Abstract
Background
Fatigue is a multidimensional construct that has significant implications for physical function in chronic non-cancer pain populations but remains relatively understudied. The current study characterized the independent contributions of self-reported ratings of pain intensity, sleep disturbance, depression, and fatigue to ratings of physical function and pain-related interference in a diverse sample of treatment-seeking individuals with chronic pain.
Methods
These relationships were examined as a path modeling analysis of self-report scores obtained from 2,487 individuals with chronic pain from a tertiary care outpatient pain clinic.
Results
Our analyses revealed unique relationships of pain intensity, sleep disturbance, and depression with self-reported fatigue. Further, fatigue scores accounted for significant proportions of the relationships of both pain intensity and depression with physical function and pain-related interference, and accounted for the entirety of the unique statistical relationship between sleep disturbance and both physical function and pain-related interference.
Conclusions
Fatigue is a complex construct with relationships to both physical and psychological factors that has significant implications for physical functioning in chronic non-cancer pain. The current results identify potential targets for future treatment of fatigue in chronic pain, and may provide directions for future clinical and theoretical research in the area of chronic non-cancer pain.
Perspective
Fatigue is an important physical and psychological variable that factors prominently in the deleterious consequences of pain intensity, sleep disturbance, and depression for physical function in chronic non-cancer pain.
Keywords: Fatigue, depression, chronic pain, sleep, physical functioning, CHOIR
Chronic pain contributes significantly to physical dysfunction, which increases health care costs in the United States through decreased work productivity and higher health care utilization.20 On an individual level, pain demonstrates deleterious effects on physical functioning through several mechanisms, including pain intensity,21 sleep problems,31 and depression.27 Another key mechanism is fatigue, which commonly accompanies pain but has a multifactorial etiology, including pain intensity, inflammatory processes, and psychological factors. Fatigue has been defined as an overwhelming and persistent feeling of exhaustion that interferes with one’s ability to function.10 Fatigue contributes to physical dysfunction in chronic illness39 and chronic pain populations,7,33 but remains a relatively poorly understood construct.
Fatigue in chronic non-cancer pain
To date, scientific inquiry has primarily examined fatigue in specific chronic medical and neurological populations, such as cancer,24 HIV,16 and multiple sclerosis.42 However, a significant co-morbidity between chronic non-cancer pain conditions and fatigue has been noted.12,17,37 Fatigue is a significant complaint in individuals with rheumatoid arthritis,41 osteoarthritis,33 fibromyalgia,29 chronic low back pain,17 and chronic abdominal pain,8 and complaints of fatigue predict poorer physical functioning in these conditions.29,33 Despite its implications for functioning, however, fatigue has remained relatively understudied and may not be thoroughly considered in the context of chronic pain treatment outside of certain pain disorders. Some of the difficulty in the use of fatigue as a clinical target may stem from its multifactorial nature.
Contributors to fatigue in chronic non-cancer pain
Fatigue has been defined as a multidimensional construct, comprised of both physical and psychological factors.45 In chronic pain, a positive relationship between pain intensity and fatigue has been reported,50 though this finding has not been replicated in all studies.37 Psychological factors also contribute to fatigue in chronic pain. An indirect effect of pain on fatigue has been noted through sleep disruption.18 Similarly, there is a notable relationship between depression and fatigue in chronic pain. Reports of low energy or significant fatigue are a diagnostic criterion for depression, 3 and elevated rates of depression have been consistently reported across a variety of pain populations.32 Further, longitudinal studies have identified a reciprocal relationship between depression and fatigue, such that individuals with increased fatigue appear to be at increased risk of developing a future major depressive episode.1 Similarly, individuals with depression are more likely to report significant fatigue in the future.44 Further, there may be significant sex-based differences in experience of pain,23,47 depression,43,47 and fatigue4 due to biological or psychosocial differences, further highlighting the complexity of fatigue in chronic pain.
Study hypotheses
Given that fatigue, pain, sleep disturbance, and depression have disruptive, but potentially overlapping, influences on physical functioning,21 it is important to examine whether these variables have unique implications for physical functioning. The current study utilized data from the Stanford-NIH Open Source Pain Registry, using a set of open-source tools for assessing patient-reported health status (Patient Reported Outcome Measurement Information System; PROMIS).10 The Stanford-NIH Open Source Pain Registry, derived from a larger, comprehensive study known as the Stanford-NIH Open Source Health Registry, collects longitudinal data using set measures (including PROMIS) that are collected at initial clinic visits and at fixed intervals thereafter, as well as follow-up appointments. Data from the Stanford-NIH Open Source Pain Registry were used to assess fatigue, depression, sleep disturbance, physical function, and pain-related interference in a diverse sample of individuals with chronic pain conditions. We expected that pain intensity, sleep disturbance, and depression would independently contribute to severity of fatigue. Further, consistent with previous studies, we expected that fatigue would account for a significant degree of the relationships of pain intensity, sleep interference, and depression with ratings of pain-related interference and physical function.
Methods
All procedures were approved by the Institutional Review Board at the Stanford University School of Medicine, and all patients provided informed consent prior to completing any measures from the data registry.
Participants
Data were collected from 2,487 patients who presented for initial medical evaluations between September 2012 and May 2014 at the Stanford Pain Management Center, a large, tertiary care pain clinic. The sample was 62.6% female (N = 1,556). The predominant ethnicity in the current sample was Caucasian (63.2% of the overall sample), followed by Asian (7.2%), African American (3.4%), Native Hawaiian or Pacific Islander (0.7%), and American Indian or Alaska Native (0.4%). Nearly one fifth of the sample (19.7%) reported an ethnicity of “Other,” and 5.4% of the sample did not endorse an ethnicity. Median education in the patient sample was a completed Associate’s Degree or equivalent occupational or technical program certification. Mean age in the current sample was 49.7 years (range 18 to 93 years). Regarding pain diagnoses, the largest proportion of patients were referred to the pain clinic for thoracolumbar pain (20.7% of the sample), followed by other musculoskeletal pain (11.5%), fibromyalgia and/or myofascial pain (8.8%), orofacial pain (7.8%), nerve pain (7.2%), neck pain (7.0%), abdominal pain (4.1%), and pelvic pain (4.1%). At the time of the initial visit, 1791 patients (92.1% of the sample) carried a single pain diagnosis, 183 patients (7.4% of the sample) had 2 pain diagnoses, 11 patients (0.4% of the sample) had 3 pain diagnoses, and 2 patients (0.1% of the sample) carried 4 pain diagnoses. Pain diagnosis information was unavailable for 500 patients in the current sample.
Procedures
At clinic check-in for their initial medical appointments, patients were provided a tablet computer that allowed them to complete a series of questionnaires. PROMIS measures were administered using a computerized adaptive testing (CAT) approach;9,22 rather than assessing a set number of items per subscale, the CAT approach identifies the optimal items within each domain based on prior responses from the respondent. CAT assessments are considered superior to traditional standard scale assessments due to the smaller number of items needed for effective assessment of each construct, as well as increased reliability of measurement.28 The Stanford-NIH Open Source Pain Registry includes computerized automated testing versions of the PROMIS measures adapted with an in-house algorithm (SNAPL-CAT). SNAPL-CAT was implemented using the same CAT algorithm as the Northwestern University Assessment Center, which has provided open access to PROMIS instruments.22
Measures
Pain Intensity
Pain intensity was assessed using an 11-point visual analog scale,15 ranging from 0–10. Respondents used this scale to rate their average pain intensity over the past 7 days. VAS have demonstrated validity as an assessment of pain in chronic pain studies.26
PROMIS Instruments
Item banks for Pain Interference, Physical Function, Fatigue, Depression, and Sleep Disturbance from the NIH Toolbox PROMIS11 were administered to patients at each clinic visit. PROMIS instruments are based on an Item Response Theory-based assessment that utilizes item-level responses rather than composite scale responses.2,25 PROMIS measures are normed against the U.S. population and have a mean of 50 points and a standard deviation of 10 points.11 CAT-based administrations frequently administer a substantially smaller number of items but yield superior efficiency in domain assessment and greater precision (i.e. lower standard error) compared to traditional, non-adaptive testing forms.19 Higher scores on average pain intensity, fatigue, sleep disturbance, and depression signified greater severity of these symptoms. Similarly, higher scores on PROMIS Pain Interference reflect greater severity of pain-related interference in functioning. However, higher scores on PROMIS Physical Function reflected a greater level of physical functioning. Questions were framed according the experience of symptoms or functioning over the past 7 days.
Analytic Plan
Path models were estimated using the Mplus software34 to test the indirect effects of average pain intensity, sleep disturbance, and depression on pain interference and physical function scores through fatigue scores. Each indirect (i.e., mediated) effect was reported as a standardized coefficient. Indirect effects were calculated using a 1000-draw bootstrap-estimated product of the coefficients of the predictor-mediator path (the a path) and the mediator-outcome path (the b path). However, as we could not establish evidence for the temporal ordering of the variables in our mediation model, it is more appropriate to employ the term “intervening variable” to describe the mediator in these models, following the recommendations of MacKinnon and colleagues.30 With this caution in mind, however, the analytic approach henceforth will be referred to as mediation analyses. Analysis results are reported using both unstandardized and standardized path coefficients. Inclusion of standardized path coefficient models was deemed to be necessary in order to provide a common measurement metric against which the size of each path could be compared. As Mplus does not provide significance values for standardized path coefficient models, however, we also opted to include unstandardized path coefficient models, in order to provide estimates of statistical significance for each examined path.
As the examined models were nearly recursive and would thus yield only fit indices suggesting near-perfect fit of the data, we opted not to include these measures in our manuscript. Pain intensity, depression, and sleep disturbance scores were freed to co-vary based on a theoretical likelihood of shared variance between these variables. Covariates representing age, gender, and pain diagnosis associated with initial clinic visits were included in the estimation of all paths. As an exploratory step, differences in each path were computed using Wald chi-square difference tests between each of the 5 largest pain diagnosis groups in our sample. This step was taken in order to articulate some of the potential differences in interrelationships between study variables. Significant Wald chi-square difference values suggest a significant difference between two variables, and are noted accordingly in the Results section. As noted previously, due to the possibility of sex-based differences in our variables, we have chosen to report our study variables separately by gender (see Table 1).
Table 1.
Means and Standard Deviations of Study Variables by Gender.
| Study Variable | Full Sample (N=2487) | Male (N=931) | Femaile (N=1556) | Gender Differences T-Test |
|---|---|---|---|---|
| VAS Average Pain Intensity | 6.06 (2.22) | 5.81 (2.23) | 6.21 (2.20) | t(2318) = 4.216, p < .001 |
| PROMIS Sleep Disturbance | 59.10 (9.16) | 58.92 (9.06) | 59.21 (9.22) | t(1720) = .621, p = .535 |
| PROMIS Depression | 58.28 (9.44) | 57.57 (9.87) | 58.71 (9.14) | t(2433) = 2.882, p = .004 |
| PROMIS Fatigue | 63.43 (9.26) | 62.22 (9.61) | 64.17 (8.96) | t(2357) = 4.977, p < .001 |
| PROMIS Physical Function | 34.90 (7.67) | 35.49 (7.87) | 34.55 (7.53) | t(2451) = −2.944, p = .003 |
| PROMIS Pain Interference | 67.74 (6.17) | 67.42 (6.20) | 67.94 (6.15) | t(2474) = 2.024, p = .043 |
Note: PROMIS assessments are based on a mean of 50 with a standard deviation of 10.
Note: VAS scores were assessed on an 11-point scale from 0–10.
Results
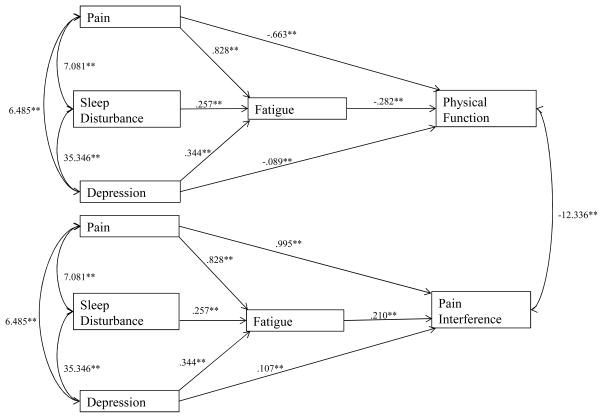
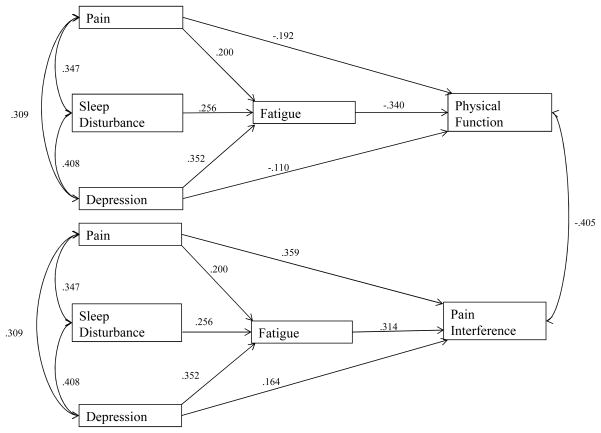
Descriptive statistics can be found in Table 1, and the total proportion of variance of each outcome variable (fatigue, physical function, and pain interference) accounted for by the overall model can be found in Table 2. Unstandardized path coefficients and their associated significance values are reported in Figure 1, and standardized path coefficients were reported for ease of interpretation and representation of the relative size of each statistical path reported in Figure 2. Our results indicated that ratings of average pain intensity, sleep disturbance, and depression had unique and statistically significant contributions to fatigue ratings over the same time period. According to the standardized path coefficients in Figure 2, depression scores demonstrated the largest effect on fatigue, followed by the effects of sleep disturbance and pain intensity.
Table 2.
Amount of Variance Accounted for in each Endogenous Variable
| Observed Variable | R2 Variance |
|---|---|
| Fatigue | 0.382 |
| Physical Function | 0.268 |
| Pain Interference | 0.433 |
Figure 1.
Path model representing indirect effects of pain, sleep disturbance, and depression on measures of pain interference and physical function through fatigue with unstandardized path coefficients and significance values.
Note: Paths from sleep disturbance to pain interference (unstandardized B = .016, p = .315) and from sleep disturbance to physical function (unstandardized B = .000, p = .995) were non-significant.
Figure 2.
Path model representing indirect effects of pain, sleep disturbance, and depression on measures of pain interference and physical function through fatigue with standardized path coefficients
Pain interference
Average pain intensity, depression, and fatigue scores also demonstrated unique and statistically significant relationships with ratings of pain interference. More specifically, pain intensity and fatigue on pain interference demonstrated larger effects on pain interference, while a smaller effect of depression was found. Fatigue was found to account for a significant proportion of the relationship between pain intensity and pain interference (ab = .063, p < .0001), between sleep disturbance and pain interference (ab = .080, p < .0001), and between depression and pain interference (ab = .111, p < .0001). However, inclusion of fatigue as a predictor of pain interference reduced the statistical relationship between sleep disturbance and pain interference to non-significance (unstandardized B = .016, p = .315). As a result, fatigue accounted for the entirety of the unique statistical relationship between sleep disturbance and pain interference scores, independent of the effects of pain intensity and depression. Pain intensity and depression maintained statistically significant relationships with pain interference, however, suggesting that fatigue ratings may be a partial, rather than full mediator of these relationships.
Physical function
Similarly, pain intensity, depression, and fatigue ratings all demonstrated unique and statistically significant inverse relationships with overall ratings of physical function. Fatigue showed the largest relative effect on physical function, followed by pain intensity, and depression. Fatigue was found to account for a significant proportion of the relationship between sleep disturbance and physical function (ab = −.087, p < .0001). The relationship between sleep disturbance and physical function, which was significant when modeled separately, was reduced to non-significance (unstandardized B = .000, p = .995) when fatigue scores were modeled as an intervening variable; this finding suggests that fatigue scores fully explained the unique relationship between sleep disturbance and ratings of physical function, independent of the effects of average pain intensity and depression. Fatigue ratings accounted for a significant proportion of the relationship between average pain intensity and physical function (ab = −.068, p < .0001) and also accounted for a significant proportion of the relationship between depression and physical function (ab = −.120, p < .0001). However, ratings of average pain intensity and depression continued to demonstrate statistically significant negative relationships with physical function when fatigue was modeled as an intervening variable, suggesting that fatigue was not a full mediator of these relationships. Inclusion of covariates representing patient age, gender, and pain diagnosis information did not change the direction or significance of any of the estimated model paths. Similarly, the direction and significance of each path in the statistical model did not vary between those individuals who did not have a documented pain diagnosis and those patients who had at least one pain diagnosis.
Diagnostic group differences
Differences in all examined paths were tested between the 5 largest pain diagnosis groups in the current study (thoracolumbar pain, musculoskeletal pain in other regions, fibromyalgia/myofascial pain, orofacial pain, and nerve pain). Wald chi-square difference tests revealed significant differences in the relationship between fatigue and physical function; a stronger relationship was found between fatigue and physical function for patients with orofacial pain (B = −.554, p < .001) than in patients with thoracolumbar pain (B = −.227, p < .001), musculoskeletal pain (B = −.203, p < .001), fibromyalgia or myofascial pain (B = −.210, p = .022), and nerve pain (B = −.343, p < .001). Additionally, a significantly stronger correlation between pain intensity and depression was noted for patients with nerve pain (r = .387, p < .001) than patients with musculoskeletal pain (r = .178, p = .004). No other significant differences were noted between study variables according to pain diagnosis.
Discussion
Fatigue is a complex construct, reflecting the confluence of physical and psychological factors relevant to chronic pain. Self-reported ratings of pain intensity, sleep disturbance, and depression independently predicted ratings of fatigue. Although comparatively greater effects were found for depression and sleep disturbance, our results suggested a small, but statistically significant effect of pain intensity on ratings of fatigue, consistent with previous studies.50 Our analyses mirror previous research with regard to the contributions of sleep dysregulation and depression to fatigue,37 but also highlight pain intensity as a small but significant contributor to fatigue.
Additionally, we found that pain intensity, fatigue, depression, and sleep disturbance contribute uniquely to physical function and pain interference in chronic pain. Our analyses highlight the importance of fatigue as an intervening factor and potential mediator of the deleterious effects of chronic pain on individual functioning. Further, the direct relationships between sleep disturbance and both physical function and pain-related interference were reduced to non-significance when fatigue ratings were included as an intervening variable. Though the cross-sectional nature of our data precludes inferences about temporal causality, significant sleep disruption appears to alter physical function primarily through increased fatigue. Notably, we found a stronger relationship between fatigue and physical function in patients with orofacial pain than in other patient groups, which was an unexpected finding. Our study is the first to connect fatigue to poorer physical function in orofacial pain. Consequently, this issue warrants additional study; greater decrements in physical functioning due to fatigue in orofacial pain may be overlooked, as fatigue may not be considered a unique vulnerability compared to other chronic pain populations.14
Our findings inform the research and clinical literature in several ways. First, our results are among the first to demonstrate unique relationships of pain intensity, depression, and sleep disturbance in chronic non-cancer pain using path modeling techniques, though similar approaches have been used in other populations.6 From a clinical standpoint, the literature regarding interventions for fatigue in chronic non-cancer pain is inconsistent, so treating individuals with these complaints can be difficult. Our model highlights several potential areas of intervention for patients complaining of significant fatigue. Interventions that affect the intensity of pain, depressive symptoms, and the quality and duration of sleep may indirectly alleviate symptoms of fatigue. However, the cross-sectional nature of our analysis suggests that temporal ordering of these effects cannot be assumed, and would be a worthy area of future study. Nevertheless, our results provide promising initial support for targeted treatment of pain, sleep, and mood as indirect means of altering complaints of fatigue in individuals with chronic non-cancer pain.
Our results also support the viability of PROMIS instruments administered through a large-scale, open-source data registry and, to our knowledge, are the first to describe relationships between these variables in an outpatient pain clinic sample. Our study utilized validated, psychometrically sound instruments developed by the National Institutes of Health and administered through the Stanford-NIH Open Source Pain Registry, a unique data registry established for use in a large outpatient pain clinic. PROMIS instruments were designed with consultation from experts from a variety of clinical areas and were designed to generalize well across patient populations.10 Similarly, PROMIS instruments are reliable and flexible while minimizing measurement error, making them well-suited for broad examination of clinical outcomes.10 Our use of IRT-based computerized adaptive testing2 allowed patients to provide reliable information about multiple domains of functioning in a timelier and more efficient manner than in traditional, static forms of assessment. Given the potential for excessive patient burden in evaluating all aspects of individual functioning in a clinical setting, it is important to evaluate the potential of efficient testing paradigms, particularly when coupled with psychometrically sound assessments like PROMIS.
Limitations
Limitations of the current study include a lack of detail regarding the effects of specific pain diagnoses. The size and diversity of our sample of individuals with chronic pain, while a strength for the purposes of statistical analysis and inference, does not provide information about the examined relationships in specific pain disorders. Although we have acknowledged some differences in our model according to pain diagnosis, many individuals in our sample carried multiple pain diagnoses. Our findings should be interpreted with this caution in mind, as the diverse nature of tertiary care pain clinic patients may not be representative of individuals who carry only a single pain diagnosis. Interpretation of these findings is further limited by the treatment-seeking nature of the sample; our analyses used only data from initial visits to the clinic. We adopted this approach in order to control for new treatments that may have been implemented after initial clinic visits. It should be noted, however, that many respondents came to the pain clinic with ongoing treatment regimens from outside providers. Thus, our findings may include a significant number of participants who were experiencing atypically elevated pain and distress that were alleviated later by adjustments to their treatment.
As the cross-sectional nature of the current model precludes interpretations regarding the causal ordering of our examined variables, our model provides at best a theoretical basis for specific interventions for fatigue. Consequently, it may be beneficial to test our findings using time-lapsed models to determine whether targeted changes in one variable may lead to changes in others. This is especially salient for the purposes of enhancing clinical utility of our findings; though it is reasonable to expect that improvements in sleep quality or depressive symptoms may lead to decreased reports of fatigue, these relationships should be substantiated in models that allow for greater causal inference.
It should also be noted that traditional definitions include substantial theoretical overlap between fatigue and depression. Particular care was taken in the development of item banks to minimize item overlap in these PROMIS instruments.40 PROMIS depression items assess negative cognitions, negative mood, positive emotion deficits, and information processing deficits, while PROMIS Fatigue items assess feelings of exhaustion and their consequences for functioning.10 Nevertheless, it should be acknowledged that fatigue is a diagnostic criterion for major depressive episodes,3 and significant fatigue may constitute a prodromal symptom of depression, as significant fatigue predicts the onset of later depression.1
Future directions
Though our results provide a more unified model of fatigue in chronic non-cancer pain than previous studies, some questions warrant additional attention. First, inflammatory dysfunction was not examined in the current study. Evidence suggests that fatigue may be more prominent in chronic pain conditions that involve dysregulation of autoimmune and inflammatory processes, such as rheumatoid arthritis,13 though this hypothesis has not always been supported.5 Future studies may benefit from examination of these disease-specific variables and their implications for fatigue, particularly in the context of examining the independence of these effects from the factors examined in the current study. There also exists a robust literature on sex-based differences in the incidence and experience of pain,23,47 depression,43,47 and fatigue.4 Though it was outside the scope of the current paper to specifically examine these factors in our path model, sex-specific factors may play a key role in the relationships between pain, emotional distress, fatigue, and physical function. For example, the relationship between pain and clinical levels of depression and anxiety may vary across men and women due to hormonal and psychological differences.43,47 We urge further attention to these factors in future research. Additionally, there is evidence that fatigue may be correlated with body mass index in some clinical pain syndromes,36,49 which may warrant inclusion in future studies.
The diverse nature of the current sample also precluded examination of the specific effects of medications, though medications like duloxetine have been shown to have notable effects on fatigue,18 while other medications may contribute to feelings of lethargy or sedation,51 thereby complicating reports of fatigue. Further, the contributions of pain intensity, sleep disturbance, and depression may not generalize to other aspects of fatigue, such as post-exertional malaise.38 Similarly, there may be aspects of fatigue that reflect depletion of psychological resources necessary for continued self-regulation, which are not accessible through retrospective self-report.48 It is reasonable to consider that the demands of chronic pain may contribute to prolonged depletion of self-regulatory processes,35 though this aspect of fatigue may be better evaluated through experimental studies.46
Conclusions
Fatigue in chronic non-cancer pain is a confluence of both physical and psychological factors and appears to be a key determinant for physical functioning in individuals with chronic non-cancer pain. The current study utilized data from a unique open-source data registry that integrates psychometrically sound PROMIS assessments to assess these relationships, which were modeled using a path modeling approach. Sleep disturbance, depression, and pain intensity demonstrated unique relationships with fatigue, and fatigue explained a significant degree of the relationship between these variables and physical function and pain-related physical difficulties in a diverse sample of individuals with chronic pain conditions. Our results highlight the importance of considering multiple physical and psychological factors in the evaluation and treatment of fatigue in chronic non-cancer pain, as well as the need for temporally-based models that allow for causal inference in order to provide clinically and theoretically useful information about fatigue.
Appendix 1.
Full List of Pain Diagnoses
| Pain Diagnosis | N | % of Sample |
|---|---|---|
| Thoracolumbar Pain | 514 | 20.7% |
| Other Musculoskeletal Pain | 285 | 11.5% |
| Fibromyalgia/Myofascial Pain | 217 | 8.8% |
| Orofacial Pain | 195 | 7.8% |
| Nerve Pain | 180 | 7.2% |
| Neck Pain | 173 | 7.0% |
| Pelvic Pain | 102 | 4.1% |
| Abdominal Pain | 101 | 4.1% |
| Systemic Disease/Disorder | 90 | 3.6% |
| CRPS | 64 | 2.6% |
| Centralized Pain | 45 | 1.8% |
| Other Rheumatological Pain | 20 | 0.8% |
| Chest Pain | 19 | 0.8% |
| Substance Use Disorder | 19 | 0.8% |
| Cardiac Pain | 14 | 0.6% |
| Sleep Disorder | 14 | 0.6% |
| Other Neurological Condition | 9 | 0.4% |
| Dermatological Pain | 6 | 0.3% |
| Vascular Pain | 5 | 0.2% |
| Urologic Pain | 4 | 0.2% |
| Other Pulmonary Condition | 3 | 0.1% |
| Congenital Pain | 2 | 0.1% |
CRPS = Complex Regional Pain Syndrome
Note: Diagnosis information was unavailable for 500 patients in the current sample
Note: Patients could carry more than one diagnosis
Highlights.
Data were sampled from an open-source data registry in a tertiary care pain clinic
PROMIS measures were used to assess physical and psychological functioning
Path analysis techniques were used to model statistical relationships
Pain intensity, sleep disturbance, and depression uniquely contribute to fatigue
Fatigue is a mediator between pain and both physical function and pain interference
Acknowledgments
The authors wish to acknowledge funding from the National Institutes of Health (NIH HHSN 271201200728P), as well as the Redlich Pain Endowment.
Footnotes
Disclosures: The authors have no financial conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Addington A, Gallo J, Ford D, Eaton W. Epidemiology of unexplained fatigue and major depression in the community: the Baltimore ECA follow-up, 1981–1994. Psychol Med. 2001;31:1037–1044. doi: 10.1017/s0033291701004214. [DOI] [PubMed] [Google Scholar]
- 2.Amtmann D, Cook KF, Jensen MP, Chen WH, Choi S, Revicki D, Cella D, Rothrock N, Keefe F, Callahan L, Lai JS. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150:173–182. doi: 10.1016/j.pain.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Association AP. Diagnostic and statistical manual of mental disorders: DSM-IV-TR®. American Psychiatric Pub; 2000. [Google Scholar]
- 4.Bensing JM, Hulsman RL, Schreurs KM. Gender differences in fatigue: biopsychosocial factors relating to fatigue in men and women. Med Care. 1999;37:1078–1083. doi: 10.1097/00005650-199910000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Bergman MJ, Shahouri SS, Shaver TS, Anderson JD, Weidensaul DN, Busch RE, Wang S, Wolfe F. Is fatigue an inflammatory variable in rheumatoid arthritis (RA)? Analyses of fatigue in RA, osteoarthritis, and fibromyalgia. J Rheumatol. 2009;36:2788–2794. doi: 10.3899/jrheum.090561. [DOI] [PubMed] [Google Scholar]
- 6.Bol Y, Duits AA, Lousberg R, Hupperts RM, Lacroix MH, Verhey FR, Vlaeyen JW. Fatigue and physical disability in patients with multiple sclerosis: a structural equation modeling approach. J Behav Med. 2010;33:355–363. doi: 10.1007/s10865-010-9266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bombardier CH, Buchwald D. Chronic fatigue, chronic fatigue syndrome, and fibromyalgia: disability and health-care use. Med Care. 1996;34:924–930. doi: 10.1097/00005650-199609000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Burke P, Elliott M, Fleissner R. Irritable bowel syndrome and recurrent abdominal pain: A comparative review. Psychosomatics. 1999;40:277–285. doi: 10.1016/S0033-3182(99)71219-3. [DOI] [PubMed] [Google Scholar]
- 9.Cella D, Gershon R, Lai J-S, Choi S. The future of outcomes measurement: item banking, tailored short-forms, and computerized adaptive assessment. Qual Life Res. 2007;16:133–141. doi: 10.1007/s11136-007-9204-6. [DOI] [PubMed] [Google Scholar]
- 10.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, Cook K, Devellis R, DeWalt D, Fries JF, Gershon R, Hahn EA, Lai JS, Pilkonis P, Revicki D, Rose M, Weinfurt K, Hays R, Group PC. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63:1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, Ader D, Fries JF, Bruce B, Rose M, Group PC. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45:S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creavin ST, Dunn KM, Mallen CD, Nijrolder I, Windt DA. Co-occurrence and associations of pain and fatigue in a community sample of Dutch adults. Eur J Pain. 2010;14:327–334. doi: 10.1016/j.ejpain.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Davis MC, Zautra AJ, Younger J, Motivala SJ, Attrep J, Irwin MR. Chronic stress and regulation of cellular markers of inflammation in rheumatoid arthritis: implications for fatigue. Brain Behav Immun. 2008;22:24–32. doi: 10.1016/j.bbi.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Leeuw R, Studts JL, Carlson CR. Fatigue and fatigue-related symptoms in an orofacial pain population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:168–174. doi: 10.1016/j.tripleo.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 16.Ferrando S, Evans S, Goggin K, Sewell M, Fishman B, Rabkin J. Fatigue in HIV illness: relationship to depression, physical limitations, and disability. Psychosom Med. 1998;60:759–764. doi: 10.1097/00006842-199811000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Feuerstein M, Carter RL, Papciak AS. A prospective analysis of stress and fatigue in recurrent low back pain. Pain. 1987;31:333–344. doi: 10.1016/0304-3959(87)90162-X. [DOI] [PubMed] [Google Scholar]
- 18.Fishbain DA, Hall JA, Risser RC, Gonzales JS. Does pain cause the perception of fatigue in patients with chronic pain? Findings from studies for management of diabetic peripheral neuropathic pain with duloxetine. Pain Pract. 2009;9:354–362. doi: 10.1111/j.1533-2500.2009.00294.x. [DOI] [PubMed] [Google Scholar]
- 19.Fries JF, Cella D, Rose M, Krishnan E, Bruce B. Progress in assessing physical function in arthritis: PROMIS short forms and computerized adaptive testing. J Rheumatol. 2009;36:2061–2066. doi: 10.3899/jrheum.090358. [DOI] [PubMed] [Google Scholar]
- 20.Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13:715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Geisser ME, Strader Donnell C, Petzke F, Gracely RH, Clauw DJ, Williams DA. Comorbid somatic symptoms and functional status in patients with fibromyalgia and chronic fatigue syndrome: sensory amplification as a common mechanism. Psychosomatics. 2008;49:235–242. doi: 10.1176/appi.psy.49.3.235. [DOI] [PubMed] [Google Scholar]
- 22.Gershon R, Rothrock NE, Hanrahan RT, Jansky LJ, Harniss M, Riley W. The development of a clinical outcomes survey research application: Assessment CenterSM. Qual Life Res. 2010;19:677–685. doi: 10.1007/s11136-010-9634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132:S26–S45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hann DM, Jacobsen PB, Martin SC, Kronish LE, Azzarello LM, Fields KK. Fatigue in women treated with bone marrow transplantation for breast cancer: a comparison with women with no history of cancer. Support Care Cancer. 1997;5:44–52. doi: 10.1007/BF01681961. [DOI] [PubMed] [Google Scholar]
- 25.Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res. 2009;18:873–880. doi: 10.1007/s11136-009-9496-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, Fainsinger R, Aass N, Kaasa S. Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. 2011;41:1073–1093. doi: 10.1016/j.jpainsymman.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Holzberg AD, Robinson ME, Geisser ME, Gremillion HA. The effects of depression and chronic pain on psychosocial and physical functioning. Clin J Pain. 1996;12:118–125. doi: 10.1097/00002508-199606000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Kao MCJ, Cook K, Olson G, Pacht T, Darnall BD, Weber SC, Mackey SC. SNAPL-CAT: Catalyzing the rate-limiting step of big data psychometrics with item-response theory and advanced computerized adaptive testing (poster presentation). American Medical Informatics Associations (AMIA) 2014 Joint Summits on Translational Science; 2014; San Francisco, CA.. [Google Scholar]
- 29.Kop WJ, Lyden A, Berlin AA, Ambrose K, Olsen C, Gracely RH, Williams DA, Clauw DJ. Ambulatory monitoring of physical activity and symptoms in fibromyalgia and chronic fatigue syndrome. Arthritis Rheum. 2005;52:296–303. doi: 10.1002/art.20779. [DOI] [PubMed] [Google Scholar]
- 30.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCracken L, Iverson G. Disrupted sleep patterns and daily functioning in patients with chronic pain. Pain Res Manag. 2001;7:75–79. doi: 10.1155/2002/579425. [DOI] [PubMed] [Google Scholar]
- 32.Miller LR, Cano A. Comorbid chronic pain and depression: who is at risk? J Pain. 2009;10:619–627. doi: 10.1016/j.jpain.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Murphy SL, Smith DM, Clauw DJ, Alexander NB. The impact of momentary pain and fatigue on physical activity in women with osteoarthritis. Arthritis Care Res. 2008;59:849–856. doi: 10.1002/art.23710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muthén LK, Muthén BO. Mplus: Statistical Analysis with Latent Variables; User’s Guide;[Version 5] Muthén & Muthén;; 2007. [Google Scholar]
- 35.Nes LS, Roach AR, Segerstrom SC. Executive functions, self-regulation, and chronic pain: a review. Ann Behav Med. 2009;37:173–183. doi: 10.1007/s12160-009-9096-5. [DOI] [PubMed] [Google Scholar]
- 36.Neumann L, Lerner E, Glazer Y, Bolotin A, Shefer A, Buskila D. A cross-sectional study of the relationship between body mass index and clinical characteristics, tenderness measures, quality of life, and physical functioning in fibromyalgia patients. Clin Rheumatol. 2008;27:1543–1547. doi: 10.1007/s10067-008-0966-1. [DOI] [PubMed] [Google Scholar]
- 37.Nicassio PM, Moxham EG, Schuman CE, Gevirtz RN. The contribution of pain, reported sleep quality, and depressive symptoms to fatigue in fibromyalgia. Pain. 2002;100:271–279. doi: 10.1016/S0304-3959(02)00300-7. [DOI] [PubMed] [Google Scholar]
- 38.Nijs J, Almond F, De Becker P, Truijen S, Paul L. Can exercise limits prevent post-exertional malaise in chronic fatigue syndrome? An uncontrolled clinical trial. Clin Rehabil. 2008;22:426–435. doi: 10.1177/0269215507084410. [DOI] [PubMed] [Google Scholar]
- 39.Penninx BW, Leveille S, Ferrucci L, Van Eijk J, Guralnik JM. Exploring the effect of depression on physical disability: longitudinal evidence from the established populations for epidemiologic studies of the elderly. Am J Public Health. 1999;89:1346–1352. doi: 10.2105/ajph.89.9.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): depression, anxiety, and anger. Assessment. 2011;18:263–283. doi: 10.1177/1073191111411667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pollard L, Choy E, Gonzalez J, Khoshaba B, Scott D. Fatigue in rheumatoid arthritis reflects pain, not disease activity. Rheumatology (Oxford) 2006;45:885–889. doi: 10.1093/rheumatology/kel021. [DOI] [PubMed] [Google Scholar]
- 42.Schreurs KM, de Ridder DT, Bensing JM. Fatigue in multiple sclerosis: reciprocal relationships with physical disabilities and depression. J Psychosom Res. 2002;53:775–781. doi: 10.1016/s0022-3999(02)00326-4. [DOI] [PubMed] [Google Scholar]
- 43.Silverstein B. Gender difference in the prevalence of clinical depression: the role played by depression associated with somatic symptoms. Am J Psychiatry. 1999;156:480–482. doi: 10.1176/ajp.156.3.480. [DOI] [PubMed] [Google Scholar]
- 44.Skapinakis P, Lewis G, Mavreas V. Temporal relations between unexplained fatigue and depression: longitudinal data from an international study in primary care. Psychosom Med. 2004;66:330–335. doi: 10.1097/01.psy.0000124757.10167.b1. [DOI] [PubMed] [Google Scholar]
- 45.Smets E, Garssen B, Bonke Bd, De Haes J. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 46.Solberg Nes L, Carlson CR, Crofford LJ, de Leeuw R, Segerstrom SC. Self-regulatory deficits in fibromyalgia and temporomandibular disorders. Pain. 2010;151:37–44. doi: 10.1016/j.pain.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 47.Tsang A, Von Korff M, Lee S, Alonso J, Karam E, Angermeyer MC, Borges GL, Bromet EJ, Demytteneare K, de Girolamo G, de Graaf R, Gureje O, Lepine JP, Haro JM, Levinson D, Oakley Browne MA, Posada-Villa J, Seedat S, Watanabe M. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain. 2008;9:883–891. doi: 10.1016/j.jpain.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 48.Vohs KD, Glass BD, Todd Maddox TW, Markman AB. Ego depletion is not just fatigue: evidence from a total sleep deprivation experiment. Soc Psychol Personal Sci. 2011;2:166–173. [Google Scholar]
- 49.Yunus MB, Arslan S, Aldag JC. Relationship between body mass index and fibromyalgia features. Scand J Rheumatol. 2002;31:27–31. doi: 10.1080/030097402317255336. [DOI] [PubMed] [Google Scholar]
- 50.Zautra AJ, Fasman R, Parish BP, Davis MC. Daily fatigue in women with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Pain. 2007;128:128–135. doi: 10.1016/j.pain.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 51.Zenz M, Strumpf M, Tryba M. Long-term oral opioid therapy in patients with chronic nonmalignant pain. J Pain Symptom Manage. 1992;7:69–77. doi: 10.1016/0885-3924(92)90116-y. [DOI] [PubMed] [Google Scholar]