Abstract
Recent studies have shown a positive association of cancer and obesity, but the morphological and molecular mechanisms involved in this relationship are still unknown. This study analysed the impact of long-term obesity on rat prostate, focusing on stromal changes. Male adult Wistar rats were treated with high-fat diet to induce obesity, while the control group received a balanced diet. After 30 weeks of feeding, the ventral prostate was analysed by immunohistochemistry for cell proliferation, smooth muscle α-actin, vimentin, chondroitin sulphate and metalloproteinases (MMP-2 and 9). The content of androgen receptor (AR), oestrogen receptors (ERs) and vascular endothelial growth factor (VEGF) was measured by Western blotting, and activity of catalase and Glutathione-S-Transferase (GST) were quantified by enzymatic assay. Long-term obesity decreased testosterone plasma levels by 70% and resulted in stromal prostate hyperplasia, as evidenced by increased collagen fibres. Such stromal hyperplasia was associated with increased number of blood vessels and raised VEGF content, and increased expression of chondroitin sulphate, vimentin, α-actin and MMP-9. In spite of the high cell density in prostate, the proliferative activity was lower in the prostates of obese rats, indicating that hyperplasia was established during the early phases in this obesity model. AR levels increased significantly, whereas the ERα decreased in this group. Moreover, the levels of catalase and GST were changed considerably. These findings indicate that long-term obesity, besides disturbing the antioxidant control, causes intense stromal remodelling and release of factors that create an environment that can promote proliferative disorders in the gland, culminating with diffuse hyperplasia.
Keywords: androgen receptor, hyperplasia, MMP-9, obesity, prostate, stromal remodelling
Obesity is a condition resulting from excessive accumulation of adipose tissue in the body, which is correlated to a potential harm to health (Kopelman 2000). The latest study conducted between 2008 and 2009 by the Ministry of Health shows that in Brazil, 35% of the population is overweight, and 16% is obese (IBGE, 2014). Excessive consumption of saturated fat is the main factor responsible for the incidence of cardiovascular disease and also for some types of cancer (Deroo & Korach, 2006). Several metabolic changes are closely associated with obesity, affecting the functioning of biological systems as a whole, including hypertension, glucose intolerance, insulin resistance and type 2 diabetes (de Santana et al., 2008).
A high-fat diet has been identified as one of the factors involved in the onset of prostate cancer and benign prostate hyperplasia (BPH), which has been considered a new metabolic disease (Corona et al. 2014; Vignozzi & Maggi 2014; Vignozzi et al. 2014). Not only the amount of fat but also the quality of fat appears to be related to prostate carcinogenesis. Various population studies have indicated an increased prostate cancer risk with high intake of saturated fat (Crowe et al. 2008; Pelser et al. 2013). Moreover, chronic inflammation (a main characteristic of obese individual) has emerged as an important risk factor for BPH and cancer development (Fibbi et al. 2010). Other metabolic disorders related to metabolism also are implicated in prostate changes. Recent studies show that obesity and hyperinsulinaemia is strongly associated with increased prostate volume and prostatic hyperplasia (Vikram et al. 2011). A recent investigation showed that metabolic syndrome induced by high-fat diet in rabbits causes prostate fibrosis and inflammation (Vignozzi et al. 2012). Furthermore, Ribeiro et al. (2012b) demonstrated that diet-induced obesity causes increased cell proliferation and modifies signalling pathways such as PI3K and the oestrogen receptor in the rat prostate. Although the negative effects of high-fat diets have recently been described for different systems, including the prostate, little is known about the molecular and morphological mechanisms that are modified by obesity culminating in the establishment of proliferative disorders in the prostate.
Some elements of the extracellular matrix such as collagen, glycosaminoglycans and metalloproteinases (MMPs) are important modulators of tissue homeostasis. Imbalance in these components may stimulate cell proliferation, migration, angiogenesis and malignant development in various tissues including the prostate (Bruni-Cardoso et al. 2010). The effect of hormonal and metabolic changes in the prostate stromal composition of rodents and humans is well described in the literature (Tuxhorn et al. 2002; Vilamaior et al. 2006; Ribeiro et al. 2008, 2009). Vignozzi et al. (2013) demonstrated that metabolic disorders such as dislipidaemia and hyperinsulinaemia and the presence of advanced glycated end-products can stimulate secretion on stromal cells. However, information regarding the effects of obesity on extracellular matrix deposition and/or remodelling is scarce, especially in relationship to long-term obesity. Therefore studies exploring the effects of obesity on the reorganization of the extracellular matrix that underlie the putative alterations in cell proliferation, differentiation, migration and subsequent malignant progression are necessary and could improve the prevention strategies for prostate cancer because this relationship has been increasingly discussed in epidemiological studies.
Material and methods
Experimental design
Twenty adult male Wistar rats (12 weeks old) were purchased from the Bioterism Center of the Campinas State University (UNICAMP, Campinas, Brazil). They were kept under controlled conditions of light and temperature and received rations and water ad libitum. Experimental procedures were in accordance with the guidelines of the Commission on Ethics in Animal Experiments (CEEA/UNESP 2008–005369). Obesity was induced by a high-fat diet (20% protein, 37% carbohydrate, 20% fat) for 30 weeks. Control rats were treated with a balanced diet (22% protein, 48% carbohydrate, 4% fat) for a similar period. The high-fat and balanced diets were standardized by the Laboratory of Experimental Clinical Medicine, Faculty of Medicine of Botucatu (UNESP) and purchased from Agroceres (Rio Claro, SP, Brazil) (Lima-Leopoldo et al. 2011;. According to this protocol, a period of 14 weeks is necessary for obesity induction in rats fed with this diet. However, the purpose of this experiment was to verify the effects of long-term exposure to obesity after 30 weeks of feeding. Control and obese rats were euthanized by CO2 inhalation followed by decapitation. Body weight and blood glucose levels were monitored during the experimental period. Epididymal and retroperitoneal fat deposits were removed and weighed after decapitation. The ventral lobe of the prostate was removed, weighed and then fixed by soaking in Methacarn solution (methanol, acetic acid and chloroform, 6:3:1) and prepared for Paraplast embedding. Sections were stained by haematoxylin-eosin (HE) and picrosirius-haematoxylin for collagen evaluation.
Serum hormone analysis
Blood samples were collected by cardiac puncture immediately after CO2 inhalation, and this procedure was followed by decapitation. Plasma was separated by centrifugation and stored at −20°C for subsequent assays. Quantification of testosterone (T) and estradiol was performed using the Modular Analyzer for Immunoassay of Chemiluminescence ECI (Johnson and Johnson, Langhorne, PA, USA) (Weeks et al., 1986). Ten animals were used from each group, and the test was performed in triplicate. The intra- and interassay variations were 4.6% and 4.3% respectively.
Immunohistochemistry
Immunohistochemical tests were performed in the prostate to evaluate the presence and distribution of smooth muscle cells (smooth muscle cell α-actin), fibroblasts (vimentin), chondroitin sulphate, metalloproteinases (MMP-2 and MMP-9) and cellular proliferation (proliferating cell nuclear antigen PCNA).
Briefly, paraffin sections were blocked for non-specific protein interactions through Background Sniper blocking reagent (Biocare Medical, Concord, MA, USA) for 10 min. Subsequently, the endogenous peroxidase was blocked by 3% H2O2 in methanol for 30 min. The primary antibodies mouse anti-CS56 (Sigma, St Louis, MO, USA), mouse anti-MMP-2 (sc13595; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-MMP-9 (ab75375; Abcam, Cambridge, UK), mouse anti-PCNA (sc-59; Santa Cruz Biotechnology), mouse anti-α actin (sc-32251, Santa Cruz Biotechnology), mouse anti-vimentin (sc-6260; Santa Cruz Biotechnology) were incubated on the slides at 4°C overnight at 1:100 dilution in 1% BSA. The reaction was revealed by the polymer LSAB (Dako, Glostrup Denmark) conjugated with horseradish peroxidase for 45 min at room temperature. Then, the peroxidase–antibody link was visualized with diaminobenzidine, and the incubation time and concentration were specific for each antibody.
A minimum of 50 images per group was quantified using a 40× objective lens to determine the frequency of each stromal component labelled by immunohistochemistry and also the collagen staining. For these measurements, 10 representative images of α-actin, vimentin and MMPs immunohistochemistry and Picrosirius-haematoxylin staining were captured per animal (n = 5) of each experimental group, totalling 50 sampled images/group. Quantification was performed with enhanced contrast and the threshold limit feature of the image j software (National Institute of Health, USA). Thus, the integrated density of pixels was systematically measured as a percentage of labelled area.
For proliferation analysis, PCNA-positive and PCNA-negative epithelial cells were counted in each microscopic field using a 40× objective lens. At least 3000 cells were counted in each group using five animals per group and 10 images per prostate section. The mean frequency (%) of proliferative cells is represented in relation to total counted cells.
Western blotting
Prostate samples were homogenized at 4°C in cell lysis buffer (20 mm Tris-HCl, 150 mm NaCl, 1% Triton 6100, 2% sodium dodecyl sulphate [SDS]) containing 100 mm phenylmethanesulfonyl fluoride, 100 mm sodium orthovanadate and a protease inhibitor cocktail (1:1000, Sigma). Lysates were centrifuged at 13 000 × g at 4°C for 15 minutes, and the supernatants were collected. Protein amount was quantified by Bradford assay reagent (Bio-Rad, Hercules, CA, USA). Equal amounts of protein (100 mg) were separated by SDS–polyacrylamide gel electrophoresis on 10% polyacrylamide Tris-glycine gels and electroblotted onto nitrocellulose membranes. Non-specific sites were blocked with 5% nonfat dry milk in TBS with 1% Tween for 1 h at room temperature and then probed with the primary antibodies (Santa Cruz Biotechnology, Palo alto, USA): rabbit anti-AR – 1:400 (sc-816); mouse anti- VEGF (sc-7269); rabbit anti-ER α (sc-542); rabbit anti-ERβ (sc-8974); mouse anti-β-actin – 1:500) overnight at 4°C. Membranes were subsequently incubated with a specific secondary horseradish peroxidase–conjugate antibody for 1 h at room temperature. Detection of antigen-bound antibody was carried out with ECL Prime Chemiluminescent Reagent (RPN2232SK; GE Healthcare, Cleveland, OH, USA) followed by film exposure. As internal control, each membrane was stripped of the first antibody and reprobed with β-actin. Quantification of bands was carried out by densitometry with the mentioned Image J software, and the protein values were normalized with β-actin one.
MMP activity in Gelatin Zymography
Five ventral prostates per group were homogenized in the same buffer described for Western blotting analysis. The homogenates were incubated for 2 h at 4°C and centrifuged at 2000 g for 15 min at 4°C. The supernatants were reserved, and the pellets were suspended once again in the same solution, heated to 60°C for 5 min and centrifuged at 2000 g for 15 min at 4°C. The protein content in the pool of the supernatants was quantified by the Bradford method (Bradford, 1976). Twenty micrograms of protein from each sample was electrophoresed on 10% SDS polyacrylamide gel containing 0.1% gelatin (used as the substrate) at 4°C under non-reducing conditions. After electrophoresis, the gel was washed twice and then gently shaken in 2.5% Triton X-100 for 30 min at room temperature to remove the SDS. The gel was incubated overnight in a 50 mm Tris-HCl buffer pH 7.4, containing 10 nm ZnCl2 and 0.1 m NaCl, at 37°C. The gel was then stained with Coomassie brilliant blue R (0.5% dye in 20% methanol and 10% acetic acid) for 1 h. Unstained bands indicating gelatinolytic activity were seen after slight destaining with 30% ethanol and 10% acetic acid. Quantitative assessment of band intensity was carried out by densitometry, using the software image j. The activity determined for the control group was used as the reference (100%). The experiments were performed in triplicate.
Activity of antioxidant enzymes
The left ventral lobes were weighed and homogenized with 1:5 vol. of buffer (Tris-HCl, 1 mm EDTA, 1 mm DTT, 0.5 m sucrose, 0.15 m KCl, 1 mm PMSF, pH 7.4) and centrifuged at 10,000 g for 20 min at 4°C. The supernatant was centrifuged at 40,000 g for an additional 60 min at 20°C, and the supernatant fraction was collected and used for the measurement of catalase (CAT) and glutathione-S-transferase (GST). The total protein content in the samples was determined by the modified Lowry method, using bovine serum albumin as a standard (Peterson 1977). The CAT activity was quantified at 240 nm by the decomposition of 10 nm H2O2 according to the method of Beutler (1975). The GST activity was determined by measuring the increase in absorbance at 340 nm after incubating reduced glutathione (200 mm GSH) and 1-chloro-2,4-dinitrobenzene (200 mm CNDB) as substrates according to Keen et al. (1976). The specific molar extinction constant (ε) was used to estimate the levels of enzyme activity in U/mg protein (ε = 0.071 for CAT and ε = 9.6 for GST).
Determination of lipid peroxidation levels
The malondialdehyde (MDA) level, an indicator of free-radical generation that increases at the end of lipid peroxidation, was estimated using the double-heating method of Draper and Hadley (1990). For the MDA quantification in the ventral prostate, 100 ml of the resulting solution from the tissue homogenization in buffer (1:5 vol.) prepared as described above was used. Next, 300 ml of 0.4% thiobarbituric acid solution diluted in 0.2 m HCl was added to the samples and incubated for 60 min at 90°C in a dry block. Subsequently, the coloured derivative of the MDA–TBA complex was extracted with 1 ml of n-butanol followed by centrifugation at 3500 g and quantification at 532 nm. The MDA estimation was based on a standard calibration curve of tetramethoxypropane (TMP) previously prepared using the same procedure as used for samples. The data are expressed as nmol TBARs/g tissue.
Statistical analysis
Numerical data were tested for normal distribution using Shapiro–Wilk test. Differences between groups were assessed with unpaired two-sided Student's t-test were used for comparison of means of normally distributed parameters. In all other cases, Mann–Whitney's U-test was used for comparisons between groups. Significance was considered when P < 0.05.
Results
Long-term obesity increases glucose and insulin levels, decreases testosterone and changes prostate volume
Long-term treatment with a high-fat diet promoted a body weight gain of 39% in rats (Table1). An increase of 60% was also noted in fat deposit which is represented by epididymal and retroperitoneal fat weight (Table1). These parameters characterize the obesity context in the group treated with a high-fat diet. The absolute weight of ventral prostate increased by 45% (Table1). Analysis of blood glucose showed a slight but significant raise in the obese group while serum insulin levels increased 85%, indicating insulin resistance in these animals (Table1). Regarding sex-steroid hormones, serum testosterone levels decreased significantly (over threefold) while estradiol levels did not change in animals fed a high-fat diet.
Table 1.
Biometric and hormonal parameters (mean ± standard error) in control and obese rats
| Control | Obese | |
|---|---|---|
| Body weight (g) | 375 ± 28 | 521 ± 16* |
| Fat deposits (mg) | 14.2 ± 1.4 | 22.7 ± 3.6* |
| Prostate weight (mg) | 371 ± 36 | 688 ± 97* |
| Insulin (ng/ml) | 1.3 ± 0.2 | 2.4 ± 0.4* |
| Glucose (mg/dl) | 103 ± 3.5 | 164 ± 22* |
| Testosterone (ng/dl) | 242 ± 41 | 71 ± 9.8* |
| Estradiol (pg/dl) | 15 ± 4 | 7 ± 0.9 |
Significant difference between groups (Student's t-test, P < 0.05).
Prostate histological alterations in obese rats
The prostate of control animals exhibited acini with typical characteristics and high epithelial folds in the distal region of the gland. The obese group showed varied morphological changes in the ventral prostate: some areas showed intense epithelial atrophy, and others, mainly closest to the distal region, had notable epithelial hyperplasia (Figure1a and b). Figure1 (c) shows the acinar epithelial hyperplasia, in which a large number of cells are observed compared with the control group. Furthermore, frequent foci of prostatic intra-epithelial neoplasia were noted (Figure1d) in these obese animals. In the stromal region, the prostates of the obese group exhibited a larger number of cells and blood vessels compared to control prostates (Figure1e and f). The analysis of capillary density confirmed this result showing a significant increase of 75% in obese rats (control, 2.5 ± 0.2 vs. obese, 4.3 ± 0.4 blood vessels/microscopic field, P = 0.00001). Furthermore, there were a greater number of collagen fibres in the prostates of the obese group, mainly in the peritubular and interacinar stroma (Figure2a, b, g).
Figure 1.
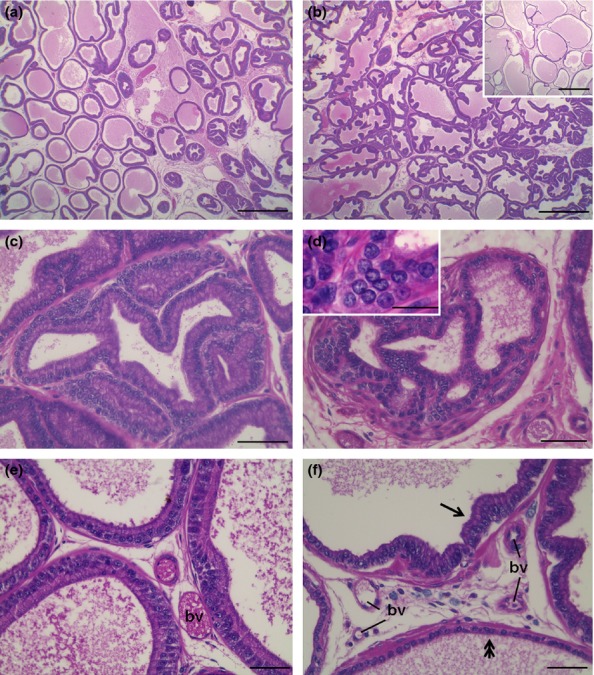
Morphological analysis in haematoxylin-eosin of the ventral prostate of control (a, e) and obese rats (b, c, d, f). (a) The prostate of control rats shows wide acini in the intermediate portion and folded epithelium in distal parts of the gland. (b) Prostatic epithelial hyperplasia was the most common find in the prostate of obese rats. However, some portions in the intermediary acini exhibited intense atrophy (inset). (c) Detail of epithelial hyperplasia. (d) Prostatic intra-epithelial neoplasia exhibiting atypical pleomorphic nuclei (inset). (e) Interacinar region of the prostate in the control group showing epithelium with typical secretory cells and scarce stromal materials. (f) In obese group, the interacinar region presents greater cell density and blood vessels (bv) and the epithelium show both hyperplasic (arrow) and atrophic (double arrow) changes. Scale bars: a, b: 50 μm; c–f: 200 μm.
Figure 2.
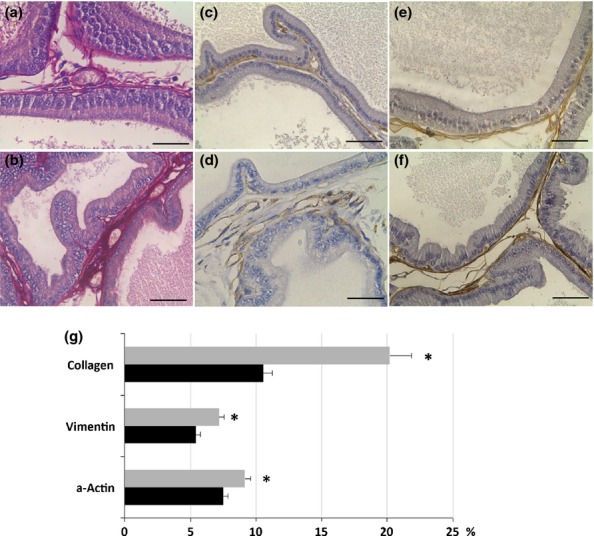
Distribution of collagen (a, b), vimentin (c, d) and α-actin (e, f) in the ventral prostate of control (a, c, e) and obese rats (b, d, f). (g) Relative frequency (%) of these parameters in the ventral prostate of control (black bars) and obese rats (grey bars). There is a notable increase in collagen, fibroblasts (vimentin), smooth muscle cells (α-actin) in the prostate of animals treated with a high-fat diet. The values are presented as Mean ± standard deviation. Student's t-test *P < 0.05. Scale bars: 50 μm
Prostate changes in obese rats is associated with increased cell number and MMP frequency
The stromal cellularity observed in HE analysis was confirmed with immunohistochemistry where there was a significant increase of 32% in the distribution of fibroblasts (vimentin immunostain) (Figure2c, d, g) and of 21% in the smooth muscle cells (alpha smooth muscle actin immunostain) in obese rats (Figure2e, f, g). The presence of myofibroblasts was evaluated by double immunostain using vimentin and alpha smooth muscle actin antibodies, but those cells were not found in the prostate of either control or obese rats. The relative distribution of the reaction for MMPs in the prostatic stroma of the obese group presented a 43% increase for MMP2 and a threefold increase for MMP9 (Fig.3a–d, g). The distribution of both elements was quite diffuse in the prostate in both groups, varying by the largest amount in the obese group. The frequency of areas exhibiting chondroitin sulphate (CS) increased by 147% in the prostates of obese rats (Figure3e, f, g). CS distribution was predominantly in the subepithelial region of acini in the control group, but it was more diffuse and dense in the obese group. Regarding cell proliferation, the immunohistochemistry revealed interesting data. Although the number of PCNA-positive cells had not changed, the percentage of these proliferating cells was reduced due to the drastic increase in the total number of cells counted in each microscopic field found in ventral prostate of the obese group (Table2).
Figure 3.
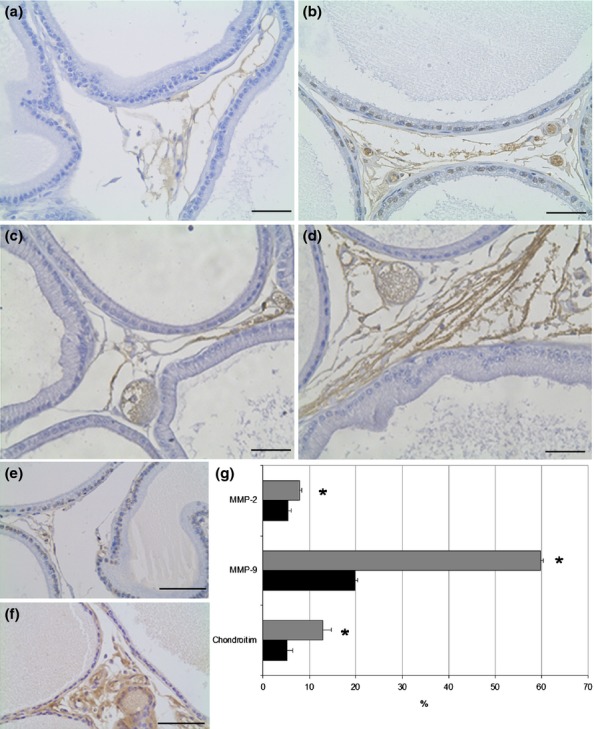
Distribution of MMP-2 (a, b), MMP-9 (c, d) and chondroitin sulphate (e, f) in the ventral prostate of control (a, c, e) and obese rats (b, d, f). The distribution of MMP-9 is higher than MMP-2 in the rat ventral prostate and obesity increases the amount of these enzymes in the region between acini (b, d). The chondroitin sulphate is increased in obese rats and shows subepithelial localization besides the interacinar distribution. Scale Bars: 50 μm. (g) Relative frequency (%) of immunohistochemistry for MMPs and chondroitin sulphate in the ventral prostate of control (black bars) and obese rats (grey bars). Student's t-test *P < 0.05.
Table 2.
Relative frequency (%) of PCNA-positive cells and total number of cells per microscopic field (mean ± standard error) in the ventral prostate of control and obese rats. (Student's t-test, P < 0.05)
| Cells | Control | Obese | P value |
|---|---|---|---|
| Total Cells | 156 ± 7.9 | 195 ± 11.1 | 0.0062 |
| PCNA-positive cells | 3.4 ± 0.3 | 3.1 ± 0.4 | 0.63 |
| % PCNA-positive cells | 2.4 ± 0.2 | 1.6 ± 0.1 | 0.043 |
High-fat treatment increases MMP-9 activity in the ventral prostate
The zymography of prostate extracts in control and obese groups showed bands with gelatinolytic activity at 64, 72 and 90 kDa, corresponding to the intermediate and pro-MMP-2, and active MMP-9 respectively (Figure4). The analysis of the band density showed that MMP-2 did not change in obese animals, but that there was a 100% increase in MMP-9 compared to the control group (Figure4).
Figure 4.
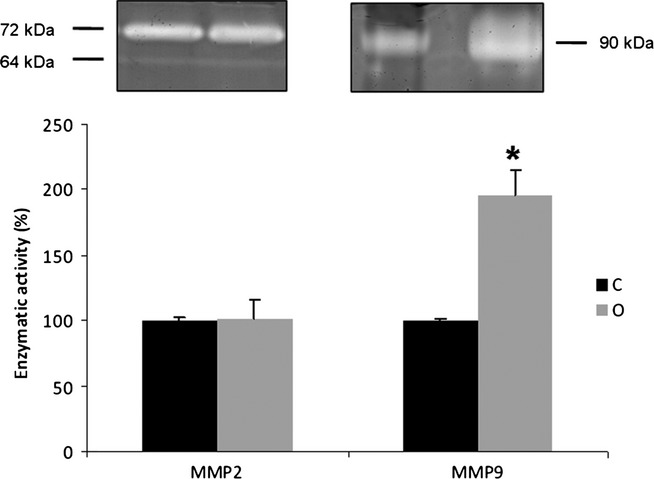
Zymography gel of ventral prostate extracts (n = 4) for control (C) and obese rats (O). The gelatinase activity was detected in bands with molecular weight of 64 and 72 kDa for the intermediate- and pro-forms of MMP-2, respectively, and 90 kDa for the active form of MMP-9. There was no change in the content of MMP-2, but we noted a sharp increase of MMP-9. The values represent the densitometry analysis of the digested bands. Mann–Whitney's U-test, *P < 0.05
Increased AR and VEGF and decreased ERs prostatic content caused by obesity
Obesity caused a 125% increase in the AR and 53% in the VEGF content in the ventral prostate (Figure5). In relation to oestrogen receptors, ERα reduced while ERβ did not change in rats treated with the high- fat diet over the long term (Figure5).
Figure 5.
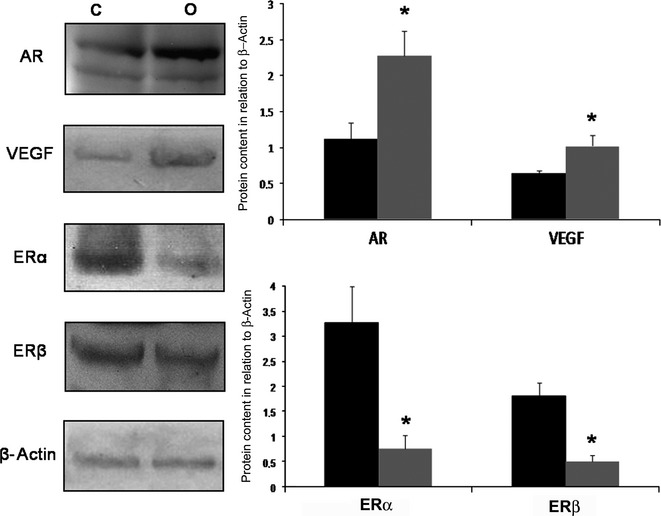
Western Blotting of ventral prostate of control (C) and obese rats (O) for AR, VEGF, ERα and ERβ. Obesity caused a significant increase in the prostatic content of AR and VEGF. However, the levels of ERα and ERβ in the prostate drastically reduced after a high-fat diet. Black bars- control group; Grey bars- Obese group. Graphs represent densitometry analysis of Western blotting where bands were normalized with β-actin. Mann–Whitney's U-test, *P < 0.05. Five animals per group were used for each electrophoresis and blotting analysis.
High-fat diet decreases Catalase activity and increases MDA content in the rat prostate
The enzymatic activity of catalase decreased by 38% in the prostates of the obese group. Moreover, the GST activity increased by about threefold in those animals when compared to the control group (Figure6). Concerning MDA levels, there was an increment of 21% on the prostate of obese rats (Figure6).
Figure 6.
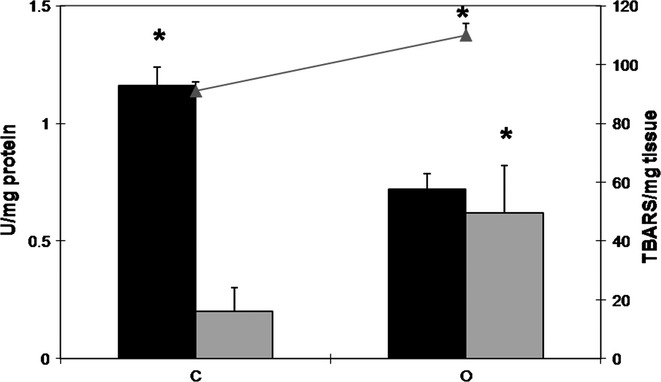
Activity of catalase (black bars) and GST (grey bars) (U/mg protein; black bars) and MDA levels (grey line- TBARS/mg tissue) in the ventral prostate extracts of control (C) and obese (O) rats. The values are presented as mean ± standard deviation. Mann–Whitney's U-test, *P < 0.05. Five animals per group were used for each biomarker of oxidative stress.
Discussion
Long-term obesity in rats causes epididymal and retroperitoneal fat accumulation (central obesity), leading to the dramatic increase in body weight observed in this investigation. Blood glucose and serum insulin levels were higher in the obese rats, which are strongly related to insulin resistance (IR). In a previous study, where the high-fat diet was administered for 15 weeks, we demonstrated the establishment of IR, but glucose levels did not change significantly as occurred in the present study (Ribeiro et al. 2012a). Thus, the long-term high-fat diet causes a lower tissue sensitization to insulin, which leads to blood glucose elevation, showing that longer periods of diet are needed for the most striking glycaemic changes.
Obesity and its associated metabolic scenario triggered different responses in the ventral prostate which exhibited some acini with epithelial atrophy and others showing marked hyperplasia. Furthermore, although a reduction in cell proliferation was noted, the density of epithelial cells was much higher in the obese prostate. This atrophic change may be an effect of exposure to low testosterone concentrations caused by obesity, because prostate epithelial cells are androgen dependent and, in its absence, the gland atrophies and undergoes apoptosis (Morrissey et al. 2002; Góes et al. 2007; Ribeiro et al. 2006). On the other hand, the hyperplasia noted in parts of the gland may be due to cell proliferation that occurs with prolonged exposure to obesity in response to hyperinsulinaemia, in addition to AR overexpression. Indeed, diet-induced obesity for shorter periods increases drastically cell proliferation in ventral prostate of rats (Ribeiro et al. 2012b). These results are in accordance with Vignozzi et al. (2012) which demonstrated low circulating T associated with increased expression of AR in the prostate of rabbits in a model of metabolic syndrome induced by 4% high-fat diet. Our data indicate that after long-term obesity, cells habituate a high-glucose, high-insulin and low-T environment, leading to a reduction in cell proliferation, but the hyperplasia was already established due to high proliferative activity in the early stages of obesity.
Oestrogen receptors can also stimulate proliferation in the prostate, where ERα is involved in gland carcinogenesis while ERβ acts as protector of the prostatic epithelium acting as a tumour suppressor (Hartman et al. 2012). Studies from Prins et al. (2001) showed that the prostate of ER-/-βERKO mice develops hyperplasia and neoplasia. In this investigation we observed reduced levels of ERα while ERβ did not change in the obese group. Although we have not found malignant lesions which could be a consequence of decreased ERα expression, a previous study from our group (Ribeiro et al. 2012b) found that 15 weeks of obesity did not change ERα expression but increased ERβ in ventral prostate. Thus, it can be suggested that ERβ could be gradually decreasing along the duration of obesity from 15 to 30 weeks, requiring longer period of exposure to lose its protective role. This finding, associated with increased AR content and hyperplasia, may be an important factor in establishment of prostate pathologies in obese individuals after longer exposures. This knowledge may be important for the understanding of the relationship between obesity and prostate cancer.
Benign prostatic hyperplasia is a disease frequently found in elderly males and is characterized by a higher content of cells and stromal elements, which causes compression of the urethra and inconvenience to the patient (Shapiro et al. 1992). Obesity promoted an increase in the number of fibroblast, smooth muscle cells and collagen fibres in the rat prostatic stroma, which could imply a rigidity of the gland favouring the establishment of benign prostatic hyperplasia. The excess of fibroblasts could be responsible for the elevation of extracellular matrix deposition, because they are primarily responsible for the synthetic activity in the connective tissues. This research also showed a significant increase in the distribution of chondroitin sulphate in the prostate of obese animals. The same finding was observed in the prostate of diabetic and castrated animals in which there is an androgen deficiency and hyperglycaemia, as well as in rabbits fed with high-fat diet (Vignozzi et al. 2012; Morrissey et al. 2002; Ribeiro et al. 2009). CS has quite diverse functions in tissues such as storing growth factors, and stimulation of proliferation, differentiation and cell migration (Zimmermann et al. 1994; Romberger 1997). It has been found that in benign hyperplasia and prostate cancer, there is a fourfold increase in levels of CS (Rowley 1999; Goulas et al. 2000; Tuxhorn et al. 2002). Therefore, this investigation shows for the first time the impact of testosterone imbalance arising from obesity and glucose levels in this prostate glycosaminoglycan which can create a microenvironment that favours cells growth and neovascularization, promoting the establishment of proliferative disorders.
The largest amount and activity of MMPs associated with collagen and CS deposition is related with the stromal remodelling processes that may occur in the prostate of obese animals. Also, it draws attention to the increase in VEGF and blood vessels in the prostate after a high-fat diet. VEGF is an important factor in angiogenesis because it enhances vascular permeability and promotes new vessel growth (Mott & Werb 2004). The MMPs comprise a family of enzymes that degrade the extracellular matrix and play a crucial role in the physiological cellular processes (Bruni-Cardoso et al. 2008; Gill & Parks 2008). The proteolytic cleavage of the extracellular matrix components plays an important role in the malignant development, not only as a result of stromal remodelling, but also by the release of growth factors which stimulate angiogenesis, migration and cell proliferation (Xu et al. 2001; Kim et al. 2011). An example is the increased expression of MMP-2 and MMP-9 and angiogenesis in various tumours (Curran & Murray 1999; Bergers et al. 2000). Thus, it is likely that the large deposits of collagen and CS provide elements whose cleavage by MMPs releases several growth factors, including VEGF, which act in prostate stimulating the secretion of extracellular matrix elements, proliferation and angiogenesis, and also contributing to the glandular hyperplasia observed in obese individuals.
Oxidative stress is a consequence of aerobic life and the extent of damage caused by reactive oxygen species (ROS) is controlled by efficient enzymes of antioxidant defence (Cooke et al., 2003). Increasing evidence shows that the cumulative production of ROS by endogenous or exogenous insults plays a role in ageing-related diseases and cancer (Scott & King 2004; Arsova-Sarafinovska et al. 2009). The reduction in the catalase activity shows an impairment of the control of oxidative stress in the prostate of obese rats. On the other hand, increased levels of total GST activity indicate a greater action of this enzyme in response to high levels of ROS, as already described in diabetic mice prostate (Gobbo et al. 2012). Also, the excess fat caused by obesity stimulates lipid peroxidation increasing the formation of MDA in the prostate. Prostate cancer has been increasingly associated with obesity, and epidemiological studies show that obese individuals have more aggressive tumours and a greater risk of death than lean ones (Stewart & Freedland 2011; Rundle et al. 2013). However, studies on the mechanisms that trigger the gland carcinogenesis are still preliminary and need to be better elucidated. Thus, this investigation demonstrated that obesity affects the enzymes that control oxidative stress in rat prostate, which may be associated with the carcinogenesis process.
Previous investigations (Ribeiro et al. 2012a,b) showed that obesity in the short term causes epithelial changes that are more significant, but the present study proposes that after long-term high-fat diet, the stromal compartment is the most affected. It is evident that longer period of exposure to the obesogenic environment is necessary for the establishment of changes in the extracellular matrix. We propose that obesity causes glandular hyperplasia, angiogenesis associated with deposition and remodelling of the ECM proteins. Also, there was an imbalance in the sex-steroid receptors and in the enzymes of oxidative stress control caused by long-term high-fat diet. Therefore, we believe that the obesogenic environment, besides interfering with the reproductive function of the gland, also creates a stromal environment conducive to the development of proliferative disorders of the prostate, corroborating epidemiological studies linking obesity with increased incidence of prostate cancer.
Acknowledgments
The authors are grateful for the technical assistance of Mr Fabrício Faria Araújo, Mrs Ester Cristina Borges Araújo and the Laboratory of Microscopy and MicroAnalysis of São Paulo State University.
Conflict of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Arsova-Sarafinovska Z, Eken A, Matevska N, et al. Increased oxidative/nitrosative stress and decreased antioxidant enzyme activities in prostate cancer. Clin. Biochem. 2009;42:1228–1235. doi: 10.1016/j.clinbiochem.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Bergers G, Brekken R. McMahon G. Matrix metalloproteinase- 9 triggers the angiogenic switch during carcinogenesis. Nat. Cell. Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler E. Red Cell Metabolism: a Manual of Biochemical Methods. 3rd edn. New York: Grune and Straton; 1975. p. 342. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bruni-Cardoso A, Vilamaior PS, Tabga SR. Carvalho HF. Localized matrix metalloproteinase (MMP)-2 and MMP-9 activity in the rat ventral prostate during the first week of postnatal development. Histochem. Cell Biol. 2008;129:805–815. doi: 10.1007/s00418-008-0407-x. [DOI] [PubMed] [Google Scholar]
- Bruni-Cardoso A, Lynch CC, Rosa-Ribeiro R, Matrisian LM. Carvalho HF. MMP-2 contributes to the development of the mouse ventral prostate by impacting epithelial growth and morphogenesis. Dev. Dyn. 2010;239:2386–2392. doi: 10.1002/dvdy.22382. [DOI] [PubMed] [Google Scholar]
- Cooke MS, Evans MD, Dizdaroglu M. Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 2003;17:1195–1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- Corona G, Vignozzi L, Rastrelli G, Lotti F, Cipriani S. Maggi M. Benign prostatic hyperplasia: a new metabolic disease of the aging male and its correlation with sexual dysfunctions. Int. J. Endocrinol. 2014;2014:329456. doi: 10.1155/2014/329456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe FL, Key TJ, Appleby PN, Travis RC, Overvad K, Jakobsen MU, Johnsen NF, Tjønneland A, Linseisen J, Rohrmann S, Boeing H, Pischon T, Trichopoulou A, Lagiou P, Trichopoulos D, Sacerdote C, Palli D, Tumino R, Krogh V, Bueno-de-Mesquita HB, Kiemeney LA, Chirlaque MD, Ardanaz E, Sánchez MJ, Larrañaga N, González CA, Quirós JR, Manjer J, Wirfält E, Stattin P, Hallmans G, Khaw KT, Bingham S, Ferrari P, Slimani N, Jenab M, Riboli E. Dietary fat intake and risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2008;87(5):1405–13. doi: 10.1093/ajcn/87.5.1405. [DOI] [PubMed] [Google Scholar]
- Curran S. Murray GI. Matrix metalloproteinases in tumour invasion and metastasis. J. Pathol. 1999;189:300–308. doi: 10.1002/(SICI)1096-9896(199911)189:3<300::AID-PATH456>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Deroo BJ. Korach KS. Estrogen receptors and human disease. J. Clin. Invest. 2006;116:561–570. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper HH. Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- Fibbi B, Penna G, Morelli A, Adorini L. Maggi M. Chronic inflammation in the pathogenesis of benign prostatic hyperplasia. Int. J. Androl. 2010;33:475–488. doi: 10.1111/j.1365-2605.2009.00972.x. [DOI] [PubMed] [Google Scholar]
- Gill SE. Parks WC. Metalloproteinases and their inhibitors: regulators of wound healing. Int. J. Biochem. Cell Biol. 2008;40:1334–1347. doi: 10.1016/j.biocel.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbo MG, Ribeiro DL, Taboga SR, de Almeida EA. Góes RM. Oxidative stress markers and apoptosis in the prostate of diabetic rats and the influence of vitamin C treatment. J. Cell. Biochem. 2012;113:2223–2233. doi: 10.1002/jcb.24092. [DOI] [PubMed] [Google Scholar]
- Góes RM, Zanetoni C, Tomiosso TC, Ribeiro DL. Taboga SR. Surgical and chemical castration induce differential histological response in prostate lobes of Mongolian gerbil. Micron. 2007;38:231–236. doi: 10.1016/j.micron.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Goulas A, Hatzichristou DG, Karakiulakis G, Mirtsou-Fidani V, Kalinderis A. Papakonstantinou E. Benign hyperplasia of the human prostate is associated with tissue enrichment in chondroitin sulphate of wide size distribution. Prostate. 2000;44:104–110. doi: 10.1002/1097-0045(20000701)44:2<104::aid-pros2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Hartman J, Ström A. Gustafsson JÅ. Current concepts and significance of estrogen receptor β in prostate cancer. Steroids. 2012;77:1262–1266. doi: 10.1016/j.steroids.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Keen JH, Habig WH. Jakoby WB. Mechanism for several activities of the glutathione S-transferases. J. Biol. Chem. 1976;251:6183–6188. [PubMed] [Google Scholar]
- Kim SH, Turnbull J. Guimond S. Extracellular matrix and cell signaling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J. Endocrinol. 2011;209:139–151. doi: 10.1530/JOE-10-0377. [DOI] [PubMed] [Google Scholar]
- Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- Lima-Leopoldo AP, Leopoldo AS, Sugizaki MM, et al. Myocardial dysfunction and abnormalities in intracellular calcium handling in obese rats. Arq. Bras. Cardiol. 2011;97:232–240. doi: 10.1590/s0066-782x2011005000061. [DOI] [PubMed] [Google Scholar]
- Morrissey C, Buser A, Scolaro J, O'Sullivan J, Moquin A. Tenniswood M. Changes in hormone sensitivity in the ventral prostate of aging sprague-dawley rats. J. Androl. 2002;23:341–351. [PubMed] [Google Scholar]
- Mott JD. Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr. Opin. Cell Biol. 2004;16:558–564. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelser C, Mondul AM, Hollenbeck AR. Park Y. Dietary fat, fatty acids, and risk of prostate cancer in the NIH-AARP diet and health study. Cancer Epidemiol. Biomarkers Prev. 2013;22:697–707. doi: 10.1158/1055-9965.EPI-12-1196-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson GL. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Prins GS, Birch L, Couse JF, Choi I, Katzenellenbogen B, Korach KS. Estrogen imprinting of the developing prostate gland is mediated through stromal estrogen receptor alpha: studies with alphaERKO and betaERKO mice. Cancer Res. 2001;61:6089–6097. [PubMed] [Google Scholar]
- Ribeiro DL, Caldeira EJ, Cândido EM, Manzato AJ, Taboga SR. Cagnon VH. Prostatic stromal microenvironment and experimental diabetes. Eur. J. Histochem. 2006;50:51–60. [PubMed] [Google Scholar]
- Ribeiro DL, Marques SF, Alberti S, et al. Malignant lesions in the ventral prostate of alloxan-induced diabetic rats. Int. J. Exp. Pathol. 2008;89:276–283. doi: 10.1111/j.1365-2613.2008.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro DL, Taboga SR. Góes RM. Diabetes induces stromal remodelling and increase in chondroitin sulphate proteoglycans of the rat ventral prostate. Int. J. Exp. Pathol. 2009;90:400–411. doi: 10.1111/j.1365-2613.2009.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro DL, Pinto ME, Maeda SY, Taboga SR. Góes RM. High fat-induced obesity associated with insulin resistance increases the FGF-2 content and causes stromal hyperplasia on the rat ventral prostate. Cell Tissue Res. 2012a;349:577–588. doi: 10.1007/s00441-012-1420-x. [DOI] [PubMed] [Google Scholar]
- Ribeiro DL, Pinto ME, Rafacho A, et al. High-fat diet obesity associated with insulin resistance increases cell proliferation, estrogen receptor, and PI3K proteins in rat ventral prostate. J. Androl. 2012b;33:854–865. doi: 10.2164/jandrol.111.016089. [DOI] [PubMed] [Google Scholar]
- Romberger DJ. Fibronectin. Int. J. Biochem. Cell Biol. 1997;29:939–943. doi: 10.1016/s1357-2725(96)00172-0. [DOI] [PubMed] [Google Scholar]
- Rowley DR. What might a stromal response mean to prostate cancer progression? Cancer Metastasis Rev. 1999;17:411–419. doi: 10.1023/a:1006129420005. [DOI] [PubMed] [Google Scholar]
- Rundle A, Jankowski M, Kryvenko ON, Tang D. Rybicki BA. Obesity and future prostate cancer risk among men after an initial benign biopsy of the prostate. Cancer Epidemiol. Biomarkers Rev. 2013;22:898–904. doi: 10.1158/1055-9965.EPI-12-0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Santana IA, Moura GS, Vieira NF, Cipolotti R. Metabolic syndrome in patients with prostate cancer. Sao Paulo Med J. 2008;126:274–278. doi: 10.1590/S1516-31802008000500006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JA. King GL. Oxidative stress and antioxidant treatment in diabetes. Ann. N. Y. Acad. Sci. 2004;1031:204–213. doi: 10.1196/annals.1331.020. [DOI] [PubMed] [Google Scholar]
- Shapiro E, Hartanto V. Lepor H. Quantifying the smooth muscle content of the prostate using double immunoenzymatic staining and color assisted image analysis. J. Urol. 1992;147:1167–1170. doi: 10.1016/s0022-5347(17)37508-0. [DOI] [PubMed] [Google Scholar]
- Stewart SB. Freedland SJ. Influence of obesity on the incidence and treatment of genitourinary malignancies. Urol. Oncol. 2011;29:476–486. doi: 10.1016/j.urolonc.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD. Rowley DR. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin. Cancer Res. 2002;8:2912–2923. [PubMed] [Google Scholar]
- Vignozzi L. Maggi M. Prostate cancer: intriguing data on inflammation and prostate cancer. Nat Rev Urol. 2014;11:369–370. doi: 10.1038/nrurol.2014.143. [DOI] [PubMed] [Google Scholar]
- Vignozzi L, Morelli A, Sarchielli E, et al. Testosterone protects from metabolic syndrome-associated prostate inflammation: an experimental study in rabbit. J. Endocrinol. 2012;212:71–84. doi: 10.1530/JOE-11-0289. [DOI] [PubMed] [Google Scholar]
- Vignozzi L, Gacci M, Cellai I, et al. Fat boosts, while androgen receptor activation counteracts, BPH-associated prostate inflammation. Prostate. 2013;73:789–800. doi: 10.1002/pros.22623. [DOI] [PubMed] [Google Scholar]
- Vignozzi L, Rastrelli G, Corona G, Gacci M, Forti G. Maggi M. Benign prostatic hyperplasia: a new metabolic disease? J. Endocrinol. Invest. 2014;37:313–322. doi: 10.1007/s40618-014-0051-3. [DOI] [PubMed] [Google Scholar]
- Vikram A, Kushwaha S. Jena GB. Relative influence of testosterone and insulin in the regulation of prostatic cell proliferation and growth. Steroids. 2011;76:416–423. doi: 10.1016/j.steroids.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Vilamaior PSL, Tabora SR. Carvalho HF. Postnatal growth of the ventral prostate in Wistar Rats: a stereological and morphometrical study. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2006;288:885–892. doi: 10.1002/ar.a.20363. [DOI] [PubMed] [Google Scholar]
- Weeks I, Sturgess ML. Woodhead JS. Chemiluminescence immunoassay: an overview. Clin. Sci. 1986;70:403–408. doi: 10.1042/cs0700403. [DOI] [PubMed] [Google Scholar]
- Xu P, Wang Y, Piao Y, et al. Effects of matrix proteins on the expression of matrix metalloproteinase-2, -9, and -14 and tissue inhibitors of metalloproteinases in human cytotrophoblast cells during the first trimester. Biol. Reprod. 2001;65:240–246. doi: 10.1095/biolreprod65.1.240. [DOI] [PubMed] [Google Scholar]
- Zimmermann DR, Dours-Zimmermann MT, Schubert M, Bruckner- Tuderman L. Versican is expressed in the proliferation zone in the epidermis and in association with the elastic network of the dermis. J. Cell Biol. 1994;124:817–825. doi: 10.1083/jcb.124.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]


