Abstract
In the 25 years, as the first of the syndecan family was cloned, interest in these transmembrane proteoglycans has steadily increased. While four distinct members are present in mammals, one is present in invertebrates, including C. elegans that is such a powerful genetic model. The syndecans, therefore, have a long evolutionary history, indicative of important roles. However, these roles have been elusive. The knockout in the worm has a developmental neuronal phenotype, while knockouts of the syndecans in the mouse are mild and mostly limited to post-natal rather than developmental effects. Moreover, their association with high-affinity receptors, such as integrins, growth factor receptors, frizzled and slit/robo, have led to the notion that syndecans are coreceptors, with minor roles. Given that their heparan sulphate chains can gather many different protein ligands, this gave credence to views that the importance of syndecans lay with their ability to concentrate ligands and that only the extracellular polysaccharide was of significance. Syndecans are increasingly identified with roles in the pathogenesis of many diseases, including tumour progression, vascular disease, arthritis and inflammation. This has provided impetus to understanding syndecan roles in more detail. It emerges that while the cytoplasmic domains of syndecans are small, they have clear interactive capabilities, most notably with the actin cytoskeleton. Moreover, through the binding and activation of signalling molecules, it is likely that syndecans are important receptors in their own right. Here, an overview of syndecan structure and function is provided, with some prospects for the future.
Keywords: cytoskeleton, glycosaminoglycan, heparan sulphate, proteoglycan
Background
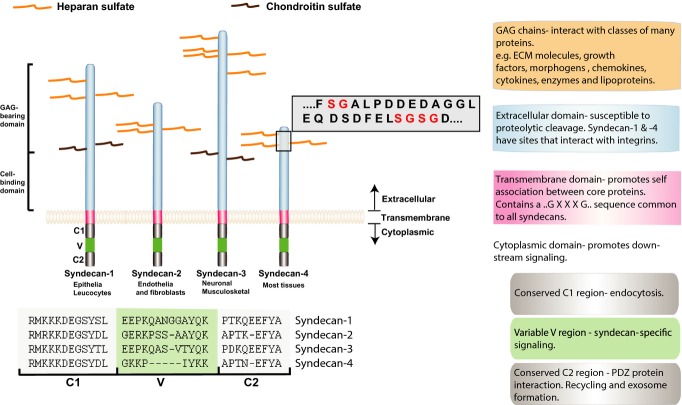
Syndecans comprise a small family of transmembrane proteoglycans. In mammals, there are four distinct genes, while all invertebrate members of the Bilateria possess one. They have, therefore, a long evolutionary history. With the cloning of the first member, syndecan-1, by Merton Bernfield's group in 1989 (Saunders et al. 1989), other members were cloned in the following few years and interest in them has steadily grown. It soon became apparent that they could support cell adhesion, and now it is known that all four mammalian members can interact with the actin cytoskeleton (Fig.1). This was perhaps unsurprising, as heparan sulphate proteoglycans (HSPGs) had been proposed to be important in cell adhesion (e.g. Culp et al. 1986) long before the first syndecan was cloned and indeed even before integrins were identified (Tamkun et al. 1986; see also Hynes 2004). During the 1980s, it became apparent that there were two classes of HSPGs on many cell types, matrix proteoglycans with hydrophilic properties and cell surface HSPGs with hydrophobic characteristics (Woods et al. 1985). It is now appreciated that while syndecans are typical type I membrane proteins, a second family, the glypicans, are endowed with glycosylphosphatidylinositol (GPI) anchors. There are six mammalian glypicans and, apart from bearing heparan sulphate chains, are unrelated to the syndecans. So far, the glypicans are not widely implicated in cell adhesion, but rather appear to have major roles in the binding of a wide range of growth factors, cytokines, chemokines and other polypeptide regulators and cooperate with high-affinity receptors (Filmus & Capurro 2014).
Figure 1.
Schematic of the mammalian syndecans, illustrating structure and interactions.
With the identification and cloning of syndecans, we became interested in how these HSPGs participate in adhesion and signalling. This work continues, but here, we summarize some of the historical aspects, syndecan signalling properties and what we have learned from genetic knockouts. Syndecans are frequently implicated in inflammation and tumour biology. Their pathogenesis also involves regulation of cell adhesion and important roles for heparan sulphate-interacting proteins. Therefore, we include a summary of recent work together with some future perspectives.
Syndecans too become associated with other receptors, notably integrins, and so came to be known as coreceptors. For a while, interest was not surprisingly focussed on integrins, as their involvement in focal adhesion formation and migration was quickly realized. Integrin knockout mice were shown to have, in most cases, profound developmental problems and often these resulted in embryonic or perinatal mortality. A side effect of the enormous interest in integrins was that syndecans were assumed to have minor supporting roles, rather than key functions.
Syndecan knockouts
The situation was not, perhaps, helped by the finding that syndecan-1 and syndecan-4 null mice had almost no developmental defects (Ishiguro et al. 2000; Stepp et al. 2002). As syndecan-1 is enriched in many epithelia, it was discovered that the proteoglycan had roles in post-natal repair, notably in the epithelial layers of the skin and cornea (Pal-Ghosh et al. 2008). The syndecan-4 null was also described to have defects in post-natal repair, involving in this case, granulation tissue angiogenesis and fibroblast migration (Echtermeyer et al. 2001). Long-term potentiation defects were noted in the syndecan-3 null mouse, the proteoglycan being notably enriched in neural tissue (Hienola et al. 2006). In Caenorhabditis elegans, syndecan is also widely present in the developing and adult nervous system, perhaps indicative that syndecan-3 is most closely related to the invertebrate members. The invertebrate syndecan is involved in diverse cellular processes during development.
Syndecan has been genetically linked to slit/robo signalling, in both Drosophila and C. elegans where it is responsible for cell migration and axon guidance during development. Mutants defective in syndecan display phenotypes such as inappropriate axon crossover at the CNS midline (Fig.2), defects in myotube formation and mispositioned cell bodies; these phenotypes can be rescued by expressing syndecan in the affected cells and not the surrounding tissue, suggesting that syndecan acts as a cell autonomously (Steigemann et al. 2004; Gysi et al. 2013). The question of whether syndecan acts as a coreceptor or as a simple ligand gatherer is debated. In one study, it was shown that slit and its robo receptor specifically bind to syndecan suggesting that syndecan acts as a coreceptor (Johnson et al. 2004). However, in another study was shown that the extracellular domain of syndecan is sufficient to mediate slit signalling and surprisingly only the chondroitin chains were needed for signalling (Chanana et al. 2009). Along similar lines, a third study showed that the cytoplasmic domain was dispensable for migration and fusion of dorsal branch cells in Drosophila (Schulz et al. 2011).
Figure 2.
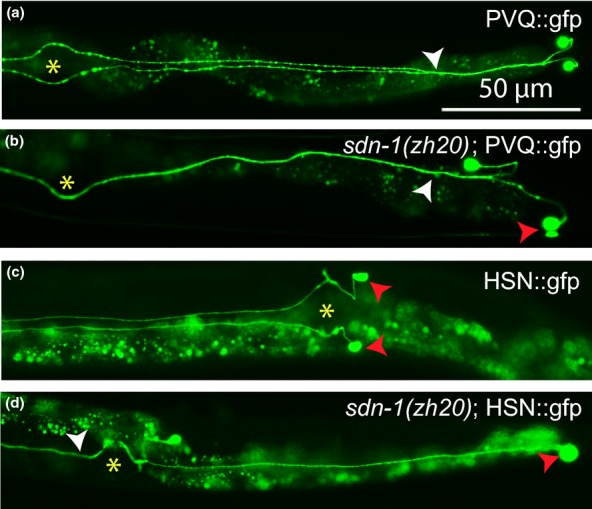
Migration and axon guidance defects in sdn-1 (zh20) null mutants of C. elegans. Genetic null mutants of SDC1, the sole syndecan gene in C. elegans, show migration and axon guidance defects in the hermaphrodite-specific neurons (HSNs) and the PVQ interneurons. In the figure, posterior is to the right and anterior to the left. GFP is expressed in the PVQ neurons (a–b) and in the HSNs (c–d). Wild-type PVQ neurons are born in the tail of the animal and extend their axons from tail to head. Axons extend to each side of the ventral nerve cord (see white arrow). In mutant worm, one of the PVQ neuronal cell bodies is mispositioned (red arrow) and axons fail to extend to each side of the ventral nerve cord (white arrow). Wild-type HSNs are born in the tail and neuronal cell bodies migrate towards the midbody of the animal (red arrow), Axons then extend to the head of the animal on each side of the ventral nerve cord. In mutant animal, one of the neuronal cell bodies failed to migrate to the midbody (red arrow) and axons fail to extend to each side of the ventral nerve cord (white arrow). Yellow stars denote position of vulva.
Together with the invertebrate glypican (lon-2), sdn-1 is responsible for correct guidance of D-type motor axons (no other HSPGs involved) – connecting syndecan with the unc-6/netrin signalling pathway in C. elegans (Gysi et al. 2013). Unexpectedly, an enhancement of the defect was observed when combining sdn-1 mutation (no enhancement combined with lon-2) with mutants of the heparan sulphate modifying enzyme machinery, suggesting that the sulphation pattern of the syndecan was not important for correct axon guidance. Furthermore, in a screen for genetic enhancers of the ventral-to-dorsal distal tip cell migration defect, seen in netrin mutants, an allele of sdn-1 was identified. The migration defect was partially reversed when mutation in Wnt and FGF genes was introduced, suggesting that syndecan could affect netrin signalling through dysregulation of growth factor pathways (Schwabiuk et al. 2009).
Syndecans are commonly substituted with heparan sulphate chains, in the case of syndecan-4, three are present. In some cases, syndecans may additionally or alternately carry chondroitin/dermatan sulphate chains (Deepa et al. 2004). Glypicans also predominantly bear heparan sulphate chains. Many genetic experiments in invertebrates and the mouse show that heparan sulphate is required in development. For example, the Ext1 and 2 proteins, which, in combination, comprise the major polymerase responsible for heparosan synthesis, were shown to be essential (Lin et al. 2000; Stickens et al. 2005). Deletion of the heparan sulphate 2-O-sulphotransferase leads to renal agenesis in the mouse (Bullock et al. 1998). The absolute requirement for heparan sulphate is not surprising, given the wide range of growth factors and morphogens that bind this glycosaminoglycan. However, these data highlight a sharp contrast with the HSPG core proteins, where in the mouse at least, no single knockout has a severe developmental phenotype. It is certainly true that knockouts of glypican-3 or glypican-6 core proteins have clear phenotypes, and there are known mutations in these genes in humans giving rise to disease (Pilia et al. 1996; Campos-Xavier et al. 2009). For syndecans, however, no mutations with human disease relevance are known.
Lower vertebrates have more marked phenotypes where syndecans are repressed by morpholinos, but here, at least in the bony fish, there appears to have been a secondary loss of the syndecan-1 gene (Chakravarti & Adams 2006). Perhaps the further loss of a syndecan by morpholinos in a background of only three family members has more pronounced effects. It does, however, suggest that there may be redundancy across the syndecan core proteins, and perhaps even that glypicans may substitute for syndecans. However, the latter seems less likely given their quite different protein structure and lack of cytoplasmic domain. It is certainly the case that in tissue culture at least, most cell types possess more than one syndecan. Syndecan-4 is most widespread, being present in mesenchymal cells, epithelia, endothelia and cells of the immune system. The other three syndecans appear to be more tissue restricted, but most data suggest they are more abundant in developing tissues where they may have redundant functions explaining the lack of phenotypes when knocked out. Double syndecan knockouts in mice have not yet been reported but are probably imminent. These may be very helpful to address functional relationships and potential redundancy between family members. For now, the molecular basis for redundancy has not been explained and it is the case that while many years of research implicate syndecans in a wide variety of diseases, including vascular disease, arthritis and cancer (Echtermeyer et al. 2009; Iozzo & Sanderson 2011; Herum et al. 2013), the molecular basis for their functions has only slowly revealed themselves.
Syndecan signalling
The cytoplasmic domains of syndecans are small, some 40 amino acids or less (Fig.1). Unsurprisingly, therefore, they have no intrinsic enzymatic activity. Some years ago, we suggested a nomenclature to partition the cytoplasmic domains into conserved and variable regions. The membrane-proximal region (C1 region) is highly conserved across all syndecans and has been shown to participate in linkage to the actin-associated cytoskeleton. Interactions with ezrin–radixin–moesin (ERM) proteins, tubulin, cortactin and Src have been noted (Kinnunen et al. 1998). The recent work of Chen and Williams (2013) reveals an interesting role of the MKKK sequence immediately adjacent to the transmembrane domain. It alone is required for clustering and endocytosis of syndecan-1 from membrane lipid rafts. Extracellular-regulated signal kinase (ERK) activation moves syndecan-1 into rafts where interactions with tubulin are lost, but cortactin interactions, subsequent to syndecan tyrosine phosphorylation, are gained. Endocytosis then follows.
A second highly conserved region of syndecan cytoplasmic domains, the C2 region, is located at the C-terminus and comprises a hydrophobic motif that interacts with PDZ domain containing proteins. These can include syntenin (also known as MDA-9), synectin (also known as GIPC1) and Ca2+/calmodulin-associated serine/threonine kinase (CASK; Multhaupt et al. 2009). PDZ proteins are numerous, and several different types may coexist in the same cells, but how interactions with receptor targets, such as syndecans, are regulated is poorly understood. Syntenin has many receptor targets other than syndecans, and little is known about how interactions are controlled. Work with syntenin suggests that interactions are important for syndecan trafficking and recycling (Zimmermann et al. 2005), but most recently, an interesting new concept has emerged (Baietti et al. 2012; Ghossoub et al. 2014). Syndecan–syntenin–ALIX complexes have been shown to regulate exosome formation. These signalling particles are thought to be important in cellular communication and are attracting much attention, for example in tumour–host crosstalk. More recently, exosome formation by tumour cells has also been shown to be accelerated by heparanase activity, which cleaves heparan sulphate chains (Thompson et al. 2013). In turn, this may impact exosome composition as well as secretion rate. Roles for the small G protein Arf6 and its target phospholipase D are now also implicated in syntenin-based exosome biogenesis (Ghossoub et al. 2014).
Between the two conserved regions (C1 and C2) is a variable (V) region that is distinct to each syndecan. Nevertheless, the sequence for each syndecan V region is conserved across species, for example zebrafish, avian and mammalian syndecan-4 V regions are highly homologous (Whiteford et al. 2008). On the basis of sequences, it would be expected that C1 and C2 functions are common to all syndecans, while V region interactions and functions are distinct to each syndecan. However, V region functions have been difficult to ascertain for all syndecans except that of syndecan-4, where quite extensive progress has been made. Widely used techniques such as yeast 2-hybrid analyses have yielded PDZ protein interacting partners for syndecan C2 regions, but little for V regions. In the light of recent work, however, required phosphorylation events in syndecan cytoplasmic V regions may explain these results. There are highly conserved tyrosine residues in syndecan cytoplasmic domains, and Tyr180 in syndecan-4, for example, determines the proteoglycan's roles in integrin internalization and function (Morgan et al. 2013). Similarly, syndecan-4 V region has a conserved serine residue immediately adjacent to tyr180, which is potentially phosphorylated by protein kinase Cδ (Murakami et al. 2002). This influences not only cell adhesion and migration, but also alters the conformation of the entire cytoplasmic domain (Koo et al. 2006). In so doing, affinity for inositol phospholipid and protein kinase Cα is reduced.
In 1997, we demonstrated that syndecan-4 V region could bind and activate PKCα, and in the following year showed that this interaction was dependent on phosphatidylinositol 4, 5 bisphosphate (Oh et al. 1997, 1998). Substrates for this kinase in the context of syndecan-4's role in cell adhesion have been proposed and include p190RhoGAP and RhoGDI (Bass et al. 2008; Dovas et al. 2010). These events may link PKC to Rho G proteins, known to be essential in focal adhesion and microfilament bundle assembly (Nobes & Hall 1999; Dovas et al. 2006), which is logical given data suggesting that syndecan-4 is a promoter of these structures (Couchman 2010).
The V region of syndecan-4 has also been shown to interact with a protein called syndesmos (Denhez et al. 2002), although beyond interactions with the focal adhesion protein paxillin, its role is presently uncertain. Lastly, α-actinin interacts directly with syndecan-4 V region, and syndecan-4 null fibroblasts show disrupted α-actinin patterns in concert with a loss of large microfilament bundles and fewer focal adhesions (Okina et al. 2012). It has been suggested that serine179 phosphorylation favours interactions with α-actinin at the expense of interactions with PKCα (Chaudhuri et al. 2005), while other data do not support this hypothesis (Okina et al. 2012).
There still remains a considerable challenge with respect to the V regions of other syndecans, including those of invertebrates. It is interesting that the sequences of the V regions of C. elegans and Drosophila are not very homologous, suggesting that their binding partners may be distinct. The structure of the syndecan cytoplasmic domains is also unknown for all except syndecan-4, which has a twisted clamp motif involving two parallel peptides interacting with each other. Whether this is the same for all syndecans needs to be addressed. Finally, the V region of syndecan-4 binds an inositol phospholipid, which by extrapolation suggests that the cytoplasmic domain lies along the inner face of the plasma membrane rather than projecting into the cytosol. This has not been experimentally verified, and whether other syndecans are similarly organized is unknown, but so far, none has been shown to interact with phospholipids in the same way. Their sequences are not, perhaps, consistent with lipid interactions.
Glypicans, as they are not transmembrane, do not have direct linkage to the cytoskeleton and may occupy distinct microdomains on the cell surface, as is seen with GPI anchored molecules. It is perhaps unlikely that they can signal independently, but they have important roles in development and in some rare genetic diseases (Pilia et al. 1996). Moreover, glypican-3 may be a key cell surface receptor in cancers such as hepatocellular carcinoma (Filmus & Capurro 2013).
Overall, the current data suggest that the C1 and C2 domains common to all syndecans are involved in trafficking, while the V regions have distinct functions. The ancestral syndecan appears to have functions in the nervous system, mirrored perhaps by mammalian syndecan-3. However, the V regions of these syndecans have largely unknown interactions. Given the power of the invertebrate genetic models, it seems likely that deeper understanding will come from that quarter.
Syndecans and inflammation
For some years, there has been interest in syndecan involvement in disease. While no mutations in human syndecans have been described with disease relevance, inflammation has become an important focus (Götte 2003; Alexopoulou et al. 2007; Teng et al. 2012). As with many other aspects of syndecan biology, most attention has been paid to syndecan-1. Several models of acute and chronic inflammation have been studied, and it appears that a major function of syndecan-1 is to negatively regulate the adhesion and migration of leucocytes. Endothelial syndecan-1 can serve in this capacity (Teng et al. 2012; Zhang et al. 2013). A recent report suggests that syndecan-1 as a component of the endothelial glycocalyx of arteries is an important mechanosensor, and consistent with other reports, its absence leads to increased leucocyte adhesion (Voyvodic et al. 2014). In studies of aortic aneurysm, on the other hand, macrophages bearing syndecan-1 had an important role in damping the inflammatory environment (Xiao et al. 2012).
Studies of inflammation, with the attendant increase in local proteinase expression, are inextricably linked to the shedding of syndecans from the cell surface. All syndecans appear to be exquisitely sensitive to cleavage, particularly in a membrane-proximal region of the core protein (Manon-Jensen et al. 2013). In addition, syndecan core proteins are sensitive to a large number of proteinases, notably the metalloproteinases. Therefore, an important consideration in the role of syndecans in inflammation is that shedding will likely occur, to release a large portion of the core protein with glycosaminoglycan chains attached. Whether this truncates signalling through the core protein is not confirmed but likely. Further processing of the remnant core proteins in their transmembrane domains by presenilin/γ-secretase has been shown for syndecan-3 (Schulz et al. 2003). The released ectodomain can serve as a competitive inhibitor of cell surface proteoglycan but may also diffuse into the local environment where it can sequester a number of inflammatory factors, many of which are heparin binding (Elenius et al. 2004; Sarrazin et al. 2011). This is, to a large extent, an anti-inflammatory response (Teng et al. 2012). However, with respect to cancer, syndecan-1 can support the pathogenic process by amplifying growth factor and cytokine signalling.
In the mammalian genome, there is a single heparanase gene, encoding an enzyme that specifically cleaves heparan sulphate chains (Fux et al. 2009; Peterson & Liu 2013). The mechanisms of cleavage and substrate specificity in terms of heparan sulphate fine structure are under investigation. It appears that the enzyme does not have precise substrate specificity, but is influenced by the sulphation in the vicinity of the cleavage site (Peterson & Liu 2013). Such cleavage can, however, leave significant portions of the polysaccharide attached to the core protein. Heparanase is now intensively studied because it is implicated in the pathogenesis of several diseases. In addition, however, it has also been demonstrated that heparanase cleavage of the syndecan heparan sulphate chains can engender enhanced sensitivity of the core protein to shedding (Ramani et al. 2012).
The closest homologue to syndecan-1 is syndecan-3, which is enriched in neural tissue but also in the musculoskeletal system. A recent study has shown up an interesting dichotomy of roles across a range of chronic inflammatory diseases. In a murine model of rheumatoid arthritis and using genetic deletion of syndecan-3, the proteoglycan was shown to be pro-inflammatory in the joint where it enhanced chemokine signalling and leucocyte recruitment. By contrast, in CXCL1-induced skin and muscle inflammation, syndecan-3 had an opposite anti-inflammatory role (Kehoe et al. 2014). Tissue context is clearly important in the pathobiology of syndecans.
While syndecan-2 has received little attention with respect to inflammation, perhaps because a knockout mouse has not yet been reported, syndecan-4 has been examined with respect to skeletal and vascular disease in particular. Expression of syndecan-4 is rapidly upregulated in inflammation, at least in part because the gene has an NF-κB response element. Therefore, tumour necrosis factor-α, interleukin-1β and lipopolysaccharide all trigger substantial increases in syndecan-4 expression (Strand et al. 2013). Equally, syndecan-4 can be shed in the inflammatory milieu, much as syndecan-1. In a model of pulmonary inflammation, syndecan-4 expression was shown to be anti-inflammatory (Tanino et al. 2012) and a similar conclusion was reached in a study of myocardial infarction (Xie et al. 2012). Indeed, therapeutic potential for syndecan-4 in suppressing inflammation and fibrosis, promoting neovascularization and improved cardiac function was suggested (Xie et al. 2012).
In a study of osteoarthritis, it was noted that the syndecan-4 null mouse was protected from cartilage degeneration. In part, this was ascribed to decreased aggrecanase (ADAMTS-5) activity at the cell surface, but also indirect regulation of MMP-3 expression (Echtermeyer et al. 2009). ADAMTS-5 was shown to interact with the heparan sulphate of syndecan-4, in which wild-type animals were upregulated in arthritic disease. This study utilized both null animals, but also injected ‘blocking’ antibodies against syndecan-4. This is one of many reports using such an approach, yet in some ways, this is a mysterious phenomenon. ADAMTS-5 interacts with the heparan sulphate chains, but core protein-directed antibodies are shown to ‘block’. How does this happen? In an immunocompetant animal, perhaps the antibody–syndecan complex is removed by leucocytes or the antibody cross-links the syndecan and the patched receptor is then internalized. In the Echtermeyer study, however, there is a possible clue. The antibodies were prepared against a membrane-proximal core protein region, so perhaps shedding was targeted and blocked. It would be interesting to establish the underlying mechanism.
Lastly, in a more recent study of bone fracture repair, syndecan-4 null mice were shown to have delayed repair, with the finger of suspicion pointed at an elevated inflammatory response (Bertrand et al. 2013). This study also sheds light on possible redundancy between syndecans in development. As has been noted many times previously, development in the single null mouse is not impaired, but post-natal repair can be compromised. Here, the authors show that in the absence of syndecan-4 and syndecan-2 is upregulated in the developing cartilage. However, it is important to note in conclusion that apart from the potential for compensatory expression of alternate syndecans in development, post-natal repair inevitably involves inflammation that, as summarized here, influences both syndecan expression and function.
With the advent of sophisticated immune systems and a closed vasculature, vertebrates have clearly expanded the roles of syndecans, which may be related to the two rounds of gene duplication events that took place at the invertebrate–chordate boundary (Chakravarti & Adams 2006). Moreover, vascular tissues and many leucocytes are rich sources of syndecans. Although unclear at the molecular level today, it will be interesting to understand the regulatory mechanisms that extend from roles in invertebrate neural development, on the one hand, to the complex processes of acquired and innate immunity in vertebrates.
Syndecans and tumour biology
Syndecans have prominent roles in cell–cell and cell–ECM interactions as well as in regulating cell adhesion and motility, processes which are central to cancer progression (Beauvais & Rapraeger 2004). In addition, syndecan ectodomains can be shed into the ECM by proteases (Manon-Jensen et al. 2010) and in soluble form could compete with growth factors such as fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF) for binding to cell surface receptors (Fears & Woods 2006). As proteinases including metzincins are frequently upregulated in tumour cells, syndecan shedding has received increasing attention. This feature of syndecans was shown to facilitate myeloma tumour progression (Yang et al. 2002).
Syndecan-1 has long been suggested to be a prognostic marker for some tumour types. Given that loss of syndecan-1 is often associated with loss of E-cadherin, it is thought to be a regulator of epithelial to mesenchymal transition in transformed epithelial cells with considerable alterations in cell morphology, growth and motility (Iozzo & Sanderson 2011; Kato et al. 1995; Sun et al. 1998). This is a likely explanation as to why low syndecan-1 expression associates with higher tumour grade and poor patient survival as well as poor prognosis in head and neck, lung and colorectal cancer (Teng et al. 2012). In contrast, high levels of syndecan-1 in breast cancer and particularly in the tumour stroma, myeloma, pancreatic and lung cancer increase tumour aggressiveness and forecast a poor outcome (Teng et al. 2012). In myeloma, syndecan-1 enhances tumorigenesis through the regulation of cell survival, adhesion and migration (Khotskaya et al. 2009; Yang et al. 2002). Consistent with this, syndecan-1 depletion in myeloma cells led to growth arrest and apoptosis (Khotskaya et al. 2009). Furthermore, neo-angiogenesis and disseminated growth of myeloma cells were commensurately inhibited when the mice were injected with syndecan-1 depleted myeloma cells, indicating that syndecan-1 is important to trigger the tumour metastasis. Conversely, head and neck squamous cell carcinoma (HNSCC) cells expressing high levels of syndecan-1 exhibited reduced motility and invasiveness in collagen I matrices compared with HNSCC cells expressing low levels of syndecan-1 (Ishikawa & Kramer 2010). Thus, roles for syndecan-1 as a stimulatory or inhibitory factor are clearly specific to each cancer type, but precisely why there are such tumour-specific differences remains to be elucidated.
Accumulating evidence also suggests that syndecan-2 modulates several key cellular processes in tumourigenesis, such as cell adhesion, migration, apoptosis and metastasis (Beauvais & Rapraeger 2004; Iozzo & Sanderson 2011). Similar to syndecan-1, syndecan-2 also has dual-function properties: either acting as a tumour suppressor or tumour promoter depending on a cancer type (Fears & Woods 2006; Iozzo & Sanderson 2011). Syndecan-2 is reported to be upregulated in colon cancer, Lewis lung carcinoma, ovarian tumours, prostate cancer, melanoma, osteosarcoma and glioma, where it may regulate cell shape, adhesion and migration. Syndecan-2 functioning as a tumour suppressor is best illustrated in Lewis lung carcinoma cells (Munesue et al. 2002, 2007). We have very recently provided evidence that syndecan-2-regulated breast cancer cell morphology is highly dependent on Rho-GTPases (Lim & Couchman 2014). A crosstalk between syndecan-2 and p190ARhoGAP in regulation of breast cancer cell actin cytoskeleton and cell migration has been identified. Syndecan-2 appears to be a novel regulator of p190ARhoGAP activity and distribution, which, in turn, regulates localized RhoA activation. On the other hand, syndecan-2 suppresses roles of syndecan-4 in regulating the distribution of p190ARhoGAP in these tumour cells, highlighting the specific effects of syndecan-2 and syndecan-4 in the regulation of cytoskeletal dynamics. These data suggest that at least in some cells, syndecans can establish a hierarchy, one member suppressing the function of another.
Syndecan-4 is the only ubiquitously expressed syndecan in mammals. Although its expression is relatively low compared to the other syndecans, its role is pivotal and diverse in cancer biology. Aberrant expression of syndecan-4 is reported in breast cancer, melanoma, hepatocellular carcinoma and malignant mesothelioma. Syndecan-4 functions in breast carcinoma are currently unclear. It may, in fact, be a good prognostic marker in patients positive for oestrogen and progesterone receptors (Lendorf et al. 2011), where more treatment options are available. In this study, expressions of syndecan-4 and syndecan-1 were shown to be independent indicators for prognosis in breast carcinoma. Patient tumour tissue immunohistochemistry showed that syndecan-4 was expressed mostly in the cytoplasm but could also be found in the nucleus as well as the tumour stroma. In addition, association of syndecan-4 with growth factors such as FGF2 has been implicated in melanoma progression. FGF2 signalling is downregulated in the absence of syndecan-4 leading to an increase of melanoma cell motility and defects in fibronectin adhesion (Chalkiadaki et al. 2009).
Transport of receptors to the nucleus is certainly not unknown, but syndecan translocation to this site has been somewhat controversial. While there are now several reports that this may occur (Kovalszky et al. 2014; Stewart & Sanderson 2014), further detailed and careful analysis is required. What still remains completely unclear is the pathway that transports syndecans to the nucleus and whether truncated or whole proteoglycans reach this site. If these early reports are verified, the most important aspect to be resolved is what role it may have, bearing in mind that knockouts of syndecans are mild. It would be interesting if it transpires that nuclear translocation is a corollary of pathogenesis.
Future perspectives
Since 1989, there have been over 2500 publications concerning the structure, distribution and function of syndecans in vertebrate cells and tissues, as well as genetic analyses in invertebrates and vertebrates. They are now implicated in many diseases, though whether they contribute to, or are a consequence of, pathogenesis is for the most part unclear. In the main, however, it is clear that syndecans are more than ligand gatherers. Apart from many studies that show ternary complexes between ligand, heparan sulphate and high-affinity receptors, syndecans have the capacity to regulate cell function through their cytoplasmic domains. The differences in actin cytoskeletal architecture between syndecan-4 null and matching wild-type cells are clear and readily corrected by transfection of wild-type syndecan-4 cDNA into the null cells. However, cytoplasmic truncation mutant cDNAs will not do so (Gopal et al. 2010; Okina et al. 2012). Moreover, to date, it appears that syndecan signalling does not impact transcription significantly, but more the morphology and behaviour of cells. The concept of dual regulation then emerges where ligands promote syndecans to control the behavioural response of cells, while the same ligands interacting with specific high-affinity receptors drive transcriptional regulation.
There is much to be learned. Syndecans have been somewhat enigmatic and intractable, but it is likely that a combination of genetics and molecular cell biology and more attention from investigators across the spectrum of biomedicine will reveal much important information on this major group of cell surface proteoglycans. The cytoplasmic domain functions of syndecans remain mostly unclear, with the possible exception of syndecan-4. Redundancy between vertebrate syndecan core proteins is also not well understood although it is very apparent. We are now investigating a possible common signalling mechanism shared by all syndecans, including those of invertebrates. A second shared property of all syndecans is the ease with which they are shed from the cell surface by a wide array of proteinases. It is not yet fully apparent whether this property is important and what roles it may have. Structural analysis of the core proteins is still primordial, but undeniably complicated by the glycosaminoglycan chains which are both heterogeneous yet an aid to retaining solubility in biochemical preparations. Are the heparan sulphate chains distinct in terms of fine structure when expressed on different syndecan core proteins in vivo, or does redundancy extend to the polysaccharide also? This is a difficult question, made even more complex by postexpression modifications by sulphatases and heparanase. Heparanase is implicated in the pathogenesis of several diseases, including tumour progression. This argues that syndecans may not be bystanders but important players. Syndecans are potentially unique in that not only do they have important roles as synthesized and transported to the cell surface, but also new properties can emerge from partial catalysis at the cell surface or in recycling pools. Sulfatases, heparanase and sheddases may all impart modified functions to syndecans but the biological rationale that underlies these events remains to be fully understood. Much more needs to be understood about how syndecans function in inflammation, proliferation, apoptosis and even epigenetic events if nuclear targeting of syndecans is relevant to disease.
Acknowledgments
Some of the work referenced here was supported by the Danish National Research Foundation, Novo Nordisk Foundation, Lundbeck Foundation and The Danish Natural Science Research Council (to JRC). The authors were also supported by The University of Copenhagen. All authors have contributed to, critically reviewed and approved this article. None of the authors has any conflict of interest to declare.
References
- Alexopoulou AN, Multhaupt HA. Couchman JR. Syndecans in wound healing, inflammation and vascular biology. Int. J. Biochem. Cell Biol. 2007;39:505–528. doi: 10.1016/j.biocel.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Baietti MF, Zhang Z, Mortier E, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012;14:677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- Bass MD, Morgan MR, Roach KA, Settleman J, Goryachev AB. Humphries MJ. p190RhoGAP is the convergence point of adhesion signals from alpha 5 beta 1 integrin and syndecan-4. J. Cell Biol. 2008;181:1013–1026. doi: 10.1083/jcb.200711129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvais DM. Rapraeger AC. Syndecans in tumor cell adhesion and signaling. Reprod. Biol. Endocrinol. 2004;2:3. doi: 10.1186/1477-7827-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand J, Stange R, Hidding H, et al. Syndecan 4 supports bone fracture repair, but not fetal skeletal development, in mice. Arthritis Rheum. 2013;65:743–752. doi: 10.1002/art.37817. [DOI] [PubMed] [Google Scholar]
- Bullock SL, Fletcher JM, Beddington RS. Wilson VA. Renal agenesis in mice homozygous for a gene trap mutation in the gene encoding heparan sulfate 2-sulfotransferase. Genes Dev. 1998;12:1894–1906. doi: 10.1101/gad.12.12.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Xavier AB, Martinet D, Bateman J, et al. Mutations in heparan sulfate-proteoglycan glypican 6 (GPC6) impair endochondral ossification and cause recessive omodysplasia. Am. J. Hum. Genet. 2009;84:760–770. doi: 10.1016/j.ajhg.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarti R. Adams JC. Comparative genomics of the syndecans defines an ancestral genomic context associated with matrilins in vertebrates. BMC Genom. 2006;7:83. doi: 10.1186/1471-2164-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalkiadaki G, Nikitovic D, Berdiaki A, et al. Fibroblast growth factor-2 modulates melanoma adhesion and migration through a syndecan-4-dependent mechanism. Int. J. Biochem. Cell Biol. 2009;41:1323–1331. doi: 10.1016/j.biocel.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Chanana B, Steigemann P, Jäckle H. Vorbrüggen G. Reception of Slit requires only the chondroitin-sulphate-modified extracellular domain of Syndecan at the target cell surface. Proc. Natl Acad. Sci. USA. 2009;106:11984–11988. doi: 10.1073/pnas.0901148106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri P, Colles SM, Fox PL. Graham LM. Protein kinase Cdelta-dependent phosphorylation of syndecan-4 regulates cell migration. Circ. Res. 2005;97:674–681. doi: 10.1161/01.RES.0000184667.82354.b1. [DOI] [PubMed] [Google Scholar]
- Chen K. Williams KJ. Molecular mediators for raft-dependent endocytosis of syndecan-1, a highly conserved, multifunctional receptor. J. Biol. Chem. 2013;288:13988–13999. doi: 10.1074/jbc.M112.444737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couchman JR. Transmembrane signaling proteoglycans. Annu. Rev. Cell Dev. Biol. 2010;26:89–114. doi: 10.1146/annurev-cellbio-100109-104126. [DOI] [PubMed] [Google Scholar]
- Culp LA, Laterra J, Lark MW, Beyth RJ. Tobey SL. Heparan sulphate proteoglycan as mediator of some adhesive responses and cytoskeletal reorganization of cells on fibronectin matrices: independent versus cooperative functions. Ciba Found. Symp. 1986;124:158–183. doi: 10.1002/9780470513385.ch10. [DOI] [PubMed] [Google Scholar]
- Deepa SS, Yamada S, Zako M, Goldberger O. Sugahara K. Chondroitin sulfate chains on syndecan-1 and syndecan-4 from normal murine mammary gland epithelial cells are structurally and functionally distinct and cooperate with heparan sulfate chains to bind growth factors. A novel function to control binding of midkine, pleiotrophin, and basic fibroblast growth factor. J. Biol. Chem. 2004;279:37368–37376. doi: 10.1074/jbc.M403031200. [DOI] [PubMed] [Google Scholar]
- Denhez F, Wilcox-Adelman SA, Baciu PC, et al. Syndesmos, a syndecan-4 cytoplasmic domain interactor, binds to the focal adhesion adaptor proteins paxillin and Hic-5. J. Biol. Chem. 2002;277:12270–12274. doi: 10.1074/jbc.M110291200. [DOI] [PubMed] [Google Scholar]
- Dovas A, Yoneda A. Couchman JR. PKCalpha-dependent activation of RhoA by syndecan-4 during focal adhesion formation. J. Cell Sci. 2006;119:2837–2846. doi: 10.1242/jcs.03020. [DOI] [PubMed] [Google Scholar]
- Dovas A, Choi Y, Yoneda A, et al. Serine 34 phosphorylation of rho guanine dissociation inhibitor (RhoGDIalpha) links signaling from conventional protein kinase C to RhoGTPase in cell adhesion. J. Biol. Chem. 2010;285:23296–23308. doi: 10.1074/jbc.M109.098129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echtermeyer F, Streit M, Wilcox-Adelman S, et al. Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J. Clin. Invest. 2001;107:R9–R14. doi: 10.1172/JCI10559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echtermeyer F, Bertrand J, Dreier R, et al. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat. Med. 2009;15:1072–1076. doi: 10.1038/nm.1998. [DOI] [PubMed] [Google Scholar]
- Elenius V, Götte M, Reizes O, Elenius K. Bernfield M. Inhibition by the soluble syndecan-1 ectodomains delays wound repair in mice overexpressing syndecan-1. J. Biol. Chem. 2004;279:41928–41935. doi: 10.1074/jbc.M404506200. [DOI] [PubMed] [Google Scholar]
- Fears CY. Woods A. The role of syndecans in disease and wound healing. Matrix Biol. 2006;25:443–456. doi: 10.1016/j.matbio.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Filmus J. Capurro M. Glypican-3: a marker and a therapeutic target in hepatocellular carcinoma. FEBS J. 2013;280:2471–2476. doi: 10.1111/febs.12126. [DOI] [PubMed] [Google Scholar]
- Filmus J. Capurro M. The role of glypicans in hedgehog signaling. Matrix Biol. 2014;35:248–252. doi: 10.1016/j.matbio.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Fux L, Ilan N, Sanderson RD. Vlodavsky I. Heparanase: busy at the cell surface. Trends Biochem. Sci. 2009;34:511–519. doi: 10.1016/j.tibs.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghossoub R, Lembo F, Rubio A, et al. Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat. Commun. 2014;5:3477. doi: 10.1038/ncomms4477. [DOI] [PubMed] [Google Scholar]
- Gopal S, Bober A, Whiteford JR, Multhaupt HA, Yoneda A. Couchman JR. Heparan sulfate chain valency controls syndecan-4 function in cell adhesion. J. Biol. Chem. 2010;285:14247–14258. doi: 10.1074/jbc.M109.056945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götte M. Syndecans in inflammation. FASEB J. 2003;17:575–591. doi: 10.1096/fj.02-0739rev. [DOI] [PubMed] [Google Scholar]
- Gysi S, Rhiner C, Flibotte S, Moerman DG. Hengartner MO. A network of HSPG core proteins and HS modifying enzymes regulates netrin-dependent guidance of D-type motor neurons in Caenorhabditis elegans. PLoS ONE. 2013;8:e74908. doi: 10.1371/journal.pone.0074908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herum KM, Lunde IG, Skribic B, et al. Syndecan-4 signaling via NFAT regulates extracellular matrix production and cardiac myofibroblast differentiation in response to mechanical stress. J. Mol. Cell. Cardiol. 2013;54:73–81. doi: 10.1016/j.yjmcc.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Hienola A, Tumova S, Kulesskiy E. Rauvala H. N-syndecan deficiency impairs neural migration in brain. J. Cell Biol. 2006;174:569–580. doi: 10.1083/jcb.200602043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. The emergence of integrins: a personal and historical perspective. Matrix Biol. 2004;23:333–340. doi: 10.1016/j.matbio.2004.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo RV. Sanderson RD. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J. Cell Mol. Med. 2011;15:1013–1031. doi: 10.1111/j.1582-4934.2010.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro K, Kadomatsu K, Kojima T, et al. Syndecan-4 deficiency impairs focal adhesion formation only under restricted conditions. J. Biol. Chem. 2000;275:5249–5252. doi: 10.1074/jbc.275.8.5249. [DOI] [PubMed] [Google Scholar]
- Ishikawa T. Kramer RH. Sdc1 negatively modulates carcinoma cell motility and invasion. Exp. Cell Res. 2010;316:951–965. doi: 10.1016/j.yexcr.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KG, Ghose A, Epstein E, Lincecum J, O'Connor MB. Van Vactor D. Axonal heparan sulfate proteoglycans regulate the distribution and efficiency of the repellent slit during midline axon guidance. Curr. Biol. 2004;14:499–504. doi: 10.1016/j.cub.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Kato M, Saunders S, Nguyen H. Bernfield M. Loss of cell surface syndecan-1 causes epithelia to transform into anchorage-independent mesenchyme-like cells. Mol. Biol. Cell. 1995;6:559–576. doi: 10.1091/mbc.6.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe O, Kalia N, King S, et al. Syndecan-3 is selectively pro-inflammatory in the joint and contributes to antigen-induced arthritis in mice. Arthritis. Res. Ther. 2014;16:R148. doi: 10.1186/ar4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khotskaya YB, Dai Y, Ritchie JP, et al. Syndecan-1 is required for robust growth, vascularization and metastasis of myeloma tumors in vivo. J. Biol. Chem. 2009;284:26085–26095. doi: 10.1074/jbc.M109.018473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen T, Kaksonen M, Saarinen J, Kalkkinen N, Peng HB. Rauvala H. Cortactin-Src kinase signaling pathway is involved in N-syndecan-dependent neurite outgrowth. J. Biol. Chem. 1998;273:10702–10708. doi: 10.1074/jbc.273.17.10702. [DOI] [PubMed] [Google Scholar]
- Koo BK, Jung YS, Shin J, et al. Structural basis of syndecan-4 phosphorylation as a molecular switch to regulate signaling. J. Mol. Biol. 2006;355:651–663. doi: 10.1016/j.jmb.2005.09.087. [DOI] [PubMed] [Google Scholar]
- Kovalszky I, Hjerpe A. Dobra K. Nuclear translocation of heparan sulfate proteoglycans and their functional significance. Biochim. Biophys. Acta. 2014;1840:2491–2497. doi: 10.1016/j.bbagen.2014.04.015. [DOI] [PubMed] [Google Scholar]
- Lendorf ME, Manon-Jensen T, Kronqvist P, Multhaupt HA. Couchman JR. Syndecan-1 and syndecan-4 are independent indicators in breast carcinoma. J. Histochem. Cytochem. 2011;59:615–629. doi: 10.1369/0022155411405057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HC. Couchman JR. Syndecan-2 regulation of morphology in breast carcinoma cells is dependent on RhoGTPases. Biochim. Biophys. Acta. 2014;1840:2482–2490. doi: 10.1016/j.bbagen.2014.01.018. [DOI] [PubMed] [Google Scholar]
- Lin X, Wei G, Shi Z, et al. Disruption of gastrulation and heparan sulfate biosynthesis in EXT1-deficient mice. Dev. Biol. 2000;224:299–311. doi: 10.1006/dbio.2000.9798. [DOI] [PubMed] [Google Scholar]
- Manon-Jensen T, Itoh Y. Couchman JR. Proteoglycans in health and disease: the multiple roles of syndecan shedding. FEBS J. 2010;277:3876–3889. doi: 10.1111/j.1742-4658.2010.07798.x. [DOI] [PubMed] [Google Scholar]
- Manon-Jensen T, Multhaupt HA. Couchman JR. Mapping of matrix metalloproteinase cleavage sites on syndecan-1 and syndecan-4 ectodomains. FEBS J. 2013;280:2320–2331. doi: 10.1111/febs.12174. [DOI] [PubMed] [Google Scholar]
- Morgan MR, Hamidi H, Bass MD, Warwood S, Ballestrem C. Humphries MJ. Syndecan-4 phosphorylation is a control point for integrin recycling. Dev. Cell. 2013;24:472–485. doi: 10.1016/j.devcel.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multhaupt HA, Yoneda A, Whiteford JR, Oh ES, Lee W. Couchman JR. Syndecan signaling: when, where and why? J. Physiol. Pharmacol. 2009;60(Suppl 4):31–38. [PubMed] [Google Scholar]
- Munesue S, Kusano Y, Oguri K, et al. The role of syndecan-2 in regulation of actin-cytoskeletal organization of Lewis lung carcinoma-derived metastatic clones. Biochem. J. 2002;363:201–209. doi: 10.1042/0264-6021:3630201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munesue S, Yoshitomi Y, Kusano Y, et al. A novel function of syndecan-2, suppression of matrix metalloproteinase-2 activation, which causes suppression of metastasis. J. Biol. Chem. 2007;282:28164–28174. doi: 10.1074/jbc.M609812200. [DOI] [PubMed] [Google Scholar]
- Murakami M, Horowitz A, Tang S, Ware JA. Simons M. Protein kinase C (PKC) delta regulates PKCalpha activity in a Syndecan-4-dependent manner. J. Biol. Chem. 2002;277:20367–20371. doi: 10.1074/jbc.M202501200. [DOI] [PubMed] [Google Scholar]
- Nobes CD. Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J. Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh ES, Woods A. Couchman JR. Syndecan-4 proteoglycan regulates the distribution and activity of protein kinase C. J. Biol. Chem. 1997;272:8133–8136. doi: 10.1074/jbc.272.13.8133. [DOI] [PubMed] [Google Scholar]
- Oh ES, Woods A, Lim ST, Theibert AW. Couchman JR. Syndecan-4 proteoglycan cytoplasmic domain and phosphatidylinositol 4,5-bisphosphate coordinately regulate protein kinase C activity. J. Biol. Chem. 1998;273:10624–10629. doi: 10.1074/jbc.273.17.10624. [DOI] [PubMed] [Google Scholar]
- Okina E, Grossi A, Gopal S, Multhaupt HA. Couchman JR. Alpha-actinin interactions with syndecan-4 are integral to fibroblast-matrix adhesion and regulate cytoskeletal architecture. Int. J. Biochem. Cell Biol. 2012;44:2161–2174. doi: 10.1016/j.biocel.2012.08.017. [DOI] [PubMed] [Google Scholar]
- Pal-Ghosh S, Tadvalkar G, Jurjus RA, Zieske JD. Stepp MA. BALB/c and C57BL6 mouse strains vary in their ability to heal corneal epithelial debridement wounds. Exp. Eye Res. 2008;87:478–486. doi: 10.1016/j.exer.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson SB. Liu J. Multi-faceted substrate specificity of heparanase. Matrix Biol. 2013;32:223–227. doi: 10.1016/j.matbio.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Pilia G, Highes-Benzie RM, MacKenzie A, et al. Mutations in GPC3, a glypican gene, cause the Simpson-Golabi-Behmel overgrowth syndrome. Nat. Genet. 1996;12:241–247. doi: 10.1038/ng0396-241. [DOI] [PubMed] [Google Scholar]
- Ramani VC, Pruett PS, Thompson CA, DeLucas LD. Sanderson RD. Heparan sulfate chains of syndecan-1 regulate ectodomain shedding. J. Biol. Chem. 2012;287:995299–61. doi: 10.1074/jbc.M111.330803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrazin S, Lamanna WC. Esko JD. Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 2011;1:3. doi: 10.1101/cshperspect.a004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders S, Jalkanen M, O'Farrell S, Bernfield M. Molecular cloning of syndecan, an integral membrane proteoglycan. J. Cell Biol. 1989;108:1547–1556. doi: 10.1083/jcb.108.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz JG, Annaert W, Vandekerckhove J, Zimmermann P, De Strooper B. David G. Syndecan 3 intramembrane proteolysis is presenilin/gamma-secretase-dependent and modulates cytosolic signaling. J. Biol. Chem. 2003;278:48651–48657. doi: 10.1074/jbc.M308424200. [DOI] [PubMed] [Google Scholar]
- Schulz JG, Ceulemans H, Caussinus E, et al. Drosophila syndecan regulates tracheal cell migration by stabilizing Robo levels. EMBO Rep. 2011;12:1039–1046. doi: 10.1038/embor.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabiuk M, Coudiere L. Merz DC. SDN-1/syndecan regulates growth factor signaling in distal tip cell migrations in C. elegans. Dev. Biol. 2009;334:235–342. doi: 10.1016/j.ydbio.2009.07.020. [DOI] [PubMed] [Google Scholar]
- Steigemann P, Molitor A, Fellert S, Jäckle H. Vorbrüggen G. Heparan sulfate proteoglycan syndecan promotes axonal and myotube guidance by slit/robo signaling. Curr. Biol. 2004;14:225–230. doi: 10.1016/j.cub.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Stepp MA, Gibson HE, Gala PH, et al. Defects in keratinocyte activation during wound healing in the syndecan-1-deficient mouse. J. Cell Sci. 2002;115:4517–4531. doi: 10.1242/jcs.00128. [DOI] [PubMed] [Google Scholar]
- Stewart MD. Sanderson RD. Heparan sulfate in the nucleus and its control of cellular functions. Matrix Biol. 2014;35:56–59. doi: 10.1016/j.matbio.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickens D, Zak BM, Rougier N, Esko JD. Werb Z. Mice deficient in Ext2 lack heparan sulfate and develop exostoses. Development. 2005;132:5055–5068. doi: 10.1242/dev.02088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand ME, Herum KM, Rana ZA, et al. Innate immune signaling induces expression and shedding of the heparan sulfate proteoglycan syndecan-4 in cardiac fibroblasts and myocytes, affecting inflammation in the pressure-overloaded heart. FEBS J. 2013;280:2228–2247. doi: 10.1111/febs.12161. [DOI] [PubMed] [Google Scholar]
- Sun D, Mcalmon KR, Davies JA, Bernfield M. Hay ED. Simultaneous loss of expression of syndecan-1 and E-cadherin in the embryonic palate during epithelial-mesenchymal transformation. Int. J. Dev. Biol. 1998;42:733–736. [PubMed] [Google Scholar]
- Tamkun JW, DeSimone DW, Fonda D, et al. Structure of integrin, a glycoprotein involved in the transmembrane linkage between fibronectin and actin. Cell. 1986;46:271–282. doi: 10.1016/0092-8674(86)90744-0. [DOI] [PubMed] [Google Scholar]
- Tanino Y, Chang MY, Wang X, et al. Syndecan-4 regulates early neutrophil migration and pulmonary inflammation in response to lipopolysaccharide. Am. J. Respir. Cell Mol. Biol. 2012;47:196–202. doi: 10.1165/rcmb.2011-0294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng YH, Aquino RS. Park PW. Molecular functions of syndecan-1 in disease. Matrix Biol. 2012;31:3–16. doi: 10.1016/j.matbio.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CA, Purushothaman A, Ramani VC, Vlodavsky I. Sanderson RD. Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. J. Biol. Chem. 2013;288:10093–10099. doi: 10.1074/jbc.C112.444562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyvodic PL, Min D, Liu R, et al. Loss of syndecan-1 induces a pro-inflammatory phenotype in endothelial cells with a dysregulated response to atheroprotective flow. J. Biol. Chem. 2014;289:9547–9559. doi: 10.1074/jbc.M113.541573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford JR, Ko S, Lee W. Couchman JR. Structural and cell adhesion properties of zebrafish syndecan-4 are shared with higher vertebrates. J. Biol. Chem. 2008;283:29322–29330. doi: 10.1074/jbc.M803505200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Couchman JR. Höök M. Heparan sulfate proteoglycans of rat embryo fibroblasts. A hydrophobic form may link cytoskeleton and matrix components. J. Biol. Chem. 1985;260:10872–10879. [PubMed] [Google Scholar]
- Xiao J, Angsana J, Wen J, et al. Syndecan-1 displays a protective role in aortic aneurysm formation by modulating T cell-mediated responses. Arterioscler. Thromb. Vasc. Biol. 2012;32:386–396. doi: 10.1161/ATVBAHA.111.242198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Wang J, Li R, et al. Syndecan-4 over-expression preserves cardiac function in a rat model of myocardial infarction. J. Mol. Cell. Cardiol. 2012;53:250–258. doi: 10.1016/j.yjmcc.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Yang Y, Yaccoby S, Liu W, et al. Soluble syndecan-1 promotes growth of myeloma tumors in vivo. Blood. 2002;100:610–617. doi: 10.1182/blood.v100.2.610. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wu C, Song J, Götte M. Sorokin L. Syndecan-1, a cell surface proteoglycan, negatively regulates initial leukocyte recruitment to the brain across the choroid plexus in murine experimental autoimmune encephalomyelitis. J. Immunol. 2013;191:4551–4561. doi: 10.4049/jimmunol.1300931. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Zhang Z, Degeest G, et al. Syndecan recycling is controlled by syntenin-PIP2 interaction and Arf6. Dev. Cell. 2005;9:377–388. doi: 10.1016/j.devcel.2005.07.011. [DOI] [PubMed] [Google Scholar]