Abstract
Earlier we reported that low molecular weight (LMW) peptides accumulate in aging human lens tissue and that among the LMW peptides, the chaperone inhibitor peptide αA66-80, derived from α-crystallin protein, is one of the predominant peptides. We showed that in vitro αA66-80 induces protein aggregation. The current study was undertaken to determine whether LMW peptides are also present in guinea pig lens tissue subjected to hyperbaric oxygen (HBO) in vivo. The nuclear opacity induced by HBO in guinea pig lens is the closest animal model for studying age-related cataract formation in humans. A LMW peptide profile by mass spectrometry showed the presence of an increased amount of LMW peptides in HBO-treated guinea pig lenses compared to age-matched controls. Interestingly, the mass spectrometric data also showed that the chaperone inhibitor peptide αA66-80 accumulates in HBO-treated guinea pig lens. Following incubation of synthetic chaperone inhibitor peptide αA66-80 with α-crystallin from guinea pig lens extracts, we observed a decreased ability of α-crystallin to inhibit the amorphous aggregation of the target protein alcohol dehydrogenase and the formation of large light scattering aggregates, similar to those we have observed with human α-crystallin and αA66-80 peptide. Further, time-lapse recordings showed that a preformed complex of α-crystallin and αA66-80 attracted additional crystallin molecules to form even larger aggregates. These results demonstrate that LMW peptide–mediated cataract development in aged human lens and in HBO-induced lens opacity in the guinea pig may have common molecular pathways.
Keywords: Crystallin, Peptide, Guinea pigs, hyperbaric oxygen, Lens, Cataract, Aggregation, Chaperone
1. Introduction
The mammalian lens protein αA-crystallin is a 20 kDa protein capable of forming homo- or hetero-oligomers with αB-crystallin, also a 20 kDa protein. The hetero-oligomer α-crystallin exists as an aggregate of ~800 kDa and has both a structural and a refractive role in mammalian lens (Groenen et al., 1994). Both αA- and αB-crystallins belong to the family of small heat shock proteins (sHSP) and are classified into HSPB-4 and HSPB-5, respectively (Ingolia and Craig, 1982; Klemenz et al., 1991; Merck et al., 1993). Like other sHSPs, αA-crystallin functions like a molecular chaperone (Horwitz, 1992; Rao et al., 1995). The chaperone function of α-crystallin is believed to be important for the maintenance of lens transparency (Horwitz et al., 1992). α-Crystallin and other lens crystallin proteins are present at high concentrations in enucleated fiber cells. Because the lens shows little protein turnover, crystallins synthesized in fiber cells must survive for a lifetime (Bassnett, 1997, 2002, 2009). The long-living crystallins are continuously challenged by protein-modifying reactions from endogenous sources, such as deamidation (Takemoto and Boyle, 2000; Voorter et al., 1988), racemization and isomerization (Fujii et al., 1994), phosphorylation (Takemoto, 1996), glycation (Argirova and Breipohl, 2002), oxidation (Linetsky et al., 2008), and truncation (Srivastava, 1988), or from environmental sources, such as ultraviolet rays (Giblin et al., 2002; Meyer et al., 2008), cosmic rays and background radiation (Klein et al., 1993; Otake and Schull, 1990). Studies have shown that certain post-translational protein modifications are associated with impaired biological function (Takemoto and Boyle, 2000) and that structurally and functionally impaired proteins are preferential targets for protease degradation (Fleshman and Wagner, 1984; Shang et al., 1994). However, altered protease activities and peptides that acquire resistance to the peptidase degradation pathway by incorporating modified amino acids persist in lens tissue over the life of an individual (Fujino et al., 2000). Ultimately, with aging, the accumulation of low-molecular weight (LMW) peptides in lens tissue begins to impair the function of crystallins and the optical quality of the lens (Harrington et al., 2004; Santhoshkumar et al., 2008). Studies also indicate that the accumulation of peptides and truncated crystallin proteins is a potential factor in the age-related increase in lens opacity and hence the development of cataract (Harrington et al., 2004; Srivastava, 1988).
In a previous study, we identified more than 25 different LMW (<3.5 kDa) peptides in human lenses and demonstrated that both cataractous lenses and aged human lenses (>70 years old) exhibit a significantly higher amount of peptide accumulation than younger lenses (Santhoshkumar et al., 2008). A more recent study identified more than 200 LMW peptides (including the low-abundance peptides) in lens tissue (Su et al, 2010). Of the 25 LMW peptides we identified, more than 60% are derived from α-crystallin. Of the α-crystallin-derived peptides, αA66-80 peptide and its truncated derivatives are most abundant, all derived from the 3-strand of native αA-crystallin (Santhoshkumar et al., 2011). Further, the αA66-80 peptide possesses a sequence that resembles a region responsible for fibril-forming by β-amyloid peptide (Santhoshkumar et al., 2011). Peptides with an aggregation propensity, such as β-amyloid 1-42 (Pike et al., 1993), prion (PrP) 118–135 (Chabry et al., 2003), and tau 306–311 (Capitaine, 1975), have been shown to be involved in protein aggregation diseases, either by aggregating among themselves or aggregating along with other interacting proteins. In a previous study, we demonstrated that the α-crystallin-derived peptide αA66-80 interacts with lens proteins and forms large light scattering high molecular weight (HMW) aggregates, similar to those found in aging and cataractous human lenses (Santhoshkumar et al., 2011). We have also demonstrated that αA66-80 readily interacts with crystallin protein and that the peptide–protein complex acts as a seed to attract other aggregation-prone proteins or even native α-, β- and γ-crystallin to form larger light scattering aggregates (Santhoshkumar et al., 2011).
To validate the peptide involvement in crystallin aggregation, we used lenses of guinea pigs subjected to hyperbaric oxygen (HBO), perhaps the closest animal model for studying human age-related cataract (Giblin et al., 1995; Simpanya et al., 2005). Human α-crystallin shares more than 95% sequence homology with guinea pig α-crystallin and HBO is known to induce increased lens nuclear light scatter in guinea pig lenses, similar to that found in early human age-related nuclear cataracts (Heys et al 2007). Lenses of guinea pigs treated extensively with HBO in vivo exhibit increased HMW crystallin aggregates in the lens nucleus held together partially by disulfide bonds (Simpanya et al., 2005). The primary evidence for this model is based on clinical observation. Patients who receive HBO therapy have been found to subsequently develop enhanced nuclear scattering and myopia (Palmquist et al., 1984). Studies in the guinea pig animal model of cataract point to a strong relationship between HBO treatment and the development of early nuclear cataract (Freel et al., 2003). Though HBO-treated guinea pig lenses show a lens pathology similar to that in human cataractous lenses, the questions of whether crystallin-derived LMW peptides are also accumulating in HBO-treated guinea pig lenses and whether the crystallin-derived peptides have any role in HBO-mediated nuclear opacity in guinea pigs remain unanswered. Therefore, the present study was undertaken to determine if crystallin-derived peptides accumulate in HBO-treated guinea pig lenses in a manner similar to that occurring in human aged and cataractous lenses. We also investigated the effect of the fibril-forming peptide αA66-80 on protein aggregation and precipitation in the HBO-treated guinea pig lens.
2. Materials and methods
Lenses of HBO-treated guinea pigs were obtained from Dr. Frank Giblin, Eye Research Institute, Oakland University, Rochester, MI. The HBO treatment was performed as described earlier (Bantseev et al., 2004; Giblin et al., 1995). Briefly, guinea pigs, initially 18 months old, were treated with 2.5 atm of 100% O2 for 2.5-hour periods three times per week, at approximately the same time of day, for a total of 84 times over 7 months. Age-matched control animals were maintained in each group. The optical properties of the lenses after HBO treatment and in control guinea pigs were documented by slit-lamp exam and the animals were sacrificed by CO2 inhalation using an Euthanex Auto CO2 System (E-Z Systems, Inc., Palmer, PA). The lenses were removed from both control and HBO-treated guinea pigs and frozen rapidly. The frozen lenses were transported to our laboratory in dry ice by overnight shipping. The lenses were stored at −70°C until use.
2.1 Isolation of LMW peptides in HBO-treated guinea pig lenses
The lenses were homogenized in phosphate buffer (50 mM, 150 mM NaCl, pH 7.4) containing 6 M urea. The homogenate was centrifuged at 16,000 × g for 1 h and urea soluble supernatant was passed through a 10 kDa cut off membrane filter (Millipore) to obtain LMW peptides. The filtrates (10 kDa cut off) of both control and HBO-treated lenses were desalted using Pep Clean C-18 spin columns (Pierce). The bound peptides were eluted by 70% acetonitrile containing 1% formic acid, and analyzed by mass spectrometry.
2.2. Analysis of LMW peptides by mass spectrometry
Matrix-assisted laser desorption time of flight mass spectrometry (MALDI-TOF MS) analysis was performed at the Proteomics Core Facility, University of Missouri, Columbia. Positive ion spectra were acquired on an AB Sciex 4700 MALDI TOF-TOF instrument (formerly Applied Biosystems, Inc.). Peptide ion spectra were acquired over the mass ranges 600–4000 Da in the reflector mode and the data were processed by data Explorer software.
2.3. Procurement and preparation of peptides
Synthetic peptides αA66-80[SDRDKFVIFLDVKHF] and the proline substitute αA66-80 (V72P) [SDRDKFPIFLDVKHF] were obtained from GenScript Corp. (Piscataway, NJ). The purity level of the synthetic peptide was more than 95% as determined by high-performance liquid chromatography (HPLC) and mass spectrometry. Before each experiment the peptide (2 mg) stock solutions were prepared freshly, in 1 ml of sterile water. All of the assays were performed in phosphate buffer (50 mM PO4, 150 mM NaCl, pH 7.2) unless otherwise specified.
2.4. Preparation of guinea pig lens extracts
HBO-treated and control guinea pig lenses were homogenized in sodium phosphate buffer in the presence of protease inhibitor cocktail set III (Calbiochem). The homogenized samples were centrifuged at 16,000 × g for 1 h to remove insoluble proteins. The protein concentration in the water soluble (WS) fraction was determined by the Bio-Rad protein assay method. The WS fractions of the nucleus and cortical regions of both HBO-treated and control lenses were prepared in a similar manner.
2.5. αA66-80 Peptide-induced aggregation and precipitation of guinea pig lens proteins
WS guinea pig lens extract (200 μg) was incubated in PO4 buffer (1 ml in the presence of different amounts of αA66-80 peptide (0, 25, 50, 75 and 100 μg) for 16 h at 37°C. At the end of incubation, protein precipitates were removed by centrifugation at 10,000 × g for 10 min and the soluble fraction was injected onto TSK G3000PWXL (7.8mm ID × 30 cm, 10 μm) gel filtration column (Tosoh Bioscience, King of Prussia, PA) connected to an HPLC system (Shimadzu, Columbia, MD) coupled to an ultraviolet detector, multi-angle light-scattering and dynamic light-scattering detectors (Wyatt Technology, Santa Barbara, CA). The flow rate was set to 0.75 mL/min and the elution was monitored using a 280-nm absorption detector. The molecular mass of eluting proteins was estimated using ASTRA software (Wyatt Technology).
2.6. Effect of αA66-80 peptide + ADH on aggregation and chaperone function of guinea pig α-crystallin
The chaperone activity of guinea pig α-crystallin (50 μg, isolated from control guinea pig lenses) was measured using heat-induced amorphously aggregating ADH (250 μg) in the presence or absence of αA66-80 (25 μg). Scrambled αA66-80 peptide was used as control peptide. The assays were carried out at 37°C in 1 ml of 50mM PO4 buffer (pH 7.4) containing 150 mM NaCl and 100mM EDTA. Light scattering was monitored at 360 nm in a Shimadzu spectrophotometer equipped with a temperature controller. In other experiments, ADH (250 μg) and guinea pig α-crystallin were incubated with different concentrations of αA66-80 peptide at 37°C for 24 h. After incubation the protein precipitates were collected by centrifugation. Precipitates were solubilized in 6M urea and analyzed by SDS-PAGE 4–20% gradient gel.
2.7. Peptide αA66-80-induced α-crystallin aggregates and attraction of other soluble crystallin proteins in guinea pig lenses
Peptide-induced α-crystallin aggregates were obtained by incubating guinea pig α-crystallin (200 μg) with 25 μg of αA66-80 peptide overnight at 37°C. Aggregates were harvested by centrifugation (10,000 × g for 10 min) and the pellets were re-suspended in PO4 buffer. The re-suspended aggregates were further incubated with fluorescently labeled αB-crystallin (αBT162C-Alexa 488, as described earlier (Santhoshkumar et al., 2011). Since the fluorescent labeling or cysteine substitution does not affect αB-crystallin solubility or chaperone function, we used the αBT162C-Alexa-488–labeled protein to monitor the progression of the aggregation process in real time. Peptide-induced aggregates and labeled αBT162C-Alexa-488 were incubated at 37°C. An aliquot of sample was removed at 0 min and 24 h, placed on a pre-cleaned glass slide and observed under the fluorescence microscope. The image was captured at 20 × magnification with the Leica microscope. Control samples were obtained by mixing αA66-80(V72P) (SDRDKFPIFLDVKHF) treated guinea pig α-crystallin with αBT162C-Alexa 488-labeled crystallin. In a separate experiment, to study the effect of peptide-induced aggregation in a crowded protein environment, increasing amounts of guinea pig lens extracts, ratio of 0 to 200 more than the αA66-80 peptide, were incubated with a fixed amount of αA66-80 peptide or a scrambled sequence of the same (25μg) in 0.2 ml PO4 buffer. After 24 h the samples were centrifuged and the amount of protein in the precipitate was estimated using Bio-Rad protein assay reagents.
2.8. Time-lapse recording of peptide αA66-80-induced α-crystallin aggregates
The mixture of αA66-80-induced α-crystallin aggregates and fluorescently labeled αB-crystallin was used to obtain a time-lapse recording of the progression of α-crystallin aggregation in HBO-treated guinea pig lenses, as described previously (Santhoshkumar et al., 2011). The sample was placed in a well on a glass slide and photographed every 2 minutes for 6 h under an Olympus IX70 inverted fluorescence microscope. MetaMorph software (Molecular Devices, Inc., Sunnyvale, CA) was used to create a movie with captured images.
3.0. Results
3.1. Comparison of water-soluble (WS) and water-insoluble (WIS) proteins in nuclear lens tissue of HBO-treated and control guinea pig lenses
HBO treatment (60–84 times) of the guinea pig is known to induce nuclear opacity (Borchman et al., 2000; Giblin et al., 1995). To evaluate the effect of HBO treatment on changes in the WS protein content in the guinea pig lens, the amount of WS and WIS lens proteins in the nuclear fraction were compared in HBO-treated and age-matched control guinea pigs. While the HBO-treated nuclear lens tissue (52.3 mg) harbored only 12.75 mg of WS protein, nuclear lens tissue (50.4 mg) of control guinea pigs had 18.75 mg of WS protein, which translates to a 30% reduction in WS proteins in the HBO-treated nuclear tissue as compared to the control lens nucleus. To determine which crystallin fraction among the lens crystallin proteins was most affected, the WS lens extracts (100 μg) were analyzed by TSK 3000 PWXL size-exclusion column and the elution profile was monitored using a 280 nm absorption detector. The profile (Fig. 1) of the control lens WS fraction showed four well-defined peaks corresponding to elution of α-, β-, γ-S and γ-crystallins. The HBO-treated lens extract showed a noticeable decrease in the α- and γ-crystallin fractions. The WS fraction of α-crystallin eluted as a broad peak between 10 min and 14 min and the profile showed a 30% to 40% decrease in α-crystallin peak intensity. These fractions were collected and the amount of protein content was determined using Bio-Rad reagents. The estimated amount of WS α-crystallin from HBO-treated guinea pig nuclear tissues was 40% (1.8 mg) less than that from control guinea pig (3.1 mg), indicating that in the HBO-treated lens nucleus, α-crystallin is a preferential target and precipitates into the WIS fraction, similar to that found in aging and cataractous human lenses (Heys et al., 2007).
Fig. 1.
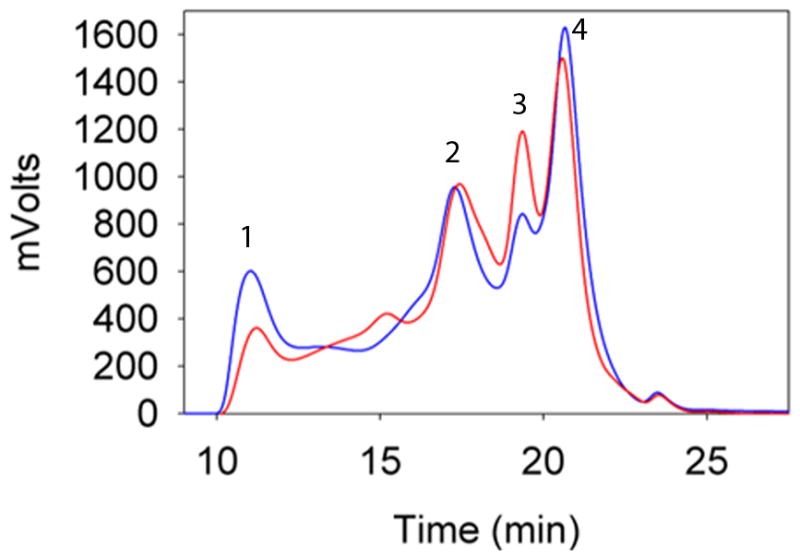
Representative elution profile of WS proteins from the lens nucleus of HBO-treated (red line) and control (blue line) guinea pig lens extracts. WS lens extract (0.1 mg in 100 μl) was injected into the TSK 3000 PWXL column and the elution was monitored using a 280 nm absorption detector. Peak 1, α-crystallin; peak 2, βH-crystallin; peak 3, βL-crystallin; peak 4, γcrystallin.
3.2. Accumulation of α-crystallin–derived LMW peptides in HBO-treated and control guinea pig lenses
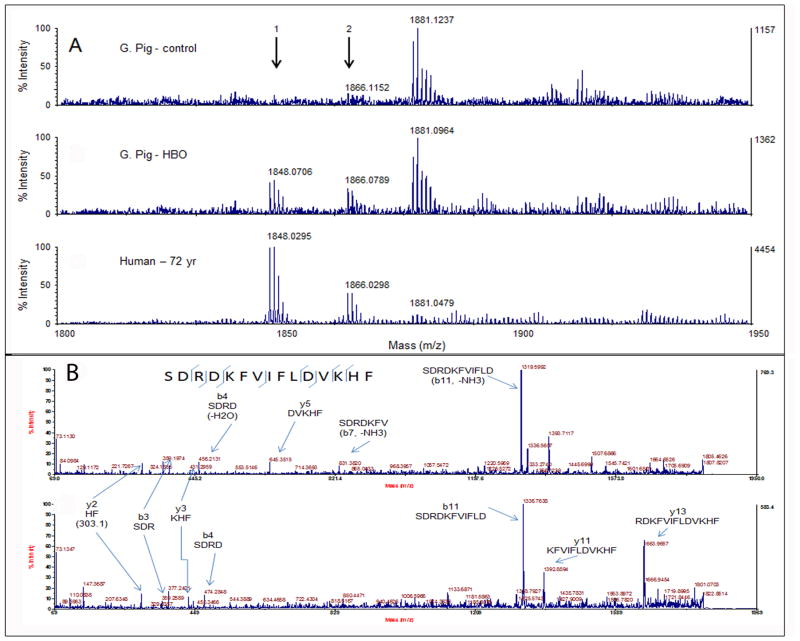
HBO-treated guinea pig lens extracts were examined to determine whether these lenses have a buildup of α-crystallin-derived LMW peptides, similar to that found in aging and cataractous human lenses (Santhoshkumar et al., 2011). LMW peptides were isolated from control and HBO-treated guinea pig lenses and subjected to MALDI TOF-MS analysis to identify <4 kDa LMW peptides. Peptides ranging from 700 Da to 4000 Da were detected. Many LMW peptides, such as those with mass ions 1204, 1388, 1405, 1447, 1865,1992, 3275 and 4136, were common in both HBO-treated and control guinea pig lens extracts. Further analysis of high-intensity ions by MS/MS revealed that 1388 corresponded to γS 167–178 (SPAVQSFRRIVE) and 1405 corresponded to βA1 (acetyl METQTVQRELE). Moreover, ions corresponding to peptide 1865 (αA66-80) and its dehydrated form, 1847, were in significantly higher concentration in HBO-treated guinea pig lenses than in control lenses. In fact, 1847 was exclusively present in HBO-treated guinea pig lens (Table 1). It has been shown that the dehydrated form of the peptide is also the N-terminal cyclized form (Lyons et al 2014). The resistance of cyclized peptide to aminopeptidase action and the interaction of the peptide with other crystallins might be the reason for the accumulation of dehydrated 1847 ion peptide in vivo. The large number of LMW peptides ions detected during MALDI-TOF MS analysis of HBO-treated and age-matched control guinea pig lenses is shown as supplementary data. The sequence of the peptides corresponding to the mass ions listed in supplementary tables 1 and 2 is yet to be determined by MS/MS analysis.
Table 1.
Crystallin derived peptides in guinea pig lens that are similar to those found in human lenses
| S. No | Mass ions | Control | HBO treated | Peptide region in Human | Peptide region in guinea pig |
|---|---|---|---|---|---|
| 1 | 1204 | + | + | γS-(169-178)d [Gene ID_1427] | γS-(169-178) [Gene ID_100307056] |
| 2 | 1388 | + | + | γS-(167-178)a, b [Gene ID_1427] | γS-(167-178) [Gene ID_100307056] |
| 3 | 1405 | + | + | βA3/A1 (30-40)c [Gene ID_1411] | βA3/A1 (30-40) [Gene ID_100379565] |
| 4 | 1447 | + | + | αA (57-69)c [GeneID_102724652] | αA (57-69) [Gene ID_100135506] |
| 5 | 1847 | − | + | αA a, b, c -(66-80) loss of H2O [GeneID_102724652] | αA-(66-80)* [Gene ID_100135506] |
| 6 | 1865 | + | + | αA-(66-80)a, b, c [GeneID_102724652] | αA-(66-80) [Gene ID_100135506] |
JBC, 2008, 283: 8477–8485;
Exp Eye Res, 2010, 91: 97–103;
Aging Cell, 2012, 11: 1125–1127;
likely loss of water
Fig. 2A shows the distribution of ion 1865 (αA66-80) and its dehydrated form 1847 in guinea pig and human lens (72 years old) extracts. Ion 1865 was at very low abundance in control guinea pig lenses (upper panel), but was increased, along with its dehydrated form, in HBO-treated guinea pig lenses (middle panel), similar to the fibril-forming peptide αA66-80 in 72-year-old human lens extracts (lower panel). MALDI TOF-TOF MS/MS analysis confirmed that the 1847 ion was the dehydrated form of 1865 ion (Fig. 2B). The calculated amount of αA66-80 peptide and its dehydrated form in HBO-treated guinea pig lenses is about 4.6 times higher in intensity than that in control guinea pig lenses, indicating that LMW peptide signature in HBO-induced guinea pig cataractous lenses is similar to that in aged human lenses and age-related cataractous human lenses.
Fig. 2.
A) MALDI qTOF- MS analysis of LMW peptides from guinea pig and human lenses. Arrows point to m/z 1865 and its dehydrated form 1847. The top panel shows a low abundance of 1865 ion in control guinea pig lens. The middle panel shows an increased amount of 1865 ion as well as its dehydrated form, 1847 ion, in HBO-treated guinea pig lenses as compared with control guinea pig lenses. Peptide ions similar to that in HBO-treated lenses are also seen in 72-year-old human lenses (lower panel). B). Top and bottom panels show MALDI TOF-TOF-MS/MS analysis of 1865 and 1847 m/z ions, confirming that the two ions originated from the same peptide (Arrows show y and b ions).
3.3. Effect of αA66-80 peptide on α-crystallin aggregation and precipitation in guinea pig lens and human lens extracts
After confirming the presence of αA66-80 peptide in guinea pig lenses, we used synthetic peptide to demonstrate its effect on protein aggregation and precipitation in guinea pig lens extracts. We previously demonstrated in human lens extracts that αA66-80 peptide induces protein aggregation and precipitation (Santhoshkumar et al., 2011). The guinea pig lens WS fraction (200 μg) was incubated in the presence of 0, 25, 50, 75 and 100 μg of αA66-80 peptide at 37°C for 30 min. The samples were centrifuged at 10,000 × g for 10 min to remove any precipitate formed during incubation and the supernatant was analyzed by a TSK 5000 PWXL size exclusion column. The elution profile showed that αA66-80 peptide caused selective precipitation of α-crystallin in a concentration-dependent manner (Fig. 3). The data demonstrate that αA66-80 peptide induces guinea pig α-crystallin to form larger aggregates, likely resulting from the interactions between peptide and crystallin, which were not able to enter the column. Such interaction was also evident from SDS-PAGE analysis of the precipitate, showing mainly α-crystallin and αA66-80 peptide (data not shown). The findings with guinea pig lens extracts suggest that peptide-induced aggregation of lens proteins is a generalized phenomenon across species, and not limited to the human lens only.
Fig. 3.
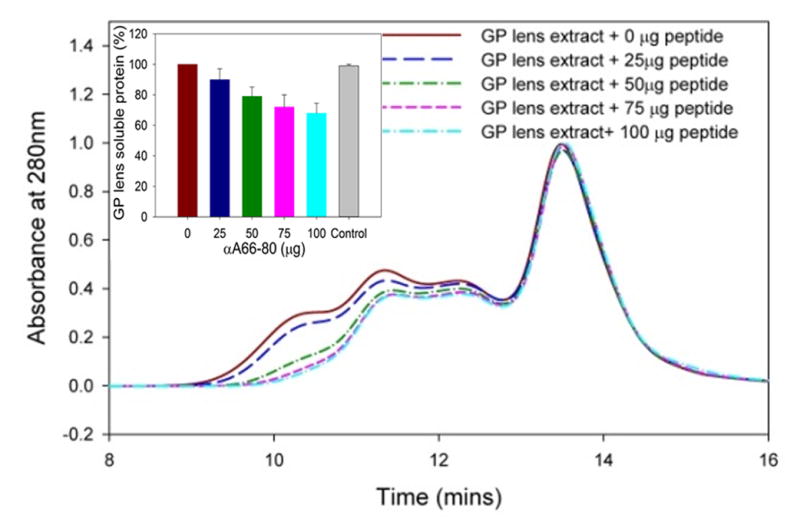
Size exclusion chromatography profile of guinea pig lens extract treated with and without αA66-80 peptide. WS guinea pig lens extract (WS 200 μg) was incubated in the presence of αA66-80 peptide (0, 25, 50, 75, 100 μg, in 100 μl) overnight (16 h) at 37°C. The samples were centrifuged at 10,000 × g for 10 min to remove precipitate, if any, and the supernatant was injected into a TSKG-5000PWXL column (7.8 mm × 30 cm). The elution of protein was monitored using a 280-nm absorbance detector. The representative profiles show that αA66-80 peptide selectively targets α-crystallin in a concentration-depended manner. The inset shows the amount of soluble crystallins recovered prior to chromatographic analysis. The amount of protein recovered with scrambled αA66-80 peptide (100 μg) as a control is shown in the gray color bar. (n=3).
3.4. Effect of the αA66-80–α-crystallin interaction on α-crystallin chaperone function in HBO-treated and control guinea pigs
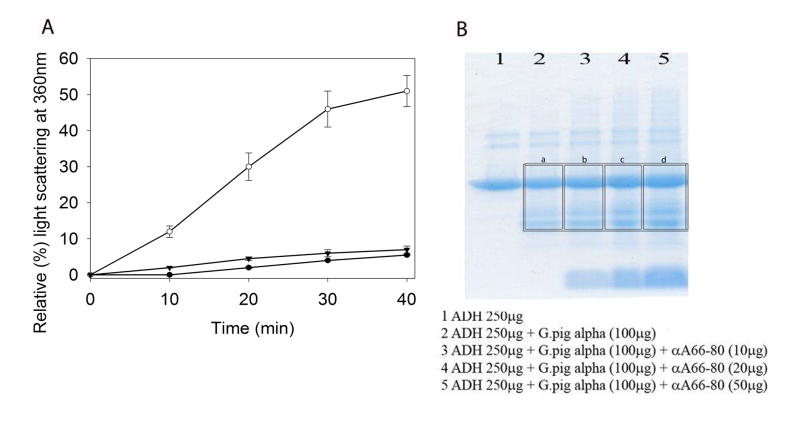
To further explore the preferential interaction of αA66-80 with α-crystallin, we investigated whether the interaction of αA66-80 peptide affects the chaperone function of guinea pig αcrystallin by performing the ADH aggregation assay, initiated by the addition of 100 mM EDTA. The addition of α-crystallin prevented amorphously aggregating ADH from forming larger light scattering aggregates (Fig. 4A) during chaperone assay, as expected. However, significant interference toward chaperone activity of α-crystallin was observed (Fig. 4A) when the sample contained αA66-80 peptide along with ADH. In contrast, when a scrambled αA66-80 peptide was used in the assay in place of αA66-80 peptide, there was no loss in α-crystallin chaperone activity toward aggregating ADH, suggesting that the effect of αA66-80 peptide was specific. We demonstrated in earlier studies that control peptides have no effect on the chaperone function of α-crystallin (Kannan et al., 2013) and the effect of αA66-80 peptide appears specific. SDS-PAGE analysis of the precipitate obtained from a separate experiment with a different ratio of peptide and a fixed amount of ADH revealed co-precipitation of αA66-80 peptide and α-crystallin. The amount of α-crystallin that precipitated correlated with the amount of peptide used during the ADH aggregation assay (Fig 4B). The data suggest that αA66-80 peptide might be playing a role in guinea pig cataract formation by selectively depleting the available α-crystallin chaperone molecules.
Fig. 4.
Fig. 4A. Inhibition of chaperone activity of guinea pig α-crystallin by αA66-80. The chaperone function of guinea pig α-crystallin (50 μg) was tested against amorphously aggregating ADH (250 μg) in the presence and absence of αA66-80 peptide. ADH + scrambled αA66-80 peptide (25 μg, used as control peptide) + guinea pig α-crystallin: -▼-; ADH + αA66-80 peptide (25 μg) + guinea pig α-crystallin: -○-; ADH + guinea pig α-crystallin: -●-. The graph is representative of the mean and standard error of three independent measurements. 4B) SDS-PAGE analysis of αA66-80-induced α-crystallin precipitate (α-crystallin isolated from HBO-treated guinea pig whole lens). 1) ADH, (250 μg); 2) ADH (250μg) + α-crystallin (100 μg); 3) ADH (250 μg) + α-crystallin (100 μg) + αA66-80 peptide (10 μg); 4) ADH (250 μg) + α-crystallin (100 μg) + αA66-80 peptide (20 μg); 5) ADH (250 μg) + α-crystallin (100 μg) + αA66-80 peptide (50 μg). Image J analysis gave relative intensity of a) 4.07, b) 4.39, c) 4.69 and d) 5.01 for the area marked in the figure. A 14% increase in the precipitation of α-crystallin was observed when 50 μg of the peptide was included with ADH+α-crystallin compared to the sample containing only ADH + α-crystallin.
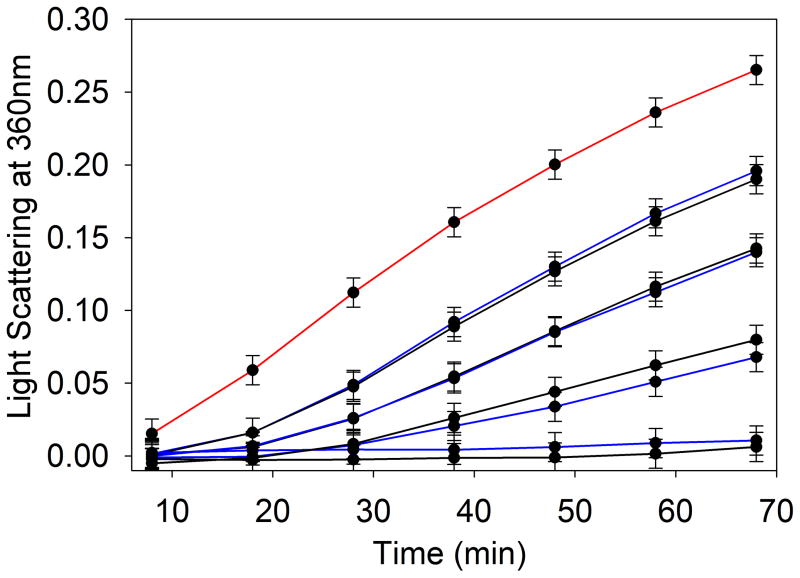
In our experiments of the chaperone activities of α-crystallin isolated from guinea pig lens nucleus of HBO-treated and control animals, the efficiency of chaperone function (protection) against amorphously aggregating ADH at 37°C was found to be similar in HBO-treated and control guinea pig lenses (Fig. 5). Although the chaperone activity of isolated α-crystallin was not significantly reduced in the HBO-treated guinea pig lens, the available chaperone molecule was reduced 40% (4.5 units) in HBO-treated guinea pig lens nucleus as compared to control guinea pig lens nucleus (7.75 units), as shown in Fig. 1.
Fig. 5.
The chaperone-like activity of HBO-treated and control guinea pig α-crystallin using amorphously aggregating ADH (250 μg). The assay was performed in 1 ml PO4 buffer (50 mM phosphate buffer containing 150 mM NaCl and 100 mM EDTA) at 37°C. Different concentrations of HBO- treated or control guinea pig α-crystallin (10, 25, 50 and 100 μg) were used. The extent of protection was estimated by monitoring the light scattering at 360 nm using a Shimadzu UV-VIS spectrophotometer equipped with a temperature-controlled multi-cell transporter. Curves: red, ADH alone; Blue, ADH plus α-crystallin (isolated from control lens nucleus); Black, ADH plus α-crystallin isolated from HBO-treated lens nucleus. (A, 10 μg; B, 25 μg; C, 50 μg; D, 100 μg of α-crystallin). The result shown is representative of two independent experiments.
3.5. αA66-80 peptide-induced aggregation of guinea pig α-crystallin in a molecular crowding environment
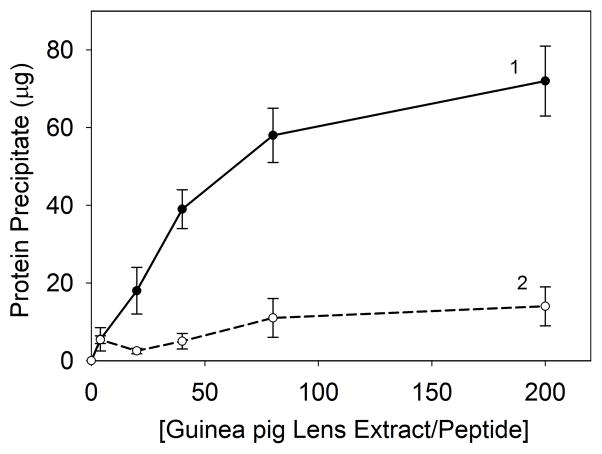
To determine the effect of αA66-80 peptide on protein at increasing concentrations, mimicking the crowded in vivo molecular environment, we incubated a fixed amount of peptide (25 μg) with 0 to 200-fold excess (wt/wt) of WS guinea pig lens proteins at 37°C for 24 h and then measured the amount of precipitated protein. While 25 μg of αA66-80 peptide, giving a1:20 ratio of peptide to α-crystallin, resulted in the precipitation of ~18 μg of guinea pig lens protein from a reaction mixture, the amount of precipitate increased to ~70 μg when the lens protein concentration was increased to provide a wt/wt ratio of 1:200 for peptide and α-crystallin. The results in Fig. 6 show that the amount of precipitate gradually increased with increasing concentrations of α-crystallin, suggesting that a molecular crowding effect augments peptide-induced α-crystallin precipitation. These data suggest that when proteins are at high concentration, as in the case of the lens, even a small amount of peptide(s) can induce aggregation and precipitation of proteins, which may not be obvious with a low concentration of protein. Our previous studies of human lenses of different ages revealed that the extent of precipitation varies depending on the age of the lens and the metastable nature of the protein (Santhoshkumar et al., 2011). Taken together, these observations suggest that the peptide-induced precipitation of lens crystallins is both concentration- and age-dependent.
Fig. 6.
Effect of protein concentration on peptide-induced aggregation of guinea pig lens extracts. Increasing amounts of lens extract were incubated with a fixed amount of αA66-80 peptide (25 μg) (-●-) or scrambled αA66-80 peptide (-○-) to obtain ratios of 1:1 to 1:200 of peptide to protein (wt/wt) in 0.2 ml of PO4 buffer containing 150 mM NaCl. After 24 h the samples were centrifuged and the amounts of precipitate were estimated using Bio-Rad protein assay reagents. The result shown is representative of three independent experiments.
3.6 Formation of αA66-80–α-crystallin complexes in guinea pig lens extracts
Studies have shown that aggregation-inducing peptides, such as β-amyloid, initially interact with native proteins to form peptide-protein complexes that in turn act as seeds to recruit other soluble proteins that, with time, lead to the formation of larger light scattering aggregates (Santhoshkumar et al., 2011). As described in the methods section, we tested whether αA66-80 peptide promotes aggregation of guinea pig lens proteins in a manner similar to that shown with human lens extracts (Santhoshkumar et al., 2011). At the start of the experiment, the addition of αBT162C-labeled α-crystallin to αA66-80 resulted in homogenous fluorescence, but, with time, α-crystallin aggregates (peptide-protein complexes) became apparent (Fig. 7C and 7D). In contrast, there was a homogenous distribution of Alexa-488 fluorescence intensity at both 0 min (Fig. 7A) and 24 h (Fig. 7B) in the samples containing proline-substituted αA66-80 peptide used as control. Such a difference suggests interactions between labeled αBT162C and αA66-80–α-crystallin aggregates. The aggregation of labelled αBT162C over α-crystallin–αA66-80 peptide complex was captured for 6 h in a glass slide following the addition of αBT162C–Alexa-488 by taking photographs every 2 min. The captured images were compressed to obtain a time-lapse recording of aggregation. The results (Fig 8B–F and Suppl video clip) confirm that, as in the human lens crystallin studies (Santhoshkumar et al., 2011), peptide-induced α-crystallin aggregate forms larger aggregate by attracting other soluble crystallin proteins. The representative pictures (Fig 8B–F) from time lapse recording of the aggregation assay show a progressive addition of labeled soluble α-crystallin to the αA66-80– α-crystallin aggregates.
Fig. 7.
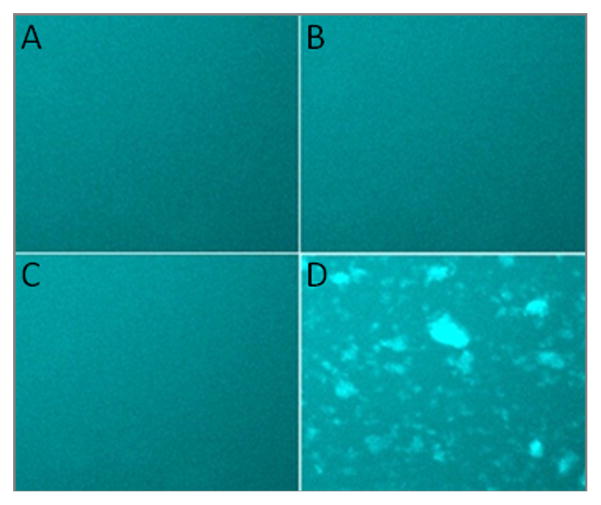
αA66-80 peptide–induced HMW aggregates of guinea pig lens extracts. Guinea pig α-crystallin (200 μg) was incubated with αA66-80 peptide (25 μg) at 37°C overnight. The aggregates were collected by centrifugation and the pellet was re-suspended with labeled αBT162C as described under methods. Seeded aggregates and soluble αB (αBT162C-Alexa 488) crystallin were mixed and incubated at 37°C. An aliquot of the sample was removed at 0 min and 24 h placed on a pre-cleaned glass slide and visualized under a fluorescence microscope. αA66-80(V72P) was used as a control peptide. A) α-crystallin–αA66-80(V72P)-aggregates + αB-crystallin-Alexa 488 at 0 h; B) α-crystallin–αA66-80(V72P)-aggregates + αB-crystallin-Alexa 488 at 24 h; C) α-crystallin–αA66-80 aggregates + αB-crystallin-Alexa 488 at 0 h; D) α-crystallin–αA66-80 aggregates + αB-crystallin-Alexa 488 at 24 h.
Fig. 8.
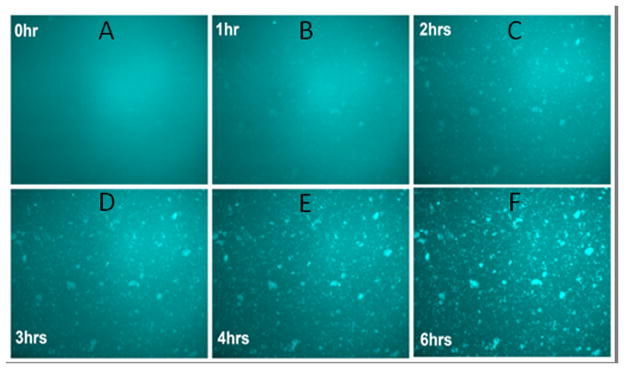
Representative figures from the time-lapse recording of αA66-80 peptide–induced HMW aggregate formation of guinea pig α-crystallin. Increased aggregation of fluorescence-labelled αB-crystallin in the presence of guinea pig α-crystallin– αA66-80 peptide aggregates was captured from a glass slide following the addition of αBT162C-Alexa 488 for 6 h. The photographs were taken every 2 min and representative pictures at specific time points are shown. A) 0 h; B) 1 h; C) 2 h; D) 3 h; E) 4 h; F) 6 h.
Discussion
α-Crystallin derived–LMW peptides and truncated crystallins are known to accumulate with age in the human lens (Grey and Schey, 2009; Santhoshkumar et al., 2008; Su et al., 2010), and the amount of LMW peptides is significantly higher in aged and cataractous human lenses than in young human lenses (Santhoshkumar et al., 2008). Other investigators have also shown the occurrence of LMW peptides in human lens and have found a parallel trend between aging and an increasing intensity of LMW peptide (Grey and Schey, 2009; Su et al., 2010). Among the 25 LMW peptides we have identified by mass spectrometric analysis, the αA66-80 peptide and its derivatives are major constituents, and are capable of forming fibrils under physiological conditions (Santhoshkumar et al., 2008). Based on our observations, we hypothesized that the accumulation of LMW peptides might accelerate age-related cataract formation because of the inherent ability of several of the peptides present in lens to cause protein aggregation. Further, we have demonstrated that the fibril-forming αA66-80 and its derivatives play a vital role in crystallin aggregation and precipitation during in vitro assays (Santhoshkumar et al., 2011). To validate our hypothesis, we examined whether these LMW peptides are also accumulating in HBO-treated guinea pig lenses, an attractive animal model for studies of age-related cataract in humans. Long-term HBO treatment is known to induce increased nuclear light scatter in the guinea pig lenses, similar to early age-related nuclear cataract in humans (Borchman et al., 2000; Giblin et al., 1995; Simpanya et al., 2005). Further, biochemical analysis of HBO-treated guinea pig lenses shows molecular changes similar to those found in the aging human lens, including loss of antioxidants, increased protein degradation, protein disulfide cross linking, crystallin aggregation and membrane abnormalities (Giblin et al., 2013; Simpanya et al.,2005).
In this study LMW peptides were isolated from whole lens tissue of control and HBO-treated guinea pig lenses and were analyzed by MALDI-TOF-MS. The data show that HBO-treated guinea pig lenses (i.e., lenses with light scattering opacity) have more LMW peptides than the control guinea pig lenses, consistent with our earlier findings in human lens tissues in which cataractous and aged human lenses had a greater accumulation of peptides than the young clear lenses (Santhoshkumar et al., 2008). Consistent with the human lens study, our study of the guinea pig cataract model demonstrates that peptide ion corresponding to human αA66-80 peptides is at a higher intensity in the HBO-treated guinea pig lens tissue than in control tissue. The amount of αA66-80 peptide was found to be 4.6 times higher in HBO-treated guinea pig lens tissue than in control guinea pig lens tissue, indicating that the accumulation and prevalence pattern of the peptide are similar in human lens opacity and in the HBO-induced model of lens opacity in guinea pigs. However, in a previously published study of the human lens (Santhoshkumar et al., 2008) we found that the aged human lens accumulates a very high amount of LMW peptides, much higher than the amount of LMW peptides found in the HBO-treated guinea pig lenses (Fig. 2). This difference could be due to the longevity of human lenses or to the minor differences between human and guinea pig crystallin subunits. (Accession: NP_001166406). Recently it was shown that the N-terminal residues in αA66-80 can spontaneously cyclize to form 2,5-diketopiperazine derivative that imparts resistance to aminopeptidase action (Lyons et al., 2014) and this is likely one of the reasons for the accumulation of the αA66-80 peptide that has lost 18 Da in mass. At this time it is not known whether the cyclized peptide aggregates by itself or induces aggregation of crystallins.
The ability of αA66-80 to induce protein aggregation was investigated using the WS fraction of guinea pig lens. Similar to human lens crystallin precipitation (Santhoshkumar et al., 2011), guinea pig α-crystallin is the preferential target for precipitation by the αA66-80 peptide in the guinea pig lens as well. In addition to α-crystallin precipitation, ~5–8% β-crystallin precipitation was also observed when the lens extract was treated with αA66-80 peptide. Researchers have reported decreased or complete absence of native α-crystallin in the nuclear region of aged human lenses (Roy and Spector, 1976) and suggested that a diminished amount of soluble α-crystallin in the nuclear region is one of the underlying molecular mechanisms for age-related lens cloudiness and cataract formation. Figures 3 and 4 support this view and also provide insights into how soluble α-crystallin selectively precipitates out from a solution in the presence of αA66-80. The hydrophobic nature of αA66-80 peptide combined with its propensity to form beta sheet structure could be a possible mediator for the interaction with chaperone molecule α-crystallin. Bis-ANS binding experiments suggest that αA66-80 peptide has hydrophobicity (Santhoshkumar et al., 2011). In a separate study we showed that Pro substitutions that disrupt the beta sheet structure decrease interaction of the peptide with crystallins (Kannan et al., 2014). In a previous study we found that αA66-80 interacts with 70–74, 75–90, 91–103, 93–107, and 164–174 regions in αB-crystallin (Kannan et al., 2013). Since αB- and αA-crystallins share more than 55% homology, the interaction of αA66-80 peptide may likewise follow a similar pattern. We know from our work (Sharma et al., 2000) and from the literature (Ghosh et al., 2005) that these regions in α-crystallin harbor important biological functions, including oligomerization, chaperone action and solubility. Therefore, it’s not surprising that peptide interaction with these segments impairs α-crystallin function and facilitates aggregation. Our earlier study (Santhoshkumar et al., 2011) and the present study together suggest that αA66-80 peptide might be involved in the depletion of available α-crystallin. The in vitro data obtained from peptide-mediated protein aggregation might reflect how protein aggregation occurs in vivo in the HBO-induced guinea pig lens opacity.
The accumulation of LMW peptides in aged and cataractous human lens tissues is well documented (Santhoshkumar et al., 2008; Su et al., 2010). The present study demonstrates the presence of LMW peptides in the cataract animal model of HBO-treated guinea pig. However, the molecular mechanism by which the LMW peptides are generated in vivo is not well understood. The long-living lens crystallins endure several post-translational modifications throughout the life of an individual. Age-related modifications in lens proteins impair their structure and function. However, structurally and functionally challenged proteins are rapidly removed by enzymatic cleavage, a major pathway for protein degradation. Several lines of evidence demonstrate that lens tissue contains a variety of proteolytic enzymes, such as calpains (Baruch et al., 2001), caspases (Andersson et al., 2000), cathepsin B and matrix metalloproteases (MMPs), ADAMs, and ADAMTs (Wride et al., 2006), secretases (Li et al., 2003), tissue plasminogen activator and the ubiquitin proteasome pathway (UPP) (Girao et al., 2005), trypsin-like proteases, multicatalytic endopeptidase, membrane-bound proteases and MMPs as well as cystatin C (Wasselius et al., 2004) and tissue inhibitors of MMPs (TIMPs).
The mechanism by which HBO treatment of guinea pigs might lead to an increased level of αA66-80 peptide in the lens (Fig. 2B) is not clear at this time. In preliminary studies using imaging mass spectrometry analysis, we found that HBO treatment of 18-month-old guinea pigs resulted in a 50% decrease in the lens nuclear levels of 11 age-related crystallin fragments, ranging in molecular weight from 6,000 to 15,000 Da (Giblin et al., ISER abstract 2012). Four of the crystallin fragments have been tentatively identified: αA1-50 (6005 Da); αA1-65 (7,721 Da); αA1-80 (9,577 Da); and αA1-101(11,978 Da). It is possible that by some yet-to-be-identified mechanism. HBO exposure in vivo can accelerate in the lens nucleus the cleavage of 6,000 to 15,000 Da peptides to LMW peptides such as αA66-80, which are capable of interacting with lens crystallins and promoting the formation of HMW aggregates or of simply shifting the kinetics of aggregation. It should be noted that the removal of αA66-80 peptide from αA1-80 results in αA1-65 found in HBO-treated lenses.
Protease-generated peptides are generally rapidly digested by lens peptidases (Sharma and Kester, 1996; Sharma and Ortwerth, 1986a, b). Surprisingly, despite the presence of active aminopeptidases in the lens, crystallin-derived peptides still accumulate in the lens. Though both enzymatic and non-enzymatic cleavages have been reported (Su et al., 2012) to be possible causes of the generation of LMW peptides in aged lens tissues, the evidence that protease action is responsible for the generation of LMW peptide is bountiful. Our recent study demonstrated that more than one protease is likely to be involved in the generation of αA66-80 peptide (Hariharapura et al., 2013). Further, the study suggested that the enzymatically cleaved peptides persist in lens tissue by acquiring resistance to peptidase action due to interaction with other lens crystallin proteins. Taken together a strategy to regulate the protease(s) and/or activation of the peptidases might prove beneficial in suppressing LMW peptide-mediated cataract formation.
In summary, the LMW peptide profile in HBO-treated guinea pig lenses exhibits similarities to that found in aged human lenses. Further, αA66-80 peptide appears to interact with crystallin protein in the HBO-treated guinea pig lens and form larger light scattering aggregates, replicating the observations made with human lens extract. Taken together, the results of this study suggest that the LMW peptide-mediated cataract pathology in age-related human cataract and in HBO-induced lens opacity in guinea pigs might have a common molecular pathway. Strategies to inhibit the generation of LMW peptides in the lens might be an avenue for the development of potential therapy to prevent age-related cataract formation.
Supplementary Material
Supplementary Table 1. LMW peptides ions from control guinea pigs lens as determined MALDI-TOF MS
Supplementary Table 2. LMW peptides ions from HBO treated guinea pigs lens as determined by MALDI-TOF MS.
The αA66-80 peptide- induced α-crystallin aggregate was placed in a well on glass slide with fluorescently labelled αBT162C–Alexa-488 protein and photographed every 2 minute for 6 h under an Olympus IX70 inverted fluorescence microscope. MetaMorph software (Molecular Devices, Inc., Sunnyvale, CA) was used to create a movie of the captured images. The recording shows progressive accumulation of fluorescently labelled αBT162C–Alexa-488 into the pre-formed aggregates.
High lights.
Crystallin-derived peptides concentration increases in lenses after HBO treatment of Guinea pigs.
Alpha A-crystallin 66–80 peptide found in HBO-induced cataract lenses inhibits chaperone activity of aloha-crystallin.
Alpha-crystallins aggregates and precipitates when treated with Alpha A-crystallin 66–80 peptide.
Crystallin-fragment induced protein aggregation appears to have a role in HBO-treatment induced Guinea pig cataract formation.
Acknowledgments
Supported by Grants EY19878 and EY 02027 from the National Eye Institute/National Institutes of Health. We thank Sharon Morey for help with the preparation of the manuscript and the Proteomics Core Facility at the University of Missouri, Columbia, for performing mass spectrometry analysis. Ed Guzman and Janet Schofding assisted with treating the guinea pigs with HBO and caring for the animals, respectively, in the Oakland University animal care facility.
Abbreviations
- ADH
alcohol dehydrogenase
- EDTA
ethylene diamine tetraacetic acid
- HBO
hyperbaric oxygen
- HMW
high molecular weight
- HPLC
high-performance liquid chromatography
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- LMW
low molecular weight
- MALDI-TOF MS
matrix-assisted laser desorption time of flight mass spectrometry
- MMPs
metalloproteases
- sHSP
small heat shock protein
- TIMPs
tissue inhibitors of metalloproteases
- UPP
ubiquitin proteasome pathway
- WIS
water insoluble
- WS
water soluble
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson M, Sjostrand J, Petersen A, Honarvar AK, Karlsson JO. Caspase and proteasome activity during staurosporin-induced apoptosis in lens epithelial cells. Invest Ophthalmol Vis Sci. 2000;41:2623–2632. [PubMed] [Google Scholar]
- Argirova MD, Breipohl W. Glycated proteins can enhance photooxidative stress in aged and diabetic lenses. Free Radic Res. 2002;36:1251–1259. doi: 10.1080/1071576021000016481. [DOI] [PubMed] [Google Scholar]
- Bantseev V, Oriowo OM, Giblin FJ, Leverenz VR, Trevithick JR, Sivak JG. Effect of hyperbaric oxygen on guinea pig lens optical quality and on the refractive state of the eye. Exp Eye Res. 2004;78:925–931. doi: 10.1016/j.exer.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Baruch A, Greenbaum D, Levy ET, Nielsen PA, Gilula NB, Kumar NM, Bogyo M. Defining a link between gap junction communication, proteolysis, and cataract formation. J Biol Chem. 2001;276:28999–29006. doi: 10.1074/jbc.M103628200. [DOI] [PubMed] [Google Scholar]
- Bassnett S. Fiber cell denucleation in the primate lens. Invest Ophthalmol Vis Sci. 1997;38:1678–1687. [PubMed] [Google Scholar]
- Bassnett S. Lens organelle degradation. Exp Eye Res. 2002;74:1–6. doi: 10.1006/exer.2001.1111. [DOI] [PubMed] [Google Scholar]
- Bassnett S. On the mechanism of organelle degradation in the vertebrate lens. Exp Eye Res. 2009;88:133–139. doi: 10.1016/j.exer.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchman D, Giblin FJ, Leverenz VR, Reddy VN, Lin LR, Yappert MC, Tang D, Li L. Impact of aging and hyperbaric oxygen in vivo on guinea pig lens lipids and nuclear light scatter. Invest Ophthalmol Vis Sci. 2000;41:3061–3073. [PubMed] [Google Scholar]
- Capitaine Y. Gastro-duodenoscopy: indications and value. Rev Med Suisse Romande. 1975;95:599–606. [PubMed] [Google Scholar]
- Chabry J, Ratsimanohatra C, Sponne I, Elena PP, Vincent JP, Pillot T. In vivo and in vitro neurotoxicity of the human prion protein (PrP) fragment P118–135 independently of PrP expression. J Neurosci. 2003;23:462–469. doi: 10.1523/JNEUROSCI.23-02-00462.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshman KR, Wagner BJ. Changes during aging in rat lens endopeptidase activity. Exp Eye Res. 1984;39:543–551. doi: 10.1016/0014-4835(84)90054-x. [DOI] [PubMed] [Google Scholar]
- Freel CD, Gilliland KO, Mekeel HE, Giblin FJ, Costello MJ. Ultrastructural characterization and Fourier analysis of fiber cell cytoplasm in the hyperbaric oxygen treated guinea pig lens opacification model. Exp Eye Res. 2003;76:405–415. doi: 10.1016/s0014-4835(03)00004-6. [DOI] [PubMed] [Google Scholar]
- Fujii N, Ishibashi Y, Satoh K, Fujino M, Harada K. Simultaneous racemization and isomerization at specific aspartic acid residues in alpha B-crystallin from the aged human lens. Biochim Biophys acta. 1994;1204:157–163. doi: 10.1016/0167-4838(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Fujino T, Watanabe K, Beppu M, Kikugawa K, Yasuda H. Identification of oxidized protein hydrolase of human erythrocytes as acylpeptide hydrolase. Biochim Biophys acta. 2000;1478:102–112. doi: 10.1016/s0167-4838(00)00004-2. [DOI] [PubMed] [Google Scholar]
- Ghosh JG, Estrada MR, Clark JI. Interactive domains for chaperone activity in the small heat shock protein, human alphaB crystallin. Biochemistry. 2005;44:14854–14869. doi: 10.1021/bi0503910. [DOI] [PubMed] [Google Scholar]
- Giblin FJ, David LL, Wilmarth PA, Leverenz VR, Simpanya MF. Shotgun proteomic analysis of S-thiolation sites of guinea pig lens nuclear crystallins following oxidative stress in vivo. Mol Vis. 2013;19:267–280. [PMC free article] [PubMed] [Google Scholar]
- Giblin FJ, Schey K, Sharma K, Simpanya F, Leverenz V. Oxygen-induced loss of age-related crystallin fragments in the guinea pig lens nucleus in vivo may contribute to high molecular weight aggregate formation. XX Biennial Meeting of the International Society of Eye Research; 2012. p. Abstract 0415.p. 143. [Google Scholar]
- Giblin FJ, Leverenz VR, Padgaonkar VA, Unakar NJ, Dang L, Lin LR, Lou MF, Reddy VN, Borchman D, Dillon JP. UVA light in vivo reaches the nucleus of the guinea pig lens and produces deleterious, oxidative effects. Exp Eye Res. 2002;75:445–458. [PMC free article] [PubMed] [Google Scholar]
- Giblin FJ, Padgaonkar VA, Leverenz VR, Lin LR, Lou MF, Unakar NJ, Dang L, Dickerson JE, Jr, Reddy VN. Nuclear light scattering, disulfide formation and membrane damage in lenses of older guinea pigs treated with hyperbaric oxygen. Exp Eye Res. 1995;60:219–235. doi: 10.1016/s0014-4835(05)80105-8. [DOI] [PubMed] [Google Scholar]
- Girao H, Pereira P, Taylor A, Shang F. Subcellular redistribution of components of the ubiquitin-proteasome pathway during lens differentiation and maturation. Invest Ophthalmol Vis Sci. 2005;46:1386–1392. doi: 10.1167/iovs.04-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey AC, Schey KL. Age-related changes in the spatial distribution of human lens alpha-crystallin products by MALDI imaging mass spectrometry. Invest Ophthalmol Vis Sci. 2009;50:4319–4329. doi: 10.1167/iovs.09-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenen PJ, Merck KB, de Jong WW, Bloemendal H. Structure and modifications of the junior chaperone alpha-crystallin. From lens transparency to molecular pathology. Eur J Biochem / FEBS. 1994;225:1–19. doi: 10.1111/j.1432-1033.1994.00001.x. [DOI] [PubMed] [Google Scholar]
- Hariharapura R, Santhoshkumar P, Krishna Sharma K. Profiling of lens protease involved in generation of alphaA-66-80 crystallin peptide using an internally quenched protease substrate. Exp Eye Res. 2013;109:51–59. doi: 10.1016/j.exer.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington V, McCall S, Huynh S, Srivastava K, Srivastava OP. Crystallins in water soluble-high molecular weight protein fractions and water insoluble protein fractions in aging and cataractous human lenses. Mol Vis. 2004;10:476–489. [PubMed] [Google Scholar]
- Heys KR, Friedrich MG, Truscott RJWRJ. Presbyopia and heat: changes associated with aging of the human lens suggest a functional role for the small heat shock protein, alpha-crystallin, in maintaining lens flexibility. Aging Cell. 2007;6:807–815. doi: 10.1111/j.1474-9726.2007.00342.x. [DOI] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad of Sci USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J, Emmons T, Takemoto L. The ability of lens alpha crystallin to protect against heat-induced aggregation is age-dependent. Curr Eye Res. 1992;11:817–822. doi: 10.3109/02713689209000754. [DOI] [PubMed] [Google Scholar]
- Ingolia TD, Craig EA. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci USA. 1982;79:2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan R, Santhoshkumar P, Mooney BP, Sharma KK. The alphaA66–80 peptide interacts with soluble alpha-crystallin and induces its aggregation and precipitation: a contribution to age-related cataract formation. Biochemistry. 2013;52:3638–3650. doi: 10.1021/bi301662w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan R, Raju M, Sharma KK. The critical role of the central hydrophobic core (residues 71–77) of amyloid-forming αA66-80 peptide in α-crystallin aggregation: a systematic proline replacement study. Amyloi. 2014;21:103–109. doi: 10.3109/13506129.2014.888994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein BE, Klein R, Linton KL, Franke T. Diagnostic x-ray exposure and lens opacities: the Beaver Dam Eye Study. Am J Public Health. 1993;83:588–590. doi: 10.2105/ajph.83.4.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemenz R, Frohli E, Steiger RH, Schafer R, Aoyama A. Alpha B-crystallin is a small heat shock protein. Proc Natl Acad Sci USA. 1991;88:3652–3656. doi: 10.1073/pnas.88.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Percontino L, Sun Q, Qazi AS, Frederikse PH. Beta-amyloid secretases and beta-amloid degrading enzyme expression in lens. Mol Vis. 2003;9:179–183. [PubMed] [Google Scholar]
- Linetsky M, Shipova E, Cheng R, Ortwerth BJ. Glycation by ascorbic acid oxidation products leads to the aggregation of lens proteins. Biochim Biophys Acta. 2008;1782:22–34. doi: 10.1016/j.bbadis.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons B, Kwan AH, Truscott R. Spontaneous cyclization of polypeptides with a penultimate Asp, Asn or isoAsp at the N-terminus and implications for cleavage by aminopeptidase. FEBS J. 2014;281:2945–2955. doi: 10.1111/febs.12833. [DOI] [PubMed] [Google Scholar]
- Merck KB, Groenen PJ, Voorter CE, de Haard-Hoekman WA, Horwitz J, Bloemendal H, de Jong WW. Structural and functional similarities of bovine alpha-crystallin and mouse small heat-shock protein. A family of chaperones. J Biol Chem. 1993;268:1046–1052. [PubMed] [Google Scholar]
- Meyer LM, Dong X, Wegener A, Soderberg P. Dose dependent cataractogenesis and Maximum Tolerable Dose (MTD(2.3:16)) for UVR 300 nm-induced cataract in C57BL/6J mice. Exp Eye Res. 2008;86:282–289. doi: 10.1016/j.exer.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Otake M, Schull WJ. Radiation-related posterior lenticular opacities in Hiroshima and Nagasaki atomic bomb survivors based on the DS86 dosimetry system. Radiation Res. 1990;121:3–13. [PubMed] [Google Scholar]
- Palmquist BM, Philipson B, Barr PO. Nuclear cataract and myopia during hyperbaric oxygen therapy. Br J Ophthalmol. 1984;68:113–117. doi: 10.1136/bjo.68.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike CJ, Burdick D, Walencewicz AJ, Glabe CG, Cotman CW. Neurodegeneration induced by beta-amyloid peptides in vitro: the role of peptide assembly state. J Neurosci. 1993;13:1676–1687. doi: 10.1523/JNEUROSCI.13-04-01676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PV, Huang QL, Horwitz J, Zigler JS., Jr Evidence that alpha-crystallin prevents non-specific protein aggregation in the intact eye lens. Biochim Biophys Acta. 1995;1245:439–447. doi: 10.1016/0304-4165(95)00125-5. [DOI] [PubMed] [Google Scholar]
- Roy D, Spector A. Absence of low-molecular-weight alpha crystallin in nuclear region of old human lenses. Proc Natl Acad Sci USA. 1976;73:3484–3487. doi: 10.1073/pnas.73.10.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhoshkumar P, Raju M, Sharma KK. alphaA-crystallin peptide SDRDKFVIFLDVKHF accumulating in aging lens impairs the function of alpha-crystallin and induces lens protein aggregation. PloS One. 2011;6:e19291. doi: 10.1371/journal.pone.0019291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhoshkumar P, Udupa P, Murugesan R, Sharma KK. Significance of interactions of low molecular weight crystallin fragments in lens aging and cataract formation. J Biol Chem. 2008;283:8477–8485. doi: 10.1074/jbc.M705876200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang F, Huang L, Taylor A. Degradation of native and oxidized beta- and gamma-crystallin using bovine lens epithelial cell and rabbit reticulocyte extracts. Curr Eye Res. 1994;13:423–431. doi: 10.3109/02713689408999870. [DOI] [PubMed] [Google Scholar]
- Sharma KK, Kester K. Peptide hydrolysis in lens: role of leucine aminopeptidase, aminopeptidase III, prolyloligopeptidase and acylpeptidehydrolase. Curr Eye Res. 1996;15:363–369. doi: 10.3109/02713689608995826. [DOI] [PubMed] [Google Scholar]
- Sharma KK, Ortwerth BJ. Aminopeptidase III activity in normal and cataractous lenses. Curr Eye Res. 1986a;5:373–380. doi: 10.3109/02713688609025176. [DOI] [PubMed] [Google Scholar]
- Sharma KK, Ortwerth BJ. Isolation and characterization of a new aminopeptidase from bovine lens. J Biol Chem. 1986b;261:4295–4301. [PubMed] [Google Scholar]
- Sharma KK, Kumar RS, Kumar GS, Quinn PT. Synthesis and characterization of a peptide identified as a functional element in alphaA-crystallin. J Biol Chem. 2000;275:3767–3771. doi: 10.1074/jbc.275.6.3767. [DOI] [PubMed] [Google Scholar]
- Simpanya MF, Ansari RR, Suh KI, Leverenz VR, Giblin FJ. Aggregation of lens crystallins in an in vivo hyperbaric oxygen guinea pig model of nuclear cataract: dynamic light-scattering and HPLC analysis. Invest Ophthalmol Vis Sci. 2005b;46:4641–4651. doi: 10.1167/iovs.05-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava OP. Age-related increase in concentration and aggregation of degraded polypeptides in human lenses. Exp Eye Res. 1988;47:525–543. doi: 10.1016/0014-4835(88)90092-9. [DOI] [PubMed] [Google Scholar]
- Su SP, Lyons B, Friedrich M, McArthur JD, Song X, Xavier D, Aquilina JA, Truscott RJ. Molecular signatures of long-lived proteins: Autolytic cleavage adjacent to serine residues. Aging Cell. 2012;11:1125–1127. doi: 10.1111/j.1474-9726.2012.00860.x. [DOI] [PubMed] [Google Scholar]
- Su SP, McArthur JD, Andrew Aquilina J. Localization of low molecular weight crystallin peptides in the aging human lens using a MALDI mass spectrometry imaging approach. Exp Eye Res. 2010;91:97–103. doi: 10.1016/j.exer.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Takemoto L, Boyle D. Increased deamidation of asparagine during human senile cataractogenesis. Mol Vis. 2000;6:164–168. [PubMed] [Google Scholar]
- Takemoto LJ. Differential phosphorylation of alpha-A crystallin in human lens of different age. Exp Eye Res. 1996;62:499–504. doi: 10.1006/exer.1996.0060. [DOI] [PubMed] [Google Scholar]
- Voorter CE, de Haard-Hoekman WA, van den Oetelaar PJ, Bloemendal H, de Jong WW. Spontaneous peptide bond cleavage in aging alpha-crystallin through a succinimide intermediate. J Biol Chem. 1988;263:19020–19023. [PubMed] [Google Scholar]
- Wasselius J, Hakansson K, Abrahamson M, Ehinger B. Cystatin C in the anterior segment of rat and mouse eyes. Acta Ophthalmol. 2004;82:68–75. doi: 10.1046/j.1600-0420.2003.00178.x. [DOI] [PubMed] [Google Scholar]
- Wride MA, Geatrell J, Guggenheim JA. Proteases in eye development and disease. Birth defects research. Part C, Embryo Today: reviews. 2006;78:90–105. doi: 10.1002/bdrc.20063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. LMW peptides ions from control guinea pigs lens as determined MALDI-TOF MS
Supplementary Table 2. LMW peptides ions from HBO treated guinea pigs lens as determined by MALDI-TOF MS.
The αA66-80 peptide- induced α-crystallin aggregate was placed in a well on glass slide with fluorescently labelled αBT162C–Alexa-488 protein and photographed every 2 minute for 6 h under an Olympus IX70 inverted fluorescence microscope. MetaMorph software (Molecular Devices, Inc., Sunnyvale, CA) was used to create a movie of the captured images. The recording shows progressive accumulation of fluorescently labelled αBT162C–Alexa-488 into the pre-formed aggregates.