Abstract
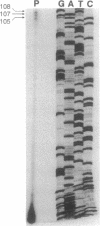
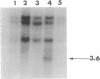
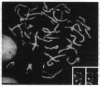
Peroxisomal acyl-CoA oxidase (ACOX; EC 1.3.3.6) is the first enzyme of the fatty acid beta-oxidation pathway, which catalyzes the desaturation of acyl-CoAs to 2-trans-enoyl-CoAs, and it donates electrons directly to molecular oxygen, thereby producing H2O2. The discovery of carcinogenic peroxisome proliferators, which markedly increase the levels of this H2O2-producing ACOX in rat and mouse liver, generated interest in peroxisomal beta-oxidation system genes. The present study deals with the structural organization of human ACOX gene. This gene spans approximately 33 kb and consists of 14 exons and 13 introns. Primer-extension analysis revealed three principal cap sites, which were mapped at 50, 52, and 53 nt upstream of the initiator methionine codon. The 5' flanking region of the ACOX gene was sequenced up to 500 bp upstream of the cap sites. This promoter region is G + C-rich and contains three copies of the "GC box" hexanucleotides. Multiple GC boxes are a characteristic feature of the rat ACOX and bifunctional protein genes of the beta-oxidation system. A + T-rich TATA-boxlike sequences, TTTATTT and TTATT, have also been identified in this human ACOX gene, but typical CCAAT motifs are absent. This ACOX gene has been mapped to chromosome 17q25 by in situ hybridization, using a biotinlabeled probe.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bout A., Franse M. M., Collins J., Blonden L., Tager J. M., Benne R. Characterization of the gene encoding human peroxisomal 3-oxoacyl-CoA thiolase (ACAA). No large DNA rearrangement in a thiolase-deficient patient. Biochim Biophys Acta. 1991 Aug 27;1090(1):43–51. doi: 10.1016/0167-4781(91)90035-k. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dreyer C., Krey G., Keller H., Givel F., Helftenbein G., Wahli W. Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992 Mar 6;68(5):879–887. doi: 10.1016/0092-8674(92)90031-7. [DOI] [PubMed] [Google Scholar]
- Green S. Receptor-mediated mechanisms of peroxisome proliferators. Biochem Pharmacol. 1992 Feb 4;43(3):393–401. doi: 10.1016/0006-2952(92)90554-v. [DOI] [PubMed] [Google Scholar]
- Göttlicher M., Widmark E., Li Q., Gustafsson J. A. Fatty acids activate a chimera of the clofibric acid-activated receptor and the glucocorticoid receptor. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4653–4657. doi: 10.1073/pnas.89.10.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijikata M., Wen J. K., Osumi T., Hashimoto T. Rat peroxisomal 3-ketoacyl-CoA thiolase gene. Occurrence of two closely related but differentially regulated genes. J Biol Chem. 1990 Mar 15;265(8):4600–4606. [PubMed] [Google Scholar]
- Ishii N., Hijikata M., Osumi T., Hashimoto T. Structural organization of the gene for rat enoyl-CoA hydratase:3-hydroxyacyl-CoA dehydrogenase bifunctional enzyme. J Biol Chem. 1987 Jun 15;262(17):8144–8150. [PubMed] [Google Scholar]
- Issemann I., Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990 Oct 18;347(6294):645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B., De Duve C. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2043–2046. doi: 10.1073/pnas.73.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa S., Hayashi H., Hijikata M., Ishii N., Furuta S., Kagamiyama H., Osumi T., Hashimoto T. Complete nucleotide sequence of cDNA and predicted amino acid sequence of rat acyl-CoA oxidase. J Biol Chem. 1987 Jun 15;262(17):8131–8137. [PubMed] [Google Scholar]
- Osumi T., Ishii N., Miyazawa S., Hashimoto T. Isolation and structural characterization of the rat acyl-CoA oxidase gene. J Biol Chem. 1987 Jun 15;262(17):8138–8143. [PubMed] [Google Scholar]
- Poll-The B. T., Roels F., Ogier H., Scotto J., Vamecq J., Schutgens R. B., Wanders R. J., van Roermund C. W., van Wijland M. J., Schram A. W. A new peroxisomal disorder with enlarged peroxisomes and a specific deficiency of acyl-CoA oxidase (pseudo-neonatal adrenoleukodystrophy). Am J Hum Genet. 1988 Mar;42(3):422–434. [PMC free article] [PubMed] [Google Scholar]
- Quan F., Korneluk R. G., Tropak M. B., Gravel R. A. Isolation and characterization of the human catalase gene. Nucleic Acids Res. 1986 Jul 11;14(13):5321–5335. doi: 10.1093/nar/14.13.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy J. K., Goel S. K., Nemali M. R., Carrino J. J., Laffler T. G., Reddy M. K., Sperbeck S. J., Osumi T., Hashimoto T., Lalwani N. D. Transcription regulation of peroxisomal fatty acyl-CoA oxidase and enoyl-CoA hydratase/3-hydroxyacyl-CoA dehydrogenase in rat liver by peroxisome proliferators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1747–1751. doi: 10.1073/pnas.83.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley J. D., Diaz M. O., Espinosa R., 3rd, Patel Y. D., van Melle E., Ziemin S., Taillon-Miller P., Lichter P., Evans G. A., Kersey J. H. Mapping chromosome band 11q23 in human acute leukemia with biotinylated probes: identification of 11q23 translocation breakpoints with a yeast artificial chromosome. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9358–9362. doi: 10.1073/pnas.87.23.9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers L., Van Veldhoven P. P., Casteels M., Eyssen H. J., Mannaerts G. P. Presence of three acyl-CoA oxidases in rat liver peroxisomes. An inducible fatty acyl-CoA oxidase, a noninducible fatty acyl-CoA oxidase, and a noninducible trihydroxycoprostanoyl-CoA oxidase. J Biol Chem. 1990 Mar 25;265(9):5242–5246. [PubMed] [Google Scholar]
- Sher T., Yi H. F., McBride O. W., Gonzalez F. J. cDNA cloning, chromosomal mapping, and functional characterization of the human peroxisome proliferator activated receptor. Biochemistry. 1993 Jun 1;32(21):5598–5604. doi: 10.1021/bi00072a015. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sternberg N. L. Cloning high molecular weight DNA fragments by the bacteriophage P1 system. Trends Genet. 1992 Jan;8(1):11–16. doi: 10.1016/0168-9525(92)90018-y. [DOI] [PubMed] [Google Scholar]
- Tolbert N. E. Metabolic pathways in peroxisomes and glyoxysomes. Annu Rev Biochem. 1981;50:133–157. doi: 10.1146/annurev.bi.50.070181.001025. [DOI] [PubMed] [Google Scholar]
- Vanhove G. F., Van Veldhoven P. P., Fransen M., Denis S., Eyssen H. J., Wanders R. J., Mannaerts G. P. The CoA esters of 2-methyl-branched chain fatty acids and of the bile acid intermediates di- and trihydroxycoprostanic acids are oxidized by one single peroxisomal branched chain acyl-CoA oxidase in human liver and kidney. J Biol Chem. 1993 May 15;268(14):10335–10344. [PubMed] [Google Scholar]
- Wei L. L., Krett N. L., Francis M. D., Gordon D. F., Wood W. M., O'Malley B. W., Horwitz K. B. Multiple human progesterone receptor messenger ribonucleic acids and their autoregulation by progestin agonists and antagonists in breast cancer cells. Mol Endocrinol. 1988 Jan;2(1):62–72. doi: 10.1210/mend-2-1-62. [DOI] [PubMed] [Google Scholar]
- Yeldandi A. V., Wang X. D., Alvares K., Kumar S., Rao M. S., Reddy J. K. Human urate oxidase gene: cloning and partial sequence analysis reveal a stop codon within the fifth exon. Biochem Biophys Res Commun. 1990 Sep 14;171(2):641–646. doi: 10.1016/0006-291x(90)91194-w. [DOI] [PubMed] [Google Scholar]
- Zhang B., Marcus S. L., Sajjadi F. G., Alvares K., Reddy J. K., Subramani S., Rachubinski R. A., Capone J. P. Identification of a peroxisome proliferator-responsive element upstream of the gene encoding rat peroxisomal enoyl-CoA hydratase/3-hydroxyacyl-CoA dehydrogenase. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7541–7545. doi: 10.1073/pnas.89.16.7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Alvares K., Huang Q., Rao M. S., Reddy J. K. Cloning of a new member of the peroxisome proliferator-activated receptor gene family from mouse liver. J Biol Chem. 1993 Dec 25;268(36):26817–26820. [PubMed] [Google Scholar]