Abstract
Aim
To evaluate the in vitro immunogenic and immunomodulatory properties of induced pluripotent stem cells (iPSCs) compared with bone marrow-derived mesenchymal stromal cells (MSCs).
Materials & methods
Mouse embryonic fibroblasts (MEFs) were isolated from C3HeB/FeJ and C57BL/6J mice, and reprogrammed to generate iPSCs. Mixed leukocyte reactions were performed using MHC-matched and -mismatched responder leukocytes and stimulator leukocytes, iPSCs or MSCs. To assess immunogenic potential, iPSCs and MSCs were used as stimulator cells for responder leukocytes. To assess immunomodulatory properties, iPSCs and MSCs were cultured in the presence of stimulator and responder leukocytes. MEFs were used as a control.
Results
iPSCs had similar immunogenic properties but more potent immunomodulatory effects than MSCs. Co-culture of MHC-mismatched leukocytes with MHC-matched iPSCs resulted in significantly less responder T-cell proliferation than observed for MHC-mismatched leukocytes alone and at more responder leukocyte concentrations than with MSCs. In addition, MHC-mismatched iPSCs significantly reduced responder T-cell proliferation when co-cultured with MHC-mismatched leukocytes, while MHC-mismatched MSCs did not.
Conclusion
These results provide important information when considering the use of iPSCs in place of MSCs in both regenerative and transplantation medicine.
Keywords: allogeneic stem cell therapy, immunogenicity, immunomodulatory, induced pluripotent stem cell, mesenchymal stromal cell, transplantation medicine
Induced pluripotent stem cells (iPSCs) were first generated in 2006 and regarded as the most promising stem cell candidate for the clinical application of regenerative therapies [1–3]. iPSCs are pluripotent, unlike mesenchymal stromal cells (MSCs), and can be used in an autologous manner, unlike pluripotent embryonic stem cells (ESCs). Additionally, iPSCs avoid the ethical concerns surrounding the isolation and use of human ESCs [4]. However, many concerns have been raised over the safety of iPSCs in terms of genetic instability, tumorigenic potential and immunogenic potential [4–10]. It has become evident that iPSC lines must be thoroughly screened for stability, safety and efficacy prior to clinical application [7,10]. Such screening, after an already lengthy generation process, makes autologous iPSC use impractical for many of the diseases that would potentially benefit from stem cell therapy. Furthermore, it has been demonstrated that genetic background affects generation of iPSCs, suggesting that autologous iPSC therapy may not be feasible for some patients regardless of timing issues [11]. For these reasons, the immunogenicity of iPSCs is of particular concern as the need for having a bank of previously screened cells has become a reality [7,10,12].
The immunogenic and immunomodula-tory properties of MSCs continue to be investigated so that these cells can be available at the time of diagnosis for immediate treatment [13–19]. Both the genetic background and age of the patient affect proliferation and differentiation rates of MSCs, suggesting that allogeneic MSC therapy may be required for some patients as for iPSCs [20–22]. Adult MSCs have low immunogenicity when used in an auto logous manner and possess significant immuno modulatory properties [14,16,23–26]. Many mechanisms for the immuno suppressive effects of MSCs have been described including inhibition of T-cell proliferation, alteration of dendritic cell maturation, induction of regulatory lymphocytes and apoptosis of CD8+ T cells [16,23,27–29]. Mesenchymal stem cells were initially believed to be immune privileged due to these immunosuppressive properties [30–33], but immune rejection of allogeneic MSCs has also been reported [34–39]. The finding that MSCs are capable of fluctuations in their MHC class I and II expression profiles is likely the cause of these conflicting results. MSCs from many species were originally described as having the phenotype of high MHC class I expression and low or negative MHC class II expression, but MSCs from mice, humans and more recently horses with high MHC II expression levels have also been described [34,35,40,41]. Additionally, both MHC class I and II expression levels on MSCs can be upregulated by proinflammatory cytokines such as IFN-γ [42,43]. These studies suggest that MSCs have a dynamic immunophenotype that can alter their immune status.
Investigation into the immunogenic properties and immune plasticity of iPSCs has just recently begun [7,12,44–47]. It is known that undifferentiated iPSCs, like ESCs, express low or absent levels of MHC class I, and are negative for MHC class II expression [47,48]. Unlike MSCs, iPSC MHC class II expression is not upregulated by differentiation or by stimulation with IFN-γ [47–50]. The extent to which MHC class I expression can change upon iPSC differentiation or stimulation with proinflammatory cytokines, however, is not understood. Several studies have shown increased MHC class I expression in iPSCs with differentiation or IFN-γ stimulation, but often to a level still much less than that of somatic cells [45,47,48]. The consequence of such a change in MHC class I expression is complex as a high expression level of MHC class I could lead to T-cell activation while a continued lack of MHC class I expression could result in iPSCs being targeted by natural killer (NK) cells in vivo [6,47]. Conflicting results have been reported for ESCs on this subject, with some groups reporting ESCs as susceptible to NK cell lysis, and others reporting that ESCs are neither susceptible to NK cell lysis nor capable of eliciting T-cell responses [6,51]. It is likely that culture conditions or differences in ESC lines could have affected these results.
It is not surprising that conflicting results have also been reported on the immunogenicity of iPSCs, as iPSCs are in many ways more variable than ESCs, particularly with the discrepancies in reprogramming methods including viral versus nonviral and integrating versus nonintegrating [44–47,49,52,53]. The first report on immunogenicity of iPSCs revealed that undifferentiated autologous (syngeneic) mouse iPSCs were immune rejected in a teratoma model study [44]. Two other reports since then have shown that both undifferentiated and differentiated syngeneic mouse iPSCs are non-immunogenic in vitro and in vivo [45,46]. To date, no studies have examined the immunomodulatory properties of iPSCs even though it is known that ESCs are capable of immunosuppression through multiple mechanisms including expression of arginase I [49,54], prevention of dendritic cell maturation [55] and up -regulation of regulatory T cells [49,56]. When considering the use of iPSCs as an alternative for MSC therapy, this information is critical. The purpose of this study, therefore, was to evaluate the in vitro immunogenic and immunomodulatory properties of iPSCs compared with adult bone marrow-derived MSCs using modified mixed leukocyte reactions (MLRs). Our hypothesis, based on prior ESC knowledge, was that undifferentiated iPSCs would have similar immunogenic and immodulatory properties as MSCs.
Materials & methods
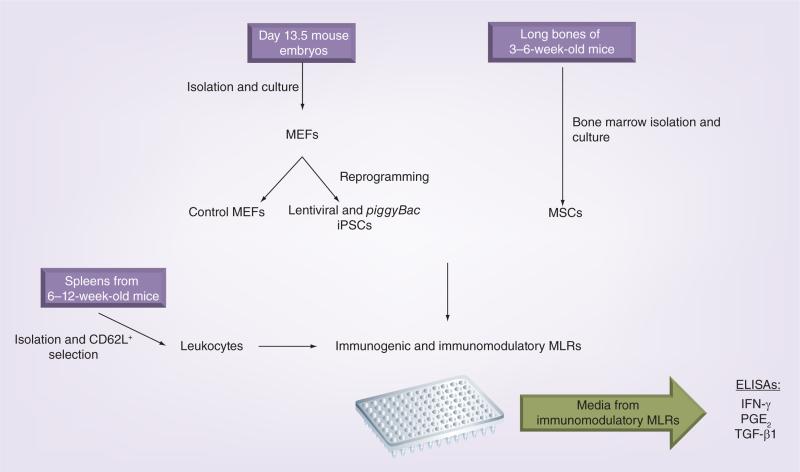
A schematic of the study design and methods is shown in Figure 1.
Figure 1. Schematic of the study design and methods used.
iPSC: Induced pluripotent stem cell; MEF: Mouse embryonic fibroblast; MLR: Mixed leukocyte reaction; MSC: Mesenchymal stem cell.
Mice
Male and female mice of the C3HeB/FeJ (MHC H2 haplotype k) and C57BL/6J (MHC H2 haplotype b) inbred strains were purchased from The Jackson Laboratory (ME, USA). For each strain, mice were bred to produce offspring needed to harvest different cell types and perform experiments. NOD.CB17-Prkdcscid/J mice, used for teratoma formation assays, were also purchased from The Jackson Laboratory. The use of mice in this study was approved by the Institutional Animal Care and Use Committee of Cornell University.
Mouse embryonic fibroblast isolation
Embryonic day 13.5 C3HeB/FeJ and C57BL/6J embryos were collected and processed to generate mouse embryonic fibroblasts (MEFs) from each strain, as previously described [11]. MEFs were cultured in MEF media (high-glucose Dulbecco's Modified Eagle's Medium [DMEM]), containing 10% fetal bovine serum (FBS), penicillin (100 units/ml), and streptomycin (100 μg/ml) and cryopreserved at passage 1 (P1) for iPSC generation and at P2 for controls in MLR experiments. MEFs to be used as feeder cells from each strain were culture expanded, irradiated with 30 Gy from a Cs-137 source, and cryopreserved.
Lentiviral reprogramming of MEFs
Lentiviral supernatant generation and reprogramming of MEFs was performed as previously described by our laboratory [11] using vectors for doxycycline-inducible transgene expression of the mouse factors Oct4, Sox2, Klf4 and c-Myc. All plasmids were purchased from Addgene (MA, USA). Briefly, P1 MEFs from each strain were thawed and cultured in MEF media for 24–48 h, after which they were trypsinized and counted. The P2 MEFs were seeded onto gelatin-coated six-well tissue culture plates at a density of 6.75 × 103 cells/cm2 in MEF media and allowed to adhere for 24 h. Culture media was replaced with fresh MEF media supplemented with viral super-natant for an additional 24 h. Following incubation with viral media, MEFs were trypsinized and passaged onto 60 mm tissue culture plates seeded with feeder cells of the same strain. Culture media was changed to ESC media (KnockOut™ DMEM (Gibco, NY, USA) supplemented with 15% KnockOut™ Serum Replacement (Gibco), recombinant LIF, MEM non-essential amino acids solution (100 μm), 2 mM GlutaMAX™ (Gibco), 0.1 mM 2-mercaptoethanol, penicillin (100 units/ml), streptomycin (100 μg/ml) and doxycycline (2 μg/ml; Sigma, MO, USA). Media was refreshed daily during reprogramming.
piggyBac reprogramming of MEFs
Passage 2 MEFs were transfected with the Nucleofector® II electroporation device (Amaxa Biosystems, MD, USA) set on program A-023. Each electroporation was performed in a 2-mm cuvette (Amaxa Bio-systems) with 2 × 106 cells and a DNA mixture of 1 μg each of the piggyBac plasmids PB-TET-MKOS, PB-CAG-rtTA and PB-CAG-GFP (kindly provided by the laboratory of Dr Nagy [57]), as well as 1 μg of the transposase expression vector pCyL43 (Wellcome Trust Sanger Institute, Cambridge, UK) in a total volume of 100 μl Ingenio® electroporation solution (Mirius Bio, WI, USA). Following electroporation, cells from each cuvette were seeded onto a 100-mm tissue culture plate in MEF media. After 24 h, culture media was changed to ESC media.
iPSC line generation
Lentiviral and piggyBac iPSC colonies were picked with pipette tips and culture expanded on feeder cells in ESC media, as previously described [11]. Lentiviral iPSC colonies were picked on day 7–11 of reprogramming, while piggyBac iPSC colonies were picked on day 17–22 post-transfection. Doxycycline was removed from media around P7 and doxycycline-independent cell lines were then further expanded (P10-P12) in order to reach cell numbers necessary for teratoma formation assays and cryopreservation of stock from each strain. In preparation for MLR experiments, iPSC cell lines from each strain were further cultured in modified RPMI 1640 media containing 10% FBS, 0.1 mM 2-mercaptoethanol, penicillin (100 units/ml), streptomycin (100 μg/ml), and ESGRO® LIF (1 μl/ml; Millipore, MA, USA). Following transition to modified RPMI 1640 media, teratoma assays were again performed.
Teratoma formation & histological ana lysis
iPSC lines from each strain were trypsinized, pelleted and suspended at 1 × 107 cells/ml in a 1:3 solution of Matrigel™ (BD Biosciences, CA, USA) to MEF media. Of this cell suspension, 150 μl (1.5 × 106 cells) was injected subcutaneously into the flank of a NOD. CB17-Prkdcscid/J mouse [11]. For each cell line, a total of two to four injections were performed. A total of 4–5 weeks post injection, tumors were surgically dissected, fixed in 4% paraformaldehyde, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. All histologic sections were reviewed by a board- certified veterinary pathologist (Teresa L Southard) for teratoma formation.
Bone marrow harvest & isolation of MSCs
Ten female mice 3–6 weeks of age from each strain were euthanized, prepared with ethanol and processed for bone marrow harvest according to a protocol kindly provided by the laboratory of Dr Rocky S Tuan [Pers. Comm.]. Hindlimbs were skinned, disarticulated from the pelvis, and placed in a petri dish with MEMα media with nucleosides and l-glutamine (Gibco) containing 10% FBS, penicillin (100 units/ml), streptomycin (100 μg/ml) and Fungizone® (0.25 μg/ml; Gibco). All muscle and tissue was removed from the bone using a scalpel blade and placed in a second petri dish with phosphate-buffered saline (PBS). Next, the ends of the long bones were cut off so that the marrow cavity was exposed. The marrow cavity of each bone was flushed with MEMα media using a 27-g needle and 12-ml syringe into an empty petri dish. The bone marrow cell suspension was then passed through a 70-μm cell strainer (BD Biosciences, CA, USA), pelleted, resuspended in red blood cell lysis buffer (0.84% NH4Cl), and incubated for 2 min on ice. Following the incubation, cells were washed with MEMα media, counted and seeded at 25 × 106 cells/ml onto 100-mm tissue culture plates with MEMα media. After 5 h of incubation, media was removed, plates were gently washed to remove non-adherent cells, and new MEMα media containing FGF-2 (5 ng/ml) added. For the next 72 h, media exchange occurred every 12 h; afterwards media exchange occurred every 72 h. When cells were approximately 80% confluent, they were trypsinized at room temperature for 2 min, counted, and seeded at 5000 cells/cm2 onto T-75 flasks with MEMα media containing FGF-2 (5 ng/ml). Cells were expanded to P2 and P3, and stocks were cryopreserved for immunophenotyping and MLR experiments.
Immunophenotyping of MEFs, iPSCs & MSCs
Mouse embryonic fibrobasts (MEFs), iPSCs and MSCs were immunophenotyped for expression levels of MHC class I and II. MSCs were additionally phenotyped for a panel of positive (CD44, CD29) and negative (CD45, CD117) markers using flow cytometry [58–60]. Leukocytes were used as a control cell type. MHC class I (rat anti-mouse; PE-conjugated), MHC class II (rat ant-mouse; PE-Cy5-conjugated) and CD29 (hamster anti-mouse; PE-conjugated) antibodies were purchased from eBioscience (Affymetrix, CA, USA). CD44 (rat anti-mouse; FITC-conjugated), CD45 (rat anti-mouse; PerCP-Cy5.5) and CD117 (rat anti-mouse; APC-conjugated) antibodies were purchased from BD Biosciences. Cells were pelleted in aliquots containing approximately 1 × 106 cells on 96-well V-bottom plates and treated with a 10-min blocking step using anti-mouse CD16/CD32 (Fc Block™; BD Biosciences) at 1:100 in PBS. Cells were pelleted and resuspended in conjugated primary antibody and incubated for 45 min at 4°C. Cells were then washed, resuspended in PBS and analyzed on a FACSCalibur (Becton Dickinson Immunocytometry Systems, CA, USA) flow cytometer equipped with 488-μm argon and 635-μm red diode lasers and BD Cell Quest™ ana lysis software (BD Biosciences). Cells exposed to appropriately conjugated rat or hamster IgG were used as negative isotype controls. Data were collected on 2 × 104 cells for each sample.
Splenocyte isolation & leukocyte purification
Spleens were aseptically harvested from C3HeB/FeJ and C57BL/6J female mice 6–12 weeks of age and dissociated in RPMI 1640 media (Gibco) using a cell dissociation sieve equipped with a 40-mesh screen (Sigma-Aldrich). The resultant splenocyte suspension was passed through a 100-μm cell strainer (BD Biosciences), pelleted, resuspended in red blood cell lysis buffer (0.84% NH4Cl) and incubated for 5 min at room temperature with rocking. Following red blood cell lysis, the suspension was washed with PBS, pelleted and purified using Lympholyte®-M density gradient centrifugation (Cedarlane Laboratories, NC, USA) according to the manufacturer's directions to obtain leukocytes. Cells destined to be stimulator leukocytes in MLRs were aliquoted at this time. The remaining leukocyte suspension was plated onto 100-mm tissue culture plates in RPMI 1640 medium (Gibco) containing 10% FBS, 0.1 mM 2-mercaptoethanol, penicillin (100 units/ml) and streptomycin (100 units/ml). After 2 h, nonadherent cells were removed from plates, pelleted and counted. Non-adherent leukocytes were then positive selected for CD62L, a naive T-cell marker [61–64], using MACS CD62L microbeads and LS columns (Miltenyi Biotec, CA, USA), according to manufacturer directions. Adherent leukocytes (containing antigen-presenting cells [APCs]) were dissociated using Accumax® cell dissociation solution (Innovative Cell Technologies Inc, CA, USA), counted and aliquoted. All leukocytes were used fresh in MLRs.
Modified one-way MLRs
Modified one-way MLRs were performed in duplicate in 24-well tissue culture plates using MHC-matched and -mismatched C3HeB/FeJ responder leukocytes and C3HeB/FeJ and C57BL/6J stimulator leukocytes, MEFs, iPSCs and MSCs. In order to assess immunogenic potential of cells, MEFs, iPSCs and MSCs were used as stimulator cells for responder leukocytes. In order to assess the immunomodulatory properties of cells, MEFs, iPSCs and MSCs were cultured in the presence of stimulator and responder leukocytes. MHC-matched stimulator leukocytes were used to establish baseline T-cell proliferation and MHC-mismatched stimulator leukocytes were used as positive MLR controls. MEFs were considered the negative control in immunomodulatory potential studies. C3HeB/FeJ responder leukocytes were labeled with 5(6)-carboxyfluorescein diacetate N-succinimidyl ester (CFSE [0.13 μg/ml], Sigma- Aldrich) and examined at four different concentrations (2 × 105, 4 × 105, 8 × 105 and 1.2 × 106 cells/well). The proliferative ability of responder cells was verified via mitogen stimulation with phytohemaglutinin (PHA-P [5 μg/ml], Sigma-Aldrich). Stimulator MEFs, iPSCs and MSCs were plated 24 h prior to addition of responder leukocytes in their appropriate media such that all cells would be approximately 80% confluent by the end of the experiment. MEFs were seeded at 1 × 104 cells/well, iPSCs (removed from feeders) at 7.5 × 104 cells/well, and MSCs at 3 × 104 cells/well. Stimulator leukocytes were irradiated with 9 Gy from a Cs-137 source to inhibit proliferation and plated at 1.6 × 106 cells/well. Responder APCs (adherent cells during isolation) were plated at 1 × 105 cells/well. Importantly, responder leukocytes and APCs were mixed with stimulator leukocytes prior to plating due to concern that the 3D nature of the iPSC colonies could interfere with responder and stimulator cell contact. The resultant ratios of responder:stimulator cells was based on previously published experimental protocols and determined to be optimal for these studies in preliminary experiments [65–67]. Cultures were maintained for 5 days with modified RPMI 1640 media (1.5 ml/well) containing 10% FBS, 0.1 mM 2-mercaptoethanol, penicillin (100 units/ml), streptomycin (100 μg/ml), and ESGRO® LIF (1 ul/ml; Millipore, MA, USA). Media were not exchanged over the 5 days. Following culture, leukocytes were aspirated from wells and stained with a hamster anti-mouse APC-conjugated CD3 antibody (Abcam, MA, USA). The antibody staining process for flow cytometry ana lysis was performed as described above for immunophenotyping.
Proliferation of gated CFSE-labeled CD3+ responder T cells was evaluated via CFSE attenuation using flow cytometry. Cells were first gated on FL4 so that only CD3+ cells (T cells) were then examined on FL1 for CFSE attenuation. Nonstimulated responder T cells were used to set the boundary of nonproliferating cells such that all cells to the left (lower fluorescence intensity on FL1) of that boundary were determined to be proliferating. Because the number of cell counts in the proliferating T-cell gate was measured, data was collected on the entirety of each sample.
MLRs were performed in a total of three separate experiments. MEFs from two different embryos of each strain were tested in addition to three iPSC lines (two lentiviral and one piggyBac) from each strain and batched MSCs from each strain. Due to naturally occurring variation in leukocyte responses between mice and experiments, the relative T-cell proliferation was reported as the fold change from that of MHC-matched MLR for the immunogenic potential experiments (i.e., looking for an increase from baseline T-cell proliferation potential if immunogenic) and as the percentage proliferation of MHC-mismatched MLR for the immunomodulatory potential experiments (i.e., looking for a decrease from positive control T-cell proliferation if immunomodulatory).
Measurement of cytokine concentrations in MLR media
Media from immunomodulatory MLR experiments were harvested after centrifugation to pellet leukocytes for flow cytometry and stored at -80°C in aliquots with protease inhibitors (Complete Protease Inhibitor Cocktail Tablets, Roche, IN, USA). Media from control MHC-mismatched MLRs and MHC-mismatched MLRs cultured in the presence of matched or mismatched MCSs or iPSCs were pooled according to experimental group and assayed for active IFN-γ, prostaglandin E2 (PGE2), and TGF-β1 concentrations using the IFN-γ Quantikine ELISA kit (R&D Systems, MN, USA), the Prostaglandin E2 ELISA kit (Abcam, MA, USA) and the TGF β1 Quantikine ELISA kit (R&D Systems), respectively.
Statistical analyses
Mixed leukocyte reaction (MLR) data for lentiviral (average of the two lines tested) and piggyBac iPSC lines were first compared using two-sample t-tests. All MLR data were normalized by log transformation and analyzed with ana lysis of covariance (ANCOVA), with experiment as a covariate, followed by a Tukey multiple comparisons test. All ELISA data were determined to be normally distributed via the Shapiro–Wilk test and were analyzed by ana lysis of variance (ANOVA) followed by a LSD multiple comparisons test. Analyses were performed using Statistix 9 software (Analytical Software, FL, USA) and significance was set at p ≤ 0.05.
Results
iPSC line generation & validation
Multiple doxycycline-independent lentiviral and piggyBac iPSC lines were established from each strain and early passage stocks were cryopreserved. Two doxycycline-independent lentiviral iPSC lines and one doxycycline piggyBac iPSC line from each strain was tested after expansion in ESC media and then again after expansion in modified RPMI 1640 media. These lines were capable of producing teratomas in NOD.CB17-Prkdcscid/J mice by 5 weeks post injection (Supplementary Figure 1; please see online at www.futuremedicine.com/doi/full/10.2217/rme.14.29), thereby confirming pluripotency and lack of alteration due to the change in media.
Immunophenotyping
MEFs had a phenotype of MHC class I positive (low) and MHC class negative, while iPSCs had a phenotype of both MHC class I and II negative (Supplementary Table 1). Mesenchymal stromal cells (MSCs) were positive for expression of MHC class I, CD44 and CD29, and negative for expression of MHC class II, CD45 and CD117 (Supplementary Figure 1).
Modified one-way MLRs
There were no significant differences in responder T-cell proliferation when stimulated by lentiviral iPSCs or piggyBac iPSCs, or when stimulated by MHC-mismatched leukocytes in the presence of lentiviral iPSCs or piggyBac iPSCs. Cells were therefore considered one group and are collectively referred to as iPSCs for the remainder of the results.
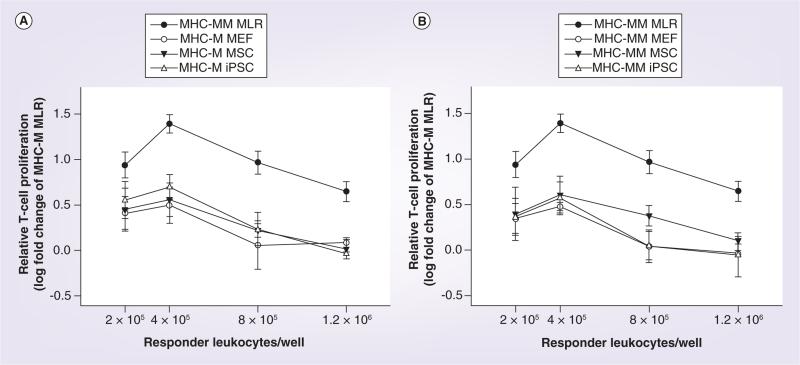
As predicted based on MHC class II expression, all cell types tested (MEFs, iPSCs and MSCs) had low immunogenicity when either MHC-matched or MHC -mismatched with responder leukocytes (Figures 2 & 3). MHC-mismatched MSCs resulted in the highest levels of responder T-cell proliferation compared with MEFs and iPSCs, but these levels still did not reach those of the positive control of MHC-mismatched leukocytes (Figure 3E–H).
Figure 2. Immunogenicity (A) of MHC-matched, and (B) MHC-mismatched MEFs, MSCs and iPSCs as determined by responder T-cell proliferation in modified one-way mixed leukocyte reactions.
Data are presented as the log fold change of MHC-matched MLR, which was considered the baseline responder T-cell proliferation value. Bars represent mean ± SD from a total of three separate experiments performed with multiple cell lines.
MHC-M: MHC-matched; MHC-MM: MHC-mismatched; MLR: Mixed leukocyte reaction; MSC: Mesenchymal stromal cell.
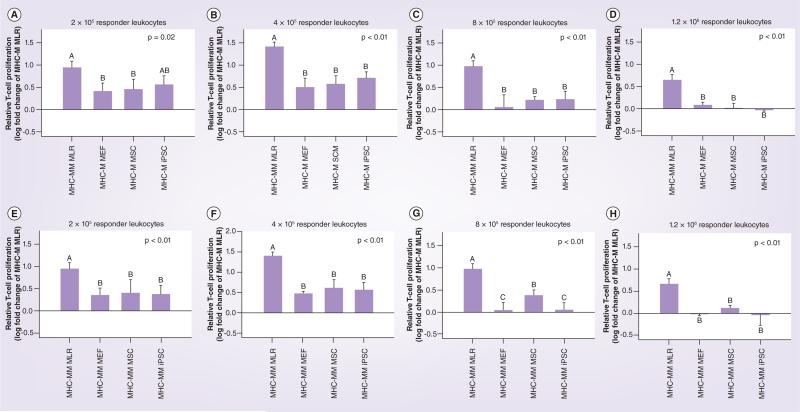
Figure 3. Immunogenicity of mouse embryonic fibroblasts, mesenchymal stem cells and induced pluripotent stem cell as determined using modified one-way mixed leukocyte reactions and with data reported as the relative T-cell proliferation, which was calculated as the log fold change from MHC-matched MLR.
MLR results as shown in Figure 2 are displayed here for MHC-matched cells (A-D) and MHC-mismatched cells (E–H) at the different responder leukocyte concentrations tested. Bars represent mean ± SD from a total of three separate experiments performed with multiple cell lines. Superscript letters indicate significant differences between groups by analysis of covariance, with experiment as a covariate, followed by a Tukey multiple comparisons test, p ≤ 0.05.
iPSC: Induced pluripotent stem cell; MEF: Mouse embryonic fibrobast; MHC-M: MHC-matched; MHC-MM: MHC-mismatched; MLR: Mixed leukocyte reaction; MSC: Mesenchymal stromal cell
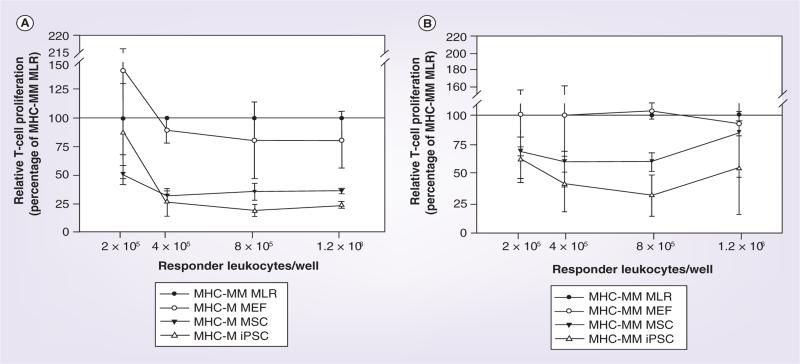
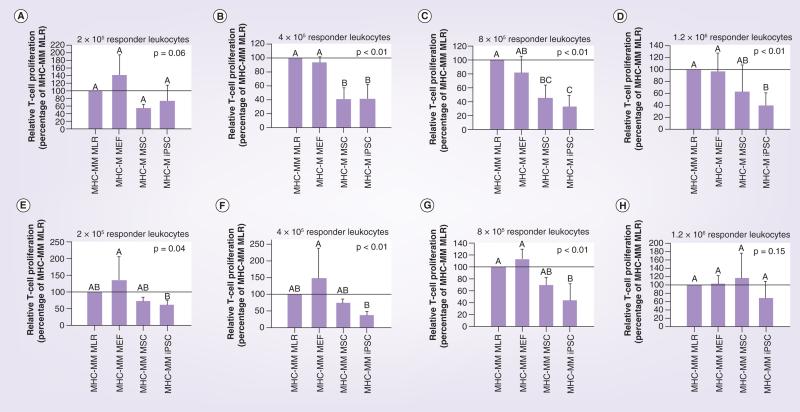
At the majority of responder leukocyte concentrations tested, both iPSCs and MSCs cultured in the presence of MHC-mismatched responder and stimulator leukocytes (MHC-mismatched MLR) resulted in a reduction of responder T-cell proliferation from that observed for the MHC-mismatched MLR baseline value (Figures 4 & 5). Importantly, MEFs cultured in the presence of MHC-mismatched responder and stimulator leukocytes were unable to reduce responder T-cell proliferation in any of responder leukocyte concentrations tested. Reduction of responder T-cell proliferation was greatest when iPSCs and MSCs were MHC-matched with responder leukocytes compared with when MHC-mismatched with responder leukocytes. MHC-matched iPSCs resulted in significantly decreased responder T-cell proliferation compared with both MHC-mismatched MLR and the negative control of MEFs at the three highest responder leukocyte concentrations (Figure 5B–D). While MHC-matched MSCs resulted in statistically equivalent responder T-cell proliferation compared with iPSCs for the same three responder leukocyte concentrations, mean T-cell proliferations were greater than for iPSCs and in some cases also equivalent to MHC-mismatched MLR and/or the negative control of MEFs (Figure 5B–D). When MHC-mismatched cell types were evaluated, only iPSCs were able to reduce responder T-cell proliferation significantly from MHC-mismatched MLR at the responder leukocyte concentration of 8 × 105 cells (Figure 5G). Once again, while MHC-mismatched MSCs resulted in statistically equivalent responder T-cell proliferation compared with iPSCs at this concentration, the mean T-cell proliferation was greater than for iPSCs and also equivalent to that of MEFs and the positive control of MHC- mismatched leukocytes (Figure 5G).
Figure 4. Immunomodulatory potential of (A) MHC-matched and (B) MHC-mismatched MEFs, MSCs and iPSCs as determined by responder T-cell proliferation in modified one-way mixed leukocyte reactions in which MHC-mismatched leukocytes were cultured in the presence or absence of these cells.
Data are presented as the percentage proliferation of the control MHC-mismatched MLR as a decrease in proliferation is indicative of immunomodulation. Bars represent mean ± SD from a total of three separate experiments performed with multiple cell lines. A reference line has been placed at 100% to denote the positive control of the MHC-mismatched MLR. iPSC: Induced pluripotent stem cell; MEF: Mouse embryonic fibrobast; MHC-M: MHC-matched; MHC-MM: MHC-mismatched;
MLR: Mixed leukocyte reaction; MSC: Mesenchymal stromal cell.
Figure 5. Immunomodulatory potential of mouse embryoinc fibroblasts, mesenchyamal stem cells and induced pluripotent stem cell as determined using modified one-way mixed leukocyte reactions in which MHC-mismatched leukocytes were cultured in the presence or absence of these cells.
Data are reported as the relative T-cell proliferation, which was calculated as the percentage proliferation of the MHC-mismatched MLR alone. MLR results as shown in Figure 4 are displayed here for MHC-matched cells (A–D) and MHC-mismatched cells (E–H) at the different responder leukocyte concentrations tested. Bars represent mean ± SD from a total of three separate experiments performed with multiple cell lines. Reference lines have been placed at 100% to denote the positive control of the MHC-mismatched MLR. Superscript letters indicate significant differences between groups by ANCOVA, with experiment as a covariate, followed by a Tukey multiple comparisons test, p ≤ 0.05. iPSC: Induced pluripotent stem cell; MEF: Mouse embryonic fibrobast; MHC-M: MHC-matched; MHC-MM: MHC-mismatched; MLR: Mixed leukocyte reaction;
MSC: Mesenchymal stromal cell.
Measurement of cytokine concentrations in MLR media
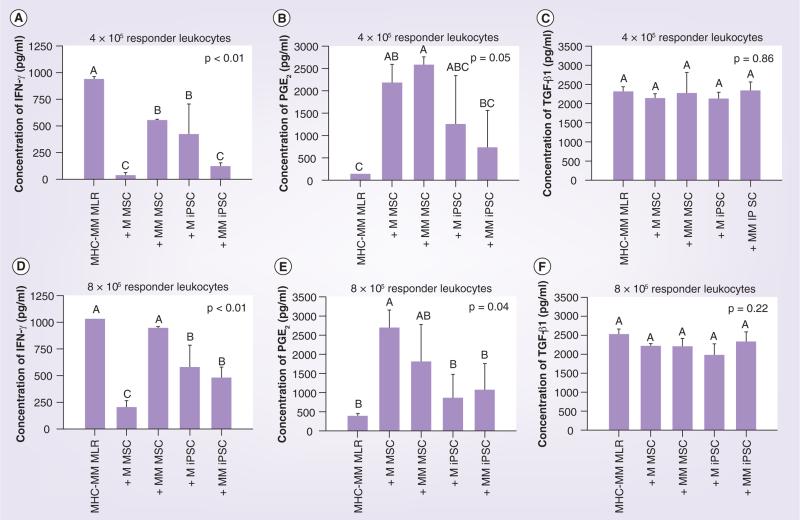
Based on the immunomodulatory results described above, only media from the immunomodulatory MLR experiments at the responder leukocyte concentrations of 4 × 105 and 8 × 105 cells were assayed. At both responder leukocyte concentrations tested, there was a significant reduction in the concentration of IFN-γ in the MLR media when MHC-mismatched leukocytes were cultured in the presence of matched MSCs, matched iPSCs or mismatched iPSCs. While the presence of matched MSCs resulted in the lowest mean IFN-γ for all groups tested, the presence of mismatched MSCs had a significant but lesser effect at the responder leukocyte concentration of 4 × 105 cells and no significant effect at the responder leuko cyte concentration of 8 × 105 cells (Figure 6A & D). At both responder leukocyte concentrations tested, the presence of either matched or mismatched MSCs resulted in the highest mean PGE2 concentrations compared with control and both matched and mismatched iPSCs. For mismatched MSCs, however, such concentrations were only significantly greater than control at responder leukocyte concentration of 4 × 105 cells. Although mean PGE2 concentrations were higher in media from MHC-mismatched MLRs cultured in the presence of either matched or mismatched iPSCs compared with control MHC- mismatched MLRs, these results were not statistically significant (Figure 6B & E). No signifi-cant differences in media TGF-β1 concentrations were found for any of the cell types tested (Figure 6C & F).
Figure 6. Immunomodulatory mixed leukocyte reaction experiment media concentrations of (A & D) IFN-γ, (B & E) PGE2 and (C & F) TGF-β1 for control MHC-mismatched MLRs and for MHC-mismatched MLRs cultured with matched or mismatched MSCs or iPSCs.
Media from experiments using responder leukocyte concentrations of 4 × 105 cells (A–C) and 8 × 105 cells (D–F) were examined based on the significant immunomodulatory results found at these concentrations as shown in Figures 4 & 5. Bars represent mean ± SD from a total of three pooled samples of each cell type from each MLR experiment. Superscript letters indicate significant differences between groups by ANOVA, followed by a LSD multiple comparisons test; p ≤ 0.05.
iPSC: Induced pluripotent stem cell; MEF: Mouse embryonic fibrobast; MHC-M: MHC-matched; MHC-MM: MHC-mismatched;
MLR: Mixed leukocyte reaction; MSC: Mesenchymal stromal cell; PGE2: Prostaglandin E2.
Discussion
In this study we directly compared iPSCs to MSCs in terms of immunogenicity and immunomodulatory capability using MLRs. Our comparisons revealed that iPSCs generated through both lentiviral and piggyBac reprogramming methods have similar immunogenic properties as MSCs and may possess more potent immunomodulatory properties than MSCs in vitro. Co-culture of MHC-mismatched leukocytes with MHC-matched iPSCs resulted in significantly less responder T-cell proliferation than observed for MHC-mismatched leukocytes alone at more responder leukocyte concentrations tested than was observed for co-culture of MHC-mismatched leukocytes with MHC-matched MSCs. In addition, MHC-mismatched iPSCs were able to significantly reduce responder T-cell proliferation at the responder leukocyte concentration of 8 × 105 cells when co-cultured with MHC-mismatched leukocytes while MHC- mismatched MSCs were not.
None of the cells (MEFs, iPSCs or MSCs) tested in this study were irradiated for use in MLRs due to the fact that iPSCs died following even very low doses (100–200 rads) of gamma irradiation. This finding suggests that iPSCs undergo p53-independent apoptosis in response to DNA damage as described for ESCs [68–70] rather than p53-mediated cell cycle arrest as is well described for somatic cells [71]. For this reason, preliminary experiments were performed to determine the proper seeding density of all cell types such that they were approximately 80% confluent on the 5th (final) day of MLR culture. This method is different than most previously described for MSC immunology studies examining MSC effects in MLRs in which MSCs are irradiated and then plated at different ratios to responder leukocytes [26,42]. It is possible that because the cells were growing that they could have either not reached or surpassed the optimal cell:leukocyte ratio for immunomodulation in some instances. The fact that both MHC-matched iPSCs and MSCs were able to significantly downregulate responder T-cell proliferation to as much as 25–30% of that of the MHC-mismatched MLR, however, suggests a broad enough range of responder leukocyte cell concentrations was covered to confidently determine whether the cells were causing immunomodulation.
Due to the nature of MLRs, it must also be considered that T-cell proliferation may have been falsely diminished due to T-cell competition with growing cells for nutrients in the media. At no point during the experiments, however, did the media appear exhausted in color. More importantly, co-culture with MEFs, which were rapidly growing and just as confluent as the other cell types by the end of the experiments, did not result in reduced T-cell proliferation. This argues against nutrient competition or depletion as a reason for the reduced responder T-cell proliferation observed in MLR co-cultures with iPSC and ESCs. Thus, MEFs were a critical experimental control for these immunomodulatory experiments. Another potential concern is that the leukocyte media used included leukemia inhibitory factor (LIF), which has been shown to have a role in MSC-mediated immunosuppression [24]. LIF is commonly used in iPSC media to maintain pluripotency and prevent differentiation [1,2,11]. It was used in these experiments for that reason and also to avoid use of feeder cells with iPSCs culture, which would have further complicated experimental design and interpretation [72]. The same leukocyte media with LIF was used for all MLR experiments, which should have prevented any biases between cell type comparisons and against MHC-matched and mismatched MLR controls. Again, the finding that control MEFs did not cause significant downregulation of responder T-cell proliferation argues against this concern.
The finding that iPSCs and MSCs that were MHC-matched with respect to the responder leukocytes resulted in a greater reduction in responder T-cell proliferation compared with iPSCs and MSCs that were MHC mismatched is interesting. Engraftment studies evaluating the effect of MSCs have previously demonstrated similar findings, with only syngeneic (MHC-matched) MSCs resulting in enhanced engraftment [17,38]. Previous MLR studies evaluating the immunosuppressive effects of ESCs, however, have found no difference in using MHC-matched or mismatched ESCs with the responder leukocytes [73]. The reasons for this discrepancy between iPSCs and MSCs is unclear, but could be due to specific immunosuppressive mechanisms employed by cells or due to differences in MHC antigen expression between cell types with iPSCs expressing very low or negligible levels of MHC class I and MSCs expressing high levels of MHC I. Of note is the fact that although MLR co-cultures with mismatched MSCs resulted in high levels of PGE2 in the media that were fairly consistent with that of matched MSCs, mismatched MSCs were unable to produce the same decrease in IFN-γ concentration as was observed in media from MLR co-cultures with matched MSCs. This result suggests that perhaps the immune stimulus of mismatched MSCs was strong enough to cause responder T-cell IFN-γ secretion despite the expected downregulation or inhibition of IFN-γ by PGE2 secreted from the MSCs [16]. Future studies evaluating the kinetics of responder T-cell proliferation during such MLR co-cultures may prove useful for distinguishing between increased immunogenicity and decreased immunomodulatory potential of the cells.
It is also an important finding that the significant decrease in media IFN-γ concentrations in MLRs co-cultured with iPSCs did not correlate well with the very modest increases in PGE2 concentrations in the same media. This suggests that iPSCs are inhibiting responder T-cell IFN-γ secretion via a mechanism other than PGE2. Further studies must be performed to elucidate this result, including examination of other soluble factors potentially expressed by iPSCs and MSCs into the media during MLRs such as TGF-β2, IL-6, IL-10, IDO and LIF [13,14,16,24,32,47,74], as well as examination of arginase-I expression by iPSCs, which could be responsible for the inhibition of responder T-cell IFN-γ secretion as previously described for ESCs [49,54]. Evaluation of the gene expression levels of these soluble factors by different cell types would also be of great interest because media additives such as LIF could be affecting protein expression and because proteins could be rapidly degrading in the culture system. While this would be difficult to perform in the MLR co-culture system described in this study in which all cell types are mixed together within a well, it is possible that the cell types could be sorted at the end of the culture system. Once suspect factors have been identified, the next essential study would be to inhibit these factors and determine whether or not responder T-cell proliferation is restored in the MLR co-culture system.
Perhaps the greatest question raised by this study is whether or not iPSCs can retain their immunogenic and immunomodulatory properties upon differentiation as it is unlikely that undifferentiated iPSCs will be used in human clinical applications due to concerns of teratoma formation. Follow-up studies must focus on differentiating iPSC lines into specific cell types and then re-evaluating their MHC class I and II expression in addition to their immunogenic and immunomodulatory properties in MLRs. Soluble factor release into the media by these cells during MLRs must also be re-assessed and compared with levels pre-differentiation. Once these in vitro studies have been completed, in vivo studies evaluating the immunogenic and immunomodulatory effect of iPSCs outside the controlled environment of the MLR must be performed. Such studies will be critical for consideration of iPSC use in the place of MSCs for both regenerative medicine and transplant medicine [7,12,52].
Executive summary.
Introduction (rationale & aim)
The immunologic properties of induced pluripotent stem cells (iPSCs) require investigation as it has become evident that banked iPSCs will be needed for most clinical applications due to cell generation time and time associated with screening for both efficacy and safety.
The aim of this study was to evaluate the in vitro immunogenic and immunomodulatory properties of iPSCs compared with adult bone marrow-derived MSCs using modified mixed leukocyte reactions.
Materials & methods
In order to assess immunogenic potential, iPSCs and MSCs were used as stimulator cells for responder leukocytes. In order to assess immunomodulatory properties, iPSCs and MSCs were cultured in the presence of stimulator and responder leukocytes. MEFs were used as a control.
Results
iPSCs generated through both lentiviral and piggyBac reprogramming methods had similar immunogenic properties and more potent immunomodulatory properties than MSCs in vitro.
Co-culture of major histocompatibility complex (MHC)-mismatched leukocytes with MHC-matched iPSCs resulted in significantly less responder T-cell proliferation than MHC-mismatched leukocytes alone at more responder leukocyte concentrations tested than was observed for co-culture of MHC-matched leukocytes with MHC-matched MSCs.
MHC-mismatched iPSCs were able to significantly reduce responder T-cell proliferation at the responder leukocyte concentration of 8 × 105 cells when co-cultured with MHC-mismatched leukocytes, while MHC-mismatched MSCs were not.
A significant decrease was found in media IFN-γ concentrations in MLRs co-cultured with iPSCs; however, this decrease did not correlate well with the modest increase in prostaglandin E2 concentration in the same media.
Discussion & conclusion
iPSCs are presumably inhibiting responder T-cell IFN-γ secretion via a mechanism other than prostaglandin E2 as for MSCs.
Further studies must be performed in order to determine whether iPSCs retain their immunogenic and immunomodulatory properties upon differentiation into specific cell or tissue types.
This information is critical when considering the use of iPSCs in the place of MSCs for both regenerative medicine and transplant medicine.
Acknowledgements
The authors would like to thank Robert Munroe for his help with animal care and for his contributions to the experimental design. The authors would also like to thank Gina Kemp for her help with animal care and handling.
This work was supported by Empire State Stem Cell Fund contract no. C024400 (LA Fortier and JC Schimenti) and NIH grant no. 1K08AR060875-01 (LV Schnabel). Finally, the authors would like to acknowledge use of the Cornell University Irradiator Core Facility and support from National Center for Research Resources grant #S10RR023781.
Footnotes
Author contributions
All authors contributed to the study design. LV Schnabel, CM Abratte, JM Cassano and JA Cross performed the experiments. TL Southard carried out histologic assessments on the teratoma assays. All authors contributed to data analysis and interpretation. LV Schnabel and LA Fortier were responsible for drafting the manuscript. All authors revised the manuscript and approved the final version.
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, Narita M, Yokura M, et al. Human induced pluripotent stem cells on autologous feeders. PLoS ONE. 2009;4(12):e8067. doi: 10.1371/journal.pone.0008067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun N, Longaker MT, Wu JC. Human iPS cell-based therapy: considerations before clinical applications. Cell Cycle. 2010;9(5):880–885. doi: 10.4161/cc.9.5.10827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hussein SM, Batada NN, Vuoristo S, et al. Copy number variation and selection during reprogramming to pluripotency. Nature. 2011;471(7336):58–62. doi: 10.1038/nature09871. [DOI] [PubMed] [Google Scholar]
- 6.Tang C, Drukker M. Potential barriers to therapeutics utilizing pluripotent cell derivatives: intrinsic immunogenicity of in vitro maintained and matured populations. Semin. Immunopathol. 2011;33(6):563–572. doi: 10.1007/s00281-011-0269-5. [DOI] [PubMed] [Google Scholar]
- 7.Okita K, Nagata N, Yamanaka S. Immunogenicity of induced pluripotent stem cells. Circ. Res. 2011;109(7):720–721. doi: 10.1161/RES.0b013e318232e187. [DOI] [PubMed] [Google Scholar]
- 8.Drukker M. Immunological considerations for cell therapy using human embryonic stem cell derivatives. StemBook. 2008 doi/10.3824/stembook.1.14.1 (Epub ahead of print) [PubMed] [Google Scholar]
- 9.de Almeida PE, Ransohoff JD, Nahid A, et al. Immunogenicity of pluripotent stem cells and their derivatives. Circ. Res. 2013;112(3):549–561. doi: 10.1161/CIRCRESAHA.111.249243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Jiang Z, Han Y, Cao X. Induced pluripotent stem cell (iPSCs) and their application in immunotherapy. Cell. Mol. Immunol. 2014;11(1):17–24. doi: 10.1038/cmi.2013.62. [Most recent review of induced pluripotent stem cells (iPSCs) and the controversy that exists in the field over iPSC immunogenicity and potential clinical applications.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schnabel LV, Abratte CM, Schimenti JC, et al. Genetic background affects induced pluripotent stem cell generation. Stem Cell. Res. Ther. 2012;3(4):30. doi: 10.1186/scrt121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fairchild PJ. The challenge of immunogenicity in the quest for induced pluripotency. Nat. Rev. Immunol. 2010;10(12):868–875. doi: 10.1038/nri2878. [DOI] [PubMed] [Google Scholar]
- 13.Waterman RS, Tomchuck SL, Henkle SL, et al. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS ONE. 2010;5(4):e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bunnell BA, Betancourt AM, Sullivan DE. New concepts on the immune modulation mediated by mesenchymal stem cells. Stem Cell. Res. Ther. 2010;1(5):34. doi: 10.1186/scrt34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol. Ther. 2009;17(6):939–946. doi: 10.1038/mt.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 17.Stagg J. Immune regulation by mesenchymal stem cells: two sides to the coin. Tissue Antigens. 2007;69(1):1–9. doi: 10.1111/j.1399-0039.2006.00739.x. [DOI] [PubMed] [Google Scholar]
- 18.Stagg J, Galipeau J. Immune plasticity of bone marrow-derived mesenchymal stromal cells. Handb. Exp. Pharmacol. 2007;(180):45–66. doi: 10.1007/978-3-540-68976-8_3. [DOI] [PubMed] [Google Scholar]
- 19.Griffin MD, Ritter T, Mahon BP. Immunological aspects of allogeneic mesenchymal stem cell therapies. Hum. Gene Ther. 2010;21(12):1641–1655. doi: 10.1089/hum.2010.156. [DOI] [PubMed] [Google Scholar]
- 20.Peister A, Mellad JA, Larson BL, et al. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103(5):1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 21.Baxter MA, Wynn RF, Jowitt SN, et al. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22(5):675–682. doi: 10.1634/stemcells.22-5-675. [DOI] [PubMed] [Google Scholar]
- 22.Majors AK, Boehm CA, Nitto H, et al. Characterization of human bone marrow stromal cells with respect to osteoblastic differentiation. J. Orthop. Res. 1997;15(4):546–557. doi: 10.1002/jor.1100150410. [DOI] [PubMed] [Google Scholar]
- 23.Nasef A, Mathieu N, Chapel A, et al. Immunosuppressive effects of mesenchymal stem cells: involvement of HLA-G. Transplantation. 2007;84(2):231–237. doi: 10.1097/01.tp.0000267918.07906.08. [DOI] [PubMed] [Google Scholar]
- 24.Nasef A, Mazurier C, Bouchet S, et al. Leukemia inhibitory factor: Role in human mesenchymal stem cells mediated immunosuppression. Cell. Immunol. 2008;253(1–2):16–22. doi: 10.1016/j.cellimm.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Nasef A, Zhang YZ, Mazurier C, et al. Selected Stro-1-enriched bone marrow stromal cells display a major suppressive effect on lymphocyte proliferation. Int. J. Lab. Hematol. 2009;31(1):9–19. doi: 10.1111/j.1751-553X.2007.00997.x. [DOI] [PubMed] [Google Scholar]
- 26.Puissant B, Barreau C, Bourin P, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br. J. Haematol. 2005;129(1):118–129. doi: 10.1111/j.1365-2141.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- 27.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 28.Jiang XX, Zhang Y, Liu B, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105(10):4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 29.Plumas J, Chaperot L, Richard MJ, et al. Mesenchymal stem cells induce apoptosis of activated T cells. Leukemia. 2005;19(9):1597–1604. doi: 10.1038/sj.leu.2403871. [DOI] [PubMed] [Google Scholar]
- 30.Djouad F, Plence P, Bony C, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102(10):3837–3844. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- 31.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 2002;30(1):42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 32.Meisel R, Zibert A, Laryea M, et al. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103(12):4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 33.Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363(9419):1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 34.Eliopoulos N, Stagg J, Lejeune L, et al. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood. 2005;106(13):4057–4065. doi: 10.1182/blood-2005-03-1004. [DOI] [PubMed] [Google Scholar]
- 35.Potian JA, Aviv H, Ponzio NM, et al. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J. Immunol. 2003;171(7):3426–3434. doi: 10.4049/jimmunol.171.7.3426. [DOI] [PubMed] [Google Scholar]
- 36.Zangi L, Margalit R, Reich-Zeliger S, et al. Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells. 2009;27(11):2865–2874. doi: 10.1002/stem.217. [DOI] [PubMed] [Google Scholar]
- 37.Griffin MD, Ryan AE, Alagesan S, et al. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: what have we learned so far? Immunol. Cell Biol. 2013;91(1):40–51. doi: 10.1038/icb.2012.67. [DOI] [PubMed] [Google Scholar]
- 38.Nauta AJ, Westerhuis G, Kruisselbrink AB, et al. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108(6):2114–2120. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Badillo AT, Beggs KJ, Javazon EH, et al. Murine bone marrow stromal progenitor cells elicit an in vivo cellular and humoral alloimmune response. Biol. Blood Marrow Transplant. 2007;13(4):412–422. doi: 10.1016/j.bbmt.2006.12.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones EA, Kinsey SE, English A, et al. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002;46(12):3349–3360. doi: 10.1002/art.10696. [DOI] [PubMed] [Google Scholar]
- 41.Schnabel LV, Pezzanite LM, Antczak DF, et al. Equine bone marrow-derived mesenchymal stromal cells are heterogeneous in MHC class II expression and capable of inciting an immune response in vitro. Stem Cell. Res. Ther. 2014;5(1):13. doi: 10.1186/scrt402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan WK, Lau AS, Li JC, et al. MHC expression kinetics and immunogenicity of mesenchymal stromal cells after short-term IFN-gamma challenge. Exp. Hematol. 2008;36(11):1545–1555. doi: 10.1016/j.exphem.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 43.Romieu-Mourez R, Francois M, Boivin MN, et al. Regulation of MHC class II expression and antigen processing in murine and human mesenchymal stromal cells by IFN-gamma, TGF-beta, and cell density. J. Immunol. 2007;179(3):1549–1558. doi: 10.4049/jimmunol.179.3.1549. [DOI] [PubMed] [Google Scholar]
- 44••.Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–216. doi: 10.1038/nature10135. [First report of potential for immune rejection of autologous/syngeneic iPSCs.] [DOI] [PubMed] [Google Scholar]
- 45••.Guha P, Morgan JW, Mostoslavsky G, et al. Lack of immune response to differentiated cells derived from syngeneic induced pluripotent stem cells. Cell Stem Cell. 2013;12(4):407–412. doi: 10.1016/j.stem.2013.01.006. [Key study to challenge the findings of Zhao et al. and report a lack of immunogenicity of both undifferentiated and differentiated autologous/syngeneic iPSCs.] [DOI] [PubMed] [Google Scholar]
- 46••.Araki R, Uda M, Hoki Y, et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 2013;494(7435):100–104. doi: 10.1038/nature11807. [Another key study to challenge the findings of Zhao et al. and report a lack of immunogenicity of both undifferentiated and differentiated autologous/syngeneic iPSCs.] [DOI] [PubMed] [Google Scholar]
- 47•.Chen HF, Yu CY, Chen MJ, et al. Characteristic expression of major histocompatibility complex and immune privilege genes in human pluripotent stem cells and the derivatives. Cell Transplant. 2013 doi: 10.3727/096368913X674639. doi:10.3727/096368913X674639 (Epub ahead of print). [Key study to report on the expression of immune privilege genes by both undifferentiated and differentiated iPSCs.] [DOI] [PubMed] [Google Scholar]
- 48.Suarez-Alvarez B, Rodriguez RM, Calvanese V, et al. Epigenetic mechanisms regulate MHC and antigen processing molecules in human embryonic and induced pluripotent stem cells. PLoS ONE. 2010;5(4):e10192. doi: 10.1371/journal.pone.0010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pearl JI, Kean LS, Davis MM, et al. Pluripotent stem cells: immune to the immune system? Sci. Transl. Med. 2012;4(164):164ps25. doi: 10.1126/scitranslmed.3005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kadereit S, Trounson A. In vitro immunogenicity of undifferentiated pluripotent stem cells (PSC) and derived lineages. Semin. Immunopathol. 2011;33(6):551–562. doi: 10.1007/s00281-011-0265-9. [DOI] [PubMed] [Google Scholar]
- 51.Bonde S, Zavazava N. Immunogenicity and engraftment of mouse embryonic stem cells in allogeneic recipients. Stem Cells. 2006;24(10):2192–2201. doi: 10.1634/stemcells.2006-0022. [DOI] [PubMed] [Google Scholar]
- 52.Kaneko S, Yamanaka S. To be immunogenic, or not to be: that's the iPSC question. Cell Stem Cell. 2013;12(4):385–386. doi: 10.1016/j.stem.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 53.Pearl JI, Lee AS, Leveson-Gower DB, et al. Short-term immunosuppression promotes engraftment of embryonic and induced pluripotent stem cells. Cell Stem Cell. 2011;8(3):309–317. doi: 10.1016/j.stem.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yachimovich-Cohen N, Even-Ram S, Shufaro Y, et al. Human embryonic stem cells suppress T cell responses via arginase I-dependent mechanism. J. Immunol. 2010;184(3):1300–1308. doi: 10.4049/jimmunol.0804261. [DOI] [PubMed] [Google Scholar]
- 55.Mohib K, Allan D, Wang L. Human embryonic stem cell-extracts inhibit the differentiation and function of monocyte-derived dendritic cells. Stem Cell.Rev. 2010;6(4):611–621. doi: 10.1007/s12015-010-9185-7. [DOI] [PubMed] [Google Scholar]
- 56.Mohib K, AlKhamees B, Zein HS, et al. Embryonic stem cell-derived factors inhibit T effector activation and induce T regulatory cells by suppressing PKC-theta activation. PLoS ONE. 2012;7(3):e32420. doi: 10.1371/journal.pone.0032420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nagy K, Sung HK, Zhang P, et al. Induced pluripotent stem cell lines derived from equine fibroblasts. Stem Cell. Rev. 2011;7(3):693–702. doi: 10.1007/s12015-011-9239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res. Ther. 2007;9(1):204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meirelles Lda S, Nardi NB. Murine marrow-derived mesenchymal stem cell: isolation, in vitro expansion, and characterization. Br. J. Haematol. 2003;123(4):702–711. doi: 10.1046/j.1365-2141.2003.04669.x. [DOI] [PubMed] [Google Scholar]
- 60.Baddoo M, Hill K, Wilkinson R, et al. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J. Cell. Biochem. 2003;89(6):1235–1249. doi: 10.1002/jcb.10594. [DOI] [PubMed] [Google Scholar]
- 61.Cieri N, Camisa B, Cocchiarella F, et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood. 2013;121(4):573–584. doi: 10.1182/blood-2012-05-431718. [DOI] [PubMed] [Google Scholar]
- 62.Lu SY, Liu KY, Liu DH, et al. High frequencies of CD62L(+) naive regulatory T cells in allografts are associated with a low risk of acute graft-versus-host disease following unmanipulated allogeneic haematopoietic stem cell transplantation. Clin. Exp. Immunol. 2011;165(2):264–277. doi: 10.1111/j.1365-2249.2011.04418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang YJ, Zhao XY, Huo MR, et al. Expression profiles of adhesion molecules on naive T cells in bone marrow grafts of healthy donors treated with granulocyte colony-stimulating factor. Transpl. Immunol. 2009;21(4):228–233. doi: 10.1016/j.trim.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 64.Chang YJ, Zhao XY, Huo MR, et al. Expression of CD62L on donor CD4(+) T cells in allografts: correlation with graft-versus-host disease after unmanipulated allogeneic blood and marrow transplantation. J. Clin. Immunol. 2009;29(5):696–704. doi: 10.1007/s10875-009-9293-9. [DOI] [PubMed] [Google Scholar]
- 65.Kruisbeek AM. Isolation of mouse mononuclear cells. Curr. Protoc. Immunol. 2001;39:3.1.1–3.1.5. doi: 10.1002/0471142735.im0301s39. [DOI] [PubMed] [Google Scholar]
- 66.Kruisbeek AM, Shevach E, Thornton AM. Proliferative assays for T cell function. Curr. Protoc. Immunol. 2004;60:3.12.1–3.12.20. doi: 10.1002/0471142735.im0312s60. [DOI] [PubMed] [Google Scholar]
- 67.Muul LM, Heine G, Silvin C, et al. Measurement of proliferative responses of cultured lymphocytes. Curr. Protoc. Immunol. 2011 doi: 10.1002/0471142735.im0710s94. Chapter 7, Unit 7.10. [DOI] [PubMed] [Google Scholar]
- 68.Aladjem MI, Spike BT, Rodewald LW, et al. ES cells do not activate p53-dependent stress responses and undergo p53-independent apoptosis in response to DNA damage. Curr. Biol. 1998;8(3):145–155. doi: 10.1016/s0960-9822(98)70061-2. [DOI] [PubMed] [Google Scholar]
- 69.You Y, Browning VL, Schimenti JC. Generation of radiation-induced deletion complexes in the mouse genome using embryonic stem cells. Methods. 1997;13(4):409–421. doi: 10.1006/meth.1997.0547. [DOI] [PubMed] [Google Scholar]
- 70.You Y, Bergstrom R, Klemm M, et al. Chromosomal deletion complexes in mice by radiation of embryonic stem cells. Nat. Genet. 1997;15(3):285–288. doi: 10.1038/ng0397-285. [DOI] [PubMed] [Google Scholar]
- 71.Pellegata NS, Antoniono RJ, Redpath JL, et al. DNA damage and p53-mediated cell cycle arrest: a reevaluation. Proc. Natl Acad. Sci. USA. 1996;93(26):15209–15214. doi: 10.1073/pnas.93.26.15209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu J, Wang F, Tang Z, et al. Role of leukaemia inhibitory factor in the induction of pluripotent stem cells in mice. Cell Biol. Int. 2010;34(8):791–797. doi: 10.1042/CBI20090484. [DOI] [PubMed] [Google Scholar]
- 73.Koch CA, Geraldes P, Platt JL. Immunosuppression by embryonic stem cells. Stem Cells. 2008;26(1):89–98. doi: 10.1634/stemcells.2007-0151. [DOI] [PubMed] [Google Scholar]
- 74.DelaRosa O, Lombardo E. Modulation of adult mesenchymal stem cells activity by Toll-like receptors: implications on therapeutic potential. Mediators Inflamm. 2010;2010:865601. doi: 10.1155/2010/865601. [DOI] [PMC free article] [PubMed] [Google Scholar]