Abstract
The expression of the water channel protein aquaporin (AQP)-5 in adult rodent and human lenses was recently reported using immunohistochemistry, molecular biology, and mass spectrometry techniques, confirming a second transmembrane water channel that is present in lens fibre cells in addition to the abundant AQP0 protein. Interestingly, the sub-cellular distribution and level of post-translational modification of both proteins changes with fibre cell differentiation and location in the adult rodent lens. This study compares the sub-cellular distribution of AQP0 and AQP5 during embryonic and postnatal fibre cell development in the mouse lens to understand how the immunolabelling patterns for both AQPs observed in adult lens are first established. Immunohistochemistry was used to map the cellular and sub-cellular distribution of AQP5 and AQP0 throughout the lens in cryosections from adult (6 weeks to 8 months) and postnatal (0-2 weeks) mouse lenses and in sections from paraffin embedded mouse embryos (E10-E19). All sections were imaged by fluorescence confocal microscopy. Using antibodies directed against the C-terminus of each AQP, AQP5 was abundantly expressed early in development, being found in the cytoplasm of cells of the lens vesicle and surrounding tissues (E10), while AQP0 was detected later (E11), and only in the membranes of elongating primary fibre cells. During the course of subsequent embryonic and postnatal development the pattern of cytoplasmic AQP5 and membranous AQP0 labelling was maintained until postnatal day 6 (P6). From P6 AQP5 labelling became progressively more membranous initially in the lens nucleus and then later in all regions of the lens, while AQP0 labelling was abruptly lost in the lens nucleus due to C-terminal truncation. Our results show that the spatial distribution patterns of AQP0 and AQP5 observed in the adult lens are established during a narrow window of post natal development (P6-P15) that precedes eye opening and coincides with regression of the hyaloid vascular system. Our results support the hypothesis that, in the older fibre cells, insertion of AQP5 into the fibre cell membrane may compensate for any change in the functionality of AQP0 induced by truncation of its C-terminal tail.
Keywords: lens, water channel, aquaporin, cataract, development
1. Introduction
The transparent ocular lens focuses light on to the retina to form a sharp image. To function as an effective optical element, the adult lens has several unique anatomical features that minimise light scattering. An ordered array of tightly-packed cells minimises extracellular space, blood vessels are absent from lens tissue, and cell organelles are degraded at a specific stage of lens fibre cell differentiation (Bassnett and Beebe. 1992, Bassnett. 1995). The highly ordered structure of the adult lens is first established during embryonic development of the eye. Briefly, the mouse lens develops after the formation of the optic vesicle from the developing forebrain by invagination of the lens placode and optic vesicle at E10.5, forming the lens pit and optic cup, respectively. The lens vesicle forms from the lens pit, and detaches from the optic cup (E11.5-12.5). Cells in the posterior lens vesicle elongate to form primary fibre (PF) cells, and make contact with the anterior epithelium (E13.5). The lens continues to grow pre- and post-partum by the continuous addition of secondary fibre cells, which arise from epithelial cells located at the lens equator that differentiate and elongate to internalise the PF cells (Lovicu and McAvoy. 2005). Because all cells are retained in the lens an inherent age gradient is established, with the oldest PF cells first laid down in the embryo located in the lens centre or nucleus, being surrounded by anucleated mature fibre (MF) cells, which are in turn internalised by nucleated differentiating secondary fibre (DF) cells.
During embryonic and postnatal development nutrients are supplied to the developing lens by a network of capillaries, termed the hyaloid vascular system (HVS). In the mouse, the HVS is first recognised at E10.5, and is completely formed by E13.5. For the developing lens, the most important part of the HVS is the tunica vasculosa lentis, which surrounds the posterior part of the lens. Its apoptotic regression is thought to begin as early as E17.5, and is completed around postnatal day 16 (Mitchell et al. 1998). While the elimination of the HVS promotes transparency in the lens post eye opening by removing a potential light scattering element from the optical pathway, the absence of a blood supply places limits on the delivery of nutrients to, and removal of waste from, the lens that could compromise its ability to maintain its transparency. To circumvent this constraint the adult lens is proposed to operate an internal microcirculation that utilises spatial differences in ion channels and transporters to generate ion and water fluxes that move into the lens via the extracellular space, and leave the lens via an intracellular route mediated by gap junction channels (Mathias et al. 1997, Mathias et al. 2007). It has been proposed that these circulating fluxes deliver nutrients to the deeper lying fibre cells faster than can be achieved by passive diffusion alone. To facilitate this water movement, the lens expresses three isoforms of the aquaporin (AQP) family of water channels; AQP0, AQP1 and AQP5 that exhibit isoform specific differences in water permeability and regulation.
AQP1 is a constitutively active water channel with a high permeability to water. In the lens it is first detected at E17.5 (Varadaraj et al. 2007), and remains exclusively expressed in the membranes of anterior epithelial cells (Hamann et al. 1998). AQP0 is first detected in primary fibre cells at E11.5 (Varadaraj et al. 2007) and is abundantly expressed in lens fibre cell membranes (Broekhuyse et al. 1976). Relative to other AQPs, AQP0 is a poor water channel when tested in oocytes using the rat and mouse AQP0 sequence (Yang and Verkman. 1997), and is thought to also function as a junctional or adhesion protein (Costello et al. 1989, Michea et al. 1995, Palanivelu et al. 2006, Zampighi et al. 1989). Furthermore, in the rat lens, AQP0 sub-cellular distribution changes with fibre cell age (Figure 1A) and the protein undergoes post-translational truncation (Grey et al. 2009). In cortical fibre cells, antibodies directed against the AQP0 C-terminus label the cell membrane. In DF cells undergoing nuclear degradation, AQP0 coalesces into large plaque-like structures. As cell nuclei degrade, a loss of labelling with this antibody is evident, possibly due to epitope masking. In MF cells, the AQP0 C-terminus antibody labels cell membranes strongly, however, in the lens nucleus the signal is lost, indicating truncation of the AQP0 C-terminus, a finding which has been confirmed by mass spectrometry (Grey et al. 2009). C-terminal truncation removes the ability of AQP0 to interact with cytoskeletal proteins (Lindsey Rose et al. 2006, Wang and Schey. 2011), and functional modulators such as AKAP2 (Gold et al. 2012), and possibly calmodulin (Nemeth-Cahalan and Hall. 2000). While it has been shown via high resolution cryo-electron microscopy that AQP0 C-terminal truncation occludes the water pore in AQP0 junctions assembled in vitro (Gonen et al. 2004, Harries et al. 2004, Palanivelu et al. 2006), water permeability is maintained in truncated forms in AQP0 expressed in exogenous systems (Ball et al. 2003, Kumari and Varadaraj. 2014). Regardless of this inconsistency, C-terminal truncation must change AQP0 functionality in the lens nucleus relative to the cortex.
Figure 1. Immunolabelling patterns of AQP0 and AQP5 in adult rat lenses.
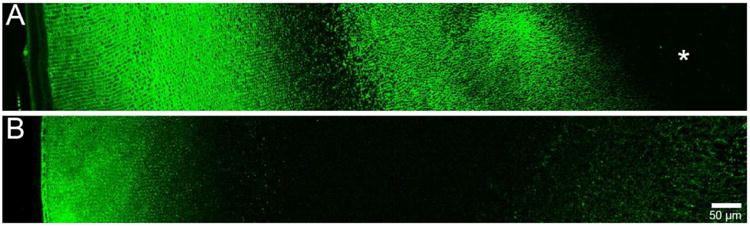
Using antibodies directed against the C termini of AQP0 (A) and AQP5 (B), the spatial distributions of each protein in the adult rat lens are shown. AQP0 is predominantly membranous throughout the lens, and undergoes truncation in the lens nucleus (asterisk). AQP5 is predominantly cytoplasmic in the lens cortex, and associated with cell membranes in the nucleus. Adapted from (Grey et al. 2009)
AQP5 is a regulated water channel that shuttles to the membrane in salivary glands. Recently, the expression of AQP5 protein in adult lens fibre cells has been confirmed (Bassnett et al. 2009, Wang et al. 2008) and its sub-cellular distribution mapped using confocal microscopy (Grey et al. 2013, Kumari et al. 2012). Interestingly, AQP5 sub-cellular distribution also changed with fibre cell age, albeit in contrast to AQP0. In rat lens epithelial and DF cells, AQP5 was localised to the cell cytoplasm, while in MF cells, AQP5 was found in the cell membrane (Figure 1B). In the mouse lens, the sub-cellular distribution of AQP5 may be determined by changes to its phosphorylation status that are driven by phosphokinase A (Kumari et al. 2012). Furthermore, AQP5 may function to preserve osmotic balance and transparency in the lens under hyperglycaemic stress (Kumari and Varadaraj. 2013). Clearly the role that AQP5 plays in the maintenance of lens transparency remains to be elucidated.
Since the sub-cellular distribution of AQP5 and the truncation of AQP0 differed in different regions of the adult lens, we have in this study utilised immunolabelling with epitope specific antibodies to systematically compare the temporal and spatial distribution of AQP5 to AQP0 during embryonic and post natal development. This comparison showed that AQP5 was expressed at an earlier stage in lens development than AQP0, and that it was predominantly located in the cell cytoplasm of embryonic lenses. However by P6, AQP5 was increasingly found localised to the cell membranes of MF cells, while AQP0 in this central region of the mouse lens was abruptly truncated. Together these results show that the spatial distribution patterns observed for AQP0 and AQP5 in the adult lens are established during a narrow window of post natal developments (P6 to P15) that coincides with withdrawal of the HVS. These observations support our earlier hypothesis that membrane insertion of AQP5 compensates for any change in the function of AQP0 induced in the lens nucleus by truncation of the AQP0 C-terminus (Grey et al. 2013).
2. Materials and Methods
2.1 Animals
Mouse embryos were a kind gift from Drs Robb de Iongh and Frank Lovicu. At least 10 tissue sections from two mice at each embryonic stage (E10, E11, E14, E15, E16, E18.5) were analysed. For the crucial early post-natal period, at least 15 tissue sections from four mice at each stage (P3, P6, P9, P15) were analysed. For adult mice, at least 15 tissue sections from three mice at each stage (6 week, 3 month, 8 month) were analysed. Stages representative of significant distribution changes to AQP5 and AQP0 are presented in Results.
2.2 Reagents
An affinity purified anti-AQP5 antibody (Cat #: AB15858), directed against the final 17 amino acids (rat sequence) of the C-terminus of the protein, was obtained from Millipore (Billerica, MA). Affinity purified anti-AQP0 (Cat #: AQP01-A), directed against the final 17 amino acids (human sequence) of the C-terminus of the protein, was obtained from Alpha Diagnostic International, Inc. (San Antonio, TX). A polyclonal antibody directed against the whole AQP0 protein, developed in the Schey Lab, was used to detect full-length and truncated forms of AQP0. Secondary antibodies (goat anti-Rabbit Alexa 488), and wheatgerm agglutinin conjugated to a fluorophore (WGA-TRITC) for labelling of the cell membrane, were obtained from Thermo Fisher Scientific (Waltham, MA). For fluorescently labelling lens fibre cell nuclei, DAPI was obtained from Sigma-Aldrich (St Louis, MO). Phosphate buffered saline (PBS) was prepared fresh from PBS tablets. Unless otherwise stated all other chemicals were from Sigma. All experiments described were carried out in accordance with the EU Directive 2010/63/EU for animal experiments, and approved by the University of Auckland Animal Ethics Committee (#001303).
2.3 Tissue preparation
Embryos were obtained from superovulated FVB/N mice (E10 and E11) or wild type C57 black mice (E16 and post natal stages). Embryos were fixed in 10% neutral buffered formalin (r.t., 2 hrs) then washed three times in 70% ethanol. The tissue was then dehydrated in a series of graded ethanol washes, prior to clearing in xylene. Finally, tissue was embedded in paraffin wax, and 5 μm-thick paraffin sections collected using a microtome (Ultracut UCT, Leica Microsystems, Germany). Sections were deparaffinised by washing with xylene followed by a wash in 100% ethanol and then water. An antigen retrieval step, using treatment in citrate buffer (pH 6) in a pressure cooker for 1 hr followed by 20 mins in PBS, was applied prior to immunolabelling.
For postnatal and adult mouse tissue, lenses were removed from the different ages of C57 Black mice immediately following death, fixed with 0.75% paraformaldehyde at room temperature (r.t.) for 24 hours, and prepared for cryosectioning using published protocols (Jacobs et al. 2003). For sectioning, whole lenses were mounted on, and encased in, optimal cutting temperature (OCT) compound (Tissue-Tek; Sakura Finetek, Zoeterwoude, The Netherlands) and snap frozen in liquid nitrogen for 15-20s. Lenses were cryosectioned at −19°C using a cryostat (CM3050, Leica Microsystems, Germany). 14 μm-thick equatorial cryosections were transferred onto microscope slides and subjected to immunohistochemistry protocols.
2.4 Immunolabelling
Immunolabelling was carried out according to established protocols (Grey et al., 2009). Briefly, sections were first incubated in blocking solution (3% bovine serum albumin, 3% normal goat serum in PBS) for 1 hour (r.t.). After washing in PBS, sections were incubated in primary antibody in blocking solution (1:100) overnight at 4 °C. Slides were washed in PBS and incubated for 1.5 hours, in the dark (r.t.), with fluorescent secondary antibodies in blocking solution (1:200) containing 0.125ug/ml DAPI. Where necessary, sections were then incubated with WGA-TRITC in PBS (1:100) for 1 hour (r.t.) to label cell membranes. Slides were coverslipped using VectaShield HardSet™ anti-fade mounting medium (Vector Laboratories, Burlingame, CA).
2.5 Confocal Microscopy
Images of each fluorophore-staining pattern were acquired using a laser scanning confocal microscope (Olympus FV1000, Tokyo, Japan) equipped with FluoView 2.0b software. For presentation, labelling patterns were pseudo-coloured and combined using Adobe Photoshop software (version CS6, Adobe Systems Inc., San Jose, CA).
2.6 MALDI-TOF mass spectrometry of mouse AQP0
Lenses were extracted from 6-week-old C 57/black mice immediately following death, and dissected in 37 °C PBS into cortex and nucleus regions. Tissue was homogenised in buffer containing 10 mM Tris and 1 mM EDTA. Membrane and cytoplasmic fractions were separated via centrifugation at 16,000g for 30 min at 4 °C in an Eppendorf 5402 centrifuge. The supernatant was discarded, and the pellet containing lens membrane proteins washed in 4 M urea, 7 M urea, and 100 mM NaOH to remove bound crystallin proteins. Membrane proteins were then washed with water prior to delipidation in 95% EtOH at −20 °C overnight. Finally, membrane proteins were washed in water, solubilised in 7:2 formic acid:isopropanol, and spotted onto a MALDI plate using saturated 2,5-dihydroxybenzoic acid in 70% ACN/0.1% TFA as matrix. Samples were analysed using a Voyager-DE™ PRO MALDI-TOF mass spectrometer (Applied Biosystems, Inc., Foster City, CA). Prior to data collection, a linear external calibration was performed using the mass calibrants: bovine insulin (Mr=5734), Escherichia coli thioredoxin (Mr=11,674) and equine apomyoglobin (Mr=16,952). For presentation, acquired MALDI mass spectra underwent smoothing and baseline subtraction using Data Explorer software.
3. Results
Regional differences in the subcellular distribution and C-terminal truncation of AQP5 and AQP0 were evident in adult rat lenses (see Introduction, Figure 1) when the expression of the two AQPs was mapped by immunohistochemistry using isoform specific antibodies that recognise the C-terminal tails of the two water channels (Grey et al. 2009) which have been used previously (AQP0, (Grey et al. 2009)) and verified using AQP5 knock out animals (Kumari et al. 2012). In the present study, maps of the cellular distribution of AQP5 during embryonic and postnatal lens development were collected for the first time, and compared to AQP0 distributions to understand how the distribution of both AQPs are established in the adult lens. The high resolution imaging approach also allowed the sub-cellular distribution of AQP5 and AQP0 in lens development to be established, allowing a model of how the two AQPs may contribute to water transport in the developing lens to be advanced.
3.1 Comparison of AQP0 and AQP5 expression patterns during embryonic development
At embryonic stage E10 (the earliest stage studied), the developing mouse eye contains the lens vesicle. While AQP0 expression was not detected in the lens vesicle at E10 (Figure 2A, B), it was detected in the cell membranes of elongating PF cells at E11 (Figure 2C), indicating that this is a significant lens developmental event to potentially enhance membrane water permeability and/or cell adhesion in PF cells. This onset of AQP0 protein expression is in agreement with that found in a previous investigation (Varadaraj et al. 2007). In contrast, AQP5 protein was strongly expressed in the lens vesicle and optic cup at E10 (Figure 2D), indicating that AQP5 protein translation occurs earlier in mouse lens development than AQP0. Interestingly, AQP5 sub-cellular distribution in lens vesicle cells was cytoplasmic at E10 and E11 (Figure 2E, F), suggesting that it does not contribute to trans-membrane water permeability at this early developmental stage.
Figure 2. AQP0 and AQP5 protein distribution in the lens vesicle.
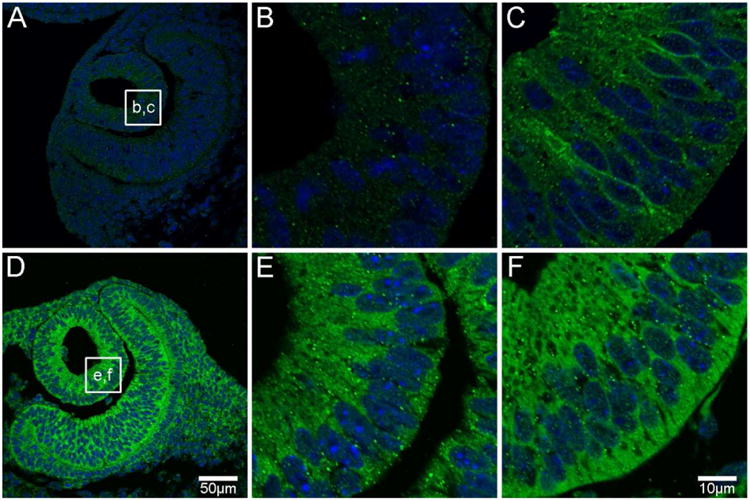
At developmental stage E10, AQP0 protein expression is not evident (A, B), while AQP5 is expressed predominantly in the cytoplasm of lens vesicle cells (D, E). By E11, AQP0 protein expression is evident in the membrane of elongating primary fibre (PF) cells (C). AQP5 continues to be expressed in the cytoplasm of PF cells (F). AQP proteins were labelled with specific antibodies (green) and cell nuclei were labelled with DAPI (blue). Boxes in A and D show locations of zoomed up images at E10 (B, E) and E11 (C, F).
This pattern of membranous AQP0 and cytoplasmic AQP5 labelling that is initially established at E11, was maintained throughout subsequent stages of embryonic lens development (E12 to birth ∼E18.5). An example of the observed spatial distribution of AQP0 and AQP5 during this period of development is shown for E16 (Figure 3). AQP0 was localised to the membranes of all fibre cells in the bulk of the lens, but not the overlying anterior epithelium (Figure 3A). Higher magnification images confirmed its membranous localisation in DF cells in the outer cortex (Figure 3B), and MF (Figure 3C) and PF cells (Figure 3D) that comprise the lens nucleus. In contrast, AQP5 was localised to both epithelial and fibre cells and was predominantly cytoplasmic (Figure 3E). The highest signal intensity of this cytoplasmic labelling for AQP5 was detected in DF cells located in the lens cortex (Figure 3F). While lower signal intensities were observed in deeper regions of the lens that contained MF (Figure 3G) and PF cells (Figure 3H), this labelling in the lens nucleus remained cytoplasmic. In summary, AQP0 was first expressed at E11 and its distribution throughout the embryonic mouse lens was characterised by an abundant membranous signal for AQP0 in lens fibre cells, which indicates that during embryonic development there is no truncation of AQP0. In contrast, AQP5 was detected in epithelial and fibre cells prior to the onset of AQP0 expression, but was predominantly cytoplasmic in all regions of the embryonic lens and, like AQP0, it was not truncated.
Figure 3. AQP0 and AQP5 protein distribution in the embryonic lens.
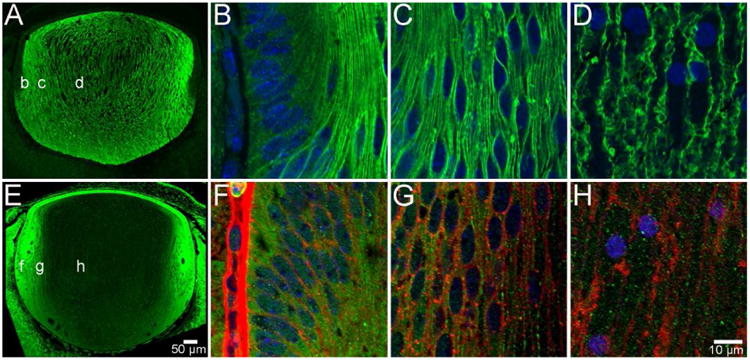
At developmental stage E16, AQP0 is expressed exclusively in fibre cells in all regions of the lens, but is not detected in the anterior epithelium (A). Furthermore, it is localised to the cell membrane of DF cells in the periphery (B), cortex (C) and PF cells in the nucleus (D). In contrast, AQP5 is localised to the cytoplasm of lens epithelial cells and fibre cells in all regions of the lens (E). This distribution continues in DF cells in the periphery (F), cortex (G), and PF cells in the nucleus (H), where cell membranes have been labelled with WGA (red) for clarity. AQP proteins were labelled with specific antibodies (green) and cell nuclei were labelled with DAPI (blue).
3.2 Comparison of AQP0 and AQP5 expression patterns during postnatal development
While embryonic tissue sections were prepared from paraffin embedded tissue, cryosections prepared from postnatal tissue were used to investigate AQP spatial distributions in postnatal development. To test whether the different tissue preparation strategies might alter the immunolabelling patterns of AQP0 or AQP5, lenses from postnatal day 3 were prepared using both protocols. No differences between the immunolabelling patterns obtained using paraffin and cryosections were observed, confirming that the different tissue fixation and preparation methods did not affect AQP immunolabelling (data not shown).
Similar distributions of AQP0 and AQP5 to those observed in late embryonic development were detected in equatorial cryosections from P3 lenses (Figure 4). Using the antibody directed against the AQP0 C terminus, AQP0 signal was detected in the membrane of DF(Figure 4B, C), MF (Figure 4D), and PF cells (Figure 4E), with no evidence of signal decrease or loss in the lens nucleus. AQP5 was localised predominantly to the fibre cell cytoplasm in the epithelium and DF (Figure 4F, G), MF (Figure 4H) and PF (Figure 4I) cells.
Figure 4. AQP0 and AQP5 protein distribution in P3 lenses.
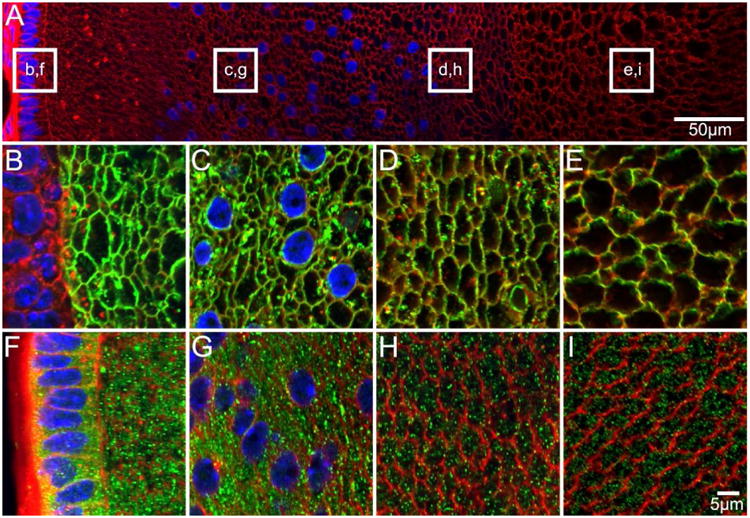
An overview of an equatorial section from a P3 lens labelled with the membrane marker WGA (A, red) to indicate where higher magnification images for AQP0 (B-E, green) and AQP5 (F-I, green) were obtained. AQP0 is detected in fibre cell membranes of all lens regions, while AQP5 is detected predominantly in the fibre cell cytoplasm in all lens regions.
Towards the end of the first week of postnatal development (P6), changes in lens AQP immunolabelling first became evident (Figure 5). Using an antibody directed against the AQP0 C-terminus, AQP0 was detected in the fibre cell membranes in all lens regions (Figure 5B-E, F-I). Interestingly, the signal intensity for AQP0 membrane labelling decreased in fibre cells located in the nucleus (Figure 5E, I) relative to more peripheral lens fibre cells using identical imaging parameters, which suggested that the AQP0 C-terminus was undergoing truncation in the lens nucleus, a finding which has previously been reported in the adult rat lens (Grey et al. 2009). In contrast to AQP0, AQP5 labelling localised predominantly to the cytoplasm of lens epithelial cells (Figure 5J, N) and fibre cells in all regions of the lens (Figure 5K-M, O-Q), although the AQP5 labelling became less diffuse and more punctate in the cytoplasm and began to associate with the cell membranes of DF cells (Figure 5K-L, O-P).
Figure 5. AQP0 and AQP5 protein distribution in early postnatal (P6) lenses.
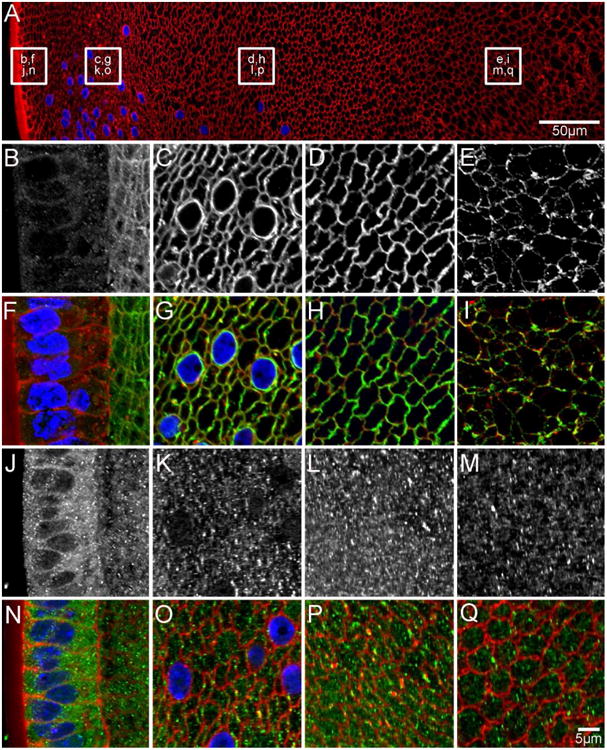
An overview of an equatorial section from a P6 lens labelled with the membrane marker WGA (A, red) to indicate where higher magnification images for AQP0 (B-E in greyscale, F-I in green) and AQP5 (J-M in greyscale, N-Q in green) were obtained. AQP0 localises to the fibre cell membrane in all lens regions. In the nucleus, using an antibody to the AQP0 C-terminus (E/I) the initial truncation of the AQP0 C-terminus is evident due to the lower signal intensity observed in the region. In contrast, AQP5 is predominantly cytoplasmic in all lens regions, although some labelling associated with the membranes of DF cells in the lens cortex is starting to become evident (K/L, O/P). Cell nuclei (blue) are labelled with DAPI.
These changes in the relative distributions of AQP0 and AQP5, first initiated at P6 continued throughout the period of postnatal development and representative immunolabelling patterns of lens fibre cell AQPs during this period are shown for P15 (Figure 6). Labelling for AQP0 remained localised to fibre cell membranes in peripheral and cortical lens regions (Figure 6B-E, G-J), but now with significant loss of signal intensity in the lens nucleus indicating extensive truncation of the C-terminal tail of AQP0 (Figure 6F, K). While AQP5 remained localised predominantly to the cytoplasm of the most superficial DF cells in the lens outer cortex (Figure 6L-M, Q-R), its distribution in the deeper cortex and nucleus was markedly different. Signal for AQP5 was localised to both the cell membrane and cytoplasm in the cortex (Figure 6N, S), and exclusively to the fibre cell membrane in discrete puncta in the deeper cortex (Figure 6O, T), and in more continuous membrane regions in the lens nucleus (Figure 6P, U). This indicates a translocation of the AQP5 protein from the cytoplasm to the membrane in the anucleated MF and PF cells that make up the lens nucleus has occurred during postnatal development.
Figure 6. AQP spatial distributions continue to change in postnatal (P15) development.
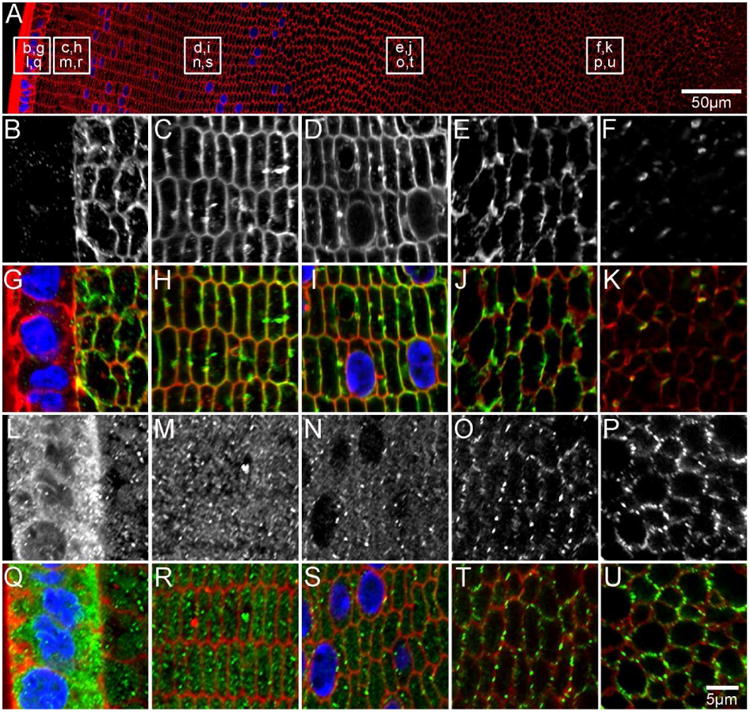
An overview of an equatorial section from a P15 lens labelled with the membrane marker WGA (A, red) to indicate where higher magnification images for AQP0 (B-F in greyscale, G-K in green) and AQP5 (L-P in greyscale, Q-U in green) were obtained. Full-length AQP0 is absent in the epithelium (B/G), and is associated with the fibre cell membrane in the lens cortex (C-E/H-J). A loss of antibody labelling in the lens nucleus is indicative of AQP0 C-terminal truncation (F/K). AQP5 is cytoplasmic in lens epithelium (L/Q) and DF cells in the lens periphery (M/R). In the cortex (N/S), AQP5 is localised to both the cytoplasm and cell membrane, while it is associated exclusively with the cell membrane in the inner cortical (O/T) and nuclear (P/U) regions. Cell nuclei (blue) are labelled with DAPI.
3.3 Comparison of AQP0 and AQP5 expression patterns in the adult lens
To confirm that signal loss using the antibody directed against the AQP0 C-terminus was due to truncation, immunolabelling patterns in the cortex and nucleus of P6, P15, and 6 week-old mouse lenses were compared (Figure 7). Labelling for the AQP0 C-terminal antibody was detected in the mouse cortex as expected at P6 (Figure 7A), P15 (Figure 7E) and 6 weeks (Figure 4I), and was decreased at P6 (Figure 7B and Figure 5 E/I) and absent at P15 (Figure 7F) and 6 weeks (Figure 7J). Using a polyclonal antibody directed against the whole AQP0 protein, labelling was detected in the cortex (Figure 7C, G, K) and nucleus (Figure 7D, H, L) at each stage of post-natal development analysed. To validate the immunolabelling results, 6 week-old mouse lenses were dissected into cortex and nucleus, and enriched for membrane proteins using centrifugation. MALDI mass spectrometry of these preparations detected full-length AQP0 in the mouse cortex (Figure 7M) at the predicted m/z 28194 +/- 0.03%. In the mouse nucleus, abundant signal for C-terminally truncated AQP0 was detected, in addition to some full length AQP0 (Figure 7N). The most abundant forms of truncated AQP0 detected were 1-234 and 1-238, which is similar to the forms of AQP0 detected in the rat lens nucleus (Grey et al. 2009). The forms of AQP0 detected, along with their predicted and observed m/z are presented in Table 1.
Figure 7. AQP0 is truncated in the postnatal mouse lens nucleus.
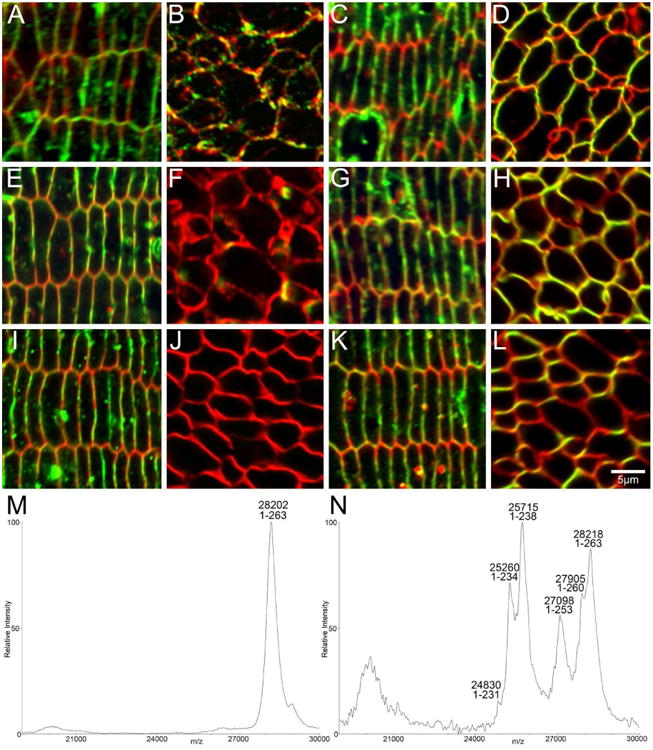
High magnification confocal microscopy of equatorial sections of P6 (A-D), P15 (E-H), and 6 week-old (I-L) mouse lenses show AQP0 (green) and cell membrane marker WGA (red) in DF cells and MF cells in the lens nucleus. Using an antibody to the AQP0 C-terminus, signal is detected in DF cells in each age (A, E, I), while signal is reduced in the P6 lens nucleus (B) and is lost in the lens nucleus of P15 (F) and 6 week-old mice (J). Using a polyclonal antibody to the whole protein, signal for AQP0 in the cortex (C, G, K) and nucleus (D, H, L) is maintained in each age, indicating that the reduction/loss of signal observed in the lens nucleus using the AQP0 C-terminus antibody is due to truncation. MALDI mass spectrometry of cell membrane preparations of microdissected lens regions of a 6 week-old mouse showed that full-length AQP0 was detected in the cortex (M), while truncated forms of AQP0 were the most abundant signals detected in the lens nucleus (N), thereby validating the differential labelling observed with the two antibodies in the nucleus.
Table 1. Predicted and observed m/z values for mouse AQP0.
| Mouse AQP0 form | Predicted m/z | Observed m/z | % error |
|---|---|---|---|
| 1-263 Cortex | 28194 | 28202 | 0.03 |
| 1-263 Nucleus | 28194 | 28218 | 0.09 |
| 1-260 | 27882 | 27905 | 0.08 |
| 1-253 | 27085 | 27098 | 0.05 |
| 1-238 | 25673 | 25715 | 0.12 |
| 1-234 | 25232 | 25260 | 0.11 |
| 1-231 | 24831 | 24830 | 0.00 |
After 6 weeks of postnatal development, when mice are considered young adults, both AQP0 C-terminal truncation in the lens nucleus and AQP5 translocation to the fibre cell membrane had continued (Figure 8). C-terminal labelling for AQP0 was decreased in the inner cortex (Figure 8E) and was absent from the lens nucleus (Figure 8F), indicating further C-terminal truncation had occurred with advancing age. In contrast, AQP5 translocation to the fibre cell membrane, and a lack of cytoplasmic signal, was evident in cortical regions (Figure 8I) as well as in inner cortical and nuclear regions (Figure 8J, K). Since ongoing changes in the immunolabelling patterns of the two fibre cell AQPs in postnatal lens development were observed, the distributions of these two proteins were investigated in an aged mouse lens (8 months, Figure 9).
Figure 8. AQP5 is localised to cortical fibre cell membranes in adult mouse lenses.
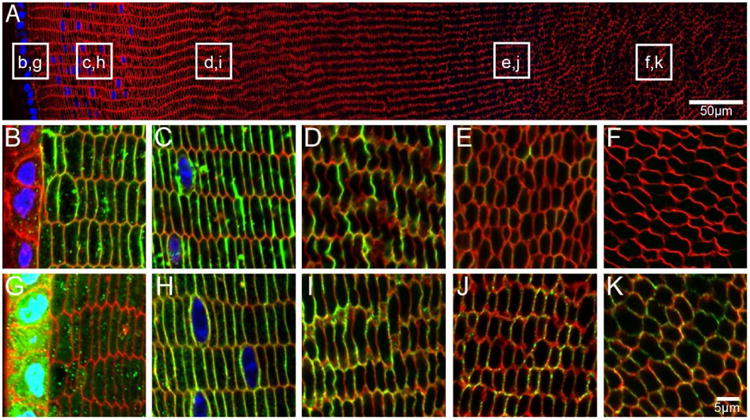
An overview of an equatorial section of a 6 week-old mouse lens labelled with WGA (red, cell membranes) and DAPI (blue, nuclei) indicates where higher magnification images of AQP0 (B-F, green) and AQP5 (G-K, green) are taken from. AQP0 is localised to the fibre cell membrane in peripheral, cortical and inner cortical fibre cells. Signal for AQP0 is absent in the nucleus indicating C-terminal truncation. In contrast, AQP5 labelling is cytoplasmic in peripheral fibre cells, but is localised to the fibre cell membrane in all other lens regions.
Figure 9. AQPs are localised to fibre cell membranes in aging mouse lenses.
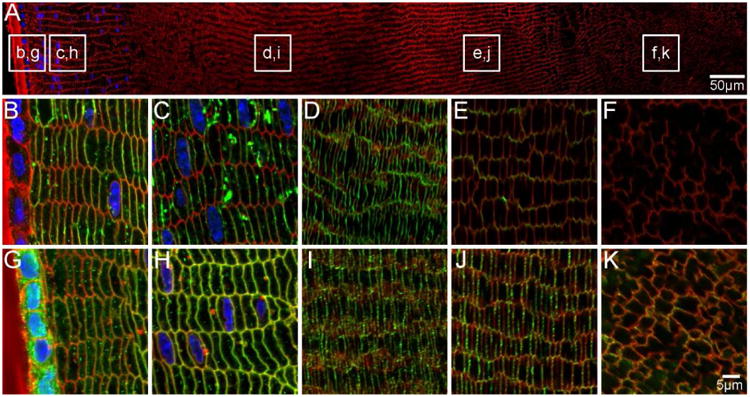
An overview of an equatorial section of an 8 month-old mouse lens labelled with WGA (red, cell membranes) and DAPI (blue, nuclei) indicates where higher magnification images of AQP0 (B-F, green) and AQP5 (G-K, green) are taken from. AQP0 is localised to fibre cell membranes in peripheral and cortical lens fibres. Weaker signal in the inner cortex may indicate truncation of the AQP0 C-terminus (E). In the nucleus AQP0 is truncated, as indicated by a lack of signal (F). In contrast, AQP5 is localised to the fibre cell membranes throughout the lens (G-K). Some cytoplasmic labelling is maintained in epithelial and peripheral lens fibres (G).
The spatial distribution of AQP0 in the aged mouse lens appeared similar to the younger adult lens in that AQP0 was localised to the membrane of peripheral and cortical lens fibre cells (Figure 9B-D). A low level of signal was detected in the inner cortex (Figure 9E), and no signal was detected in fibre cells of the lens nucleus (Figure 9F). In contrast, AQP5 was detected predominantly in the fibre cell membrane in all lens regions, while cytoplasmic signal was also detected in the lens epithelium and peripheral fibre cells (Figure 9G). Since no evidence of signal loss that could be attributed to AQP5 C-terminal truncation in the lens nucleus was detected, this would suggest that the function of AQP5 is maintained in this region of the lens. However, further post-translational modifications that alter function cannot be ruled out and will require further investigation. Regardless of the functional implications, our data show that while AQP5 translocation to the fibre cell membrane initially coincides with truncation of the C-terminus of AQP0 in the postnatal lens nucleus, in adult lenses AQP5 continues to translocate to the fibre cell membrane throughout lens aging in the absence of further AQP0 truncation in the lens cortex.
4. Discussion
The goal of the present study was to determine how the spatial differences in the expression patterns of AQP0 and AQP5 observed in adult lens fibre cells (Figure 1) are initially established, and to determine whether they changed during lens development, differentiation and growth. To achieve this we have performed immunohistochemical mapping of the spatial distributions of both AQPs in embryonic (Figures 2&3), postnatal (Figures 4, 5&6) and old mouse lenses (Figures 8&9), using antibodies directed against the C-terminus of the two water channels. While AQP0 expression during mouse lens development has been reported previously (Varadaraj et al., 2007), this is the first study to examine AQP5 during embryonic and postnatal lens development. Consistent with Varadaraj et al., AQP0 expression was first detected at E11 in the membranes of primary fibre cells that had started to elongate to fill the lens vesicle (Figure 2C). AQP5 preceded AQP0 expression and was detected at E10, but only in the cell cytoplasm (Figure 2D&E). This pattern of membranous AQP0, and cytoplasmic AQP5 labelling was maintained throughout embryonic development (Figure 3) and into the early postnatal period and suggests that due to its cytoplasmic location AQP5 does not contribute significantly to the water permeability of fibre cell membranes during this period of development. From P6 a change in the relative labelling patterns of the two AQPs began to emerge. A loss of AQP0 labelling (Figure 5&6) by the C-terminal antibody was observed in the nucleus, that was subsequently confirmed to be due to loss of the epitope via truncation of the C-terminus of AQP0 (Figure 7), was mirrored by an increase in the association of AQP5 with the membranes of PF and MF cells first in the nucleus and then progressively in DF cells in the outer cortex. These changes in the observed expression patterns for AQP5 and AQP0 are correlated with key milestones in lens development in Table 2.
Table 2. Observed AQP0 and AQP5 sub-cellular distribution changes correlated to major milestones in lens development.
| Age | Milestone | HVS status | Protein Expression Patterns | |||
|---|---|---|---|---|---|---|
| AQP0 | AQP5 | |||||
| Cortex | Nucleus | Cortex | Nucleus | |||
| E11.5 | Lens Vesicle Formation | Forming | M | n/a | C | n/a |
| E13.5 | Vesicle Lumen Disappears | Present | M | M | C | C |
| E17.5 | AQP1 protein expressiona | Present | M | M | C | C |
| P0 | Birth | Regressing | M | M | C | C |
| P14 | Eye Opening | Regressing | M | T | C | M |
| P21 | Weaning | Absent | M | T | C/M | M |
| P30 | Maximal AQP1 expressiona | Absent | M | T | C/M | M |
| P42 | Animal reaches adulthood | Absent | M | T | M | M |
data from (Varadaraj et al., 2007)
C = cytoplasmic, M = membranous, T = truncated
From our observations it is evident that both AQPs are subjected to distinctly different post translational modifications that are abruptly initiated during the period of post natal development that precedes eye opening. It has been extensively reported in a variety of species that AQP0 in MF cells undergoes a series of cleavage events that removes up to residues 232-263 (rat) (Grey et al. 2009, Schey et al. 1999) or 247-263 (human) (Schey et al. 2000) from its C-terminal tail. Such a large truncation would be expected to change either the properties of AQP0, or its interaction with other proteins. Indeed it has been proposed that AQP0 C-terminal truncation occludes the water pore, (Gonen et al. 2004, Harries et al. 2004, Palanivelu et al. 2006), however, water permeability was shown to be maintained in C-terminally truncated AQP0 up to residue 243 (Ball et al. 2003, Kumari and Varadaraj. 2014). The major truncation products observed in the rodent lens, (AQP0)1-234 and (AQP0)1-238, (see Figure 7 and (Grey et al. 2009)) did not traffic to the membrane and therefore their ability to transport water could not be effectively tested. However, irrespective of the direct consequence of AQP0 C-terminal truncation on its inherent water permeability, the interaction of AQP0 with cytoskeletal elements, and/or regulatory proteins such as AKAP2 and calmodulin that bind to the C-terminus of AQP0, would be lost in MF cells in the lens nucleus and suggests that truncation of AQP0 changes its functionality relative to the cortex. In this study we show for the first time that AQP0 truncation begins at P6 (Figure 5E), suggesting that this is a developmentally programmed truncation and not the result of age-related, non-enzymatic peptide backbone cleavage, as has been reported for AQP0 in other species (Schey et al. 2000). The observed AQP0 C-terminal truncation is mirrored by a translocation of AQP5 to the membranes of MF cells (Figure 6). This sequence of events suggests that the membrane insertion of AQP5 into the membrane may be compensating for a loss in functionality provided by full length AQP0 in the nucleus of the lens. Although AQP5 is less abundant than AQP0 (Grey et al. 2013), because it is 20× more permeable to water than AQP0 (Yang and Verkman. 1997), it is interesting to speculate that AQP5 insertion maintains or even increases water permeability in the lens nucleus.
While the translocation of AQP5 from the cytoplasm to the membranes of fibre cells first occurs at P6 when AQP0 is first truncated in the lens nucleus, with advancing age AQP5 also translocates to the cell membranes of cortical and peripheral DF cells in regions where AQP0 C-terminal truncation does not occur (Figure 8). Thus we can define two temporally and spatially resolved zones of AQP5 translocation. The first involves an initial translocation event that starts around P6 and results in the cytoplasmic pool of AQP5, originally expressed during the previous phases of embryonic and postnatal development, to be inserted into the membranes of MF cells in the nucleus, and then later into the membranes of DF cells in the cortex. The second zone occurs as a result of the differentiation of epithelial cells into fibre cells, a process that continues throughout life and adds additional layers of fibres cells to the lens. Post eye opening these newly derived fibre cells contain a substantial pool of cytoplasmic AQP5 labelling which is maintained as the DF cells elongate towards both poles of the lens and increasingly become internalised by the formation of additional superficial layers of younger DF cells. However, at a discrete stage in this continuous process of fibre cell differentiation there is a translocation of AQP5 to the cell membrane.
Although our data clearly show that during distinct phases of lens development, differentiation and growth AQP5 undergoes translocation from a cytoplasmic pool to the membrane, the signalling pathways that trigger this transition remain unclear. In other tissues, however, there is evidence that AQP5 can increase water permeability by being shuttled to the plasma membrane. In rat salivary glands, AQP5 translocates to the apical membrane in response to elevated intracellular calcium via an acetycholine-mediated pathway (Ishikawa et al. 1998). Elevated intracellular calcium is also proposed to mediate AQP5 translocation to the membrane in sweat glands (Inoue et al. 2013). In MDCK cells exposed to a PKA agonist, mouse AQP5 membrane localisation decreased, suggesting that phosphorylation regulates the sub-cellular localisation of AQP5 (Kumari et al. 2012). Peptides that encompass the two predicted phosphorylation sites of AQP5 (S156, T259) were not detected in the mouse, bovine, or human lens membrane proteome by mass spectrometry (Bassnett et al. 2009, Grey et al. 2013, Wang et al. 2008). Therefore, further investigation is required to establish the phosphorylation status of cytoplasmic and fibre cell membrane-localised AQP5 in order to understand the mechanism for the redistribution of the protein in the developing and aging mouse lens observed in the present study.
Our temporal and spatial mapping of the relative changes in the subcellular distribution and modification of AQP0 and AQP5 raises questions about how changes in the expression of the two water channels affects the overall function of the lens. A theoretical model of the lens microcirculation system predicts (Donaldson et al. 2010, Mathias et al. 1997, Mathias et al. 2007), and recent experiments (Candia et al. 2012, Gao et al. 2011, Vaghefi et al. 2011) have confirmed, that a circulating water flux enters the lens at its poles and exits the lens via an intracellular pathway mediated by gap junction channels that directs the water flux to the lens equator. Since in this system water follows ion fluxes and crosses cell membranes via water channels, changes in the sub-cellular localisation and functionality of AQPs in different regions of the lens may contribute to the directionality of the water fluxes generated by the circulation system. From previous and the present study, AQP0 expression was only detected from E11, while AQP5 was detected at E10, but only in the cell cytoplasm, suggesting that AQP5 does not contribute to cell membrane water permeability at this early developmental stage. Given that the size of the E10/E11 lens is small, and that during embryonic and early postnatal development the lens is nourished by the HVS, this suggests that nutrient delivery to the developing lens can be achieved in the absence of a microcirculation system. This is consistent with a previous study that proposed the lens microcirculation was not initiated until later in embryonic development (E17.5), when AQP1 protein is first expressed in the lens epithelium (Varadaraj et al. 2007). The same study suggested that the primary function of AQP0 in lens development is structural, providing cell-to-cell adhesion to facilitate development of the ordered cellular architecture of the adult lens, while AQP0 takes on a water transport role later in postnatal development to maintain homeostasis in the growing lens fibre cell mass. This hypothesis is consistent with studies that show that AQP0 is essential for the ordered development of the lens since mutations in, or removal of, the AQP0 gene lead to significant lens structural defects (Al-Ghoul et al. 2003, Shiels et al. 1991).
As a result of our mapping of the spatial distribution of AQP0 and AQP5 we can now add to this hypothesis. During embryonic development, full-length AQP0 is expressed throughout fibre cell membranes in all lens regions, while AQP5 is cytoplasmic in all lens regions. The most significant spatial distribution changes and modifications to lens fibre cell AQPs take place during postnatal development to accommodate the growing lens fibre cell mass and to prepare for eye opening. At P6, AQP0 C-terminal truncation and AQP5 translocation to the membranes is initiated in MF cells located in the lens nucleus (Figure 5). In parallel to the changes that are occurring to the AQP0, Evans et al., have reported similar changes to the gap junction protein, Cx50, during early postnatal development in the mouse lens (Evans et al. 1993). Cx50, together with Cx46, form the intercellular pathway that directs ion and water fluxes from the lens nucleus to surface cells at the lens equator (Jacobs et al. 2004). At around P2 the large broad side Cx50 gap junction plaques seen in embryonic fibre cells begin to disperse into progressively smaller structures within the lens nucleus, and then at around P6 Cx50 in this region undergoes a truncation of its C-terminus (Evans et al. 1993). By comparing the gap junctional permeability measured in Cx46 and Cx50 knock out and knock in lenses it has been shown that the truncation of Cx50 renders it non-functional in the lens nucleus (Baldo et al. 2001, Wang et al. 2012). This sequence of events means that intercellular communication in the adult lens nucleus is solely mediated by Cx46 (Baldo et al. 2001). While these changes to key proteins that mediate circulating fluxes in the adult lens are being established the HVS begins to regress (Table 2, (Mitchell et al. 1998)). The loss of the HVS results in a switch from a vascular tissue to an avascular tissue that is solely dependent on the internal microcirculation system to maintain homeostasis and transparency of the lens post eye opening.
In conclusion, our comparative mapping of the spatial distributions of AQP5 and AQP0 at sub-cellular resolution in embryonic and postnatal lenses by immunohistochemistry has revealed that the distinct spatial distributions of the two AQPs observed in the adult lens are abruptly established during a narrow window of postnatal development and coincide with the regression of the HVS prior to eye opening. We propose that these changes in the distribution of AQPs are critical for the development of the lens microcirculation system that enables the avascular adult lens to maintain its transparency. Taken together our results suggest that the truncation of AQP0 and the translocation of AQP5 in MF cells act to increase membrane water permeability in the centre of the adult mouse lens, and that with advancing age the contribution of AQP5 to overall water permeability in the lens increases. To test these hypotheses, novel assays to measure the water permeability of DF and MF cells will be required.
Highlights.
AQP5 protein is expressed earlier in development than AQP0
In the embryo, AQP5 is cytoplasmic while AQP0 is membranous
Postnatally, AQP5 translocates to mature fibre cell membranes and AQP0 is cleaved
Acknowledgments
The authors thank Associate Professor Robb de Iongh (University of Melbourne) and Professor Frank Lovicu (University of Sydney) for supplying embryonic mouse tissue used in this study. Dr Grey was support by a Foundation for Research Science and Technology Post-doctoral Fellowship, while Ms Petrova was the recipient of a University of Auckland Doctoral Scholarship. The authors acknowledge the support of NIH (Grant #EY13462).
Abbreviations
- AQP
aquaporin
- PF
primary fibre
- DF
differentiating fibre
- MF
mature fibre
- PBS
phosphate-buffered saline
- WGA
wheat germ agglutinin
- Cx
connexin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Ghoul KJ, Kirk T, Kuszak AJ, Zoltoski RK, Shiels A, Kuszak JR. Lens structure in MIP-deficient mice. Anatomical Record. 2003;273:714–30. doi: 10.1002/ar.a.10080. [DOI] [PubMed] [Google Scholar]
- Baldo GJ, Gong X, Martinez-Wittinghan FJ, Kumar NM, Gilula NB, Mathias RT. Gap junctional coupling in lenses from alpha(8) connexin knockout mice. J Gen Physiol. 2001;118:447–456. doi: 10.1085/jgp.118.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball LE, Little M, Nowak MW, Garland DL, Crouch RK, Schey KL. Water permeability of C-terminally truncated aquaporin 0 (AQP0 1-243) observed in the aging human lens. Investigative Ophthalmology and Visual Science. 2003;44:4820–8. doi: 10.1167/iovs.02-1317. [DOI] [PubMed] [Google Scholar]
- Bassnett S. The fate of the Golgi apparatus and the endoplasmic reticulum during lens fiber cell differentiation. Investigative Ophthalmology and Visual Science. 1995;36:1793–803. [PubMed] [Google Scholar]
- Bassnett S, Beebe DC. Coincident loss of mitochondria and nuclei during lens fiber cell differentiation. Developmental Dynamics. 1992;194:85–93. doi: 10.1002/aja.1001940202. [DOI] [PubMed] [Google Scholar]
- Bassnett S, Wilmarth PA, David LL. The membrane proteome of the mouse lens fiber cell. Molecular Vision. 2009;15:2448–63. [PMC free article] [PubMed] [Google Scholar]
- Broekhuyse RM, Kuhlmann ED, Stols AL. Lens membranes II. Isolation and characterization of the main instrinsic polypeptide (MIP) of bovine fiber membranes. Experimental Eye Research. 1976;23:365–371. doi: 10.1016/0014-4835(76)90135-4. [DOI] [PubMed] [Google Scholar]
- Candia OA, Mathias R, Gerometta R. Fluid circulation determined in the isolated bovine lens. Invest Ophthalmol Vis Sci. 2012;53:7087–7096. doi: 10.1167/iovs.12-10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello MJ, McIntosh TJ, Robertson JD. Distribution of gap junctions and square array junctions in the mammalian lens. Invest Ophthalmol Vis Sci. 1989;30:975–989. [PubMed] [Google Scholar]
- Donaldson PJ, Musil LS, Mathias RT. Point: A critical appraisal of the lens circulation model--an experimental paradigm for understanding the maintenance of lens transparency? Invest Ophthalmol Vis Sci. 2010;51:2303–2306. doi: 10.1167/iovs.10-5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CW, Eastwood S, Rains J, Gruijters WT, Bullivant S, Kistler J. Gap junction formation during development of the mouse lens. European Journal of Cell Biology. 1993;60:243–9. [PubMed] [Google Scholar]
- Gao J, Sun X, Moore LC, White TW, Brink PR, Mathias RT. Lens intracellular hydrostatic pressure is generated by the circulation of sodium and modulated by gap junction coupling. Journal of General Physiology. 2011;137:507–20. doi: 10.1085/jgp.201010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MG, Reichow SL, O'Neill SE, Weisbrod CR, Langeberg LK, Bruce JE, Gonen T, Scott JD. AKAP2 anchors PKA with aquaporin-0 to support ocular lens transparency. EMBO Mol Med. 2012;4:15–26. doi: 10.1002/emmm.201100184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonen T, Sliz P, Kistler J, Cheng Y, Walz T. Aquaporin-0 membrane junctions reveal the structure of a closed water pore. Nature. 2004;29:193–7. doi: 10.1038/nature02503. [DOI] [PubMed] [Google Scholar]
- Grey AC, Li L, Jacobs MD, Schey KL, Donaldson PJ. Differentiation-dependent modification and subcellular distribution of aquaporin-0 suggests multiple functional roles in the rat lens. Differentiation. 2009;77:70–83. doi: 10.1016/j.diff.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey AC, Walker KL, Petrova RS, Han J, Wilmarth PA, David LL, Donaldson PJ, Schey KL. Verification and Spatial Localization of Aquaporin-5 in the Ocular Lens. Experimental Eye Research. 2013;108C:94–102. doi: 10.1016/j.exer.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann S, Zeuthen T, La Cour M, Nagelhus EA, Ottersen OP, Agre P, Nielsen S. Aquaporins in complex tissues: distribution of aquaporins 1-5 in human and rat eye. American Journal of Physiology. 1998;274:C1332–45. doi: 10.1152/ajpcell.1998.274.5.C1332. [DOI] [PubMed] [Google Scholar]
- Harries WE, Akhavan D, Miercke LJ, Khademi S, Stroud RM. The channel architecture of aquaporin 0 at a 2.2-A resolution. Proc Natl Acad Sci U S A. 2004;101:14045–14050. doi: 10.1073/pnas.0405274101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R, Sohara E, Rai T, Satoh T, Yokozeki H, Sasaki S, Uchida S. Immunolocalization and translocation of aquaporin-5 water channel in sweat glands. J Dermatol Sci. 2013;70:26–33. doi: 10.1016/j.jdermsci.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Eguchi T, Skowronski MT, Ishida H. Acetylcholine acts on M3 muscarinic receptors and induces the translocation of aquaporin5 water channel via cytosolic Ca2+ elevation in rat parotid glands. Biochem Biophys Res Commun. 1998;245:835–840. doi: 10.1006/bbrc.1998.8395. [DOI] [PubMed] [Google Scholar]
- Jacobs MD, Donaldson PJ, Cannell MB, Soeller C. Resolving morphology and antibody labeling over large distances in tissue sections. Microscopy Research and Techniques. 2003;62:83–91. doi: 10.1002/jemt.10360. [DOI] [PubMed] [Google Scholar]
- Jacobs MD, Soeller C, Sisley AM, Cannell MB, Donaldson PJ. Gap junction processing and redistribution revealed by quantitative optical measurements of connexin46 epitopes in the lens. Invest Ophthalmol Vis Sci. 2004;45:191–199. doi: 10.1167/iovs.03-0148. [DOI] [PubMed] [Google Scholar]
- Kumari SS, Varadaraj K. Aquaporin 5 knockout mouse lens develops hyperglycemic cataract. Biochemical and Biophysical Research Communications. 2013;441:333–338. doi: 10.1016/j.bbrc.2013.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari SS, Varadaraj K. Intact and N- or C-terminal end truncated AQP0 function as open water channels and cell-to-cell adhesion proteins: End truncation could be a prelude for adjusting the refractive index of the lens to prevent spherical aberration. Biochimica Et Biochimica Acta. 2014;1840:2862–2877. doi: 10.1016/j.bbagen.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari SS, Varadaraj M, Yerramilli VS, Menon AG, Varadaraj K. Spatial expression of aquaporin 5 in mammalian cornea and lens, and regulation of its localization by phosphokinase A. Molecular Vision. 2012;18:957–67. [PMC free article] [PubMed] [Google Scholar]
- Lindsey Rose KM, Gourdie RG, Prescott AR, Quinlan RA, Crouch RK, Schey KL. The C terminus of lens aquaporin 0 interacts with the cytoskeletal proteins filensin and CP49. Invest Ophthalmol Vis Sci. 2006;47:1562–1570. doi: 10.1167/iovs.05-1313. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Developmental Biology. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Mathias RT, Kistler J, Donaldson P. The lens circulation. Journal of Membrane Biology. 2007;216:1–16. doi: 10.1007/s00232-007-9019-y. [DOI] [PubMed] [Google Scholar]
- Mathias RT, Rae JL, Baldo GJ. Physiological properties of the normal lens. Physiological Reviews. 1997;77:21–50. doi: 10.1152/physrev.1997.77.1.21. [DOI] [PubMed] [Google Scholar]
- Michea LF, Andrinolo D, Ceppi H, Lagos N. Biochemical evidence for adhesion-promoting role of major intrinsic protein isolated from both normal and cataractous human lenses. Exp Eye Res. 1995;61:293–301. doi: 10.1016/S0014-4835(05)80124-1. [DOI] [PubMed] [Google Scholar]
- Mitchell CA, Risau W, Drexler HC. Regression of vessels in the tunica vasculosa lentis is initiated by coordinated endothelial apoptosis: a role for vascular endothelial growth factor as a survival factor for endothelium. Developmental Dynamics. 1998;213:322–33. doi: 10.1002/(SICI)1097-0177(199811)213:3<322∷AID-AJA8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Nemeth-Cahalan KL, Hall JE. pH and calcium regulate the water permeability of aquaporin 0. J Biol Chem. 2000;275:6777–6782. doi: 10.1074/jbc.275.10.6777. [DOI] [PubMed] [Google Scholar]
- Palanivelu DV, Kozono DE, Engel A, Suda K, Lustig A, Agre P, Schirmer T. Co-axial association of recombinant eye lens aquaporin-0 observed in loosely packed 3D crystals. J Mol Biol. 2006;355:605–611. doi: 10.1016/j.jmb.2005.10.032. [DOI] [PubMed] [Google Scholar]
- Schey KL, Fowler JG, Shearer TR, David L. Modifications to rat lens major intrinsic protein in selenite-induced cataract. Invest Ophthalmol Vis Sci. 1999;40:657–667. [PubMed] [Google Scholar]
- Schey KL, Little M, Fowler JG, Crouch RK. Characterization of human lens major intrinsic protein structure. Invest Ophthalmol Vis Sci. 2000;41:175–182. [PubMed] [Google Scholar]
- Shiels A, Griffin CS, Muggleton-Harris AL. Immunochemical comparison of the major intrinsic protein of eye-lens fibre cell membranes in mice with hereditary cataracts. Biochimica Et Biochimica Acta. 1991;1097:318–24. doi: 10.1016/0925-4439(91)90087-p. [DOI] [PubMed] [Google Scholar]
- Vaghefi E, Pontre BP, Jacobs MD, Donaldson PJ. Visualizing ocular lens fluid dynamics using MRI: manipulation of steady state water content and water fluxes. Am J Physiol Regul Integr Comp Physiol. 2011;301:R335–42. doi: 10.1152/ajpregu.00173.2011. [DOI] [PubMed] [Google Scholar]
- Varadaraj K, Kumari SS, Mathias RT. Functional expression of aquaporins in embryonic, postnatal, and adult mouse lenses. Developmental Dynamics. 2007;236:1319–28. doi: 10.1002/dvdy.21125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Gu S, Yin X, Weintraub ST, Hua Z, Jiang JX. Developmental truncations of connexin 50 by caspases adaptively regulate gap junctions/hemichannels and protect lens cells against ultraviolet radiation. J Biol Chem. 2012;287:15786–15797. doi: 10.1074/jbc.M111.313171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Han J, Schey KL. Spatial differences in an integral membrane proteome detected in laser capture microdissected samples. Journal of Proteome Research. 2008;7:2696–2702. doi: 10.1021/pr700737h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Schey KL. Aquaporin-0 interacts with the FERM domain of ezrin/radixin/moesin proteins in the ocular lens. Invest Ophthalmol Vis Sci. 2011;52:5079–5087. doi: 10.1167/iovs.10-6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Verkman AS. Water and glycerol permeabilities of aquaporins 1-5 and MIP determined quantitatively by expression of epitope-tagged constructs in Xenopus oocytes. J Biol Chem. 1997;272:16140–16146. doi: 10.1074/jbc.272.26.16140. [DOI] [PubMed] [Google Scholar]
- Zampighi GA, Hall JE, Ehring GR, Simon SA. The structural organization and protein composition of lens fiber junctions. J Cell Biol. 1989;108:2255–2275. doi: 10.1083/jcb.108.6.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]


