Abstract
Despite evidence of autonomic disturbances in chronic multi-symptom illnesses such as temporomandibular joint disorder (TMD) and fibromyalgia (FM), additional work is needed to characterize the role of parasympathetic reactivity in these disorders. Given the high levels of comorbidity with psychiatric disorders characterized by stronger parasympathetic reductions than controls in safe contexts (leading to higher arousal), it was hypothesized that individuals with TMD and FM would respond similarly. In this preliminary investigation, 43 women with TMD (n = 17), TMD + FM (n = 11), or neither (controls; n = 15) completed a baseline assessment of respiratory sinus arrhythmia (RSA; a measure of parasympathetic activity) followed by ongoing parasympathetic assessment during a questionnaire period. As predicted, patients showed greater parasympathetic decline in response to the questionnaire period, suggesting an autonomic stance that supports defensive rather than engagement behaviors. Individual differences in parasympathetic reduction during the questionnaire period were related to a variety of physical and psychosocial variables. Although this study has a number of key limitations, including a convenience sampling approach and the small group sizes, if replicated in larger samples, the findings would have important implications for the treatment of patients with these disorders.
Perspective
Compared to controls, individuals with temporomandibular disorders or temporomandibular disorder and fibromyalgia demonstrated greater parasympathetic reduction during psychosocial assessment, and individual differences in parasympathetic reduction predicted negative patient outcomes. Such parasympathetic reductions may betray a tendency to readily perceive danger in safe environments.
Keywords: autonomic reactivity, polyvagal theory, respiratory sinus arrhythmia, fibromyalgia, temporomandibular disorder, chronic pain
Introduction
Chronic multi-symptom illnesses such as temporomandibular disorders (TMD) and fibromyalgia (FM) often co-occur and share many features.34,54,53,7,25 There is no universal known cause of either disorder, although numerous overlapping risk factors have been identified.1,56,25 Altered functioning of the autonomic nervous system (ANS) represents one such overlapping risk factor.
The two branches of the ANS have antagonistic effects on autonomic arousal. However, arousal is under tonic inhibitory control of the parasympathetic branch via the myelinated vagus nerve (termed the “vagal brake” or “parasympathetic maintenance”), which allows for efficient upregulation of arousal via parasympathetic reduction (or “vagal withdrawal”). According to Porges’ polyvagal theory, parasympathetic maintenance promotes calm engagement, whereas vagal withdrawal facilitates quick escape from danger. 36,37,39 FM and TMD are linked to higher baseline sympathetic activity or predominance, especially at night,12,29,42,32,14,46,36 and lower baseline parasympathetic activity.44,42,12, With regard to ANS reactivity in these disorders, some evidence points toward blunted sympathetic responding coupled with greater increases in arousal,42,31,55,11 from which one might infer greater parasympathetic reduction in response to the environment. However, very little is known about the nature of parasympathetic reactivity in these conditions.42
Some reduction in parasympathetic activity in response to safe stimuli facilitates task engagement.39 However, rapid or exaggerated parasympathetic decline in response to safe stimuli is associated with hypersensitivity to environmental danger36 and has been described as a “nonspecific marker of emotional lability.”3 Consistent with this perspective, greater parasympathetic reductions in response to objectively safe laboratory stimuli have been associated with various reactive emotional disorders such as panic,52 generalized anxiety,48 and borderline personality disorder.2 Given the frequent comorbidity of FM and TMD with such disorders,34,7 we hypothesize a similar pattern of parasympathetic reactivity among individuals with TMD or FM.38
The current study examined the physiologic functioning of controls, individuals with TMD, and individuals with both TMD and FM at rest and while completing psychosocial measures. Questionnaire completion was conceptualized as an safe task that mirrors the assessment protocol used in pain clinics, where patients fill out assessment paperwork prior to examination.34 Given the objectively safe nature of this context, the polyvagal theory would specify the adaptive response as relative maintenance of parasympathetic activity to facilitate task engagement, whereas relatively greater parasympathetic reduction would represent an inappropriate defensive response related to the inaccurate perception of danger.38
Hypotheses
The following specific hypotheses were tested:
Baseline parasympathetic functioning is suppressed among individuals with TMD or TMD + FM.
Individuals with TMD and TMD + FM will exhibit greater parasympathetic decline during the assessment period. Such decline would be greater among individuals with both diagnoses than individuals with one or no diagnosis, and greater among individuals with TMD than with no diagnosis.
Individual differences in rate of parasympathetic decline during the assessment period will be associated with poorer sleep, poorer general physical and mental functioning, greater chronic pain symptom severity, greater impact of symptoms on functioning, and greater distress and depression.
Materials and Methods
Participants
Participants were 43 females between the ages of 18 and 65. Pain patients were diagnosed with TMD with or without comorbid FM. Diagnosis of TMD was made by a clinician using the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD).18,43 Patients were included if they presented with painful temporomandibular disorders. 88.3% of participants in the TMD-only group and 90.9% of participants in the TMD+FM group were diagnosed with myalgia; the majority of those participants were also diagnosed with another type of TMD pain (i.e., disc disorders or anthralgia/osteoarthritis). Diagnosis of comorbid FM was made by a Rheumatologist using 1990 ACR classification criteria and/or 2010 ACR criteria. Patients with TMD were recruited from a university-based tertiary orofacial pain clinic, from the Kentucky Women's Health Registry (KWHR), or from advertisements by flier or in the newspaper. The KWHR is a longitudinal cohort study containing self-report information including the presence of painful conditions including TMD and FM where patients agree to be contacted for studies for which they may qualify based on inclusion and exclusion criteria. Controls were recruited from the KWHR or from the community using fliers. Potential participants were excluded from the study if they were pregnant or nursing, prisoners or institutionalized, severely obese as defined by body mass index ≥ 40, current alcohol or other substance abusers, current smokers of ≥ 1 pack of cigarettes daily, taking or received oral, inhaled, or injected corticosteroids within 3 months, unable to discontinue medications that affect heart rate variability such as beta blockers, currently active axis I psychiatric diagnosis other than simple phobia as assessed by the Mini-International Neuropsychiatric Interview (MINI) structured diagnostic interview, presence of any active or unstable medical condition including chronic infection or inflammatory/autoimmune condition.
Table 1 contains descriptive data regarding demographic (and substantive) variables in the full sample and by group. Racial makeup of the sample was as follows: 95.3% Caucasian (Control Group = 93.3%; TMD Group = 100%; TMD + FIBRO Group = 90.9%) and 4.7% African American (Control Group = 6.7%; TMD Group = 0%; TMD + FIBRO Group = 9.1%).
Table 1.
Descriptive Statistics in the Full Sample and by Diagnostic Group
| Variable | Full Sample (N = 43) Mean (SD) | Controls (n = 15) Mean (SD) | TMD Only (n = 17) Mean (SD) | TMD + FM (n = 11) Mean (SD) |
|---|---|---|---|---|
| Age | 45.95 (12.40) | 46.13 (12.51) | 42.76 (12.90) | 50.64 (10.91) |
| Baseline RSA | 5.35 (1.04) | 5.64 (1.01) | 5.53 (1.21) | 5.24 (.81) |
| Rate of RSA Decline during Assessment† | −.005 (.01) | −.001 (.01)a | −.006 (.005)b | −.01 (.02)c |
| Baseline HP | .83 (.07) | .83 (.09) | .84 (.08) | .81 (.05) |
| Rate of HP Change during Assessment | −.00001 (.0009) | .0001 (.0007)a | .0002 (.0009)a | −.0006 (.001)b |
| Sleep Problems (PSQI) | 10.76 (3.42) | 9.40 (2.50)a | 10.41 (3.35)a | 13.18 (3.62)b |
| Pain (BPI) | 2.46 (2.30) | .60 (.82)a | 2.26 (1.67)b | 5.31 (1.63)c |
| Fibromyalgia Symptom Severity (WPI +SSS) | 4.90 (5.41) | 1.64 (2.34)a | 3.13 (2.62)a | 11.63 (5.46)b |
| Symptom Interference (SQIR) | 5.37 (6.37) | 1.40 (1.12)a | 3.43 (2.50)a | 13.81 (7.09)b |
| Depression (CES-D) | 48.00 (75.60) | 12.80 (24.20)a | 67.00 (86.8)b | 63.60 (89.20)b |
| Global Psychological Symptoms (SCL-90 GSI) | 53.05 (10.22) | 46.31 (8.55)a | 53.65 (8.78)b | 60.80 (9.23)c |
| Mental Health Component Score (SF-12v2 MCS) | 50.07 (8.43) | 53.93 (4.90) | 48.76 (6.94) | 46.81 (12.28) |
| Physical Health Component Score (SF-12v2 PCS) | 49.21 (11.67) | 55.73 (4.49)a | 53.06 (5.73)a | 34.36 (12.66)b |
| Perceived Stress Scale (PSS) | 19.80 (3.60) | 19.00 (3.28) | 19.58 (3.96) | 21.18 (3.40) |
SD = standard deviation; RSA = Respiratory Sinus Arrhythmia; HP = Heart Period; BPI = Brief Pain Inventory; WPI = Widespread Pain Index; SSS = Symptom Severity Score; SQIR = Symptom Interference Questionnaire – Revised; CES-D = Center for Epidemiological Studies Depression Scale; SCL-90 GSI= Symptom Checklist – 90 Global Severity Index; Differing subscripts (i.e., a, b, c) indicate significant pairwise group differences determined using pairwise contrasts of significant One-Way ANOVAs. The Tukey method was used to decrease the probability of Type I error by adjusting p values for the large number of contrasts performed.
This variable represents a reverse-scored version of an individual's raw slope of time on RSA during the assessment period. Rate of RSA decline during assessment was calculated by multiplying the raw slope of time on RSA by −1). Group values for raw slope can be determined by simply reversing the sign (+/−) of the values presented here. The FM symptom severity score (WPI + SSS) was normalized by applying a square-root transformation. All symptom variables except the SF-12v2 variables are scored such that higher scores indicate greater symptom severity.
Procedures
All procedures were reviewed and approved by the University of Kentucky institutional review board. During the initial visit, patients provided informed consent and were examined by a clinician to determine eligibility for the study. Informed consent included an explanation of the measures included in the packet, with an emphasis on the anonymous nature of responses, and also included an explanation of physiological recording procedures, emphasizing that these would be non-invasive and not painful. Patients were classified as TMD (n = 17), TMD and FM (n = 11), or control (n = 15), and the MINI was completed. If it was necessary to discontinue any medications that could affect heart rate, instructions were provided.
Participants returned to the clinical research center for autonomic and psychosocial assessment. Participants were seated in a comfortable dental exam chair in a clean room with modern decor. Sessions began with an interaction with the experimenter, who reviewed the participant's previously-provided information to assure there were no changes to the participants’ medications. The experimenter was dressed in normal “business casual” clothing and was not wearing a lab coat. Next, the experimenter connected the participant for psychophysiological recording to a Biopac ECG100C electrocardiogram amplifier module using three Ag-AgCl leads in a lead-I configuration (leads attached to the anterior shoulders and left ankle) with a sampling rate of 1,000 samples per second. After a 5 minute acclimation period, the experimenter instructed the participant to sit quietly for 15 minutes while their baseline heart rate variability (HRV) and heart period (HP) were recorded, and then left the room for this baseline period. Participants were allowed to look at an assortment of magazines during this time. Following the baseline period, the experimenter returned and oriented the participant to the psychosocial questionnaires, at which time the participant was told they would have as much time as they needed to complete the measures, while the experimenter again left the room. The equipment recorded their physiological responses for the first 15 minutes of the assessment period.
Psychological Measures
Subjective Pain Severity
Subjective pain was measured using the Pain Severity subscale of the Brief Pain Inventory.13 The pain severity items are measured on a visual analog 1-100 scale, and include a subjective measure of pain at its “worst,” “least”, “average”, and “now” (current pain).
General Physical and Mental Functioning
The 12-item Short Form Health Survey (SF-12v2) served as a measure of general health status.51 The SF-12v2 measures eight domains of health status; in the present study, we utilized the physical functioning summary score (domains 1-4) and mental health summary score (domains 5-8) as measures of general functioning. In this study the standard (i.e., 4-week recall) version was used. In the present sample, reliability was excellent for both the physical functioning (α = .94) and mental health (α = .90) summary scores.
Physical Symptom Severity and Functional Impairment
The revised Symptom Impact Questionnaire (SIQR) is a 21-item measure of the severity and impact of chronic pain syndromes.22,4 Participants are asked to rate items on three subscales: specific symptoms (e.g., pain, fatigue, stiffness), specific types of functional problems related to the symptoms (e.g., prepare a homemade meal, brush or comb your hair), and overall impact of symptoms (e.g., I was completely overwhelmed by my medical problems). The rating scale ranged from 0-10, with 0 representing none of a given symptom or functional impairment, and 10 representing the highest level of a given symptom or functional impairment. In the present study, the total score was utilized; reliability was excellent (α = .96). All outcomes presented in this paper were nearly identical when the total SIQR scale was replaced with any of the specific subscales.
Sleep Disturbance
The Pittsburgh Sleep Quality Index (PSQI)8 is an 18-item measure of sleep disturbance. Items inquire about general sleep quantity and quality, including number of hours spent in bed and asleep, number of sleep disturbances, sleep latency, sleep efficiency, and use of sleep medication. In the present study, the full scale was used rather than any particular subscale; reliability was good (α = .89).
Perceived Life Stress
The Perceived Stress Scale is a 10-item measure of nonspecific life stress in the past month. Participants rate how often they have felt or thought a certain way on a scale from 0 (Never) to 4 (Very Often). Higher scores indicate higher levels of life stress, and have been associated with a variety of negative health outcomes, including greater vulnerability to depressive symptoms and poorer health.15,16 In the present sample, reliability was good (α = .87).
Depressive Symptoms
The Center for Epidemiologic Studies Depression Scale (CES-D) is a 20-item inventory of depressive symptoms.41 The CES-D asks participants to rate their mood, thoughts, and behavior during the previous week on a 4-point Likert scale. CES-D items were scored such that higher total scores were indicative of greater depressive symptoms. In the present sample, reliability was excellent (α = .94).
General Psychological Distress
The Symptom Checklist-90-Revised (SCL-90-R) is a 90-item measure assessing the number and severity of psychological symptoms.19 Participants rate how much each symptom “distressed or bothered” them during the past week on a scale from 0 (not at all) to 4 (Extremely). Although the SCL-90-R has multiple subscales, recent evidence suggests that the Global Severity Index (GSI) scale which is obtained by averaging the scores of all items answered, may be especially predictive of psychological distress status in chronic pain.7 The test-retest reliability and the internal consistency of the SCL-90 domains is generally considered good (r = 0.78-90 and coefficient α = 0.77-90).
Physiological Measures
Physiological recordings were captured as 1-minute epochs during the baseline period (15 minutes) as well as the assessment period (15 minutes). Baseline measures of respiratory sinus arrhythmia (RSA) and HP were calculated by averaging across the 15 1-minute epochs during the baseline period. During the first 15 minutes of the assessment period, RSA and HP were conceptualized as repeated measures across 15 1-minute time points; reactivity was operationalized as the slope of time on either RSA or HP across this 15-minute period for a given individual (see “Extraction of Individual Differences in Autonomic Responses During Assessment” section below for a more detailed explanation). This method of quantifying change over time avoids typical problems associated with the interpretation of change scores and maximizes the reliability of change as an individual difference measure (Cronbach & Furby, 1970; Singer & Willett, 2003).
Respiratory Sinus Arrhythmia (RSA)
RSA represents an approximate index of vagus nerve activity—a measure of parasympathetic influence on the heart—as evidenced by the degree of oscillation present in the natural pattern of acceleration and deceleration of the heart rate across the respiratory cycle.6 In the present study, Mindware HRV 3.0.1 software (Mindware, Inc.; Gahanna, OH) was used to conduct spectral analysis of heart rate variability using electrocardiogram data to quantify high frequency heart rate variability (HF-HRV; .15-.40 Hz), which serves as a measure of RSA. In their paper outlining standards for the measurement of heart rate variability, the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology recommends the use of frequency-domain methods such as spectral analysis for short-term recordings, and states that that the duration of such recordings should be “at least 10 times the wavelength of the lower frequency bound of the investigated component” in order to provide reliable estimation of HRV at the epoch level. In the case of the high frequency component of HRV, this would require a duration of “approximately 1 minute” (p. 364). 9 Therefore, RSA was measured in 1-minute epochs. Correction of movement artifacts as well as errors in computerized marking of R-peaks was accomplished manually in Mindware HRV 3.0.1 software, again according to standards suggested by the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology.9 In many cases (n = 186 out of 2,580 total epochs measured), increased movement (perhaps associated with responding to the questionnaires or adjusting one's body for greater comfort) led to extreme movement artifacts in the data that could not be corrected in a satisfactory manner. In these cases, the epoch was entered as missing. The number of missing epochs did not significantly differ between groups (all p's greater than .46).
Heart Period (HP)
HP, which was measured as average inter-beat interval (IBI) across 15 1-minute epochs, served as a measure of overall autonomic arousal. Heart period is conceptualized as a measure of overall arousal, and is under the dual control of both sympathetic and parasympathetic input.
Analytic Plan
Preliminary analyses included screening data for skew and kurtosis, as well as one-way ANOVAs and follow-up contrasts (using the Tukey method) to compare the three groups on all outcomes. The particular contrast tests comparing baseline parasympathetic activity across the three groups represents a test of hypothesis 1—that patient groups would have lower baseline parasympaethetic activity than controls.
To test hypothesis 2—that patient groups would show greater parasympathetic decline (hyperarousal) than controls during an objectively safe psychosocial assessment—SAS PROC MIXED was used to fit two multilevel growth models (15 assessment period epochs nested within individuals). We specified the Kenward-Roger method for denominator degrees of freedom, which has been demonstrated to provide the most reliable estimates of fixed effects in small samples compared with other available methods.27 These two models were designed to test for pairwise group differences in the trajectory of RSA over time during the assessment period. Time was treated as a random effect. The first model, which compared the rates of change in RSA during the assessment period in the two pain groups to the rate of change in the control group, included the following predictors: time, TMD + FM group (coded 1 for those with TMD + FM, 0 for all others), TMD-only group (coded 1 for those with TMD only, 0 for all others), and the interactions of time with both dichotomous group variables. The second model replaced the group and interaction terms that included TMD + FM with the Control group (effectively changing the reference group to the TMD + FM group) in order to test whether the rate of RSA change over time differed between the TMD only and TMD + FM groups. Similar models were fit for HP.
Next, an individual difference variable was created that represented each participant's rate of this parasympathetic decline during the assessment period. This was achieved by saving the slope of time on RSA during the assessment for each participant in a multilevel model with only time as a predictor. This variable was then correlated with all other outcomes, which served as an initial test of Hypothesis 3—that individual differences in parasympathetic decline in a safe context would be associated with poorer outcomes. This hypothesis was further tested by regressing outcomes on this individual difference variable while controlling for baseline parasympathetic activity.
In order to reduce the likelihood of type I error given the large number of statistical tests, alpha was set at .01.
Results
Data Screening
All variables except the FM symptom severity score (WPI+SSS), which was positively skewed, met the assumptions of ordinary least squares regression. The FM symptom severity score (WPI + SSS) was normalized by applying a square-root transformation. Data were also screened for univariate outliers on RSA to rule out the possibility that extreme values of RSA (i.e., values 3.29 standard deviations from the mean; n = 32 epochs out of 2,394 valid epochs) had a substantial impact on our findings. As removal of these outliers did not change the effect sizes and did not increase p-values for hypothesis tests, outliers were retained in final models in order to avoid over-fitting models to the current sample data.
Preliminary Analyses
Descriptive information for both demographic variables and variables used in models for the full sample and by diagnostic group are located in Table 1. One-way ANOVAs and follow-up contrasts using the Tukey method were carried out to determine group differences in each variable; significant differences are denoted by differing superscripts in Table 1. Compared with controls, a diagnosis of TMD (without comorbid FM) was associated with steeper RSA decrease (more vagal withdrawal) during the assessment period, greater pain on the BPI, greater depression on the CES-D, and greater global psychological symptoms on the GSI subscale of the SCL-90. Compared with a diagnosis of TMD (without comorbid FM), individuals diagnosed with both TMD and FM had steeper declines in RSA (more vagal withdrawal) and steeper increases in HP during the assessment period, greater self-reported sleep problems on the PSQI, greater pain on the BPI, greater FM symptoms as measured by the sum of the WPI and SSS, greater symptom interference on the SIQR, greater depression on the CES-D, greater global psychological symptoms on the GSI subscale of the SCL-90, and greater physical symptoms on the physical component subscale of the SF-12v2. Compared with controls, individuals diagnosed with both FM and TMD had steeper decreases in RSA (more vagal withdrawal) and steeper increases in HP during the assessment period, greater self-reported sleep problems on the PSQI, greater pain on the BPI, greater FM symptoms as measured with the sum of the WPI and SSS, greater symptom interference on the SIQR, greater depression on the CES-D, greater global psychological symptoms on the GSI subscale of the SCL-90, and greater physical symptoms on the physical component subscale of the SF-12v2. In general, then, although the TMD group showed slightly greater problems than controls in a few areas, the TMD + FM group showed greater problems than both controls and the TMD group on the majority of outcomes. Notably, there were no significant group differences in baseline parasympathetic activity; therefore, hypothesis 1 was not supported in this sample.
Multilevel Growth Models Comparing RSA and HP Change Over Time During the Assessment Period by Group and Pain Level
Respiratory Sinus Arrhythmia
Porges’ polyvagal theory would predict that individuals with stress-related somatic syndromes such as TMD, FM, or both will show greater reduction in RSA in response to objectively safe stimuli such as psychosocial assessment. Preliminary inspection of the raw RSA data over time with fitted loess lines ruled out the presence of a quadratic, cubic, or discontinuous effect of time on RSA.44 Consistent with hypothesis 2, both interaction terms were negative, indicating that, compared with controls, there was greater reduction in RSA during the assessment period among those in the TMD-only group (γTMDONLY*TIME = −.012, SE = .003, t(37) = −2.79, p = .008) and the TMD + FM group (γTMD + FM*TIME = −.018, SE = .007, t(36) = −3.01, p = .004).
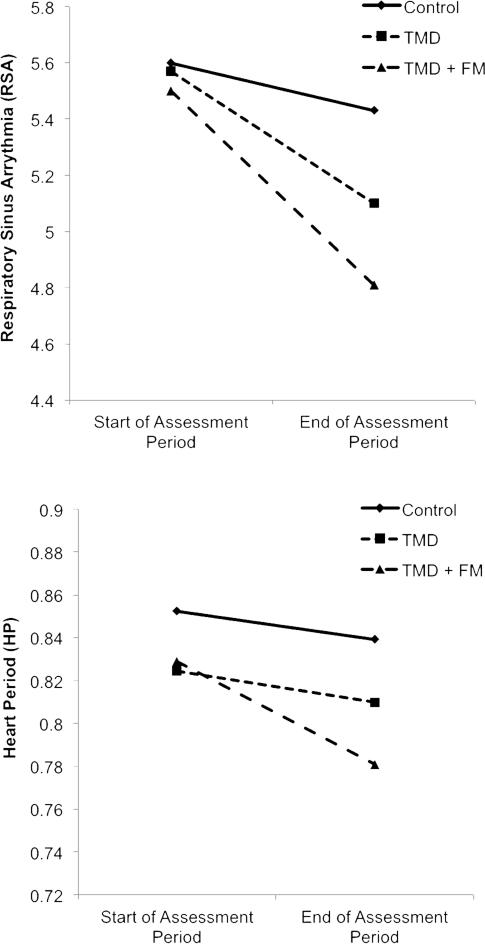
We also predicted that the TMD + FM group would show greater reduction in RSA during the assessment period than the TMD-only group. Again consistent with hypothesis 2, the interaction term comparing rate of RSA change in the TMD-only group to the rate of RSA change in the TMD + FM group was also significant (γTMD + FM*TIME = −.011, SE = .003, t(35) = −3.67, p = .0008), suggesting that the rate of RSA decline during the questionnaires was significantly different between the two diagnostic groups. See the top panel of Figure 1 for a depiction of RSA over time in the three groups.
Figure 1.
Graphs depicting mean levels of RSA and HP at the beginning and end of the assessment period by diagnostic group.
Data points depicted are mean levels of RSA or HP within a given group during the first minute (“Beginning of Assessment Period) and last minute (“End of Assessment Period”) of the assessment phase of the study. In the upper panel, the observed decrease in RSA over time indicates “vagal withdrawal”, which is the term used to describe this phenomenon in the rest of this paper.
Our final prediction regarding rate of change in RSA was that higher levels of subjective pain levels would be associated with greater parasympathetic reduction during the assessment period. To test this prediction, we fit a multilevel growth model predicting RSA from time, standardized scores on the BPI, and their interaction. Consistent with hypothesis 2, there was a significant interaction between time and pain predicting RSA (γPAIN*TIME = −.010, SE = .003, t(36) = −3.33, p = .002) such that higher levels of pain (+1 SD) were associated with greater RSA reduction during the assessment period (γTIME = −.014, SE = .004, t(36) = −3.58, p = .001), whereas there was no significant association between time and RSA among individuals with lower levels of pain (-1 SD; γTIME = −.001, SE = .003, t(36) = −.33, p = .743), suggesting maintenance of parasympathetic activity among these low-pain individuals. See the top panel of Figure 2 for a depiction of the interaction between time and pain predicting RSA.
Figure 2.
Graphs depicting the interaction between time and pain levels predicting RSA and HP during the assessment period.
Lines represent the simple effects of time at the first minute (“Beginning of Assessment Period”) and last minute (“End of Assessment Period”) of the assessment period at both high (1 SD above the sample mean) and low (1 SD below the sample mean) levels of pain as indicated in the Brief Pain Inventory. In the upper panel, the observed decrease in RSA over time indicates “vagal withdrawal”, which is the term used to describe this phenomenon in the rest of this paper.
Heart Period
Identical predictive models were also tested for HP in order to provide descriptive information about change in HP over time by group and pain level. Once again, preliminary inspection of the raw HP data over time with fitted loess lines ruled out the presence of a quadratic, cubic, or discontinuous effect of time on HP.44 In the model comparing the two diagnostic groups to the control group, there was no significant difference in HP change over time during the assessment period between the controls and those with TMD (γTMD*TIME = .0001, SE = .0002, t(39) = .51, p = .697), but there was a significant difference in rate of HP change between controls and those with TMD + FM (γTMD + FM*TIME = −.0009, SE = .0003, t(38) = −3.11, p = .004) such that individuals with TMD + FM showed a stronger decline in HP (indicating a steeper increase in heart rate) than controls. In the model comparing rate of HP change in the two diagnostic groups, the TMD + FM group showed a stronger decline in HP (again, indicating an steeper increase in heart rate) across the assessment period (γTMD + FM*TIME = −.001, SE = .0003, t(37) = −3.36, p = .002) than the TMD-only group. See the bottom panel of Figure 1 for a depiction of HP over time in the three groups. Finally, there was also a significant interaction between BPI pain and time predicting HP (γPAIN*TIME = −.0003, SE = .0001, t(38) = −3.62, p = .0009) such that HP decreased significantly over time (indicating an steeper increase in heart rate) among individuals with higher levels of pain (+1 SD; γTIME = −.0003, SE = .0001, t(37) = −3.07, p = .004) whereas HP lengthened significantly over time among individuals with lower levels of pain (indicating a much milder increase in heart rate; −1 SD; γTIME = .0003, SE = .0001, t(37) = 3.87, p = .0004). See the bottom panel of Figure 2 for a depiction of the interaction between time and pain predicting HP.
Extraction of Individual Differences in Autonomic Responses During Assessment
Subsequent study predictions required the creation of variables representing individual differences in rate of parasympathetic decline during the assessment period-- both changes in RSA and HP. For each autonomic variable, we used SAS PROC MIXED to fit a two-level multilevel growth model (with epochs from the assessment period nested within individuals) using only time as a predictor. For each individual, the slope of time predicting either RSA or HP (i.e., the rate of change) was saved as a between-person variable. Moving forward, these two variables were used to represent individual variability in autonomic responses during the assessment period.
Vagal Contribution to Changes in Heart Period by Group and Pain Level
Further work from Porges and colleagues demonstrates that, for individuals who tend to be reactive to safe stimuli, changes in overall arousal (e.g., as measured with HP/heart rate) are not as tightly controlled by parasympathetic reduction, and that modulation of arousal for such individuals is often heavily dependent on costly, inefficient increases in sympathetic activity in addition to reductions in parasympathetic activity.36,2 This results in a failure of changes in overall arousal (e.g., HP) to significantly correlate with changes in parasympathetic activity.5 Therefore, we further examined whether vagal contribution to changes in HP during the assessment would be attenuated among the diagnostic groups and among individuals with higher levels of self-reported pain. Such contribution was estimated by correlating the rate of change in RSA (raw slope) with the rate of change in HP across the assessment segment. Correlations were conducted within each diagnostic group, and among individuals above or below the median of self-reported pain. These correlational analyses evaluate whether, in each group, the observed rate of change in RSA and HP share a common vagal (parasympathetic) mechanism, or whether parasympathetic change is not significantly correlated with HP changes, in which case sympathetic influence on arousal is suspected.5 Heart period changes that are totally dependent on vagal regulation should correlate very highly with RSA changes; in contrast, changes in HP that are not tightly regulated by the vagus (i.e., sympathetically-driven changes) should show very low or nonsignificant correlations with change in RSA.
There was a positive correlation between rate of change in HP and rate of change in RSA among individuals with no diagnosis (r(15) = .67, p = .011) and among those below the median level of self-reported pain (r(23) = .46, p = .028), indicating that any changes in HP are associated with changes in parasympathetic control over the heart among these individuals (i.e., decreases in RSA were associated with shortened HP). In contrast, changes in HP and RSA were not significantly correlated within the combined diagnostic groups (i.e., individuals with TMD and individuals with TMD + FM; r(28) = .34, p = .082), within the TMD-only group (r(17) = .21, p = .422), within the TMD + FM group ( r(11) = .34, p = .297), or within the group of individuals above the median level of self-reported pain ( r(20) = .30, p = .213). Therefore, although there was greater parasympathetic reduction among individuals with chronic multi-symptom syndromes or greater pain in general, these changes in parasympathetic activity did not account well for HP changes, suggesting that HP change in response to assessment may be mediated at least in part by some other mechanism (e.g., changes in sympathetic activity).
Assessment-Related Vagal Withdrawal, Sleep Problems, Symptom Severity, Life Interference, and Depression
For all subsequent analyses, we created a reverse-scored variable that represented the rate of parasympathetic decline (i.e., rate of RSA decline) during the assessment period by multiplying the slope by −1 in order to produce a variable coded such that higher scores represented greater parasympathetic decline over time.
Zero-order correlations among primary study variables can be found in Table 2 (calculated using SAS PROC CORR. Surprisingly, baseline RSA was not significantly correlated with any other study variable. However, rate of RSA decline (i.e., parasympathetic reduction) was generally associated with more negative outcomes on nearly all other variables, while rate of HP lengthening (i.e., heart rate slowing) was associated only with better physical functioning. Outcomes were generally intercorrelated in expected ways.
Table 2.
Correlations among Primary Study Variables (N = 43)
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Baseline RSA | 1 | ||||||||||||
| 2. Rate of RSA Decline during Assessment† | .05 | 1 | |||||||||||
| 3. Baseline HP | .22 | .16 | 1 | ||||||||||
| 4. Rate of HP Change during Assessment | −.04 | −.39* | .02 | 1 | |||||||||
| 5. Self-Reported Sleep Problems (PSQI) | −.06 | .41* | −.16 | −.05 | 1 | ||||||||
| 6. Self-Reported Pain (BPI) | −.12 | .36* | −.16 | −.27 | .52* | 1 | |||||||
| 7. Fibromyalgia Symptom Severity (WPI+SSS) | −.11 | .25* | .01 | −.23 | .56* | .79* | 1 | ||||||
| 8. Symptom Interference (SIQR) | −.10 | .38* | −.15 | −.25 | .60* | .92* | .82* | 1 | |||||
| 9. Depression (CES-D) | −.17 | .32* | −.22 | .30 | .45 | .55* | .40* | .57* | 1 | ||||
| 10. Physical Functioning (SF-12v2) | .11 | −.31* | .06 | .35* | −.58* | −.76* | −.74* | −.84* | −.06 | 1 | |||
| 11. Mental Functioning (SF-12v2) | .07 | −.18 | .25 | .04 | −.16 | −.22 | −.24 | −.46 | −.75* | .12 | 1 | ||
| 12. Psychological Distress (SCL-90 GSI) | −.09 | .15 | −.08 | −.06 | .45* | .44* | .60* | .59* | .61* | −.45* | −.64* | 1 | |
| 13. Perceived Life Stress (PSS) | −.11 | −.02 | .08 | .01 | .14 | .06 | .31 | .13 | .30 | −.02 | −.16 | .45* | 1 |
Table 2 Note. RSA = Respiratory Sinus Arrhythmia; HP = Heart Period; BPI = Brief Pain Inventory; WPI = Widespread Pain Index; SSS = Symptom Severity Score; SQIR = Symptom Interference Questionnaire – Revised; CES-D = Center for Epidemiological Studies Depression Scale; SCL-90 GSI = Symptom Checklist – 90 Global Severity Index. The FM symptom severity score (WPI + SSS) was normalized by applying a square-root transformation.
Correlations with this variable represent associations with a reversed version of the slope of time on RSA (i.e., raw slope of time on RSA multiplied by −1) that indicates the rate of RSA decline during the assessment period. Associations with the raw slope can be ascertained by simply reversing the sign (+/−) of the correlation.
p < .01.
Given the evidence presented above that individuals with chronic stress-related pain syndromes and higher self-reported pain demonstrate greater parasympathetic reduction during psychosocial assessment, we moved on to test hypothesis 3, which predicts that an individual's rate of RSA reduction during assessment will be positively associated with a variety of negative outcomes in the sample as a whole, including poorer sleep as measured with the PSQI, greater fibromyalgia symptoms as measured by the WPI + SSS, greater life interference due to pain as measured with the SIQR, greater depression as measured using the CES-D, greater psychological distress as measured by the SCL-90-R Global Severity Index, greater perceived stress on the PSS, and poorer general mental and physical functioning on the SF-12v2. Using SAS PROC REG, we regressed each outcome on rate of parasympathetic decline controlling for standardized baseline RSA.
Consistent with hypothesis 3, rate of RSA decline during the assessment period predicted a variety of outcomes over and above baseline RSA, with steeper decline predicting poorer sleep on the PSQI (β = 1.18, SE = .51, t(38) = 2.31, p = .026), more fibromyalgia symptoms on the WPI + SSS (β = .34, SE = .14, t(38) = 2.31, p = .026), more symptom interference on the SIQR (β = 2.47, SE = .92, t(38) = 2.67, p = .010), greater depression on the CES-D (β = 1.56, SE = .68, t(38) = 2.27, p = .028), poorer physical functioning on the SF-12v2 (β = −3.71, SE = 1.70, t(38) = −2.17, p = .036), and poorer mental functioning on the SF-12v2 (β = −2.43, SE =1.12, t(38) = −2.16, p = .034). Rate of RSA decline was not significantly associated with perceived stress on the PSS (β = .06, SE = .57, t(38) = .10, p = .91) or general psychological distress on the Global Severity Index (β =1.62, SE = 1.68, t(38) = .96, p = .340). Therefore, individual differences in rate of parasympathetic decline during psychosocial assessment were associated with several outcomes of great relevance to chronic pain patients.
Discussion
Although some evidence indicates baseline dysregulation of the ANS in chronic multi-symptom disorders such as TMD and FM, further work is needed to characterize the role of parasympathetic reactivity. Given that (1) these illnesses tend to co-occur with psychiatric disturbances, and that (2) many psychiatric problems are characterized by relatively greater parasympathetic reduction in response to objectively safe stimuli, it was predicted that individuals with TMD or TMD + FM would demonstrate similarly greater parasympathetic reduction, and that the degree of such responses would be associated with poorer outcomes. The current study represents a preliminary attempt to test this hypothesis; as a preliminary study, it has significant limitations which are listed below.
Limitations
A small convenience sample of individuals from several clinics and the community was utilized. The sample sizes in each group were relatively small, predominantly Caucasian, and female; larger samples would increase statistical power, while more diverse samples would ensure generalizability of these findings. In particular, the control group in the present study was very small (n = 15) compared to most studies of this kind. Individuals with psychiatric disturbances were eliminated, limiting the generalizability of our findings to the general population of individuals with TMD and FM, which is characterized by psychiatric comorbidity. On the other hand, this may have reduced power to detect effects on psychological wellbeing by slightly restricting the high end of the range of psychiatric symptoms. Our study did not measure genetics, fitness level, childhood adversity, or post-traumatic symptoms, all of which represent plausible factors in the development of relatively greater parasympathetic reductions in safe situations; future studies should investigate the role of these variables. In addition, the measurement of sleep disturbance with a self-report instrument is a limitation, and future work in this area would benefit from more objective sleep measures. Although the alpha value was set at .01 rather than .05, the number of analyses presented here introduces a strong possibility of type I error. The results of hypothesis tests in the present paper should be interpreted cautiously and replicated in a larger sample.
The present paper argues that the autonomic responses of patients imply a biased evaluation of the situation as threatening when it is in fact safe; however, ongoing measurements of perceived threat were not collected, limiting the ability to draw firm conclusions about the psychological mechanisms of greater parasympathetic decline. Future studies could be improved by the inclusion of this type of manipulation check. The present study was intended to explore psychophysiological response to clinic assessment, which generally takes place while the patient is alone in a waiting or exam room. Therefore, the present findings may or may not necessarily generalize to interview assessment procedures. Relatedly, chronic pain participants may have experienced the questionnaires as more threatening or stressful for reasons other than perceived danger; constructs measured may have had greater personal relevance to patients, which may have primed negative emotions (i.e., interpersonal conflict or trauma, physical pain, cognitive fatigue, memory or concentration difficulties). This alternative explanation should be investigated in future studies.
Summary and Discussion of Findings
As predicted, individuals with TMD, TMD + FM, or high self-reported pain showed greater parasympathetic decline during a psychosocial assessment than controls or those with low pain. Further, individuals with TMD + FM showed greater decline than individuals with TMD only. Additionally, controls and women with below-average pain showed a correlation between rate of change in RSA and rate of change in HP, indicating efficient parasympathetic control over the heart with a more trivial sympathetic influence.5 Among individuals with TMD or TMD + FM diagnoses or high self-reported pain, however, this correlation was not significant, suggesting the additional influence of less efficient sympathetic reactivity on arousal (HP) similar to that found in certain psychiatric populations.36,2 These results could be interpreted to suggest that individuals with TMD or a combination of TMD and FM responded to an objectively safe environment with an inappropriately defensive autonomic stance.
The greater parasympathetic decline demonstrated in patients in the present study is consistent with a relatively sensitive stress response. Protracted stress has been linked to disease, including chronic pain.47 However, previous studies have not linked this type of parasympathetic decline in response to safe stimuli specifically to TMD or FM. The present study supports further inquiry into the nature of the disruption in stress response systems in these disorders. The results of the present study indicate both relatively greater parasympathetic reduction and a failure of such reduction to fully account for changes in arousal, indicating both parasympathetic and sympathetic contributions to changes in arousal. 40,2,5
Childhood trauma and post-traumatic stress disorder often co-occur with both FM26 and TMD17, and such experiences may explain autonomic hypersensitivity to danger among victims of such adversity. Porges’ theory postulates that developmental disturbances to the process of learning which environments are safe or unsafe cause later deficits in the accurate appraisal of contexts as safe or threatening.36 However, it is also possible that other genetic or environmental risk factors play a role in the development of both hypersensitivity to danger and risk for TMD or FM symptoms.
Also as predicted, individual differences in the rate of parasympathetic decline during the assessment period predicted poorer outcomes across the sample, including FM symptoms, sleep, life interference, depression, and general physical and mental functioning. These results held after controlling for baseline parasympathetic activity, suggesting that autonomic reactivity was a predictor of problems in this sample. As Porges36 noted, a system that is hyperreactive in this way may be especially susceptible to many problems in functioning, including those found among patients in the present sample. Although a variety of potential pathways for these effects should be considered, autonomic hypersensitivity to danger may influence symptoms in part by contributing to disturbed sleep. 33,40,24,28,49
Surprisingly, although group means suggest a trend in the expected direction, we did not replicate the finding45 that baseline parasympathetic activity is significantly lower in TMD and FM. Perhaps due to the small size of our control group, the controls in our study had slightly lower RSA than controls used in previous studies; a one-sample t-test comparing the mean baseline RSA of the control group in Solberg Nes et al.45 (Mean Baseline RSA for Controls = 6.11) indicated that the mean for controls in that study was significantly higher than the mean for controls in the present study (Mean Difference = −.89, t(14) = −3.40, p = .004). Therefore, the controls in our sample may have simply had slightly lower baseline RSA than would be expected in a healthy sample, preventing a distinction between the clinical and control groups.
Clinical Implications
A few clinical insights may be drawn from these findings. Clinicians may anticipate and be less disturbed by patients who have become distressed or defensive during assessment. Behavioral interventions targeting dysregulated autonomic functioning (e.g., paced deep breathing, relaxation techniques) and cognitive interventions that seek to reduce inaccurate perceptions of danger (e.g., safety cues, challenging perceptions of danger) may be woven into clinical protocol. 35,10,21,23,50,20 Finally, it may be helpful to know that this response to assessment pattern may be associated with a specific set of patient outcomes.
Conclusion
The present study provides the first test of Porges’38 polyvagal theory with regard to chronic multi-symptom illness. Our evidence supports the view that individuals with TMD or TMD + FM show greater parasympathetic reduction in safe settings consistent with hypersensitivity to danger. Furthermore, it appears that such reactivity may be associated with a variety of negative outcomes regardless of diagnostic status.
Highlights.
Chronic pain predicted parasympathetic dampening during psychosocial assessment.
Parasympathetic dampening during assessment predicted poorer outcomes.
Findings held after controlling for baseline parasympathetic activity.
Acknowledgments
Funded by grants from the National Institutes of Health / National Institute of General Medical Sciences (Grant # P20GM103538) the National Center for Advancing Translational Sciences, (Grant # UL1TR000117), and the National Institute of Mental Health (Grant # T32MH093315).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors of the presents study have no conflicts of interest to declare.
References
- 1.Aaron LA, Buchwald D. A review of the evidence for overlap among unexplained clinical conditions. Ann Intern Med. 2001;134:868–881. doi: 10.7326/0003-4819-134-9_part_2-200105011-00011. [DOI] [PubMed] [Google Scholar]
- 2.Austin MA, Riniolo TC, Porges SW. Borderline personality disorder and emotion regulation: Insights from the polyvagal theory. Brain Cogn. 2007;65:69–76. doi: 10.1016/j.bandc.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beauchaine T. Vagal tone, development, and gray's motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Dev Psychopathol. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- 4.Bennett RM, Friend R, Jones KD, Ward R, Han BK, Ross RL. The revised fibromyalgia impact questionnaire (FIQR): Validation and psychometric properties. Arthritis Research and Therapy. 2009;11:R120. doi: 10.1186/ar2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berntson G, Caccioppo J, Quigley K. Respiratory sinus arrhythmia: autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology. 1993;30:183–196. doi: 10.1111/j.1469-8986.1993.tb01731.x. 1993. [DOI] [PubMed] [Google Scholar]
- 6.Berntson GG, Quigley KS, Lozano D. Cardiovascular psychophysiology. Handbook of psychophysiology. Cambridge University Press; New York: 2007. [Google Scholar]
- 7.Burris JL, Evans DR, Carlson CR. Psychological correlates of medical comorbidities in patients with temporomandibular disorders. J Am Dent Assoc. 2010;141:22–31. doi: 10.14219/jada.archive.2010.0017. [DOI] [PubMed] [Google Scholar]
- 8.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 9.Camm A, Malik M, Bigger J, Breithardt G, Cerutti S, Cohen R, Kleiger R. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. task force of the european society of cardiology and the north american society of pacing and electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 10.Carlson CR, Bertrand PM, Ehrlich AD, Maxwell AW, Burton RG. Physical self-regulation training for the management of temporomandibular disorders. J Orofac Pain. 2000;15:47–55. [PubMed] [Google Scholar]
- 11.Chalaye P, Goffaux P, Bourgault P, Lafrenaye S, Devroede G, Watier A, Marchand S. Comparing pain modulation and autonomic responses in fibromyalgia and irritable bowel syndrome patients. Clin J Pain. 2012;28:519–526. doi: 10.1097/AJP.0b013e31823ae69e. [DOI] [PubMed] [Google Scholar]
- 12.Chervin RD, Teodorescu M, Kushwaha R, Deline AM, Bucksch CB, Ribbens-Grimm C, Crofford LJ. Objective measures of disordered sleep in fibromyalgia. J Rheumatol. 2009;36:2009–2017. doi: 10.3899/jrheum.090051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cleeland C, Ryan K. Pain assessment: Global use of the brief pain inventory. Ann Acad Med Singap. 1994;23:129–138. [PubMed] [Google Scholar]
- 14.Cohen H, Neumann L, Shore M, Amir M, Cassuto Y, Buskila D. Autonomic dysfunction in patients with fibromyalgia: Application of power spectral analysis of heart rate variability. 2000;29:217–227. doi: 10.1016/s0049-0172(00)80010-4. [DOI] [PubMed] [Google Scholar]
- 15.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983:385–396. [PubMed] [Google Scholar]
- 16.Cohen S, Williamson GM. Stress and infectious disease in humans. Psychol Bull. 1991;109:5–24. doi: 10.1037/0033-2909.109.1.5. [DOI] [PubMed] [Google Scholar]
- 17.de Leeuw R, Bertoli E, Schmidt JE, Carlson CR. Prevalence of traumatic stressors in patients with temporomandibular disorders. Journal of oral and maxillofacial surgery. 2005;63:42–50. doi: 10.1016/j.joms.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 18.de Leeuw R, Klasser GD. Orofacial pain: Guidelines for assessment, diagnosis, and management. Quintessence Publishing Company; Hanover Park: 2013. [Google Scholar]
- 19.Derogatis LR. SCL-90-R: Symptom checklist-90-R: Administration, scoring, and procedures manual. NCS Pearson; Cedar Rapids, IA: 1996. [Google Scholar]
- 20.Dersh J, Polatin PB, Gatchel RJ. Chronic pain and psychopathology: Research findings and theoretical considerations. Psychosom Med. 2002;64:773–786. doi: 10.1097/01.psy.0000024232.11538.54. [DOI] [PubMed] [Google Scholar]
- 21.Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Diatchenko L, Dubner R, Slade GD. Psychological factors associated with development of TMD: The OPPERA prospective cohort study. J Pain. 2013;14:T75–T90. doi: 10.1016/j.jpain.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friend R, Bennett RM. Distinguishing fibromyalgia from rheumatoid arthritis and systemic lupus in clinical questionnaires: an analysis of the revised Fibromyalgia Impact Questionnaire (FIQR) and its variant, the Symptom Impact Questionnaire (SIQR), along with pain locations. Arthritis Res Ther. 2011;13:1–10. doi: 10.1186/ar3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol Bull. 2007;133:581–624. doi: 10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- 24.Hall M, Vasko R, Buysse D, Ombao H, Qingxia C, Cashmere JD, Kupfer D, Thayer JF. Acute stress affects heart rate variability during sleep. Psychosom Med. 2004;66:56–62. doi: 10.1097/01.psy.0000106884.58744.09. [DOI] [PubMed] [Google Scholar]
- 25.Hudson JI, Goldenberg DL, Pope HG, Jr, Keck PE, Jr, Schlesinger L. Comorbidity of fibromyalgia with medical and psychiatric disorders. Am J Med. 1992;92:363–367. doi: 10.1016/0002-9343(92)90265-d. [DOI] [PubMed] [Google Scholar]
- 26.Imbierowicz K, Egle UT. Childhood adversities in patients with fibromyalgia and somatoform pain disorder. Eur J Pain. 2003;7:113–119. doi: 10.1016/S1090-3801(02)00072-1. [DOI] [PubMed] [Google Scholar]
- 27.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 53:983–997. [PubMed] [Google Scholar]
- 28.Kooh M, Martínez-Lavin M, Meza S, Martin-del-Campo A, Hermosillo G, Pineda C, Nava A, Amigo C, Drucker-Colin R. Concurrent heart rate variability and polysomnography analyses in fibromyalgia patients. Clin Exp Rheumatol. 2003;21:529–530. [PubMed] [Google Scholar]
- 29.Lerma C, Martinez A, Ruiz N, Vargas A, Infante O, Martinez-Lavin M. Nocturnal heart rate variability parameters as potential fibromyalgia biomarker: Correlation with symptoms severity. Arthritis Res Ther. 2011;13:R185. doi: 10.1186/ar3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leventhal H, Nerenz DR. A model for stress research with some implications for the control of stress disorders. In: Meichenbaum D, Jaremko ME, editors. Stress reduction and prevention. Springer; New York: 1983. pp. 5–38. [Google Scholar]
- 31.Light KC, Bragdon EE, Grewen KM, Brownley KA, Girdler SS, Maixner W. Adrenergic dysregulation and pain with and without acute beta-blockade in women with fibromyalgia and temporomandibular disorder. J Pain. 2009;10:542–552. doi: 10.1016/j.jpain.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martínez-Lavín M. Fibromyalgia as a sympathetically maintained pain syndrome. Curr Pain Headache Rep. 2004;8:385–389. doi: 10.1007/s11916-996-0012-4. [DOI] [PubMed] [Google Scholar]
- 33.Martínez-Lavín M, Hermosillo AG, Rosas M, Soto M. Circadian studies of autonomic nervous balance in patients with fibromyalgia: A heart rate variability analysis. Arthritis Rheum. 1998;41:1966–1971. doi: 10.1002/1529-0131(199811)41:11<1966::AID-ART11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 34.McWilliams LA, Higgins KS. Associations between pain conditions and borderline personality disorder symptoms: Findings from the national comorbidity survey replication. Clin J Pain. 2013;29:527–532. doi: 10.1097/AJP.0b013e31826ab5d0. [DOI] [PubMed] [Google Scholar]
- 35.Okeson JP. Bell's orofacial pains: The clinical management of orofacial pain. Quintessence Publishing; Hanover Park, IL: 2005. [Google Scholar]
- 36.Porges SW. The polyvagal theory: Neurophysiological foundations of emotions, attachment, communication, and self-regulation. W. W. Norton & Company; New York: 2011. [Google Scholar]
- 37.Porges SW. The polyvagal theory: Phylogenetic contributions to social behavior. Physiol Behav. 2003;79:503–513. doi: 10.1016/s0031-9384(03)00156-2. [DOI] [PubMed] [Google Scholar]
- 38.Porges SW. The polyvagal theory: Phylogenetic substrates of a social nervous system. International Journal of Psychophysiology. 2001;42:123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- 39.Porges SW. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology. 1995;32:301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- 40.Quartana PJ, Wickwire EM, Klick B, Grace E, Smith MT. Naturalistic changes in insomnia symptoms and pain in temporomandibular joint disorder: A cross-lagged panel analysis. Pain. 2010;149:325–331. doi: 10.1016/j.pain.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 41.Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Applied psychological measurement. 1977;1:385–401. [Google Scholar]
- 42.del Paso Reyes, Gustavo A, Garrido S, Pulgar Á , Duschek S. Autonomic cardiovascular control and responses to experimental pain stimulation in fibromyalgia syndrome. J Psychosom Res. 2011;70:125–134. doi: 10.1016/j.jpsychores.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 43.Schiffman E, Truelove E, Ohrbach R, Anderson GC, John MT, List T, Look JO. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: Recommendations of the international RDC/TMD consortium network and orofacial pain special interest group. J Orofac Pain. 2009;24:7–24. [Google Scholar]
- 44.Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford University Press; New York: 2003. [Google Scholar]
- 45.Solberg Nes L, Carlson CR, Crofford LJ, de Leeuw R, Segerstrom SC. Self-regulatory deficits in fibromyalgia and temporomandibular disorders. Pain. 2010;151:37–44. doi: 10.1016/j.pain.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 46.Staud R. Heart rate variability as a biomarker of fibromyalgia syndrome. Future. 2008;3:475–483. doi: 10.2217/17460816.3.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor SE. Mechanisms linking early life stress to adult health outcomes. Proc natl acad sci. 2010;107:8507–8512. doi: 10.1073/pnas.1003890107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of generalized anxiety disorder and worry. Biol Psychiatry. 1996;39:255–266. doi: 10.1016/0006-3223(95)00136-0. [DOI] [PubMed] [Google Scholar]
- 49.Thomas RJ, Mietus JE, Peng C, Goldberger AL, Crofford LJ, Chervin RD. Impaired sleep quality in fibromyalgia: Detection and quantification with ECG-based cardiopulmonary coupling spectrograms. Sleep Med. 2010;11:497–498. doi: 10.1016/j.sleep.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Turner JA, Mancl L, Huggins KH, Sherman JJ, Lentz G, LeResche L. Targeting temporomandibular disorder pain treatment to hormonal fluctuations: A randomized clinical trial. Pain. 2011;152:2074–2084. doi: 10.1016/j.pain.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 52.Wilhelm FH, Roth WT. Taking the laboratory to the skies: Ambulatory assessment of self - report, autonomic, and respiratory responses in flying phobia. Psychophys. 1998;35:596–606. doi: 10.1017/s0048577298970196. [DOI] [PubMed] [Google Scholar]
- 53.Wolfe F, Clauw DJ, Fitzcharles M, Goldenberg DL, Hauser W, Katz RS. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: A modification of the ACR preliminary diagnostic criteria for fibromyalgia. J Rheumatol. 2011;38:1113–1122. doi: 10.3899/jrheum.100594. [DOI] [PubMed] [Google Scholar]
- 54.Wolfe F, Smythe HA, Yunus MB, Bennet RM, Bombardier C, Goldenberg DL, Clark P. The american college of rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis & Rheumatism. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 55.Yoshihara T, Shigeta K, Hasegawa H, Ishitani N, Masumoto Y, Yamasaki Y. Neuroendocrine responses to psychological stress in patients with myofascial pain. J Orofac Pain. 2005;19:202–208. [PubMed] [Google Scholar]
- 56.Yunus M, Masi A, Aldag J. A controlled study of primary fibromyalgia syndrome: Clinical features and association with other functional syndromes. J Rheumatol. 1989;19:62–71. [PubMed] [Google Scholar]