Key Clinical Message
Megalencephalic leukoencephalopathy (MLC) is a rare neurological disorder with an autosomal recessive pattern. Clinical diagnosis was based on macrocephaly, recurrent seizure, and magnetic resonance imaging (MRI). Here we report first finding of a novel homozygous single base deletion in the MLC1 gene in an affected Iranian child causing a premature stop codon (p.L150fs.160X).
Keywords: Iranian, leukodystrophy, MLC1 gene, novel mutation
Research Letter
Megalencephalic leukoencephalopathy (MLC) is a rare disorder with unknown frequency. This is the first report of molecular prenatal test for a novel mutation in the MLC1 gene from Iran.
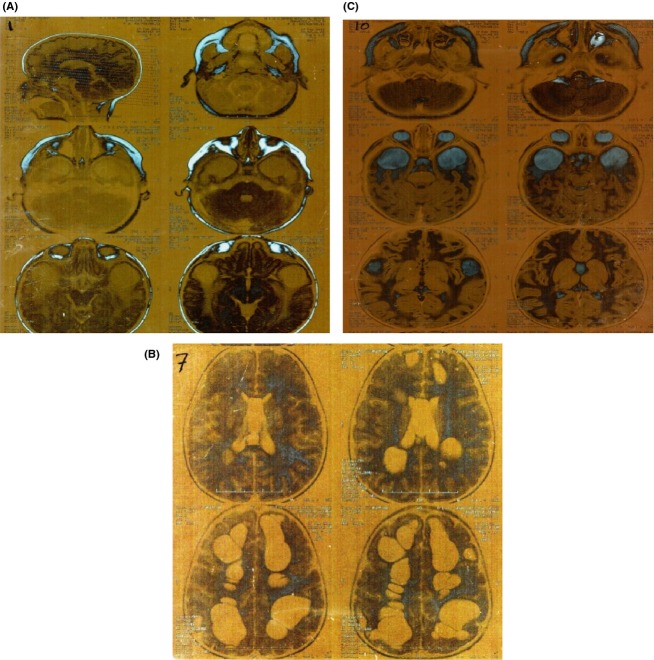
Van der Knaap et al. described first in 1995 a case of leukodystrophy with swelling and cysts. Their observations were based on clinical and neurobiological criteria 1. The reason for the swollen appearance of the white matter might be the myelin vacuolation affecting the outer myelin layers 2. However, macrocephaly and cerebral white matter abnormality is a specific neurological sign without gray matter involvement 2. Hereby, magnetic resonance imaging (MRI) can be useful tool for differential diagnosis 2. Brain MRI (without contrast) of our patient at age of 2.5 years old showed a diffuse myelination involving subcortical U fibers (Fig.1).
Figure 1.
Brain magnetic resonance imaging (MRI) was made for the patient at the age of 2.5 showing abnormal myelination in white matter.
Today, the disorder is defined as MLC with subcortical cysts associated with macrocephaly as a common sign 3. MLC is a very rare disorder with unknown frequency, although several ethic groups show more cases 3,4. Molecular genetics studies show that the mutation of two MLC1 and HEPACAM genes as being responsible for the disease.
Pathogenic mutations in the HEPACAM gene (hepatic and glial cell adhesion molecule, MIM 611642) account for ∼20% of individuals with improved MLC phenotype 5. In contrast to the MLC1 gene with exclusive autosomal recessive inheritance, some patients show monoallelic (heterozygous) HEPACAM mutation with dominant inheritance 5. These individuals have macrocephaly and mental retardation with or without autistic signs 5. Disease causing mutations in the MLC1 gene (MIM 605908) was found in ∼75% of classic MLC patients 6. MLC1 appear as an oligomeric membrane protein that is exclusively expressed in brain tissue 7,8, and localized in astrocytes junctions 7,9. Biologically, the MLC1 is postulated to be an ion transporter, however, its exact role is still unknown 7,10.
An Iranian family with a 3 years old affected child was referred to our laboratory for prenatal diagnosis of the next child, as yet unborn. Clinical diagnosis was based on macrocephaly and recurrent seizure. MRI of patient showed white matter dystrophy with several subcortical cysts. The mother was pregnant in the 6th week. With informed consent, genomic DNA from whole blood and chorionic villous sample was extracted with routine salting out method. PCR primers to amplify exons and flanking intron sequences were designed by Primer3out software program according to the gene accession number. Primer sequences and PCR condition are given in Table1. To exclude maternal cell contamination, standard VNTR (variable number tandem repeat) and STR (Short tandem repeat) markers were used for parent's samples and CVS (Chorionic villus sample). Bidirectional sequencing was performed with big dye terminator V3.1 cycle sequencing kit using an Applied Biosystem 3130 genetic analyzer (ABI Newyork USA).
Table 1.
Primer sequences of the coding exons and flanking intron sequences with appropriate PCR product length and annealing temperatures
| Exon | Sequence | Tm (C) | Size (bp) |
|---|---|---|---|
| X2-F | AAGTTGCCGATGGATGTTGT | 60.38 | 168 |
| X2-R | tttgaagaagaaaatgagcacttg | 59.93 | |
| X3-F | gtttcctcagagtggccaaa | 60.23 | 336 |
| X3-R | gtcaccagagggaccagatg | 60.53 | |
| X4-F | ctggaagcgcaaatgttaga | 59.06 | 199 |
| X4-R | acactgtctgtcagcccctc | 60.32 | |
| X5-F | gaatggcctgaagtgtggtt | 59.97 | 227 |
| X5-R | ctgtgggtgtcaggcgtc | 61.38 | |
| X6-F | gtccggtggacgctgaag | 62.89 | 229 |
| X6-R | cctggggtgatgcctctg | 62.71 | |
| X7-F | gcagtgctgagtccctgtg | 60.63 | 203 |
| X7-R | acgtgacgtttaatccagcc | 60.00 | |
| X8-F | ctccacttccttatgagccg | 59.83 | 251 |
| X8-R | tgaatgcaccaagactgagc | 59.99 | |
| X9-F | ttggaattcgacttcttcgac | 59.30 | 192 |
| X9-R | caccaagggagggctagg | 60.60 | |
| X10-F | aaaaggcagaggtttcagca | 59.99 | 324 |
| X10-R | agagcaccacatgtctggg | 59.68 | |
| X11-F | gagggagctttggtctcctg | 61.30 | 277 |
| X11-R | ccactcacctccccagtg | 60.08 | |
| X12-F | tggccctggtgaagtaacac | 60.95 | 457 |
| X12-R | TGAGAGAGGCAGGAAGAGGA | 60.21 |
Exon 1 is noncoding. The same primers were used for direct sequencing of PCR products.
As it has been illustrated in figure 2 a novel homozygous single base deletion at codon 150 was found in the affected child causing a premature stop codon (p.L150fs.160X). Consanguine parents were heterozygous for the mentioned change. To see whether this mutation is pathogenic, we checked this point mutation in 35 healthy controls. None of 35 normal genomes showed this mutation. After molecular confirmation of the disease, we performed molecular prenatal diagnosis in the 11th week of pregnancy on DNA extracted from CVS. The fetus was heterozygous and also a carrier for the novel deletion. We also advised the family to continue the pregnancy.
Figure 2.
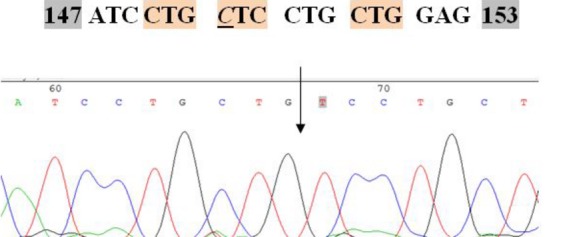
Partial sequence of the megalencephalic leukoencephalopathy 1 (MLC1) gene shows homozygous deletion of a cytosine at codon 150 that has been detected in affected child.
This is the first molecular diagnosis report of the MLC in southwest of Iran that extend the mutation spectrum of the MLC1 gene. Because of the involvement of second gene in the pathogenicity of MLC disorder and because of our finding, we suggest considering the MLC1 gene as the first choice for molecular screening of patients, at least for Iran and the Middle East. Systematic screening of high-risk pregnancies of some inherited disorders such as alpha and beta thalassemia have been done successfully for two decades in Iran. But other abnormalities are not followed, consequently. We showed here the feasibility of prenatal diagnosis of some difficult cases in relatively short time, like the present case.
Conflict of Interest
None declared.
References
- van der Knaap MS, Barth PG, Stroink H, van Nieuwenhuizen O, Arts WF, et al. Leukoencephalopathy with swelling and a discrepantly mild clinical course in eight children. Ann. Neurol. 1995;37:324–334. doi: 10.1002/ana.410370308. [PubMed: 7695231] [DOI] [PubMed] [Google Scholar]
- van der Knaap MS, Barth PG, Vrensen GF. Valk J. Histopathology of an infantile-onset spongiform leukoencephalopathy with a discrepantly mild clinical course. Acta Neuropathol. 1996;92:206–212. doi: 10.1007/s004010050510. [PubMed: 8841668] [DOI] [PubMed] [Google Scholar]
- Leegwater PA, Boor PK, Yuan BQ, van der Steen J, Visser A, Konst AA, et al. Identification of novel mutations in MLC1 responsible for megalencephalic leukoencephalopathy with subcortical cysts. Hum. Genet. 2002;110:279–283. doi: 10.1007/s00439-002-0682-x. PubMed: [11935341] [DOI] [PubMed] [Google Scholar]
- Montagna G, Teijido O, Eymard-Pierre E, Muraki K, Cohen B, Loizzo A, et al. Vacuolating megalencephalic leukoencephalopathy with subcortical cysts: functional studies of novel variants in MLC1. Hum. Mutat. 2006;27:292. doi: 10.1002/humu.9407. [PubMed: 16470554] [DOI] [PubMed] [Google Scholar]
- López-Hernández T, Ridder MC, Montolio M, Capdevila-Nortes X, Polder E, Sirisi S, et al. Mutant GlialCAM causes megalencephalic leukoencephalopathy with subcortical cysts, benign familial macrocephaly, and macrocephaly with retardation and autism. Am. J. Hum. Genet. 2011;88:422–432. doi: 10.1016/j.ajhg.2011.02.009. PMC free article: [PMC3071909] [PubMed: 21419380] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilja Boor PK, de Groot K, Mejaski-Bosnjak V, Brenner C, van der Knaap MS. Scheper GC, Pronk JC, et al. Megalencephalic leukoencephalopathy with subcortical cysts: an update and extended mutation analysis of MLC1. Hum. Mutat. 2006;27:505–512. doi: 10.1002/humu.20332. [PubMed: 16652334] [DOI] [PubMed] [Google Scholar]
- Teijido O, Martínez A, Pusch M, Zorzano A, Soriano E, Del Río JA, et al. Localization and functional analyses of the MLC1 protein involved in megalencephalic leukoencephalopathy with subcortical cysts. Hum. Mol. Genet. 2004;13:2581–2594. doi: 10.1093/hmg/ddh291. [PubMed: 15367490] [DOI] [PubMed] [Google Scholar]
- Boor PKI, de Groot K, Waisfisz Q, Kamphorst W, Oudejans CB, Powers JM, et al. MLC1: a novel protein in distal astroglial processes. J. Neuropathol. Exp. Neurol. 2005;64:412–419. doi: 10.1093/jnen/64.5.412. [PubMed: 15892299] [DOI] [PubMed] [Google Scholar]
- Teijido O, Casaroli-Marano R, Kharkovets T, Aguado F, Zorzano A, Palacín M, et al. Expression patterns of MLC1 protein in the central and peripheral nervous systems. Neurobiol. Dis. 2007;26:532–545. doi: 10.1016/j.nbd.2007.01.016. [PubMed: 17434314] [DOI] [PubMed] [Google Scholar]
- Kaganovich M, Peretz A, Ritsner M, Bening Abu-Shach U, Attali B, Navon R, et al. Is the WKL1 gene associated with schizophrenia? Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2004;125B:31–37. doi: 10.1002/ajmg.b.20115. [PubMed: 14755440] [DOI] [PubMed] [Google Scholar]