Abstract
The Akt family of serine-threonine kinases integrates a myriad of signals governing cell proliferation, apoptosis, glucose metabolism, and cytoskeletal organization. Akt affects neuronal morphology and function, influencing dendrite growth and the expression of ion channels. Akt is also an integral element of PI3Kinase-target of rapamycin (TOR)-Rheb signaling, a pathway that affects synapse assembly in both vertebrates and Drosophila. Our recent findings demonstrated that disruption of this pathway in Drosophila is responsible for a number of neurodevelopmental deficits that may also affect phenotypes associated with tuberous sclerosis complex, a disorder resulting from mutations compromising the TSC1/TSC2 complex, an inhibitor of TOR (Dimitroff et al., 2012). Therefore, we examined the role of Akt in the assembly and physiological function of the Drosophila neuromuscular junction (NMJ), a glutamatergic synapse that displays developmental and activity-dependent plasticity. The single Drosophila Akt family member, Akt1 selectively altered the postsynaptic targeting of one glutamate receptor subunit, GluRIIA, and was required for the expansion of a specialized postsynaptic membrane compartment, the subsynaptic reticulum (SSR). Several lines of evidence indicated that Akt1 influences SSR assembly by regulation of Gtaxin, a Drosophila t-SNARE protein (Gorczyca et al., 2007) in a manner independent of the mislocalization of GluRIIA. Our findings show that Akt1 governs two critical elements of synapse development, neurotransmitter receptor localization, and postsynaptic membrane elaboration.
Keywords: Akt1, glutamate receptor, Gtaxin, subsynaptic reticulum, membrane trafficking
INTRODUCTION
Synaptic plasticity requires molecular and morphological changes that allow previous activity to shape the physiological properties of synaptic communication. Secreted protein growth factors such as brain-derived neurotrophic factor play essential roles in synaptic plasticity, directing developmental and activity-dependent changes at these specialized cell junctions (Lauterborn et al., 2007). While an expanding set of growth factors are being identified as important determinants of synaptic plasticity, the molecular outputs of these signaling systems are less well understood (Rawson et al., 2003; Salinas, 2003). One signaling molecule of central importance for the integration of many growth factor inputs is the serine-threonine kinase Akt (Franke, 2008). In mammalian systems, three Akt isoforms govern a range of cellular and physiological processes from cell growth to membrane trafficking (Zhang et al., 2002; Manning and Cantley, 2007). Akt1 plays critical roles in cell growth and cell survival (Chen et al., 2001). Akt phosphorylation of AS160 influences exocytosis of glucose transporter-containing vesicles, providing an increased capacity for glucose transport across the plasma membrane (Gonzalez and McGraw, 2006; Watson and Pessin, 2006; Grillo et al., 2009). Consistent with a role of Akt in glucose uptake and homeostasis, mice null for Akt2, expressed ubiquitously in all cell types, show defects in insulin-stimulated glucose uptake (Nakatani et al., 1999; Cho et al., 2001; Bae et al., 2003; Easton et al., 2005; McCurdy and Cartee, 2005). Akt3, the isoform expressed most abundantly in the central nervous system, is essential for normal brain growth affecting both the number and size of neurons (Tschopp et al., 2005). Akt signaling is also known to govern neuronal morphology and synapse development directly (Dudek et al., 1997; Grider et al., 2009; Lee et al., 2011). Phosphorylation of the type A GABA receptor by Akt increases its localization to the synapse (Serantes et al., 2006). Akt regulates dendrite formation in Drosophila peripheral sensory neurons, demonstrating the capacity of this kinase to govern membrane processes that influence synaptic function (Parrish et al., 2009). The central role of Akt in signal integration prompted us to explore its function in the development of the Drosophila neuromuscular junction.
The Drosophila neuromuscular junction is a powerful model for molecular analysis of synapse development and plasticity. Each muscle of the larval body wall is innervated by identifiable motoneurons, and these peripheral synapses are well described at the molecular, morphological, and physiological levels (Jan and Jan, 1976; Gramates and Budnik, 1999; Ruiz-Canada and Budnik, 2006; Schuster, 2006). The Drosophila NMJ is a synapse that expands greatly during larval growth, and the dynamic matching of pre- and postsynaptic elements is critical for its assembly. The growth of the NMJ is accompanied by the expansion of a specialized postsynaptic membrane, the subsynaptic reticulum (SSR), as well as the regulated expression of specific glutamate receptor subunits. GluRIIA is critical for the functional strengthening and morphological growth of the synapse that accompanies muscle expansion during development (Petersen et al., 1997; Sigrist et al., 2002).
We have explored the function of the single Akt gene in Drosophila, Akt1, in synapse assembly and function using the NMJ as a model. We demonstrate that Akt1 is required for the developmentally regulated expansion of the SSR, in addition to regulating glutamate receptor composition. These findings demonstrate that Akt1 serves a critical role in two fundamental elements of synapse development.
MATERIALS AND METHODS
Fly Stocks
All fly strains were raised in standard cornmeal food at 25°C during embryogenesis and 30°C during larval development under a 12-h/12-h day/night cycle, unless otherwise stated. Oregon-R strain served as the wild type stock. Akt11/TM3 and Akt104226/TM3 were obtained from the Bloomington Drosophila Stock Center (BDSC). Akt104226 is a P-element insertion and hypomorphic allele. The null allele Akt11 is embryonic lethal, but Akt11/Akt104226 transheterozygotes are semi-viable and some survive to the adult stage. G14-GAL4, 24B-GAL4, Mef2-GAL4, and elav-GAL4 transposon-containing stocks (BDSC) were used for muscle and neuronal-specific expression of UAS-Akt1RNAi (Vienna Drosophila RNAi Center (VDRC) #103703), UAS-GtxRNAi (VDRC #105113), UAS-Gtaxin (from V. Budnick, University of Massachusetts (Gorczyca et al., 2007)), UAS-GluRIIA-mRFP (Kittel et al., 2006), and UAS-mCD8-GFP (BDSC #5137) constructs, respectively. Protein trap line Bsg-GFP (Flytrap #G00311) directs the expression of GFP-tagged Basigin under the control of its endogenous promoter. UAS-DicerII was used together with elav-GAL4 to increase the effectiveness of RNA interference in neurons (Dietzl et al., 2007). The temperature-sensitive GAL80 repressor, GAL80ts under tubulin promoter (Tubp-GAL80ts, from BDSC), was combined with Mef2-GAL4 line for temporal control of UAS-Akt1RNAi expression in the muscle (Zeidler et al., 2004). GAL80ts suppressed GAL4 function at the permissive temperature (18°C). At the restrictive temperature (30°C), GAL80ts released GAL4, allowing its binding to the UAS, and inducing the expression of Akt1RNAi. To inhibit the expression of Akt1 at the early developmental stage, embryos were kept at 30°C for 2 days and then raised at 18°C until they reached third instar larval stage. In contrast, animals, which Akt1 was suppressed at the late stage, were raised at 18°C until second instar stage and then shifted to 30°C for 2 days before immunohistochemistry. The constitutively active forms of Akt1 (Akt1CA) and GFP tagged Akt1CA (Akt1CA-GFP) were generated using QuikChange II Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA), resulting in the replacement of amino acids Threonine 342 (ACC) and Serine 505 (AGC) with Aspartic Acid (GAC). The Akt1CA and Akt1CA-GFP constructs were cloned into pUAST-attB vector and then integrated into the third chromosome (99F8) by site-specific P-element mediated germline transformation (Rainbow Transgenic, CA).
Immunohistochemistry and Confocal Microscopy
The third instar larval muscles were dissected in ice-cold Ca2+ free HL-3 media and fixed with either 4% paraformaldehyde for 30 min or Bouin's fixative solution for 5 min (for glutamate receptor subunits antibody immunostaining) or 15 min (for Gtaxin antibody immunostaining). All subsequent washes were performed in PBST (0.5% triton X-100 in phosphate buffered saline (PBS)). A total of 5% normal goat serum in PBST was used for sample blocking and antibody incubations. Primary antibodies mouse anti-glutamate receptor IIA antibody (1:50, 8B4D2, Developmental Studies Hybridoma Bank (DSHB), University of Iowa, Iowa City, IA), rabbit anti-glutamate receptor IIB and IIC antibodies (1:2000 from D. Featherstone, University of Illinois at Chicago), mouse anti-DsRed (1:500, Santa Cruz Biotechnology), rat anti-Syndapin (1:100, from M. Ramaswami, University of Arizona), rat anti-Gtaxin (1:200, from V. Budnick, University of Massachusetts), rabbit anti-Dorsal and Cactus antibodies (1:1000, from S. Wasserman, University of California, San Diego), mouse anti-Discs large (1:500, 4F3, DSHB), mouse anti-Cysteine string protein (1:1000, 6D6, DSHB), mouse anti-α-Spectrin (1:1000, 3A9, DSHB), and mouse anti-Bruchpilot (1:1000, nc82, DSHB) were incubated with sample for at least 12 h at 4°C. Alexa-fluorescence conjugated secondary antibodies were obtained from Life Technologies (Grand Island, NY).
Images were acquired using an Olympus Fluoview FV1000 laser scanning confocal microscope (Olympus America, Lake Success, NY). Quantification of protein levels were performed using Imaris 7.3 (Bitplane, Saint Paul, MN) and ImageJ1.42q (NIH) software for image processing and analysis. Serial images taken by confocal microscopy were reconstructed into 3D images using Imaris without any other processing. Immunoreactivity-positive voxels were then assayed by counting the total number of voxels (Abundance) and by measuring their average fluorescent intensity. Both of the values were further normalized by muscle size for each preparation.
Western Blotting Analysis
Total protein was prepared from dissected third instar larval muscle tissues in SDS-loading buffer and ran on 9% sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE), polyvinylidene difluoride (PVDF) membrane was incubated overnight with anti-phosphorylated Akt1 or anti-β-Actin antibodies (Cell Signaling Technology, MA) in blocking solution (5% w/v nonfat dry milk in 0.5% Tween-20 in Tris-buffer saline) at 4°C. Signals were amplified using horseradish peroxidase (HRP) conjugated secondary antibody and detected using Supersignal West Femto Maximum Sensitivity Substrate (Thermo Scientific, IL).
Transmission Electron Microscopy
The third instar larval muscles were dissected in ice-cold Ca2+ free HL-3 media and fixed in buffer (1.5% glutaraldehyde, 2.5% paraformaldehyde, 1.8 mM Ca2+ in 0.1M Na-cacodylate, pH 7.4) at 4°C overnight. Postfixation was done in 1% osmium tetroxide, and en bloc staining was performed with 2% uranyl acetate in dark condition. The samples were rinsed in 0.1M sodium cacodylate buffer (pH 7.4), dehydrated and infiltrated, embedded in Spurr's resin, and sectioned to 70 nm slices. The images were taken with a transmission electron microscope (JEOL1200, Tokyo, Japan) and analyzed by ImageJ1.42q (NIH).
Electrophysiology
Excitatory junction potentials (EJPs) and miniature excitatory junction potentials (mEJPs) were recorded at room temperature from muscle 6 of abdominal hemi-segment A3 in third instar larvae (Rawson et al., 2003). The third instar larvae were dissected in ice-cold Ca2+ free HL-3 media and recordings were performed with larvae in HL-3 media containing 1.2 mM Ca2+. Muscle 6 of A3 was impaled with the recording electrode and before stimulation, recordings were taken for 1 min to measure spontaneous activities (mEJPs) (Stewart et al., 1994). Following the recording of mEJPs, evoked EJPs were elicited in the same muscle with 1 Hz pulses. A total of 1 nA of current was injected for 200 ms to record plasma membrane resistance and capacitance. Recordings were acquired with Axoclamp 2B amplifier and Clampex 9.2 software (Axon Instruments, CA). Only the recordings with resting membrane potentials lower than −60 mV were included in this analysis. EJP and mEJP amplitudes and kinetics were analyzed with MiniAnalysis (Synaptosoft, Fort Lee, NJ).
Statistical Analysis
Statistical analyses for quantitative data were performed in Minitab Release 16 (Minitab, State College, PA). All data points were presented as mean ± SEM and analyzed using Student's t-tests for normally distributed data or post hoc Tukey–Kramer for pairwise comparisons of data with non-normal distributions.
RESULTS
Akt1 plays a central role in a number of signaling processes, acting both downstream and upstream of growth factor and target of rapamycin-directed events. The Akt1 kinase governs a number of cellular activities including cell proliferation, cell survival, and cytoskeleton organization [Fig. 1(A)]. Given these diverse and critical functions, we explored the role of Akt1 in synapse assembly. In addition to the well-described Akt1 mutant alleles (Staveley et al., 1998; Mozden and Rubin, 1999; Guo and Zhong, 2006), we used an Akt1RNAi transgene (Dietzl et al., 2007) to inhibit Akt1 function selectively in either motoneurons or muscle cells. To assess the level of inhibition achieved by the Akt1RNAi construct, we measured the level of phosphorylated Akt1 (active form of Akt1) by western blot. Using a muscle-directed GAL4 to drive the expression of UAS-Akt1RNAi, phosphorylated Akt1 protein was reduced to 24.2% of wild-type level in third instar larval muscle tissue [Fig. 1(B)].
Figure 1.
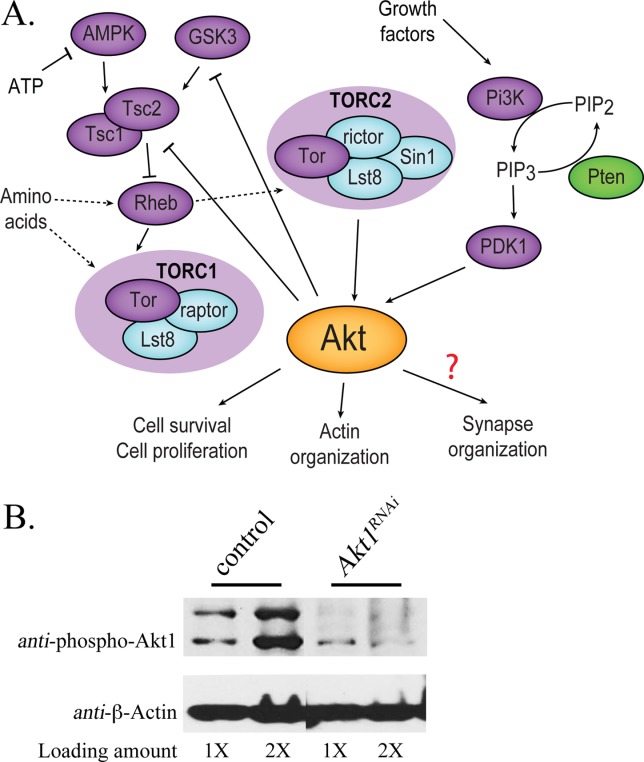
The Akt signaling system and level of Akt1 knockdown using RNA interference in Drosophila. A: In this summary, kinases Rheb, and Tsc1/2 are purple symbols, phosphatases are green, and other components of TOR1 and TOR2 complex are blue. Akt1 is activated by growth factors via Pi3K and PDK1 and by nutritional sensing through Tsc1/2 and TOR complexes. Relationships that are not fully understood or have several possible intermediary steps are shown as dashed arrows or a question mark (adapted from Dimitroff et al., 2012). B: Akt1 function was compromised by muscle-specific expression of an Akt1RNAi construct using the GAL4-UAS system. The level of phosphorylated Akt1 was measured by Western blot. Total muscle proteins were prepared from third instar larval muscles of control animals (UAS-Akt1RNAi transgene only; UAS-Akt1RNAi/+) or Akt1RNAi animals with muscle specific knockdown of Akt1 using 24B-GAL4 driver (24B-GAL4>UAS-Akt1RNAi). Akt1 was dramatically decreased in muscle tissue expressing Akt1RNAi as compared with controls. Measures of β-Actin were used as a protein loading controls. Total proteins extracted from either one (1×) or two larvae (2×) were loaded.
We began assessing the role of Akt1 in NMJ assembly by examining the distribution and level of glutamate receptor IIA (GluRIIA), one of the neurotransmitter receptor subunits at this glutaminergic synapse. Glutamate is the major excitatory neurotransmitter at the type I bouton of the Drosophila larval NMJ (Brunner and Okane, 1997; Collins and DiAntonio, 2007). The NMJ glutamate receptor (GluR) is a heterotetramer comprised of three invariant subunits: GluRIIC, D, and E. The fourth subunit, either GluRIIA or B, determines the type and the electrophysiological properties of the receptor (DiAntonio et al., 1999; Featherstone et al., 2005; Qin et al., 2005a; DiAntonio, 2006). Subunit GluRIIA and B competitively bind to GluRIIC; hence, the preferential expression of these two subunits constitutes one element of developmental plasticity exhibited by this synapse (Marrus et al., 2004). We examined the levels and distributions of GluRIIA using a well-characterized monoclonal antibody, anti-GluRIIA (Featherstone et al., 2002; Qin et al., 2005a; Karr et al., 2009). The specificity of this antibody has been well documented by showing that immunoreactive signal is lost in GluRIIA null mutant (Marrus et al., 2004). Partial loss of Akt1 function, achieved with the heteroallelic combination Akt11/Akt104226, altered GluRIIA distributions and levels, with a reduction at postsynaptic structures and the appearance of GluRIIA immunoreactivity within repeated bands throughout the muscle cells [Fig. 2 compare (A), (B) to (C), (D)]. This latter phenotype was more prominent in muscles 15 and 16 and was observed to a lesser extent in muscles 6 and 7, the postsynaptic cells typically used for electrophysiological analysis [arrowheads in Fig. 2(C,D)].
Figure 2.
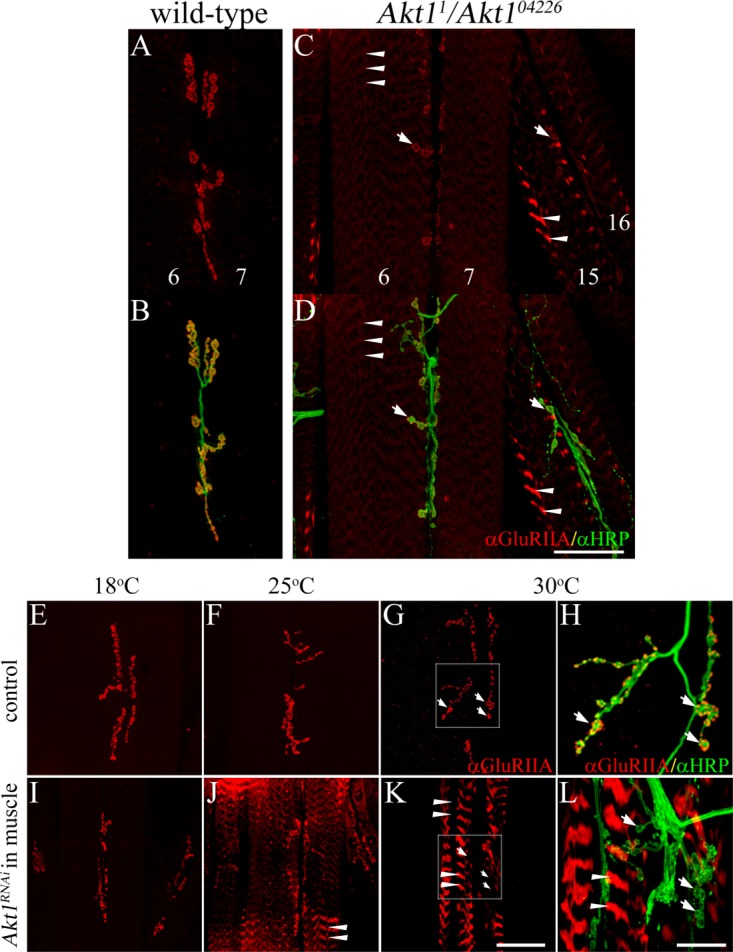
GluRIIA localization was modified in Akt1 mutants and animals with muscle-specific inhibition of Akt1. GluRIIA localization was examined in muscles 6 and 7 using monoclonal anti-GluRIIA antibody (red). Anti-HRP antibody detected neuronal projections (green). A and B: In wild-type animals, GluRIIA was located in the postsynaptic specialization that surrounds the motoneuron boutons. C and D: Akt11/Akt104226 mutants showed reduction of GluRIIA at synaptic boutons (see arrows) and redirection to intracellular bands (faint staining in muscles 6 and 7, and more prominent in muscles 15 and 16; see arrowheads). E–L: Akt1 function was compromised by muscle-specific expression of an Akt1RNAi construct using the GAL4-UAS system. UAS-Akt1RNAi/+ animals served as controls. GAL4 transcriptional activation shows temperature dependence, permitting a graded level of Akt1 blockade from 18°C (low level of inhibition) to 30°C (high level of inhibition). E–H: In control larvae, GluRIIA immunoreactivity was concentrated in the postsynaptic region surrounding boutons at all temperatures. H: Enlarged view of white box area in (G), arrows show the motoneuron boutons surrounded by GluRIIA. I–L: In Akt1RNAi expressing larval muscle (24B-GAL4>UAS-Akt1RNAi), GluRIIA mislocalization (arrowheads) was more severe with greater inhibition of Akt1 function at increasing temperature (larvae reared at 18°C (I), 25°C (J), or 30°C (K and L)). L Enlarged view of white box area in (K), arrows show synaptic boutons lacking GluRIIA immunoreactivity; arrowheads mark ectopic GluRIIA within intracellular bands. Scale bar in (A–G) and (I–K), 50 µm, in (H) and (L), 5 µm.
Directed expression of an Akt1RNAi in either muscle or neuron using the GAL4-UAS binary system (Brand and Perrimon, 1993) provided the means of assessing the cell-type specific requirements for Akt1. We used multiple muscle-specific GAL4 driver lines, G14, 24B, and Mef2 to confirm that the associated phenotypes were due to muscle-directed RNAi expression, but not expression in some alternative cell types. These GAL4 drivers showed some differences in the level of transcriptional activity, but all induced similar Akt1 knockdown phenotypes for GluRIIA localization and SSR expansion. Muscle-specific expression of Akt1RNAi produced a dramatic loss of GluRIIA at the synapse and its redistribution into intracellular bands in the muscle cell, confirming the phenotype observed in Akt11/Akt104226 mutants [Fig. 2 compare controls shown in (E–H) to muscle cell-directed Akt1RNAi animals in (I–L)]. Knockdown of Akt1 in the motoneuron had no effect on GluRIIA distribution (data not shown). GAL4-directed transcriptional activation is temperature-dependent, allowing for different levels of Akt1RNAi expression and consequently loss of Akt1 function, by simply rearing the animals at different temperatures. At 18°C, GluRIIA distributions were normal, but with decreasing levels of Akt1 function produced at 25°C and 30°C, GluRIIA was progressively lost from the postsynaptic site and increasingly localized within intracellular bands [Fig. 2 compare control animals, panels (E–H), to muscle-specific Akt1RNAi, panels (I–L); in enlarged images (H) and (L), arrows indicate synaptic boutons; arrowheads indicate GluRIIA in bands]. Although GluRIIA failed to localize to the postsynaptic specialization upon inhibition of Akt1 function, we did note a net and significantly increased level of GluRIIA within intracellular structures [Supporting Information Fig. 1(A), animals reared at 30°C]. These findings established that localization of GluRIIA was affected by reductions of Akt1 function mediated by Akt1RNAi transgene expression in the postsynaptic cell.
To further explore the mechanism of the dramatic redistribution of GluRIIA achieved by knockdown of Akt1, we examined the expression pattern of an mRFP-tagged GluRIIA derived from a UAS-transgene. This provided the opportunity to visualize the transgenic GluRIIA-mRFP by both fluorescence of the mRFP protein, and immunodetection of the polypeptide with an anti-RFP antibody, anti-DsRed. Consistent with our earlier results looking at endogenous GluRIIA, compromising Akt1 function produced loss of GluRIIA-mRFP at the synapse, detected by either mRFP fluorescence or anti-RFP antibody [Fig. 3 compare (B), (C) to (F), (G)]. Interestingly, the redistribution of GluRIIA-mRFP to intracellular bands was only detected with the anti-RFP antibody, but not by monitoring the fluorescence of the mRFP-tagged receptor subunit [Fig. 3(F,G)]. In control animals, the RFP-fluorescence pattern precisely overlaps the anti-RFP signal [Fig. 3(B,C)]. This result suggests that reduction of Akt1 function may disrupt the structural integrity of GluRIIA-mRFP, resulting in loss of its native fluorescence, whereas the RFP-epitope is found redistributed to intracellular membrane structures.
Figure 3.
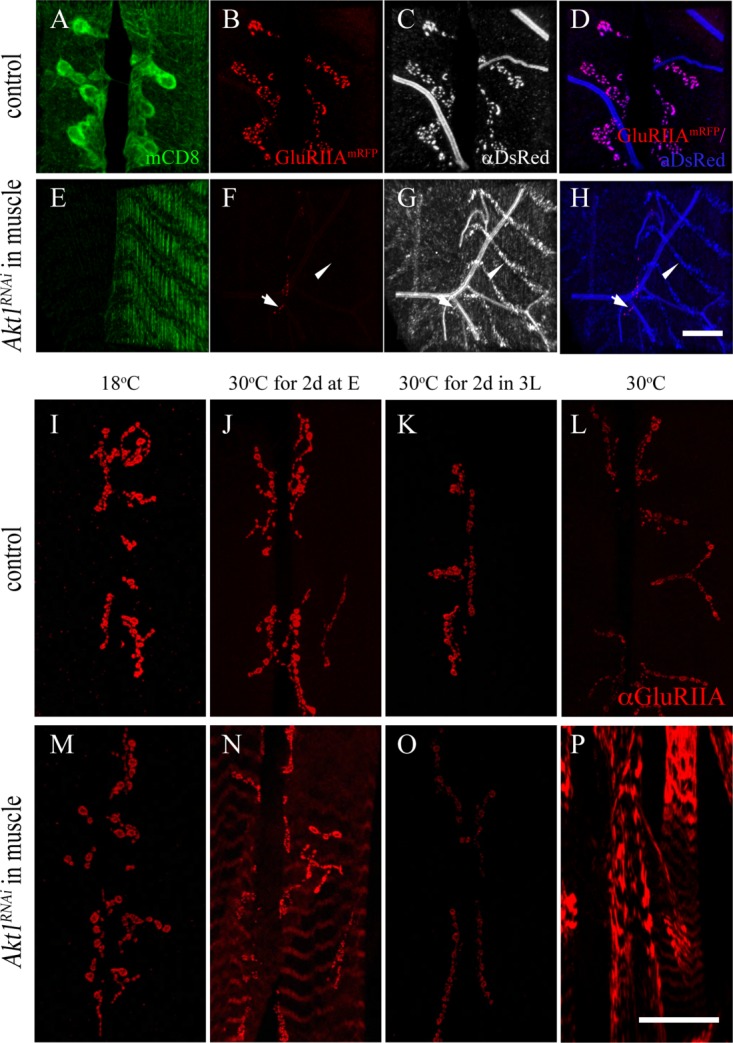
Akt1 affects GluRIIA trafficking to NMJ and is crucial in the early developmental stage. Two experiments are shown here. The first (panels A–H) shows the results from a study where the distribution of an engineered GluRIIA-RFP when Akt1 function was compromised with RNA interference. The GluRIIA-RFP was detected with either endogenous fluorescence from the mRFP or an anti-mRFP antibody (anti-DsRed). The second experiment (panels I–P) was designed to determine the developmental window when Akt1 activity was critical for GluRIIA localization. Time-limited inhibition of Akt1 function was achieved using Akt1RNAi and a temperature-sensitive GAL80 (see “Materials and Methods”). A–D: GluRIIA-mRFP (red) was colocalized with anti-DsRed signals (gray) at the NMJ in control animals (G14-GAL4, UAS-mCD8-GFP; UAS-GluRIIA-mRFP/+). E–H: GluRIIA-mRFP fluorescence was reduced significantly at the postsynaptic density (arrows) upon inhibition of Akt1 function. The redistribution of GluRIIA-mRFP protein into an intracellular compartment was detected only with anti-DsRed immunostaining in Akt1 compromised animals (G14-GAL4, UAS-mCD8-GFP; UAS-GluRIIA-mRFP>UAS-Akt1RNAi) (arrowheads). I–P: To investigate the critical periods when Akt1 is required for GluRIIA localization at the NMJ during development, the temperature-sensitive GAL80 repressor under tubulin promoter, Tubp-GAL80ts, was used along with the GAL4-UAS binary system to allow temporal spatial regulation of Akt1RNAi expression (Tubp-GAL80ts, Mef2-GAL4>UAS-Akt1RNAi). I–L: At all temperatures, control (Tubp-GAL80ts, Mef2-GAL4/+) animals showed normal GluRIIA distribution at the NMJ. M: In Tubp-GAL80ts, Mef2-GAL4/UAS-Akt1RNAi animals at the permissive temperature (18°C), when expression of Akt1RNAi is minimal on account of GAL80ts blockade of transcription, the animals displayed a normal GluRIIA distribution. N: Incubation at the restrictive temperature (30°C) for 2 days right after egg laying induced modest GluRIIA mislocalization in muscles while much of the GluRIIA remained at the NMJ. O: Temperature shift from 18°C to 30°C for 2 days at the third instar larval stage produced reduced levels of GluRIIA immunoreactivity at the NMJ but no abnormal localization. P: Animals reared at 30°C throughout the entire developmental stages displayed severe GluRIIA mislocalization. Scale bar in (A–H), 10 µm, in (I–P), 50 µm.
We have also examined the developmental window during which Akt1 is essential for GluRIIA localization by using the temperature-sensitive GAL80ts system (Zeidler et al., 2004). When a GAL80ts transgene is present with GAL4-UAS components, the GAL80 suppresses the activity of the transcriptional activator GAL4, preventing expression of the UAS-transgene, in this case, UAS-Akt1RNAi. Raising the temperature to restrictive level inactivates GAL80ts and permits expression of the Akt1RNAi. We used this system to inactivate Akt1 during different developmental stages. Reduction of Akt1 function during a 2-day window early in development (embryo-first instar larva) produced some redistribution of GluRIIA into intracellular stripes, whereas a later 2-day inactivation window in third instar larval stage merely reduced the levels of GluRIIA at the synapse [Fig. 3(I–P)]. These data suggest that the redistribution of GluRIIA observed with reduction of Akt1 throughout development is not merely the result of a failure of synaptic stabilization because the levels of GluRIIA would likely recover quickly from new synthesis (Rasse et al., 2005) but is affecting a process occurring in early development that alters GluRIIA production and delivery to the synaptic specialization.
We also examined the effect of Akt1 on two potential downstream targets, Dorsal and Cactus (Drosophila homologs of NF-κB and Iκ-B, respectively). These two proteins have recently been shown to localize to postsynaptic specializations and regulate glutamate receptor levels at the NMJ (Heckscher et al., 2007). While Dorsal and Cactus have been well characterized as transcriptional activator proteins, their activity at the NMJ is posttranscriptional, affecting the localization or stabilization of glutamate receptors in the SSR (Heckscher et al., 2007). To determine whether Akt1's effects on GluRIIA localization could be mediated at least in part by an influence on Dorsal or Cactus, the levels and distributions of these two proteins at the NMJ were evaluated. As previously described, Dorsal and Cactus were concentrated in postsynaptic specializations at type Ib boutons in control animals [Fig. 4(A,B,E,F)]. Upon RNAi knockdown of Akt1, both Dorsal and Cactus levels significantly decreased at the NMJ [Fig. 4(C,D,G,H)] [Supporting Information Fig. 1(B)]. In addition to the reduction of Dorsal levels at the NMJ, Dorsal was mislocalized in a number of animals (23.8% penetrance) and partially colocalized with GluRIIA into intracellular bands in the muscle cell [Fig. 4(L–N); arrowheads indicate the bands of GluRIIA and Dorsal, arrows indicate synaptic boutons]. Although the penetrance of this phenotype was modest, it was reproducible across three different sets of experiments. These findings showed that Akt1 affects the levels of two potential Akt1 downstream targets known to regulate GluRIIA levels, and suggest the possibility that Akt1 regulates GluRIIA at least in part via the control of Dorsal and Cactus.
Figure 4.
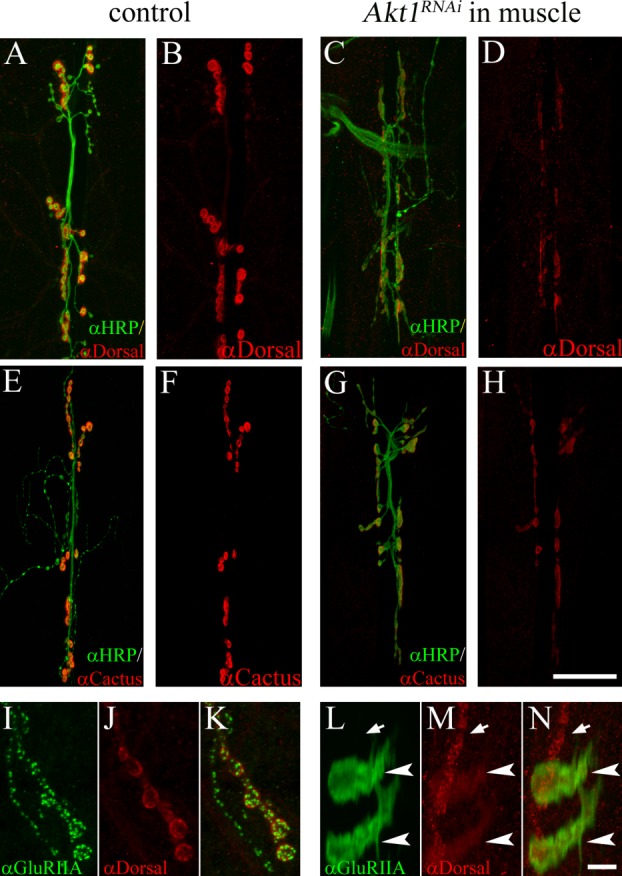
Influence of Akt1 on Dorsal and Cactus levels and distribution at the NMJ. A and B: In control animals (UAS-Akt1RNAi/+), Dorsal (detected by anti-Dorsal antibody; red) is localized to the postsynaptic specialization. Neuronal projections were labeled by anti-HRP staining (green). C and D: Akt1 function was compromised by expressing UAS-Akt1RNAi under the muscle-specific 24B-GAL4. Dorsal levels at the NMJ were significantly reduced. E and F: Cactus (red) was concentrated at the NMJ in control animals. G and H: Inhibition of Akt1 function in the muscle (24B-GAL4/UAS-Akt1RNAi) resulted in reduced levels of Cactus at the NMJ. I–K: In control animals, Dorsal immunoreactivity (red) colocalized with GluRIIA (green) immunoreactivity at the postsynaptic specialization. L–N: In muscles where Akt1 expression was inhibited, both Dorsal and GluRIIA redistributed into intracellular bands, although the effect on Dorsal was less and incompletely penetrant. Mislocalized Dorsal partially overlapped with GluRIIA (arrowheads indicate bands of Dorsal and GluRIIA; arrows indicate synaptic boutons). Scale bar in (A–H), 50 µm, in (I–N), 5 µm.
The ability of Akt1 to affect the trafficking of one glutamate receptor subunit to the postsynaptic specialization suggested the possibility that this mechanism could regulate GluR subunit composition. The distributions of glutamate receptor subunits IIB and IIC were therefore examined in animals with knockdown of Akt1 in the muscle. In the animals with reduced Akt1 function, GluRIIB, the functional alternative to IIA, remained at the synapse under conditions where GluRIIA was localized almost exclusively within intracellular bands [Supporting Information Fig. 2(H–K)]. The correct delivery of GluRIIB to the postsynaptic specialization when Akt1 function was compromised with Akt1RNAi was confirmed by showing its spatial colocalization with Bruchpilot, a presynaptic protein required for active zone function [Supporting Information Fig. 2(E–G) for control and (L–N) for Akt1 knockdown] (Wagh et al., 2006). The correct delivery of GluRIIB is consistent with the observation that these larvae were motile, and that a functional receptor must contain either GluRIIA or GluRIIB. Likewise, the essential subunit GluRIIC was appropriately localized to the postsynaptic specialization in the face of reduced Akt1 function (data not shown). Akt1 is therefore selectively regulating the delivery of GluRIIA to the synapse, and reductions in Akt1 result in mislocalization of IIA to an intracellular compartment.
The selective requirement for Akt1 function to correctly localize GluRIIA but not the other receptor subunits begs the question as to whether other proteins require Akt1 for correct targeting to the postsynaptic specialization. Therefore, we have examined three other synaptic components: Discs-Large (DLG), the homolog of mammalian PSD-95; Syndapin, an F-BAR domain-containing protein; and Basigin, a transmembrane protein located principally in the SSR. Both DLG and Syndapin promote SSR expansion (Lahey et al., 1994; Budnik et al., 1996; Guan et al., 1996; Kumar et al., 2009) and associate with SSR membrane following their translation in the cytoplasm (Thomas et al., 2000). Basigin is a synaptic transmembrane protein located principally in the postsynaptic SSR and is required for synaptic function (Besse et al., ,). Reduction of Akt1 function in the muscle to a degree that completely disrupted GluRIIA localization did not alter the selective targeting of Basigin to the synapse [Supporting Information Fig. 3, compare (A) to (B)]. DLG and Syndapin, the two cytoplasmically synthesized and SSR-associated proteins, showed normal localization to the postsynaptic specialization of the NMJ [Supporting Information Fig. 3 compare controls without GAL4 driver, (C–E) to muscle-specific 24B-GAL4>UAS-Akt1RNAi animals in (F–H)]. Quantitation of the immunofluorescence signal for these proteins did show significantly reduced levels of Basigin and Syndapin, whereas DLG signal was lower but did not achieve statistical significance (Supporting Information Fig. 4). Taken together these findings demonstrated that the mislocalization of GluRIIA upon reduction of Akt1 is specific, and does not affect the localization of other transmembrane (Basigin, GluRIIB) or cytoplasmically synthesized (Dlg, Syndapin) postsynaptic proteins.
The SSR is a complex postsynaptic membrane specialization that requires the activity of a number of proteins for its growth and maintenance, including DLG, Syndapin, and the Drosophila t-SNARE Gtaxin (Lahey et al., 1994; Budnik et al., 1996; Gorczyca et al., 2007; Kumar et al., 2009). Given that reductions in Akt1 function affected the levels of synaptic proteins Syndapin and Basigin, it was of interest to determine if Akt1 affected the elaboration of the SSR. The ultrastructure of the SSR was evaluated in animals with reduced Akt1 function using transmission electron microscopy (TEM) of NMJ synaptic boutons. At the Drosophila NMJ, the motoneuron boutons are “embedded” in the surface of the muscle cell (Jia et al., 1993). The tubulo-membranous SSR is seen as a complex set of multilayered membranes within the muscle cell and surrounding the nerve terminal [Fig. 5(A)]. The dimensions and complexity of the SSR were reduced in larvae expressing Akt1RNAi in the muscle cell without affecting the length of the presynaptic active zones [Fig. 5(A–C)]. These experiments demonstrated that Akt1 was required for the proper expansion of the SSR.
Figure 5.
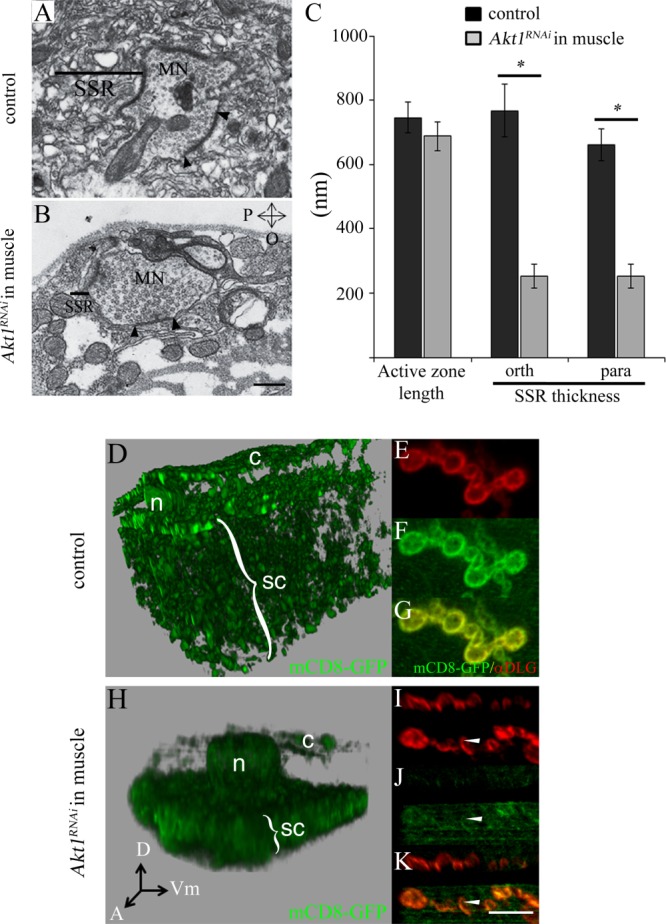
Muscle-specific inhibition of Akt1 affects the elaboration of the subsynaptic reticulum (SSR), and Akt1 is required for the integrity of the endomembrane system. A: Transmission electron microscopy shows SSR surrounding the motoneuron terminal in a control animal (UAS-Akt1RNAi/+). Bar indicates approximate dimension of SSR. For both (A) and (B), the size of the SSR was determined by measuring its thickness in two-dimensions: parallel and orthogonal to the muscle surface. B: The dimensions and complexity of the SSR were dramatically reduced in Akt1-compromised larvae (24B-GAL4>UAS-Akt1RNAi) without affecting the length of the presynaptic active zones (electron dense region, between two black arrowheads), where synaptic vesicles are released. C: Quantification of the length of presynaptic active zones, parallel and orthogonal SSR thicknesses. When compared with control animals, SSR thicknesses significant decreased in all dimensions with Akt1compromised (24B-GAL4>UAS-Akt1RNAi). “*” denotes p < 0.0005, n = 15 each. P, parallel; O, orthogonal to the axis of muscle surface. D–K: To evaluate the organization of muscle membranes, mCD8-GFP, a transmembrane protein that tags cellular membranes, was used. mCD8-GFP expressed in the muscle (G14-GAL4>UAS-mCD8-GFP) localizes to membrane compartments, including plasma membrane, t-tubules, nuclear envelope, and the endoplasmic reticulum. The panels (D) (Control; G14-GAL4,UAS-mCD8-GFP/+) and (H) (muscle-specific Akt1 knockdown: G14-GAL4, UAS-mCD8-GFP>UAS-Akt1RNAi) show 3D rendered images of serial confocal sections, representing the entire muscle cell thickness in a region where there are no synaptic boutons. The nuclear layer is located at the top of the image separating cortical (c) and subcortical (sc) membrane domains. Muscle-specific knockdown of Akt1 produced a decrease in overall muscle cell thickness and reduced the complexity of membrane compartments (H). Akt1RNAi results in a more compact subcortical membrane domain and notable reduction in the cortical membrane domain compared to control animals (D). c, the cortical membrane domain; sc, the subcortical membrane domain; n, nucleus; A, anterior; D, dorsal; and Vm, ventral midline. E–G: Visualization of the SSR by mCD8-GFP, and of the postsynaptic specialization with anti-DLG antibody (red). I–K: The extent of mCD8-GFP tagged SSR was dramatically reduced (arrowheads) by loss of Akt1 function. DLG was correctly localized and modestly reduced in comparison with mCD8-GFP in these animals. Scale bar in (A and B), 0.5 µm, in (E–G) and (I–K), 5 µm.
Gtaxin mutant shows reduced SSR elaboration as well as changes in the complex membrane architecture of the muscle cell (Gorczyca et al., 2007). This architecture was revealed by labeling all muscle membranes with a mouse transmembrane protein, mCD8-GFP, and performing 3D reconstruction of optically sectioned cells. The mCD8-GFP integral membrane protein tag uncovered a cortical membrane compartment above the muscle nuclei, and a subcortical membrane network below the nuclei intermingled with the contractile apparatus. The cortical membrane compartment was at the same level as the SSR and was greatly reduced in Gtaxin mutant (Gorczyca et al., 2007). There is evidence that DLG traffics through the cortical membrane compartment on its way to the SSR (Thomas et al., 2000; Gorczyca et al., 2007). Given that reductions of both Gtaxin and Akt1 affected SSR, we examined whether Akt1 also influenced the organization of intracellular membrane compartments, as documented for Gtaxin mutant. As previously reported, mCD8-GFP expression in the muscle cell revealed a complex set of membranous structures including a cortical domain (c), the nuclear envelope (n), and a subcortical network (sc) [Fig. 5(D,H)]. The SSR was also prominently labeled by mCD8-GFP in the muscle, as evidenced by co-localization with DLG [Fig. 5(E–G)]. Reduction of Akt1 function affected muscle cell membrane organization in a manner similar to that observed in Gtaxin mutant (Gorczyca et al., 2007). Namely, the cortical domain was nearly abolished and the subcortical domain was compressed, consistent with the much reduced muscle thickness in these animals [Fig. 5(H)]. The mCD8-GFP labeling of the SSR was also dramatically decreased, supporting the TEM findings of reduced SSR elaboration in animals with muscle-directed AktRNAi expression [Fig. 5(I–K)].
Based on the similar ultrastructural changes in the SSR and muscle membrane organization resulting from reductions in Gtaxin and Akt1 function, Gtaxin was a logical candidate as a downstream target of Akt1 activity. To explore this possibility, we examined Gtaxin levels and distribution in animals with muscle-specific expression of Akt1RNAi (Mef2-GAL4>UAS-Akt1RNAi) or a constitutively active form of Akt1 (Mef2-GAL4>UAS-Akt1CA). In wild-type animals, Gtaxin immunoreactivity is concentrated at the SSR [Fig. 6(B,C)], and muscle-directed RNAi of Akt1 greatly reduced Gtaxin levels at this postsynaptic specialization [Fig. 6(F,G)]. Gtaxin has been implicated in SSR formation not only on account of the reduction of SSR complexity in Gtaxin mutant but also from the production of ectopic, mCD8-GFP labeled membranous structures in animals overexpressing wild-type Gtaxin (Gorczyca et al., 2007). Muscle-directed expression of Akt1CA produced membranous structures with the same visible features. In these animals, Gtaxin was present at increased levels and localized to patches throughout the muscle [Fig. 6(I–K)]. These ectopic membrane elaborations were confirmed at the TEM level and are structurally similar to those documented in animals overexpressing Gtaxin in the muscle [Fig. 6(M–O)].
Figure 6.
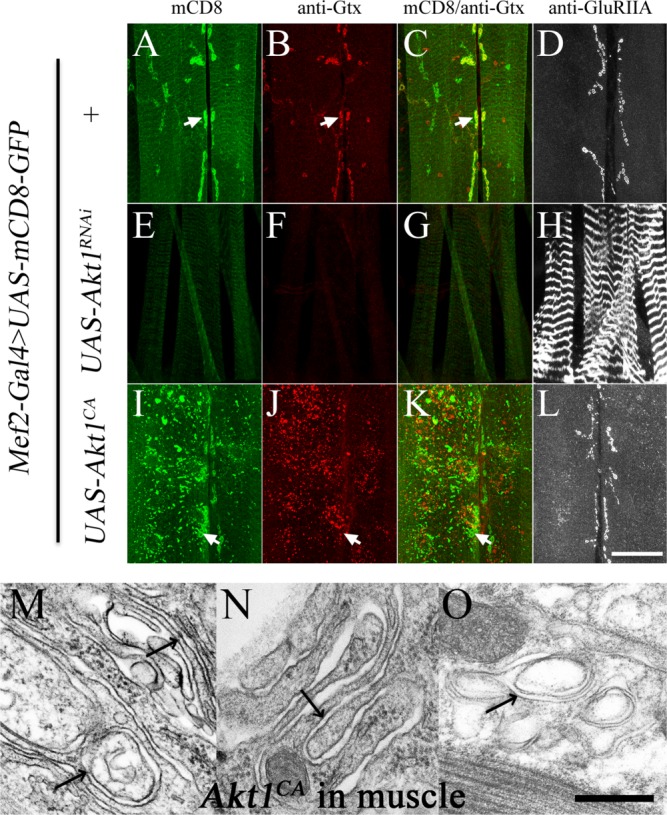
The localization and levels of Gtaxin at the postsynaptic specialization are Akt1-dependent, and overexpression of a constitutively active form of Akt1 creates ectopic membranes distant from the synaptic region. Gtaxin localization and levels were examined in animals with reduced Akt1 function or muscle directed expression of a constitutively active form of Akt1, Akt1CA. All animals in this experiment also carried the muscle-specific driver Mef2-GAL4 and UAS-mCD8-GFP transgenes. Gtaxin and GluRIIA were detected with anti-Gtx (red) and anti-GluRIIA (grayscale) antibodies. Panels (A–C), (E–G), and (I–K) are each from a single animal. Panels (D), (H), and (L) are anti-GluRIIA staining each from a single larva. A–D: Control animals had no Akt1-bearing transgene either Akt1RNAi or Akt1CA (labeled as +). Gtaxin is concentrated at the SSR, colocalizing with mCD8-GFP (arrows). GluRIIA is also highly concentrated at the NMJ specialization. E–H: Animals expressing UAS-Akt1RNAi showed dramatic reductions in the levels of mCD8-GFP at the SSR (E) and loss of Gtaxin at the synapse (F), as well as mislocalization of GluRIIA (H). I–L: Overexpression of Akt1CA caused ectopic mCD8 patches throughout the muscle (I), as well as increased levels of Gtaxin (J). The normal distribution of Gtaxin at the SSR was lost, with mislocalized Gtaxin patches evident throughout the muscle cell (J). Correct GluRIIA localization was maintained in these animals (L). M–O: Transmission electron microscope photomicrographs show ectopic membranous structures in muscles overexpressing the constitutively active form of Akt1 (Mef2-GAL4>UAS-Akt1CA). Arrows point to infoldings of multilayered membranes in the cytosol or underneath the plasma membrane. Scale bar in (A–L), 50 µm, in (M–O), 0.1 µm.
The formation of mCD8-GFP-labelled membrane patches mediated by Akt1CA was also found to be dependent on Gtaxin. The ectopic membranous patches induced by Akt1CA expression in the muscle were visualized by mCD8-mRFP and showed some features of SSR, namely concentration of α-Spectrin and DLG (Pielage et al., 2006) [Fig. 7]. Reduction of Gtaxin by RNA interference blocked the Akt1CA-mediated formation of these “ectopic” SSR structures [Fig. 7(B,D)]. The ectopic membrane patches induced by Akt1CA overexpression were not reduced by expression of a control UAS-transgene, excluding the possibility that suppression of Akt1CA function was due to titration of GAL4 proteins in GtaxinRNAi expressed animals (data not shown). Inhibition of Gtaxin by GtaxinRNAi expression in muscle induced loss of mCD8 at the SSR but DLG remained at the postsynaptic specialization [Fig. 7(F,H)]. In addition, GluRIIA localization was not disrupted by GtaxinRNAi, indicating that Gtaxin does not play a role in this aspect of Akt1 function and is consistent with published findings (Gorczyca et al., 2007) [Fig. 7(J)]. These results demonstrated that Gtaxin is required for Akt1CA-mediated formation of ectopic membranous structures. It is of interest that Gtaxin bears a consensus sequence (RXRXXS/T) for Akt1 phosphorylation, indicating a potential phosphorylation site at Serine 255, suggesting that Gtaxin could be a direct target of Akt1 activity (Datta et al., 1999; Zhang et al., 2002).
Figure 7.
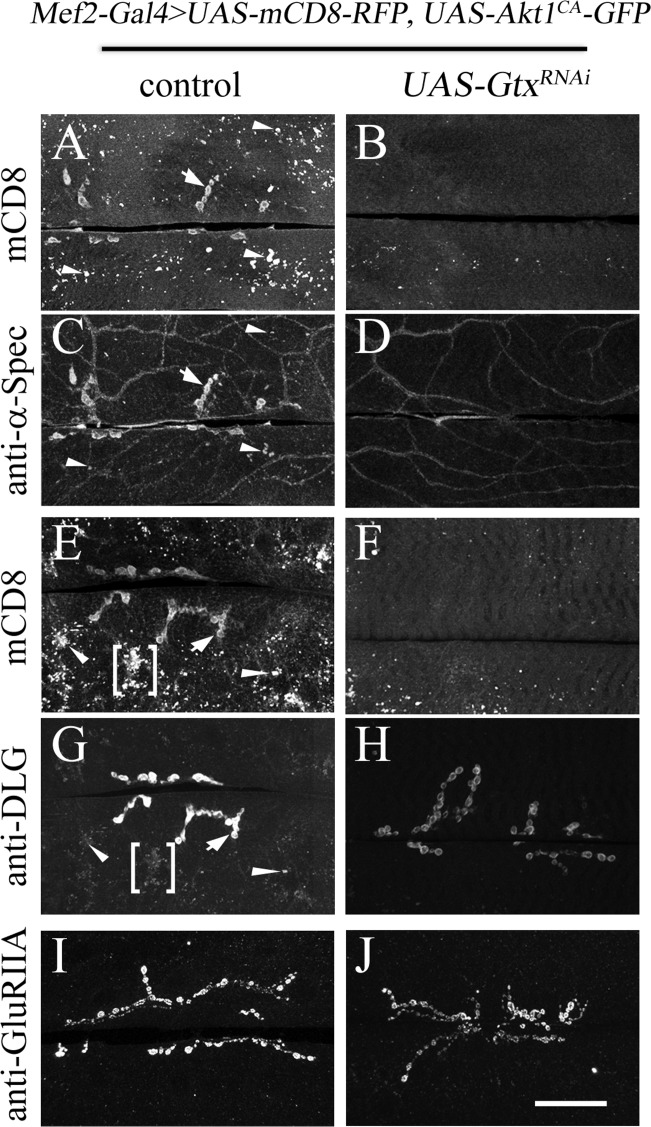
Gtaxin is required for ectopic membranous patches produced by expression of a constitutively activated form of Akt1. Synapse organization was assessed with mCD8-RFP and anti-α-Spectrin or anti-DLG antibody staining. All animals carried Mef2-GAL4, UAS-mCD8-RFP, UAS-Akt1CA-GFP, and over either OreR as a control or UAS-GtxRNAi. Either anti-α-Spectrin or anti-DLG with mCD8-RFP images for each genotype were from the same animal, with a second preparation providing the anti-GluRIIA data. A and C: In animals with muscle-directed expression of Akt1CA-GFP, ectopic patches of mCD8 were observed throughout the muscle (panel A, arrows), while leaving the SSR structure intact (on set of postsynaptic specializations shown with small arrow). Some of the ectopic mCD8 membrane patches also showed anti-α-Spectrin antibody staining (arrowheads in C). Inhibition of Gtx with RNAi abolished the mCD8 patches and greatly reduced the anti-α-Spectrin staining (panels B and D). Using anti-DLG to examine SSR structure in animals with muscle-directed expression of Akt1CA also revealed that the membranous patches show some SSR-properties as evidenced by anti-DLG colocalization (arrowheads and a bracket in panels E and G). Inhibition of Gtx produced loss of mCD8-concentrated SSR membrane but not DLG localization to the postsynaptic specialization (panels F and H). As reported earlier and confirmed here, loss of Gtx did not compromise GluRIIA localization (panels I and J). Scale bar for A–J, 50 µm.
The experiments described above establish that Akt1 is required for correct delivery of the GluRIIA subunit to the postsynaptic membrane and elaboration of the SSR. To assess the effects of Akt1 on NMJ physiology, we conducted single cell recordings on the muscles of both Akt1 mutants and animals with muscle-specific knockdown of Akt1 using RNA interference. In animals with Akt1RNAi directed to the muscle, amplitudes of miniature excitatory junctional potentials (mEJPs) were dramatically reduced, consistent with earlier reports that this measure of muscle response to spontaneous neurotransmitter release is greatly reduced [Fig. 8(A)] (Petersen et al., 1997). In larvae bearing a combination of mutant alleles (Akt11/Akt104226), mEJPs amplitudes were reduced but not to a statistically significant level [Fig. 8(A,B)]. However, Akt11/Akt104226 transheterozygotes showed significantly reduced EJP amplitudes, the response of the muscle to a single suprathreshold stimulus of the motoneuron [Fig. 8(C,D)]. The shape of the EJP was also altered in Akt11/Akt104226 animals, with measures of the EJP decay indicating a significantly decreased time to restore the membrane voltage to resting levels [Fig. 8(E)]. We noted these same changes in EJP properties in animals with Akt1 function compromised in the muscle with targeted expression of Akt1RNAi. The temperature sensitivity of the GAL4-UAS system allowed the graded reduction of Akt1 function in the muscle. At elevated temperatures, Akt1RNAi expression was higher and thus the reduction in Akt1 function was more pronounced. At 24°C, expression of Akt1RNAi in the muscle produced a significant reduction in the EJP amplitude, as observed in Akt11/Akt104226 animals [Fig. 8(F,G)]. The EJP amplitudes were not significantly altered in animals reared at 18°C where Akt1 function was compromised modestly [Fig. 8(F,G)]. However, at both temperatures, significant differences in the decay times of the EJPs in Akt1RNAi expressing animals were observed [Fig. 8(H)].
Figure 8.
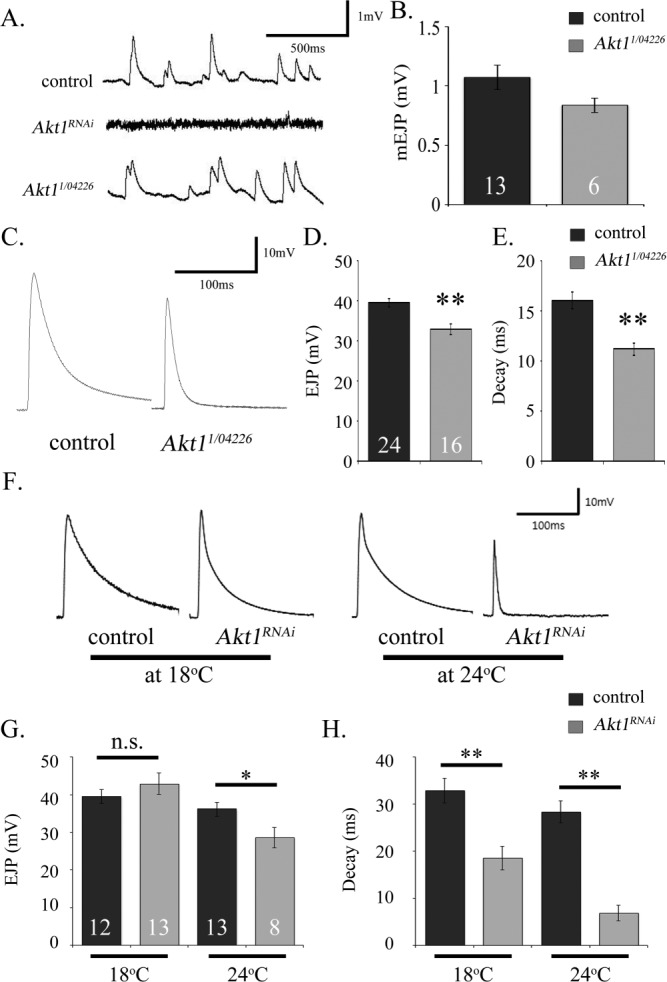
Akt1 is required for normal electrophysiological response at neuromuscular synapses. A: Representative traces of miniature excitatory junction potentials (mEJPs) for OreR strain (as control), Akt1 knockdown in the muscle, and Akt11/Akt104226 transheterozygous mutant animals. The Akt1RNAi expressing animals showed no readily detectable mEJP. B: Akt11/Akt104226 mutants, a mild hypomorphic combination of alleles, displayed somewhat reduced but not statistically significant different mEJP amplitude compared with controls (p = 0.08). C: EJP responses, detected in the muscle following motoneuron stimulation for control and Akt11/Akt104226 mutant. D and E: Akt11/Akt104226 mutant larvae exhibited significantly decreased EJP amplitudes and decay time compared to control (**p < 0.005, n = 24/16). F: Traces of EJP in control (Mef2-GAL4>UAS-mCD8-GFP) and Akt1RNAi expressing larvae (Mef2-GAL4>UAS-Akt1RNAi) reared at two different temperatures. G: EJP amplitude showed no difference at 18°C (low level of inhibition, n.s., no significant, n = 12/13), but was significantly decreased at 24°C (greater degree if Akt1 inhibition,*p < 0.05, n = 13/8). H: Similar to the electrophysiological changes observed in the Akt11/Akt104226 mutant animals, EJP decay time was abbreviated in Akt1RNAi expressing larvae, both at 18°C or 24°C (**p < 0.005).
A number of factors can influence the dynamics of the EJP, including membrane capacitance and resistance, as well as changes in voltage-gated channels in the membrane. To determine if the muscle membrane showed any changes in baseline resistance or capacitance, muscle cell responses to small current injections (1nA) were examined. Akt1RNAi expressing animals did not show any significant changes to these small current applications (data not shown), suggesting that nonvoltage-dependent membrane properties could not account for the changes we observed in the EJPs of animals with decreased Akt1 function.
It is important to note that electrophysiological changes observed in Akt1 mutant and muscle-specific Akt1 RNAi animals match the morphological and molecular alterations at the NMJ. In Akt1RNAi expressing animals at 24°C, mEJP amplitudes were decreased to a nearly undetectable level [Fig. 8(A)], consistent with the previous characterization of GluRIIA null mutant (DiAntonio et al., 1999). Furthermore, the decreased EJP decay times recorded in Akt11/Akt104226 and muscle-directed Akt1RNAi animals also mimicked a prominent phenotype of Gtaxin mutant animals (Gorczyca et al., 2007), supporting the hypothesis that changes in SSR and muscle membranous system were the reason for these physiological changes.
DISCUSSION
We explored Akt function in synapse development and function using a well-characterized model system, the Drosophila neuromuscular junction. There is a single Akt homolog in Drosophila, Akt1, facilitating the genetic and cellular studies of Akt function in synapse assembly. Our findings are summarized in Figure 9. We found that Akt1 was specifically required for the correct assembly of A-type glutamate receptors. Reductions of Akt1 function either by mutation or RNA interference resulted in a loss of GluRIIA at the synapse paired with accumulation into intracellular structures. Reduction of Akt1 influenced the levels and localization of proteins shown to affect GluRIIA, Dorsal, and Cactus. Therefore, Akt1 could affect GluRIIA at least in part via control of these proteins. Akt1 was also required for the normal expansion of a specialized postsynaptic membrane compartment, the SSR. We provide evidence that Akt1 mediates its effects on SSR via control of the t-SNARE Gtaxin. RNA interference of Gtaxin did not affect GluRIIA localization, showing that the control of SSR expansion and glutamate receptor composition mediated by Akt1 occurs via different molecular mechanisms.
Figure 9.
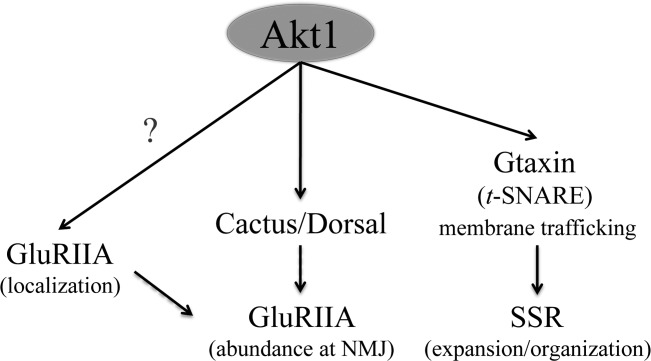
Model for Akt1's regulatory role at the NMJ. Akt1 selectively affects A-type glutamate receptor abundance at the NMJ. Compromising Akt1 function reduced the levels of Dorsal and Cactus at the NMJ, two potential Akt1 targets that regulate GluRIIA levels. Loss of Akt1 also results in GluRIIA mislocalization to intracellular membrane compartments, suggesting that other Akt1 targets are involved as well. Akt1-dependent subsynaptic membrane expansion is mediated through a separate pathway where the Drosophila t-SNARE protein Gtaxin acts downstream of Akt1 function.
The analysis of Akt1 reported here examined physiological, morphological, and cellular phenotypes, using both traditional Akt1 mutant alleles and cell-type directed knockdown achieved with either of two different UAS-Akt1RNAi lines. The results from these different genetic tools were consistent and showed that Akt1 function is critical for both GluRIIA localization and SSR expansion. In particular, combinations of Akt1 alleles resulted in the redistribution of GluRIIA into intracellular bands, a phenotype found to be even more pronounced in muscle-directed RNAi of Akt1. This remarkable phenotype was also observed in larvae expressing both Akt1RNAi and a UAS-transgene-derived GluRIIA-RFP in the muscle, the latter detected by either endogenous fluorescence or anti-RFP antibody. It was of note that fluorescent signal from the GluRIIA-RFP was reduced at the synapse but receptor mislocalization to intracellular compartments was detected only with anti-RFP antibody. Akt1-dependent events were clearly required for the proper formation of the folded RFP domain of the recombinant GluRIIA protein while the polypeptide, detected with the anti-RFP antibody was present and redirected to an alternative cellular location, as we observed for the endogenous GluRIIA. These data implicate Akt1 in processes of folding, stabilization, or assembly of GluRIIA.
A number of experiments were conducted to evaluate if Akt1 was required for the localization of specific postsynaptic proteins, or rather served a more generalized role in directing a variety of proteins to this membrane specialization. The correct localization of GluRIIB, GluRIIC, Basigin, Discs large, and Syndapin in animals with Akt1 knockdown in the muscle demonstrated that Akt1 has specific targeting functions for GluRIIA and is not a general factor for delivery of all postsynaptic proteins. Levels of these postsynaptic proteins were reduced in Akt1RNAi bearing animals, not surprisingly given the substantial size reduction in the SSR.
At the Drosophila NMJ, two types of glutamate receptors have been defined by their distinct compositions and physiological properties (DiAntonio et al., 1999; DiAntonio, 2006). The shifting between A- and B-type receptors provides a mechanism for modulating postsynaptic responses to variable presynaptic inputs during development (Sigrist et al., 2002). There is considerable evidence that modulation of GluRIIA and B representation at the NMJ is governed by different signaling systems. Coracle, a homolog of protein 4.1 in Drosophila, has been shown to specifically influence the targeting of GluRIIA but not IIB (Chen et al., 2005). A physical interaction between Coracle and GluRIIA was essential for actin-dependent trafficking of GluRIIA-containing vesicles to the plasma membrane. Conversely, DLG has been shown to be required for GluRIIB but not GluRIIA localization at the NMJ (Chen and Featherstone, 2005). Our finding supports the conclusion that A and B receptor subunits are differentially regulated and show that Akt1 serves a role in A but not B subunit control.
There is evidence that the assembly and localization of GluRIIA into the postsynaptic density at the NMJ is accomplished following delivery to the plasma membrane (Broadie and Bate, 1993; Rasse et al., 2005). This conclusion is based upon the observation that fluorescence photobleaching of the entire muscle delays accumulation of new GluRIIA to synaptic sites more so than local bleaching at the NMJ (Rasse et al., 2005). The effects of Akt1 on GluRIIA localization could therefore be mediated by either regulated delivery of GluRIIA-containing vesicles to the plasma membrane, or by affecting the localization to the postsynaptic density following insertion into the plasma membrane. The accumulation of GluRIIA into an intracellular membrane compartments argues for a trafficking-based mechanism. This model is further supported by the results from the developmental timing experiments, where Akt1 function was removed during different stages in synapse assembly. Loss of Akt1 in a 2 day window early in development produced the phenotypes observed with continuous loss of Akt1, whereas a 2 day loss in third instar did not. If Akt1 simply served to retain GluRIIA at the synapse, there should have been time for new synthesis to repopulate the NMJ. Therefore, we favor a model where Akt1 affects developmental processes required for the selective delivery of GluRIIA from the endoplasmic reticulum into functional receptor units that arrive at the plasma membrane. It is notable that in mammalian systems, Akt is critical for the insulin-stimulated exocytosis of glucose transporter containing vesicles to the plasma membrane (Gonzalez and McGraw, 2006; Grillo et al., 2009). Perhaps Akt1 governs similar exocytic processes at synapses. Akt1 signaling has also shown to be essential for AMPA receptor trafficking in hippocampal neurons, further supporting a role for Akt1 in trafficking of synaptic proteins (Qin et al., 2005b; Hou et al., 2008; Pratt et al., 2011).
A striking phenotype of animals with reduced Akt1 function in muscles was a severe reduction in the SSR and disruption of intracellular membrane organization. These phenotypes were similar to those found in a Gtaxin mutant and suggested the possibility that Akt1 and Gtaxin are involved in the same cellular process (Gorczyca et al., 2007). A number of observations reported here indicate Akt1 activity is mediated at least in part by control of Gtaxin. First, Gtaxin levels at the SSR are greatly reduced in animals with reduced Akt1 function in the muscle cells. Second, muscle-directed overexpression of a constitutively active form of Akt1 (Akt1CA) produced ectopic membranous structures; a phenotype also observed with Gtaxin overexpression and elevated levels of Gtaxin. Third, inhibition of Gtaxin blocks the effects of the constitutively active Akt1 in the muscle cell. Gtaxin does contain a consensus site for Akt1 phosphorylation and could therefore be a direct target of Akt1 kinase activity in regulating SNARE complex assembly.
The regulatory roles of Akt1 in glutamate receptor composition and postsynaptic membrane expansion could be accomplished through separate or identical downstream effectors. The fact that Gtaxin mutants did not disrupt GluRIIA distribution suggests different downstream effectors regulated by Akt1. The regulation of GluRIIA localization by Akt1 does not involve Gtaxin but could be mediated via Dorsal and Cactus. Dorsal and Cactus influence glutamate receptor delivery and are known effectors of Akt activity in mammalian cells (Heckscher et al., 2007; Dan et al., 2008). The levels of both Dorsal and Cactus were reduced in animals with knockdown of Akt1 in the muscle. Notably, in some animals expressing Akt1RNAi in the muscle, Dorsal showed an altered intracellular distribution that overlapped with the mislocalized GluRIIA. However, because Dorsal and Cactus mutants are not reported to mislocalize GluRIIA into intracellular bands, Akt1 is likely to have additional downstream targets that influence GluRIIA localization and delivery to the postsynaptic specialization.
Physiological measures of synaptic transmission showed that Akt1 function is required for normal synapse function. Akt1 transheterozygous mutants (Akt11/Akt104226) showed reduced EJP amplitudes and altered decay kinetics of the EJP. These same phenotypes were observed in animals with muscle-specific inhibition of Akt1 function, with the severity correlating to the degree of Akt1 inhibition. These changes in EJP kinetics were not accompanied by alterations of nonvoltage-dependent membrane capacitance or resistance, suggesting that voltage-gated channels contributing to EJP rise and decay times may be affected by Akt1. These findings contrast published work with Akt1 mutant animals describing changes in long-term depression but not in EJP properties (Guo and Zhong, 2006). However, we note that our physiological studies were conducted at a higher Ca2+ concentration, which could account for these different measures of EJP properties in Akt1 mutants. It is important to point out that the physiological changes we document were observed in both Akt1 mutant larvae as well as animals with RNA interference of Akt1 in the muscle cell. The physiological changes we observed in Akt1 compromised animals are logical consequences of observed changes in NMJ composition. Loss of GluRIIA-containing receptors and an overall decrease in functional GluRs at the synapse could decrease the EJP amplitude. The altered EJP decay pattern in animals with reduced Akt1 is consistent with the involvement of Gtaxin, as we have documented here. Gtaxin mutants showed similar changes in EJP decay, indicating that this feature of Akt1 mediated physiological change is associated with the consequences of compromising the function of this t-SNARE.
There is a precedent for Akt-mediated regulation of neurotransmitter receptor localization to the cell surface. The NMDA receptor subunit NR2C is developmentally regulated in cerebellar granule cells and Akt-mediated phosphorylation is critical for cell surface expression of NR2C-containing receptors (Chen, 2009). Akt has also proven to be important in the elaboration of dendritic complexity in Drosophila sensory neurons, suggesting that this kinase is of general importance in the control of nervous system receptive fields (Parrish et al., 2009). Selective control of Akt or its downstream targets could provide a powerful method of influencing synaptic transmission and the receptive properties of neurons.
Acknowledgments
We are grateful to Aaron DiAntonio, David Featherstone, Mani Ramaswami, Steven Wasserman, Vivian Budnick, Fumiko Kawasaki, Richard Ordway, and Melissa Rolls as well as to the Bloomington Drosophila stock center and Vienna Drosophila RNAi Center for Drosophila stocks and antibodies. We also acknowledge Missy Hazen for technical assistance with electron microscopy and Claire Reynolds for critical reading of the manuscript. Our study was supported by grants from USDD, Martin Lenz Harrison Endowed Chair (Department of Pediatrics, University of Minnesota), and The Pennsylvania State University.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supporting Figure 1.
Supporting Figure 2.
Supporting Figure 3.
Supporting Figure 4.
REFERENCES
- Bae SS, Cho H, Mu J, Birnbaum MJ. Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. J Biol Chem. 2003;278:49530–49536. doi: 10.1074/jbc.M306782200. [DOI] [PubMed] [Google Scholar]
- Besse F, Mertel S, Kittel R, Wichmann C, Rasse T, Sigrist S, Ephrussi A. Basigin controls neuromuscular junction growth and synaptic vesicle distribution and release. J Neurogenet. 2006;20:86–87. [Google Scholar]
- Besse F, Mertel S, Kittel RJ, Wichmann C, Rasse TM, Sigrist SJ, Ephrussi A. The Ig cell adhesion molecule Basigin controls compartmentalization and vesicle release at Drosophila melanogaster synapses. J Cell Biol. 2007;177:843–855. doi: 10.1083/jcb.200701111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene-expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Broadie K, Bate M. Innervation directs receptor synthesis and localization in Drosophila embryo synaptogenesis. Nature. 1993;361:350–353. doi: 10.1038/361350a0. [DOI] [PubMed] [Google Scholar]
- Brunner A, Okane CJ. The fascination of the Drosophila NMJ. Trends Genet. 1997;13:85–87. doi: 10.1016/s0168-9525(97)01060-3. [DOI] [PubMed] [Google Scholar]
- Budnik V, Koh YH, Guan B, Hartmann B, Hough C, Woods D, Gorczyca M. Regulation of synapse structure and function by the Drosophila tumor suppressor gene dlg. Neuron. 1996;17:627–640. doi: 10.1016/s0896-6273(00)80196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B-S. Growth factor-dependent trafficking of cerebellar NMDA receptors via protein kinase B/Akt phosphorylation of NR2C. Neuron. 2009;62:471–478. doi: 10.1016/j.neuron.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Merino C, Sigrist SJ, Featherstone DE. The 4.1 protein coracle mediates subunit-selective anchoring of Drosophila glutamate receptors to the postsynaptic actin cytoskeleton. J Neurosci. 2005;25:6667–6675. doi: 10.1523/JNEUROSCI.1527-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KY, Featherstone DE. Discs-large (DLG) is clustered by presynaptic innervation and regulates postsynaptic glutamate receptor subunit composition in Drosophila. BMC Biol. 2005;3 doi: 10.1186/1741-7007-3-1. :1 doi:10.1186/1741-7007-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, Roninson I, et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the akt1 gene. Genes Dev. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu QW, Crenshaw EB, Kaestner KH, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- Collins CA, DiAntonio A. Synaptic development: Insights from Drosophila. Curr Opin Neurobiol. 2007;17:35–42. doi: 10.1016/j.conb.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Dan HC, Cooper MJ, Cogswell PC, Duncan JA, Ting JPY, Baldwin AS. Akt-dependent regulation of NF-kappa B is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008;22:1490–1500. doi: 10.1101/gad.1662308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: A play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- DiAntonio A. Glutamate receptors at the Drosophila. 2nd ed. Vivian Budnik and Catalina Ruiz-Canada: Elsevier Academic Press Inc; 2006. pp. 165–179. neuromuscular junction. In: Fly Neuromuscular Junction: Structure and function. [Google Scholar]
- DiAntonio A, Petersen SA, Heckmann M, Goodman CS. Glutamate receptor expression regulates quantal size and quantal content at the Drosophila neuromuscular junction. J Neurosci. 1999;19:3023–3032. doi: 10.1523/JNEUROSCI.19-08-03023.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Dimitroff B, Howe K, Watson A, Campion B, Lee HG, Zhao N, O'Connor MB, et al. Diet and energy-sensing inputs affect TorC1-mediated axon misrouting but not TorC2-directed synapse growth in a Drosophila model of tuberous sclerosis. PLoS ONE 7(2): e30722.doi:10.1371/journal.pone.0030722. 2012 doi: 10.1371/journal.pone.0030722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao RJ, Cooper GM, Segal RA, et al. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS, Lee VMY, et al. Role for Akt3/Protein kinase B gamma in attainment of normal brain size. Mol Cell Biol. 2005;25:1869–1878. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone DE, Rushton E, Broadie K. Developmental regulation of glutamate receptor field size by nonvesicular glutamate release. Nat Neurosci. 2002;5:141–146. doi: 10.1038/nn789. [DOI] [PubMed] [Google Scholar]
- Featherstone DE, Rushton E, Rohrbough J, Liebl F, Karr J, Sheng Q, Rodesch CK, et al. An essential Drosophila glutamate receptor subunit that functions in both central neuropil and neuromuscular junction. J Neurosci. 2005;25:3199–3208. doi: 10.1523/JNEUROSCI.4201-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke TF. PI3K/Akt: Getting it right matters. Oncogene. 2008;27:6473–6488. doi: 10.1038/onc.2008.313. [DOI] [PubMed] [Google Scholar]
- Gonzalez E, McGraw TE. Insulin signaling diverges into Akt-dependent and -independent signals to regulate the recruitment/docking and the fusion of GLUT4 vesicles to the plasma membrane. Mol Biol Cell. 2006;17:4484–4493. doi: 10.1091/mbc.E06-07-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorczyca D, Ashley J, Speese S, Gherbesi N, Thomas U, Gundelfinger E, Gramates LS, et al. Postsynaptic membrane addition depends on the discs-large-interacting t-SNARE gtaxin. J Neurosci. 2007;27:1033–1044. doi: 10.1523/JNEUROSCI.3160-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramates LS, Budnik V. Assembly and maturation of the Drosophila larval neuromuscular junction. Int Rev Neurobiol. 1999;43:93–117. doi: 10.1016/s0074-7742(08)60542-5. [DOI] [PubMed] [Google Scholar]
- Grider MH, Park D, Spencer DM, Shine HD. Lipid Raft-targeted Akt promotes axonal branching and growth cone expansion Via mTOR and Rac1, respectively. J Neurosci Res. 2009;87:3033–3042. doi: 10.1002/jnr.22140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo CA, Piroli GG, Hendry RM, Reagan LP. Insulin-stimulated translocation of GLUT4 to the plasma membrane in rat hippocampus is PI3-kinase dependent. Brain Res. 2009;1296:35–45. doi: 10.1016/j.brainres.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan B, Hartmann B, Kho YH, Gorczyca M, Budnik V. The Drosophila tumor suppressor gene, dig, is involved in structural plasticity at a glutamatergic synapse. Curr Biol. 1996;6:695–706. doi: 10.1016/s0960-9822(09)00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo HF, Zhong Y. Requirement of Akt to mediate long-term synaptic depression in Drosophila. J Neurosci. 2006;26:4004–4014. doi: 10.1523/JNEUROSCI.3616-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckscher ES, Fetter RD, Marek KW, Albin SD, Davis GW. NF-kappa B, I kappa B, and IRAK control glutarnate receptor density at the Drosophila NMJ. Neuron. 2007;55:859–873. doi: 10.1016/j.neuron.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q, Zhang D, Jarzylo L, Huganir RL, Man HY. Homeostatic regulation of AMPA receptor expression at single hippocampal synapses. Proc Natl Acad Sci USA. 2008;105:775–780. doi: 10.1073/pnas.0706447105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Properties of larval neuromuscular-junction in Drosophila melanogaster. J Physiol. 1976;262:189–214. doi: 10.1113/jphysiol.1976.sp011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia XX, Gorczyca M, Budnik V. Ultrastructure of neuromuscular-junctions in Drosophila—Comparison of wild-type and mutants with increased excitability. J Neurobiol. 1993;24:1025–1044. doi: 10.1002/neu.480240804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr J, Vagin V, Chen KY, Ganesan S, Olenkina O, Gvozdev V, Featherstone DE. Regulation of glutamate receptor subunit availability by microRNAs. J Cell Biol. 2009;185:685–697. doi: 10.1083/jcb.200902062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittel RJ, Wichmann C, Rasse TM, Fouquet W, Schmidt M, Schmid A, Wagh DA, et al. Bruchpilot promotes active zone assembly, Ca2+ channel clustering, and vesicle release. Science. 2006;312:1051–1054. doi: 10.1126/science.1126308. [DOI] [PubMed] [Google Scholar]
- Kumar V, Fricke R, Bhar D, Reddy-Alla S, Krishnan KS, Bogdan S, Ramaswami M. Syndapin promotes formation of a postsynaptic membrane system in Drosophila. Mol Biol Cell. 2009;20:2254–2264. doi: 10.1091/mbc.E08-10-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey T, Gorczyca M, Jia XX, Budnik V. The Drosophila tumor-suppressor gene Dlg is required for normal synaptic bouton structure. Neuron. 1994;13:823–835. doi: 10.1016/0896-6273(94)90249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterborn JC, Rex CS, Kramar E, Chen LY, Pandyarajan V, Lynch G, Gall CM. Brain-derived neurotrophic factor rescues synaptic plasticity in a mouse model of fragile × syndrome. J Neurosci. 2007;27:10685–10694. doi: 10.1523/JNEUROSCI.2624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C-C, Huang C-C, Hsu K-S. Insulin promotes dendritic spine and synapse formation by the PI3K/Akt/mTOR and Rac1 signaling pathways. Neuropharmacology. 2011;61:867–879. doi: 10.1016/j.neuropharm.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrus SB, Portman SL, Allen MJ, Moffat KG, DiAntonio A. Differential localization of glutamate receptor subunits at the Drosophila neuromuscular junction. J Neurosci. 2004;24:1406–1415. doi: 10.1523/JNEUROSCI.1575-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy CE, Cartee GD. Akt2 is essential for the full effect of calorie restriction on insulin-stimulated glucose uptake in skeletal muscle. Diabetes. 2005;54:1349–1356. doi: 10.2337/diabetes.54.5.1349. [DOI] [PubMed] [Google Scholar]
- Mozden SM, Rubin GM. The Berkeley Drosophila genome project gene disruption project: Single P-element insertions mutating 25% of vital Drosophila genes. Genetics. 1999;153:1491–1491. doi: 10.1093/genetics/153.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K, Sakaue H, Thompson DA, Weigel RJ, Roth RA. Identification of a human Akt3 (protein kinase B gamma) which contains the regulatory serine phosphorylation site. Biochem Biophys Res Commun. 1999;257:906–910. doi: 10.1006/bbrc.1999.0559. [DOI] [PubMed] [Google Scholar]
- Parrish JZ, Xu PZ, Kim CC, Jan LY, Jan YN. The microRNA bantam functions in epithelial cells to regulate scaling growth of dendrite arbors in Drosophila sensory neurons. Neuron. 2009;63:788–802. doi: 10.1016/j.neuron.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SA, Fetter RD, Noordermeer JN, Goodman CS, DiAntonio A. Genetic analysis of glutamate receptors in Drosophila reveals a retrograde signal regulating presynaptic transmitter release. Neuron. 1997;19:1237–1248. doi: 10.1016/s0896-6273(00)80415-8. [DOI] [PubMed] [Google Scholar]
- Pielage J, Fetter RD, Davis GW. A postsynaptic spectrin scaffold defines active zone size, spacing, and efficacy at the Drosophila neuromuscular junction. J Cell Biol. 2006;175:491–503. doi: 10.1083/jcb.200607036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt KG, Zimmerman EC, Cook DG, Sullivan JM. Presenilin 1 regulates homeostatic synaptic scaling through Akt signaling. Nat Neurosci. 2011;14:1112–1114. doi: 10.1038/nn.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin G, Schwarz T, Kittel RJ, Schmid A, Rasse TM, Kappei D, Ponimaskin E, et al. Four different subunits are essential for expressing the synaptic glutamate receptor at neuromuscular junctions of Drosophila. J Neurosci. 2005a;25:3209–3218. doi: 10.1523/JNEUROSCI.4194-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Zhu YH, Baumgart JP, Stornetta RL, Seidenman K, Mack V, van Aelst L, et al. State-dependent Ras signaling and AMPA receptor trafficking. Genes Dev. 2005b;19:2000–2015. doi: 10.1101/gad.342205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasse TM, Fouquet W, Schmid A, Kittel RJ, Mertel S, Sigrist CB, Schmidt M, et al. Glutamate receptor dynamics organizing synapse formation in vivo. Nat Neurosci. 2005;8:898–905. doi: 10.1038/nn1484. [DOI] [PubMed] [Google Scholar]
- Rawson JM, Lee M, Kennedy EL, Selleck SB. Drosophila neuromuscular synapse assembly and function require the TGF-beta type I receptor saxophone and the transcription factor mad. J Neurobiol. 2003;55:134–150. doi: 10.1002/neu.10189. [DOI] [PubMed] [Google Scholar]
- Ruiz-Canada C, Budnik V. Introduction on the use of the Drosophila embryonic/larval neuromuscular junction as a model system to study synapse development and function, and a brief summary of pathfinding and target recognition. Int Rev Neurobiol. 2006;75:1–31. doi: 10.1016/S0074-7742(06)75001-2. [DOI] [PubMed] [Google Scholar]
- Salinas PC. Synaptogenesis: Wnt and TGF-beta take centre stage. Curr Biol. 2003;13:R60–R62. doi: 10.1016/s0960-9822(02)01429-x. [DOI] [PubMed] [Google Scholar]
- Schuster CM. Glutamatergic synapses of Drosophila neuromuscular junctions: A high-resolution model for the analysis of experience-dependent potentiation. Cell Tissue Res. 2006;326:287–299. doi: 10.1007/s00441-006-0290-5. [DOI] [PubMed] [Google Scholar]
- Serantes R, Arnalich F, Figueroa M, Salinas M, Andres-Mateos E, Codoceo R, Renart J, et al. Interleukin-1 beta enhances GABA(A) receptor cell-surface expression by a phosphatidylinositol 3-kinase/Akt pathway—Relevance to sepsis-associated encephalopathy. J Biol Chem. 2006;281:14632–14643. doi: 10.1074/jbc.M512489200. [DOI] [PubMed] [Google Scholar]
- Sigrist SJ, Thiel PR, Reiff DF, Schuster CM. The postsynaptic glutamate receptor subunit DGluR-IIA mediates long-term plasticity in Drosophila. J Neurosci. 2002;22:7362–7372. doi: 10.1523/JNEUROSCI.22-17-07362.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staveley BE, Ruel L, Jin J, Stambolic V, Mastronardi FG, Heitzler P, Woodgett JR, et al. Genetic analysis of protein kinase B (AKT) in Drosophila. Curr Biol. 1998;8:599–602. doi: 10.1016/s0960-9822(98)70231-3. [DOI] [PubMed] [Google Scholar]
- Stewart BA, Atwood HL, Renger JJ, Wang J, Wu CF. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J Comp Physiol A. 1994;175:179–191. doi: 10.1007/BF00215114. [DOI] [PubMed] [Google Scholar]
- Thomas U, Ebitsch S, Gorczyca M, Koh YH, Hough CD, Woods D, Gundelfinger ED, et al. Synaptic targeting and localization of discs-large is a stepwise process controlled by different domains of the protein. Curr Biol. 2000;10:1108–1117. doi: 10.1016/s0960-9822(00)00696-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp O, Yang ZZ, Brodbeck D, Dummler BA, Hemmings-Mieszczak M, Watanabe T, Michaelis T, et al. Essential role of protein kinase B gamma (PKB gamma/Akt3) in postnatal brain development but not in glucose homeostasis. Development. 2005;132:2943–2954. doi: 10.1242/dev.01864. [DOI] [PubMed] [Google Scholar]
- Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Durrbeck H, Buchner S, et al. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49:833–844. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Watson RT, Pessin JE. Bridging the GAP between insulin signaling and GLUT4 translocation. Trends Biochem Sci. 2006;31:215–222. doi: 10.1016/j.tibs.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Zeidler MP, Tan C, Bellaiche Y, Cherry S, Hader S, Gayko U, Perrimon N. Temperature-sensitive control of protein activity by conditionally splicing inteins. Nat Biotechnol. 2004;22:871–876. doi: 10.1038/nbt979. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zha XM, Tan Y, Hornbeck PV, Mastrangelo AJ, Alessi DR, Polakiewicz RD, et al. Phosphoprotein analysis using antibodies broadly reactive against phosphorylated motifs. J Biol Chem. 2002;277:39379–39387. doi: 10.1074/jbc.M206399200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figure 1.
Supporting Figure 2.
Supporting Figure 3.
Supporting Figure 4.


