johns et al. 2005). Here the sexual trait (male eyespan) had 5–6 QTLs. In contrast, nonsexual traits had fewer—none for male thorax length and a single QTL for female thorax length; although the number found for female eyespan variation was higher (4–5 QTLs). A further contrast was the X-linked QTL for eyespan that accounted for over a third of the observed male variation, but considerably less in females. These observations are suggestive that sexual traits may be associated with both a greater number of loci and larger effects at some loci than is the case for nonsexual traits or be determined by many loci of small effect. The generality of these observations needs further investigation.
In a final analysis, we compared the pattern of inbreeding depression across generations (Fig.2). There was an obvious plateau in the decline of thorax length as the inbreeding coefficient increased (measured as a significant quadratic term; Fig.2A). This is indicative of negative epistasis in the interaction between loci that contribute to this trait (Falconer and Mackay 1996). That is, multiple homozygosity has less effect with inbreeding. Although there was a similar response in male and female absolute eyespan, this nonlinearity disappeared after controlling for thorax length, so relative eyespan declined in a linear fashion over the 11 generations of inbreeding (Fig.2C). This is indicative that the loci contributing to eyespan have a greater degree of additivity than those contributing to thorax length (i.e., body size). This is an interesting finding as it suggests that eyespan better reflects the degree of homozygosity than does body size per se, and hence this might explain why male eyespan is used by females in their mate choice (Cotton et al. 2006). Note that it has recently been pointed out that purging of deleterious alleles caused by the “extra” selection in homozygotes because of nonadditivity could also result in a reduced response with f (Garcia-Dorado 2012). If this holds, it implies again that there is less nonadditivity in eyespan than thorax length.
The differing responses of sexual and nonsexual traits to inbreeding in D. meigenii are similar to the way these traits respond to environmental stress. The heightened response of sexual traits to environmental stress has been shown in other species of stalk-eyed fly (David et al. 1998; Cotton et al. 2004b). It suggests that similar general mechanisms may underlie the stress response in D. meigenii and other species, so that different kinds of stress in development feed through the same mechanisms, resulting in alterations to the allocation of resources to different traits. It is significant to note that the greater response of the male sexual trait is a general finding in a range of other study systems using environmental rather than genetic stress (Cotton et al. 2004a; Bonduriansky and Rowe 2005; Boughman 2007; Eraud et al. 2007; Siitari et al. 2007; Kemp 2008; Punzalan et al. 2008; McGuigan 2009; Gosden and Chenoweth 2011).
These results add to the growing body of literature studying the effect of inbreeding depression on male sexual traits. Overall, the literature provides inconsistent support for the hypothesis of greater inbreeding depression in sexual traits (see the introduction paragraph). One reason for this variation is differences in the methodology used. Most studies only consider the effect of one generation of inbreeding (e.g., Mariette et al. 2006; Drayton et al. 2007; Frommen et al. 2008; Ala-Honkola et al. 2009; Prokop et al. 2010). More inbreeding and a more severe level of genetic stress may be required to observe the detrimental effects of inbreeding as well as differences between sexual and nonsexual traits. A related possibility is that environmental factors may mask the detrimental effects of the inbreeding (reviewed in Armbruster and Reed 2005). To date, only one study has looked at the effect of inbreeding under different levels of environmental stress. Zajitschek and Brooks (2010) looked at the effect of three generations of inbreeding on male pigmentation and courtship behavior in the guppy Poecilia reticulata. They found that inbreeding depression in male pigmentation was greater in stressful environments compared to benign environments. However, inbreeding had no effect on male courtship behavior in any of the environments. Assessing how sexual traits respond to inbreeding in different environments may be a more reliable method of detecting inbreeding depression, but requires considerably greater effort. Finally, the variable response to inbreeding may be real and reflect different degrees of condition dependence between traits or species (Cotton et al. 2004a), although this remains to be investigated.
An unexpected and very interesting result was that the eyespan of F1 males predicted extinction risk of a line (i.e., the offspring of founding males). As expected, extended inbreeding over the 11 generations (f = 0.908) resulted in the extinction of the majority of lines (∼75%), with a higher rate initially. This pattern of extinction has been reported in a number of species (Frankham 1995; Bijlsma et al. 2000; Reed et al. 2002; Radwan 2003; Wright et al. 2008) and is probably explained by the random fixation of deleterious recessive alleles, with a reduced rate in the latter generations due to purging (Hedrick 1994; Wang et al. 1999). Strikingly, only the sexual trait, F1 male absolute and relative eyespan, was significantly associated with extinction risk. Lines with larger F1 male relative eyespan went extinct later than lines with smaller F1 male relative eyespan. In contrast, F1 nonsexual morphological traits (female eyespan and male and female wing length) were not associated with extinction risk. If extinction risk is correlated with the number and effect of recessive or partially recessive deleterious mutations carried by a line, then these observations suggest that male eyespan reflects this more than do nonsexual traits. This implies that male eyespan is a signal of genetic quality and that females who mate with large eyespan males are likely to gain indirect genetic benefits for their offspring. These results also demonstrate that size per se is less informative, presumably because it has a weaker association with the genetic signal (see deductions above about epistasis). The weaker prediction of extinction risk in the latter generations presumably is due to a greater impact of stochastic factors once purging has occurred.
We also expected that line fecundity would be associated with extinction risk, as observed in other studies (e.g., Radwan 2003). However, female absolute and relative fecundity did not correlate with the inbreeding coefficient at which lines went extinct. In large part, this was due to reduced statistical power when dealing with fecundity measures. Our criteria for including individuals in the fecundity analysis were restrictive, resulting in a loss of sample size and power (n = 131, power = 0.183) compared to morphological trait measurements (n = 240–319, power = 0.224–0.872). Our experience with D. meigenii and other stalk-eyed fly species is that fecundity is highly variable through time (Reguera et al. 2004). A more intensive sampling regime may have generated a more robust relationship between extant and extinct lines.
It is worth noting that not all the findings in this investigation were consistent with theoretical expectations. Fecundity is a life-history trait closely related to fitness and was expected to suffer from inbreeding depression. Contrary to predictions, female fecundity increased over the first four generations of inbreeding (f = 0.594). This was probably due to the experimental design because the subline that was selected to maintain the line was invariably the subline that was first to yield the number of males and females necessary to produce the following generation. Therefore, there was inadvertent artificial selection for highly fecund females that reproduced early and laid the most eggs.
Overall our results are consistent with those predicted by the handicap principle (Zahavi 1975; Pomiankowski 1987; Cotton et al. 2004a). If sexual traits are reliable indicators of condition they are expected to be more susceptible than nonsexual traits to the negative effects of the genetic stress caused by inbreeding (Pomiankowski and Moller 1995; Rowe and Houle 1996; Cotton et al. 2004a). Our results differ from those of another study of inbreeding using a different species of stalk-eyed fly, T. dalmanni (Prokop et al. 2010), which found that inbreeding depression in male eyespan was fully accounted by body scaling.
The contrasting results probably reflects differences in experimental design. Prokop et al. (2010) compared inbred flies after one generation of inbreeding with outbred flies, whereas we investigated the consequences of long-term inbreeding and crossing of highly inbred lines. Our approach may simply have allowed a greater accumulated response to the detrimental effects of inbreeding to become evident, although we still found a significant response in male eyespan in the first generation of inbreeding. Evidence for this is found when looking at 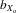 coefficients (i.e., the effect of inbreeding depression after controlling for the inbreeding period), which are relatively low in both D. meigenii and T. dalmanni (0.06 and 0.13, respectively; Prokop et al. 2010) relative to life-history traits (DeRose and Roff 1999). This suggests that the genetic benefit of mating with particular males may be limited in stalk-eyed flies. However, the present experiment was carried out in a benign environment, and our previous work has shown that the genetic signal is amplified under more stressful environmental conditions (David et al. 2000).
coefficients (i.e., the effect of inbreeding depression after controlling for the inbreeding period), which are relatively low in both D. meigenii and T. dalmanni (0.06 and 0.13, respectively; Prokop et al. 2010) relative to life-history traits (DeRose and Roff 1999). This suggests that the genetic benefit of mating with particular males may be limited in stalk-eyed flies. However, the present experiment was carried out in a benign environment, and our previous work has shown that the genetic signal is amplified under more stressful environmental conditions (David et al. 2000).
In conclusion, our results are consistent with those predicted by the handicap principle (Andersson 1986; Pomiankowski 1987; Grafen 1990; Iwasa et al. 1991; Rowe and Houle 1996; Cotton et al. 2004a; Johnstone et al. 2009). We have shown that the sexual trait (male eyespan) in D. meigenii is more affected by inbreeding depression than nonsexual traits (female eyespan and male wing length) and that this pattern persists after controlling for body size variation. We also found male eyespan to be a good predictor of extinction risk after repeated full-sibling inbreeding. The heightened responsiveness in the male sexual trait to genetic stress mirrors the response observed after applying environmental stress. Altogether, these findings provide support for the handicap hypothesis that condition-dependent sexual traits are reliable indicators both of genetic and environmental stress. The clarity of the results also establishes the utility of serial inbreeding as a simple way to assess the consequences of genetic stress for sexual traits.
Acknowledgments
The authors thank Aaron Towlson and Jennifer Small for maintaining the inbred lines. LB was supported by a Biotechnology and Biological Sciences Research Council (U.K.) Studentship. KF, NC, and AP were supported by an Natural Environment Research Council (U.K.) grant NE/G00563X/1. AP is also supported by an Engineering and Physical Sciences Research Council (U.K.) grants EP/F500351/1 and EP/I017909/1. We are very grateful to J. Wilkinson who suggested that we should cross lines, and to two anonymous reviewers for their comments on the article.
Supporting Information
Disclaimer: Supplementary materials have been peer-reviewed but not copyedited.
We present GLMM tables and effect size estimates for full models. The values may differ slightly to those in the text for models in which fewer factors were included or sexes were analyzed separately. The tables are split into six sections:
A. Effect of inbreeding on morphological traits
B. Effect of inbreeding on morphological traits, with a quadratic term
C. Effect of inbreeding on fecundity
D. Association of trait values with extinction
E. Crossing of inbred lines
F. Effect of environmental stress on morphological traits
LITERATURE CITED
- Ala-Honkola O, Uddstrom A, Pauli BD. Lindstrom K. Strong inbreeding depression in male mating behaviour in a poeciliid fish. J. Evol. Biol. 2009;22:1396–1406. doi: 10.1111/j.1420-9101.2009.01765.x. [DOI] [PubMed] [Google Scholar]
- Andersson M. Evolution of condition-dependent sex ornaments and mating preferences—sexual selection based on viability differences. Evolution. 1986;40:804–816. doi: 10.1111/j.1558-5646.1986.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Armbruster P. Reed DH. Inbreeding depression in benign and stressful environments. Heredity. 2005;95:235–242. doi: 10.1038/sj.hdy.6800721. [DOI] [PubMed] [Google Scholar]
- Aspi J. Inbreeding and outbreeding depression in male courtship song characters in Drosophila montana. Heredity. 2000;84:273–282. doi: 10.1046/j.1365-2540.2000.00655.x. [DOI] [PubMed] [Google Scholar]
- Baker RH. Wilkinson GS. Phylogenetic analysis of sexual dimorphism and eye-span allometry in stalk-eyed flies (Diopsidae) Evolution. 2001;55:1373–1385. doi: 10.1111/j.0014-3820.2001.tb00659.x. [DOI] [PubMed] [Google Scholar]
- Baker RH. Wilkinson GS. Phylogenetic analysis of correlation structure in stalk-eyed flies (Diasemopsis, Diopsidae) Evolution. 2003;57:87–103. doi: 10.1111/j.0014-3820.2003.tb00218.x. [DOI] [PubMed] [Google Scholar]
- Bijlsma R, Bundgaard J. Boerema AC. Does inbreeding affect the extinction risk of small populations?: predictions from Drosophila. J. Evol. Biol. 2000;13:502–514. [Google Scholar]
- Bolund E, Martin K, Kempenaers B. Forstmeier W. Inbreeding depression of sexually selected traits and attractiveness in the zebra finch. Anim. Behav. 2010;79:947–955. [Google Scholar]
- Bonduriansky R. Rowe L. Sexual selection, genetic architecture, and the condition dependence of body shape in the sexually dimorphic fly Prochyliza xanthostoma (Piophilidae) Evolution. 2005;59:138–151. [PubMed] [Google Scholar]
- Boughman JW. Condition-dependent expression of red colour differs between stickleback species. J. Evol. Biol. 2007;20:1577–1590. doi: 10.1111/j.1420-9101.2007.01324.x. [DOI] [PubMed] [Google Scholar]
- Bussiere LF, Hunt J, Stolting KN, Jennions MD. Brooks R. Mate choice for genetic quality when environments vary: suggestions for empirical progress. Genetica. 2008;134:69–78. doi: 10.1007/s10709-007-9220-z. [DOI] [PubMed] [Google Scholar]
- Chapman T, Pomiankowski A. Fowler K. Stalk-eyed flies. Curr. Biol. 2005;15:R533–R535. doi: 10.1016/j.cub.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Charlesworth B. Charlesworth D. The genetic basis of inbreeding depression. Genet. Res. 1999;74:329–340. doi: 10.1017/s0016672399004152. [DOI] [PubMed] [Google Scholar]
- Charlesworth D. Willis JH. The genetics of inbreeding depression. Nat. Rev. Genet. 2009;10:783–796. doi: 10.1038/nrg2664. [DOI] [PubMed] [Google Scholar]
- Costa FJV. Macedo RH. Coccidian oocyst parasitism in the blue-black grassquit: influence on secondary sex ornaments and body condition. Anim. Behav. 2005;70:1401–1409. [Google Scholar]
- Cotton S, Fowler K. Pomiankowski A. Do sexual ornaments demonstrate heightened condition-dependent expression as predicted by the handicap hypothesis? Proc. R. Soc. Lond. B. 2004a;271:771–783. doi: 10.1098/rspb.2004.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton S, Fowler K. Pomiankowski A. Condition dependence of sexual ornament size and variation in the stalk-eyed fly Cyrtodiopsis dalmanni (Diptera: Diopsidae) Evolution. 2004b;58:1038–1046. doi: 10.1111/j.0014-3820.2004.tb00437.x. [DOI] [PubMed] [Google Scholar]
- Cotton S, Fowler K. Pomiankowski A. Heightened condition dependence is not a general feature of male eyespan in stalk-eyed flies (Diptera: Diopsidae) J. Evol. Biol. 2004c;17:1310–1316. doi: 10.1111/j.1420-9101.2004.00754.x. [DOI] [PubMed] [Google Scholar]
- Cotton S, Rogers DW, Small J, Pomiankowski A. Fowler K. Variation in preference for a male ornament is positively associated with female eyespan in the stalk-eyed fly Diasemopsis meigenii. Proc. R. Soc. Lond. B. 2006;273:1287–1292. doi: 10.1098/rspb.2005.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow FC. Kimura M. Introduction to population genetics theory. New York: Harper and Row Publishers; 1970. [Google Scholar]
- David P, Hingle A, Greig D, Rutherford A, Pomiankowski A. Fowler K. Male sexual ornament size but not asymmetry reflects condition in stalk-eyed flies. Proc. R. Soc. Lond. B. 1998;265:2211–2216. [Google Scholar]
- David P, Bjorksten T, Fowler K. Pomiankowski A. Condition-dependent signalling of genetic variation in stalk-eyed flies. Nature. 2000;406:186–188. doi: 10.1038/35018079. [DOI] [PubMed] [Google Scholar]
- DeRose MA. Roff DA. A comparison of inbreeding in life-history and morphological traits in animals. Evolution. 1999;53:1288–1292. doi: 10.1111/j.1558-5646.1999.tb04541.x. [DOI] [PubMed] [Google Scholar]
- Drayton JM, Hunt J, Brooks R. Jennions MD. Sounds different: inbreeding depression in sexually selected traits in the cricket Teleogryllus commodus. J. Evol. Biol. 2007;20:1138–1147. doi: 10.1111/j.1420-9101.2006.01286.x. [DOI] [PubMed] [Google Scholar]
- Enquist M. Communication during aggressive interactions with particular reference to variation in choice behavior. Anim. Behav. 1985;33:1152–1161. [Google Scholar]
- Eraud C, Devevey G, Gaillard M, Prost J, Sorci G. Faivre B. Environmental stress affects the expression of a carotenoid-based sexual trait in male zebra finches. J. Exp. Biol. 2007;210:3571–3578. doi: 10.1242/jeb.005496. [DOI] [PubMed] [Google Scholar]
- Falconer DS. Mackay TFC. Introduction to quantitative genetics. New York: Longman Scientific and Technical; 1996. [Google Scholar]
- Frankham R. Inbreeding and extinction – a threshold effect. Conserv. Biol. 1995;9:792–799. [Google Scholar]
- Frommen JG, Luz C, Mazzi D. Bakker TCM. Inbreeding depression affects fertilization success and survival but not breeding coloration in threespine sticklebacks. Behaviour. 2008;145:425–441. [Google Scholar]
- Garcia-Dorado A. Understanding and predicting the fitness decline of shrunk populations: inbreeding, purging, mutation and standard selection. Genetica. 2012;190:1461–1476. doi: 10.1534/genetics.111.135541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason JM. Ritchie MG. Do quantitative trait loci (QTL) for a courtship song difference between Drosophila simulans and D. sechellia coincide with candidate genes and intraspecific QTL? Genetics. 2004;166:1303–1311. doi: 10.1534/genetics.166.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosden TP. Chenoweth SF. On the evolution of heightened condition dependence of male sexual displays. J. Evol. Biol. 2011;24:685–692. doi: 10.1111/j.1420-9101.2010.02205.x. [DOI] [PubMed] [Google Scholar]
- Grafen A. Sexual selection unhandicapped by the Fisher Process. J. Theor. Biol. 1990;144:473–516. doi: 10.1016/s0022-5193(05)80087-6. [DOI] [PubMed] [Google Scholar]
- Grafen A. Hails R. Modern statistics for the life sciences. Oxford, U.K: Oxford Univ. Press; 2002. [Google Scholar]
- Hedrick PW. Purging inbreeding depression and the probability of extinction – full sib mating. Heredity. 1994;73:363–372. doi: 10.1038/hdy.1994.183. [DOI] [PubMed] [Google Scholar]
- Hunt J, Brooks R, Jennions MD, Smith MJ, Bentsen CL. Bussiere LF. High-quality male field crickets invest heavily in sexual display but die young. Nature. 2004;432:1024–1027. doi: 10.1038/nature03084. [DOI] [PubMed] [Google Scholar]
- Huttunen S, Aspi J, Hoikkala A. Schlotterer C. QTL analysis of variation in male courtship song characters in Drosophila virilis. Heredity. 2004;92:263–269. doi: 10.1038/sj.hdy.6800406. [DOI] [PubMed] [Google Scholar]
- Iwasa Y, Pomiankowski A. Nee S. The evolution of costly mate preferences 2. The ‘handicap’ principle. Evolution. 1991;45:1431–1442. doi: 10.1111/j.1558-5646.1991.tb02646.x. [DOI] [PubMed] [Google Scholar]
- Johns PM, Wolfenbarger LL. Wilkinson GS. Genetic linkage between a sexually selected trait and X chromosome meiotic drive. Proc. R. Soc. Lond. B. 2005;272:2097–2103. doi: 10.1098/rspb.2005.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone RA. Sexual selection, honest advertisment and the handicap principle – reviewing the evidence. Biol. Rev. Cambridge Philos. Soc. 1995;70:1–65. doi: 10.1111/j.1469-185x.1995.tb01439.x. [DOI] [PubMed] [Google Scholar]
- Johnstone RA, Rands SA. Evans MR. Sexual selection and condition-dependence. J. Evol. Biol. 2009;22:2387–2394. doi: 10.1111/j.1420-9101.2009.01822.x. [DOI] [PubMed] [Google Scholar]
- Kemp DJ. Resource-mediated condition dependence in sexually dichromatic butterfly wing coloration. Evolution. 2008;62:2346–2358. doi: 10.1111/j.1558-5646.2008.00461.x. [DOI] [PubMed] [Google Scholar]
- Kotiaho JS. Testing the assumptions of conditional handicap theory: costs and condition dependence of a sexually selected trait. Behav. Ecol. Sociobiol. 2000;48:188–194. [Google Scholar]
- Mariette M, Kelley JL, Brooks R. Evans JP. The effects of inbreeding on male courtship behaviour and coloration in guppies. Ethology. 2006;112:807–814. [Google Scholar]
- Maynard Smith J. Harper D. Animal signals. Oxford, U.K: Oxford Univ. Press; 2003. [Google Scholar]
- McGuigan K. Condition dependence varies with mating success in male Drosophila bunnanda. J. Evol. Biol. 2009;22:1813–1825. doi: 10.1111/j.1420-9101.2009.01791.x. [DOI] [PubMed] [Google Scholar]
- Moehring AJ. Mackay TFC. The quantitative genetic basis of male mating behavior in Drosophila melanogaster. Genetics. 2004;167:1249–1263. doi: 10.1534/genetics.103.024372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousseau TA. Roff DA. Natural selection and the heritability of fitness components. Heredity. 1987;59:181–197. doi: 10.1038/hdy.1987.113. [DOI] [PubMed] [Google Scholar]
- Mundy NI. A window on the genetics of evolution: MC1R and plumage colouration in birds. Proc. R. Soc. Lond. B. 2005;272:1633–1640. doi: 10.1098/rspb.2005.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomiankowski A. Sexual selection – the handicap principle does work sometimes. Proc. R. Soc. Lond. B. 1987;231:123–145. [Google Scholar]
- Pomiankowski A. Moller AP. A resolution to the lek paradox. Proc. R. Soc. Lond. B. 1995;260:21–29. [Google Scholar]
- Prokop ZM, Les JE, Banas PK, Koteja P. Radwan J. Low inbreeding depression in a sexual trait in the stalk-eyed fly Teleopsis dalmanni. Evol. Ecol. 2010;24:827–837. [Google Scholar]
- Prokop ZM, Michalczyk L, Drobiak SM, Herdegen M. Radwan J. Meta-analysis suggests choosy females get sexy sons more than ‘‘good genes. Evolution. 2012;66:2665–2673. doi: 10.1111/j.1558-5646.2012.01654.x. [DOI] [PubMed] [Google Scholar]
- Punzalan D, Cooray M, Rodd FH. Rowe L. Condition dependence of sexually dimorphic colouration and longevity in the ambush bug Phymata americana. J. Evol. Biol. 2008;21:1297–1306. doi: 10.1111/j.1420-9101.2008.01571.x. [DOI] [PubMed] [Google Scholar]
- Radwan J. Inbreeding depression in fecundity and inbred line extinction in the bulb mite, Rhizoglyphus robini. Heredity. 2003;90:371–376. doi: 10.1038/sj.hdy.6800254. [DOI] [PubMed] [Google Scholar]
- Radwan J. Maintenance of genetic variation in sexual ornaments: a review of the mechanisms. Genetica. 2008;134:113–127. doi: 10.1007/s10709-007-9203-0. [DOI] [PubMed] [Google Scholar]
- Reed DH, Briscoe DA. Frankham R. Inbreeding and extinction: the effect of environmental stress and lineage. Conserv. Genet. 2002;3:301–307. [Google Scholar]
- Reguera P, Pomiankowski A, Fowler K. Chapman T. Low cost of reproduction in female stalk-eyed flies, Cyrtodiopsis dalmanni. J. Insect Physiol. 2004;50:103–108. doi: 10.1016/j.jinsphys.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Roff DA. Inbreeding depression: tests of the overdominance and partial dominance hypotheses. Evolution. 2002;56:768–775. doi: 10.1111/j.0014-3820.2002.tb01387.x. [DOI] [PubMed] [Google Scholar]
- Roff DA. Mousseau TA. Quantitative genetics and fitness: lessons from Drosophila. Heredity. 1987;58:103–118. doi: 10.1038/hdy.1987.15. [DOI] [PubMed] [Google Scholar]
- Rowe L. Houle D. The lek paradox and the capture of genetic variance by condition dependent traits. Proc. R. Soc. Lond. B. 1996;263:1415–1421. [Google Scholar]
- Sharp PM. The effect of inbreeding on competitive male-mating ability in Drosophila melanogaster. Genetics. 1984;106:601–612. doi: 10.1093/genetics/106.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw KL. Lesnick SC. Genomic linkage of male song and female acoustic preference QTL underlying a rapid species radiation. Proc. Natl. Acad. Sci. USA. 2009;106:9737–9742. doi: 10.1073/pnas.0900229106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan L. Pomiankowski A. Fluctuating asymmetry, spot asymmetry and inbreeding depression in the sexual coloration of male guppy fish. Heredity. 1997;79:515–523. [Google Scholar]
- Siitari H, Alatalo RV, Halme P, Buchanan KL. Kilpimaa J. Color signals in the black grouse (Tetrao tetrix): signal properties and their condition dependency. Am. Nat. 2007;169:S81–S92. doi: 10.1086/510140. [DOI] [PubMed] [Google Scholar]
- Thompson CW, Hillgarth N, Leu M. McClure HE. High parasite load in house finches (Carpodacus mexicanus) is correlated with reduced expression of a sexually selected trait. Am. Nat. 1997;149:270–294. [Google Scholar]
- Tomkins JL, Radwan J, Kotiaho JS. Tregenza T. Genic capture and resolving the lek paradox. Trends Ecol. Evol. 2004;19:323–328. doi: 10.1016/j.tree.2004.03.029. [DOI] [PubMed] [Google Scholar]
- van Oosterhout C. Trigg RE, Carvalho GR, Magurran AE, Hauser L. Shaw PW. Inbreeding depression and genetic load of sexually selected traits: how the guppy lost its spots. J. Evol. Biol. 2003;16:273–281. doi: 10.1046/j.1420-9101.2003.00511.x. [DOI] [PubMed] [Google Scholar]
- Wang JL, Hill WG, Charlesworth D. Charlesworth B. Dynamics of inbreeding depression due to deleterious mutations in small populations: mutation parameters and inbreeding rate. Genet. Res. 1999;74:165–178. doi: 10.1017/s0016672399003900. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. Genetic consequences of sexual selection in stalk-eyed flies. In: Dugatkin LA, editor. Model systems in behavioral ecology: integrating conceptual, theoretical, and empirical approaches. Princeton, NJ: Princeton Univ. Press; 2001. pp. 72–91. [Google Scholar]
- Wright LI, Tregenza T. Hosken DJ. Inbreeding, inbreeding depression and extinction. Conserv. Genet. 2008;9:833–843. [Google Scholar]
- Wright S. Evolution and the genetics of populations, volume 3. Experimental results and evolutionary deductions. Chicago, IL: The University of Chicago Press; 1977. [Google Scholar]
- Zahavi A. Mate selection – selection for a handicap. J. Theor. Biol. 1975;53:205–214. doi: 10.1016/0022-5193(75)90111-3. [DOI] [PubMed] [Google Scholar]
- Zajitschek SRK. Brooks RC. Inbreeding depression in male traits and preference for outbred males in Poecilia reticulata. Behav. Ecol. 2010;21:884–891. [Google Scholar]
- Zuk M, Thornhill R, Ligon JD. Johnson K. Parasites and mate choice in red jungle fowl. Am. Zool. 1990;30:235–244. doi: 10.1111/j.1558-5646.1990.tb05933.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
We present GLMM tables and effect size estimates for full models. The values may differ slightly to those in the text for models in which fewer factors were included or sexes were analyzed separately. The tables are split into six sections:
A. Effect of inbreeding on morphological traits
B. Effect of inbreeding on morphological traits, with a quadratic term
C. Effect of inbreeding on fecundity
D. Association of trait values with extinction
E. Crossing of inbred lines
F. Effect of environmental stress on morphological traits


