Introduction
Bipolar disorder (BD) is a major cause of social morbidity, accounting for 14.4 million disability-adjusted life years lost worldwide due to uncontrolled mood variations (Collins et al. 2011). Women of reproductive age with BD often find that mood variation can be linked to menstrual cycle phase, termed “menstrually entrained” (Rasgon et al. 2003, Blehar et al. 1998). The nature and degree of this effect, however, varies greatly between individuals (Leibenluft et al. 1999, Shivakumar et al. 2008, Teatero et al. 2013). BD is often comorbid with premenstrual syndrome (PMS) or premenstrual dysphoric disorder (PMDD), and individuals with BD who experience menstrually-entrained exacerbations exhibit more severe symptoms, more frequent relapses, and impaired therapeutic response (Cirillo et al. 2012).
Although the physiology of menstrual entrainment remains unclear, it has been suggested that hormonally influenced exacerbations of mood symptoms may be affected by cyclic variations of excitatory and inhibitory neurotransmitters across the menstrual cycle. Glutamate levels have been found to be inversely correlated with plasma estrogen and progesterone levels (Zlotnick et al. 2011, Casas et al. 2013), and accordingly glutamate levels vary across the menstrual cycle (Batra et al. 2008). Similarly, several lines of evidence point to changes in GABAergic activity that accompany alterations in steroid hormone milieu (Bäckström et al. 2003). Both the density and subunit composition of GABAA receptors in the central nervous system vary over the estrous cycle in response to fluctuations in neurosteroid levels (Maguire et al. 2005, Maguire et al. 2007, Maguire et al. 2009, and Porcu et al. 2014).
Lamotrigine, an anticonvulsant used in maintenance treatment of BD, has been implicated in the modulation of glutamatergic (Leach et al. 1986) and potentially of GABAergic (Kuzniecky et al. 2002, Wang et al. 2001) signaling, and could potentially affect menstrual cycle-related alterations in neurotransmitter activity. Hence, it is plausible to speculate that use of lamotrigine might ameliorate cyclic variation in levels of excitatory or inhibitory central neurotransmitters and thereby enhance mood stability across the menstrual cycle for women with BD.
Individual psychiatric case reports (Becker et al. 2004, Sepede et al. 2013, Skokou et al. 2013) as well as a single controlled study performed in women with epilepsy (Herzog et al. 2011) have supported the utility of lamotrigine as pharmacotherapy for menstrually entrained mood lability. However, to date no controlled study has examined the use of lamotrigine specifically for this purpose.
Mood lability in bipolar disorder is moderated by alterations in sleep, and regular sleep is crucial to maintenance of mood stability (Plante et al. 2008). Disruptions in sleep rhythms can often precipitate episodes of mood instability (Bauer et al. 2006). Self-perceived sleep quality may vary across the menstrual cycle in healthy women (Baker et al. 2004), and disruption in circadian rhythms is associated with disruption in menstrual function (Labyak et al. 2002). Thus it is possible that variations in sleep quality across the menstrual cycle could also have bearing on mood stability in women with bipolar disorder.
Lamotrigine has been reported in some studies to enhance sleep continuity and promote REM sleep (Placidi et al. 2000, Foldvary et al. 2001), suggesting that it could also have beneficial effects on sleep-related mood instability.
No controlled study to date has specifically compared lamotrigine to other therapies for the management of variability in mood and sleep over the course of the menstrual cycle in women with BD. We report on an investigation of mood and sleep stability across the phases of the menstrual cycle in women with BD taking lamotrigine, compared to mood stabilizing regimens that did not contain lamotrigine.
Hypotheses
Our first hypothesis was that variability in mood and sleep across phases of the menstrual cycle would be more prevalent and more severe in women with BD compared to women without BD. A second hypothesis was that, in those women with BD whose regimens included lamotrigine, variations in mood and sleep across menstrual cycle phases would be attenuated compared to women with BD whose regimens did not include lamotrigine.
In an additional exploratory analysis, the effects on mood and sleep of combining oral contraceptives (OCP) with lamotrigine and other psychiatric medications commonly used in our study sample were examined.
Method
Participants
Women ages 18–45 years were recruited through the Stanford Women’s Wellness and Adult Bipolar Disorder clinics, as well as via community postings, for two concurrent studies taking place at the Stanford University Department of Psychiatry. Both studies were approved by the University Institutional Review Board, and subjects provided written informed consent prior to participation. Participants were consecutively assigned to alternate study groups based on the order in which they presented to the study coordinators.
Forty-two reproductive-aged women with bipolar disorder (including BD-I, BD-II, or BD not otherwise specified) were recruited for a prospective open-label study of lamotrigine addition to standard treatment. Lamotrigine was added to the existing medication regimens of these participants and continued for six months. Three additional women who were initially included in this group were excluded from the analysis because they did not take lamotrigine for the entire study period.
Thirty control women with BD ages 18–45 who were not taking lamotrigine were recruited for a concurrent study of reproductive functioning in women with BD (Reynolds-May et al. 2014). These women maintained their existing medication regimens and did not receive lamotrigine during the course of the study. Details of the medications taken by study participants are available in Supplementary Table 2.
Finally, thirteen healthy controls were recruited through flyer and newspaper advertisements in the surrounding community. These participants had no history of mood disorder (confirmed by structured clinical interview) and had never received any psychotropic medication.
Exclusion criteria for all participants included illicit drug use in the previous six months; uncontrolled medical conditions; being peri- or post-menopause; current pregnancy, breast-feeding, or plans to get pregnant; endocrine disease (e.g., diabetes, hypothyroidism); or a mood disorder secondary to a general medical condition. Women with BD receiving psychotropic medication were required to have stable medications for at least three months prior to baseline evaluation. For the main analysis, women using hormonal contraception were excluded; however in an additional exploratory analysis the effects of combining various medications with oral hormonal contraception were examined. For this work the 72 bipolar patients from the study group described above were used as the no-oral-contraceptive group, together with an additional 19 women with bipolar disorder, recruited by the same means described previously, who were also using oral contraception (OCP).
Procedures
Initial study evaluations took place at the Center for Neuroscience in Women’s Health within the Department of Psychiatry and Behavioral Sciences at Stanford University Medical Center and at the Clinical Trials Research Unit (CTRU) at Stanford Hospital. After providing verbal and written informed consent, all subjects underwent a psychiatric evaluation (either SCID, Affective Disorders Evaluation, or submission of medical chart records for review) to verify the diagnosis of BD and to rule out psychopathology in control subjects. In some cases, diagnoses were confirmed by interviews with family members. Detailed information was collected from all patients with BD regarding current and previous psychotropic treatment. Participants were required to notify study personnel of any medication changes during the course of the study. Study procedures were approved by the Institutional Review Board of Stanford University.
Participant ratings of the studied parameters were recorded by all participants using the previously validated ChronoRecord software that was installed on a home computer (Bauer et al. 2004, Bauer et al. 2008). The ChronoRecord software is a computer-based adaptation of an established paper-based form for self-reporting, the ChronoSheet, which has been validated against two established observer-rated scales, the Young Mania Rating Scale and Hamilton Depression Rating Scale (Bauer et al. 1991). Daily ratings of mood, hours of sleep, hours spent awake in bed, medications taken and days of menstrual cycle were obtained.
Mood ratings were recorded on a scale of 0–100, with scale extremes corresponding to the most extreme mania and depression the patient ever experienced, and with higher numbers indicating greater well-being. Based upon validation studies comparing the self-ratings with clinician-rated (Bauer et al. 2004, Bauer et al. 2008), a mood entry less than 40 was considered depression, 40 to 60 euthymia, and greater than 60 hypomania/mania. Recorded adherence to prescribed regimens was very good; participants are listed as having taken a medication in Supplementary Table 3 if they recorded >80% daily adherence over at least one menstrual cycle.
The number of menstrual cycles recorded by each subject ranged from two to nine, with an average of 4.17. Luteal phase was considered to be the seven days prior to the initiation of menses, while follicular phase was recorded as the period of time between the end of menstrual phase and the beginning of the next luteal phase.
Statistical Analysis
Statistical analysis was carried out using SPSS version 20 (SPSS, Inc., Chicago, IL, USA). Paired-sample T tests were used to examine differences in average mood, sleep, and insomnia among phases of the menstrual cycle. Independent-sample T tests were used to explore differences between test groups in average mood, sleep, and insomnia within each phase of the menstrual cycle. Independent-sample tests were two-tailed; all T tests were conducted at the 0.05 significance level. Univariate ANOVA was used to explore potential interactions between hormonal contraceptives and psychotropic medications.
Results
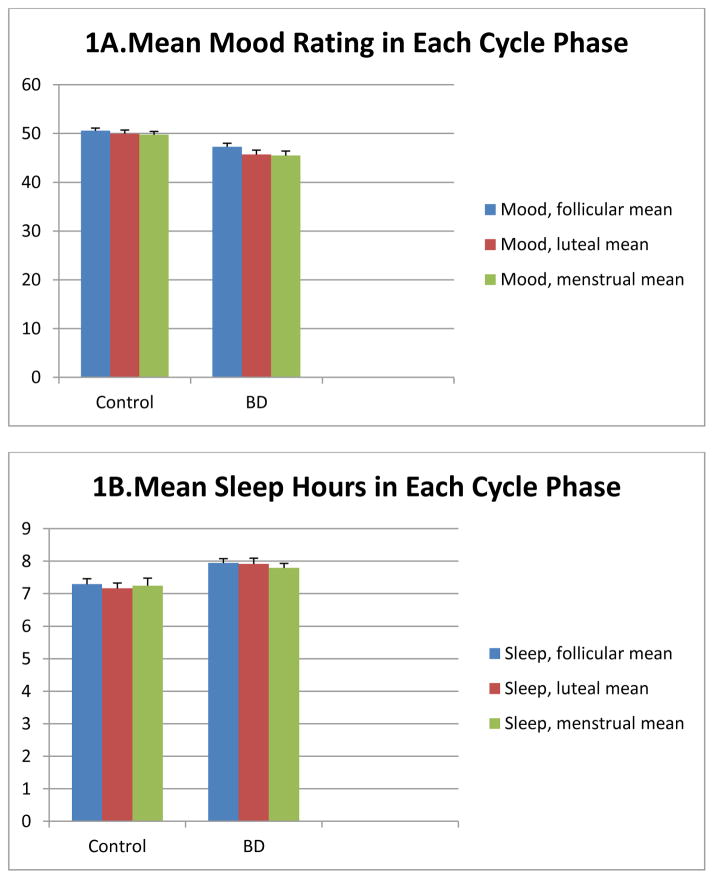
First mean mood ratings were compared between the group of all women with bipolar disorder versus the group of healthy controls in each phase of the menstrual cycle (Figure 1a). Mood ratings were higher in controls compared to women with BD (pooled without regard to psychiatric regimen) over all phases of the menstrual cycle by independent-samples T tests.
Figure 1.
Ratings of mood, hours of sleep, and hours spent awake in bed across the menstrual cycle for all women with BD compared to healthy controls.
During the follicular phase, mean mood rating in controls was 50.6 (SD 1.9), compared to a mean mood in women with BD of 47.3 (SD=6.3, t(DF) = 3.70, p<0.001). For the luteal phase, mean daily mood rating in controls was 50.0 (SD 2.6) compared to 45.7 (SD=7.9) in women with BD (t(DF)=3.59, p<0.001). For the menstrual phase, mean daily mood rating in controls was 49.8 (SD=2.4) compared to 45.5 (SD= 8.0) in women with BD (t(DF)=3.63, p<0.001). (Figure 1a.)
Mean sleep ratings between all women with bipolar disorder versus healthy controls were then compared in each phase of the menstrual cycle (Figure 1b). Sleep duration was longer in women with BD compared to controls over all three phases of the menstrual cycle by independent-samples T test. During the follicular phase, mean nightly sleep duration in controls was 7.3 hours (SD=0.6), compared to 7.9 hours (SD=1.1) in women with BD (t(DF)= −3.04, p=0.005). For the luteal phase, mean sleep duration in controls was 7.2 hours (SD=0.6), compared to 7.9 hours (SD=1.5) in women with BD (t(DF)= −3.02, p=0.004). For the menstrual phase, mean sleep duration in controls was 7.2 hours (SD=0.8), compared to 7.8 hours (SD=1.2) in women with BD; this difference was not significant (t(DF)= −1.59, p=0.116). (Figure 1b.)
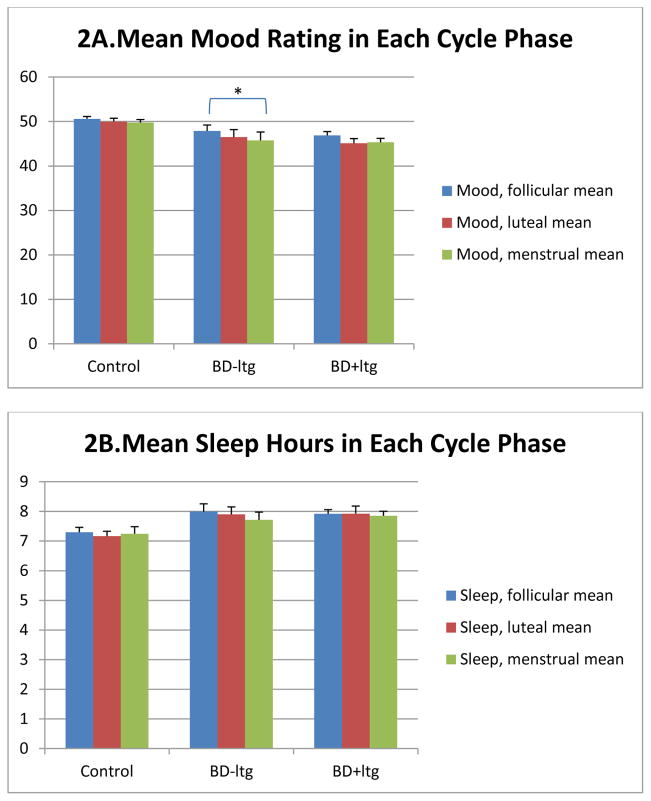
The second set of analyses focused on the effects of lamotrigine on variations in mood and sleep across menstrual cycle phases in women with BD.
It was expected that variations in mood across menstrual cycle phases would be attenuated in women with BD who were using lamotrigine compared to women with BD receiving usual care (as previously published by Becker and colleagues, 2004). Paired-sample T tests were used to compare mood ratings across the three cycle phases within each test group (Figure 2a). Among women with BD, both with and without lamotrigine, mood was lowest in the menstrual phase of the cycle. For women with BD using lamotrigine, mean follicular-phase mood rating was 46.7 (SD=5.56), not significantly different from mean menstrual-phase mood which was 45.3 (SD=5.87) or from mean luteal-phase mood (mean 45.1, SD=6.76). For women with BD not using lamotrigine, mean follicular-phase mood was 47.9 (SD=7.30), significantly different from mean menstrual-phase mood which was 45.8 (SD=10.23; p=0.033), though not from mean luteal-phase mood which was 46.5 (SD=9.28). In addition to the significant difference in mood between follicular and menstrual-phase moods in the group not using lamotrigine, standard deviations were consistently higher than those for the control and the lamotrigine group, suggesting greater within-phase variability in mood as well.
Figure 2.
Ratings of mood, hours of sleep, and hours spent awake in bed across the menstrual cycle for healthy controls, for women with BD not taking lamotrigine, and for women with BD taking lamotrigine.
By contrast, among the healthy control group of women without BD, no changes in mood were observed across phases of the cycle (Figure 2a).
Paired-sample T tests were also used to examine changes in sleep across cycle phases within each test group. Mean hours of nightly sleep did not differ significantly across cycle phases for any of the three groups (Figure 2b).
No differences in mood or sleep ratings in any menstrual cycle phase were significant among groups of bipolar women using lamotrigine versus usual care, by independent-samples T test (data not shown).
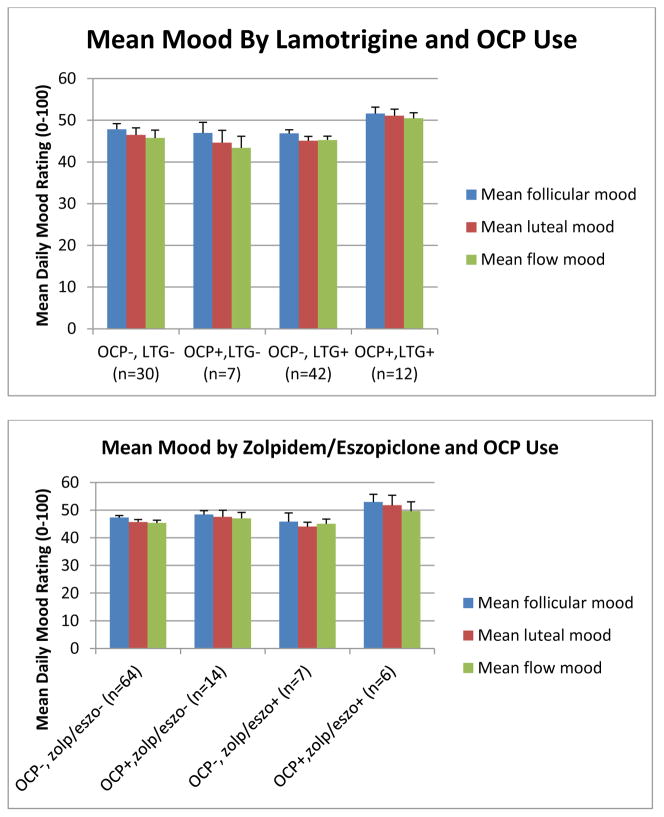
The second phase of this study involved an exploratory analysis of the interactions between OCP and various psychotropic medications used in the study sample.
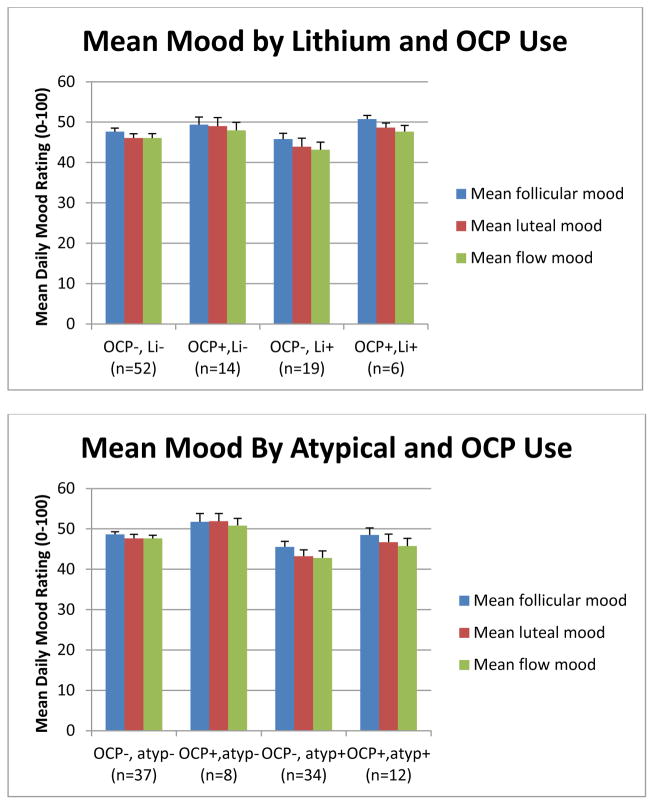
An initial graph of average mood suggested that the combination of lamotrigine and OCP resulted in consistently better overall baseline mood (Figure 3). While this effect was not significant by univariate ANOVA, it was thought that there could be a more general interaction of OCP with medications having a common receptor target, such that the significance of the OCP-lamotrigine interaction might have been masked by the variable presence or absence of other medications affecting the same target.
Figure 3.
Ratings of mean mood across the menstrual cycle for women with BD, by use of oral contraceptives and psychotropic medications.
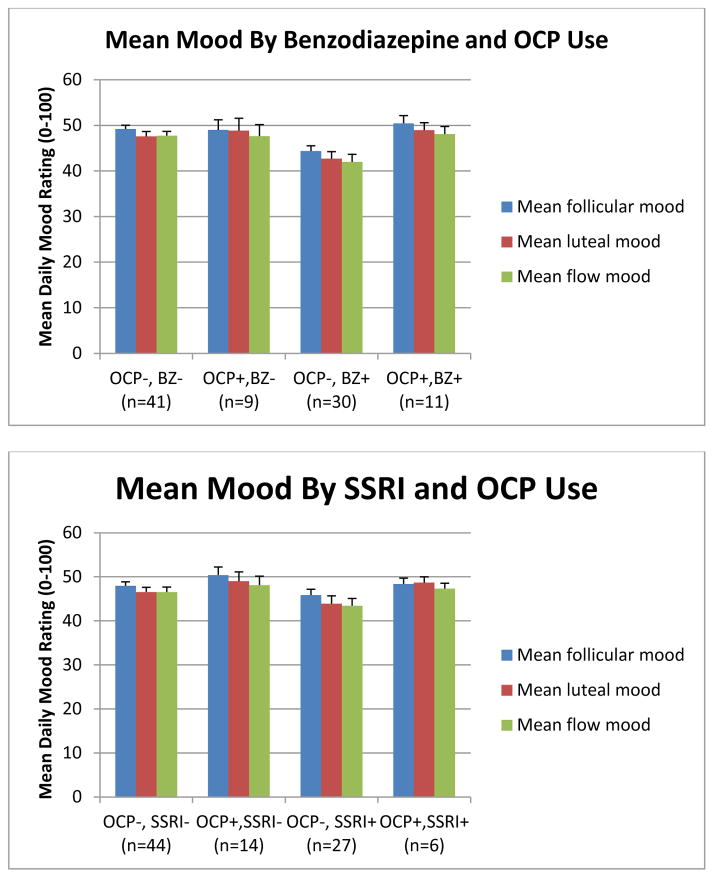
A number of other medications taken by our subject group were then tested for interactions with OCP with respect to mood effects. Graphs depicting the effects of combining benzodiazepines or zolpidem/eszopiclone with OCP followed the pattern displayed by lamotrigine. Combinations of OCP with SSRIs, bupropion, atypical antipsychotics, lithium, or valproic acid followed a different pattern, where patients in the OCP group generally had slightly better mood but this effect was independent of whether the OCP was combined with the target medication. Sample graphs are shown in Figure 3.
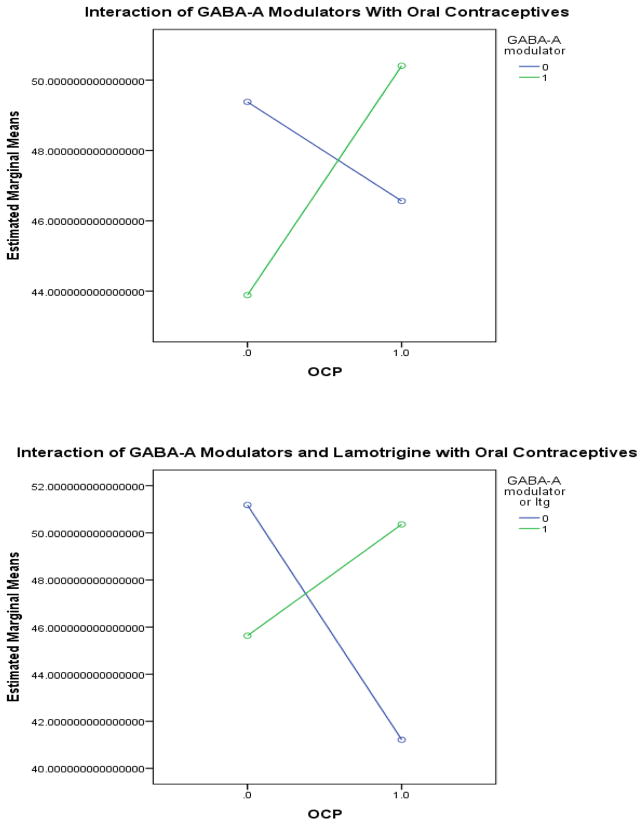
A commonality of all these medications other than lamotrigine involved action at GABAARs. (While lithium and valproic acid also act to alter GABAergic neurotransmission, they function at the GABAB receptor (Ketter et al. 2003).) Based on these data, it was hypothesized that there could be a specific interaction of OCP with medications that alter neurotransmission via GABAAR. The data were therefore recoded to include a measure of whether any medication known to affect GABAAR was used. The list of medications known to affect the GABAAR that were used in this population included benzodiazepines (Kralic et al. 2002), topiramate (Herrero et al. 2002), zonisamide (Mimaki et al. 1991), zolpidem (Kralic et al. 2002, Jia et al. 2009), and eszopiclone (Jia et al. 2009). Fifty women were using at least one of these medications and 41 women were not using any of them. The interaction between OCP and GABAAR modulators was significant by univariate ANOVA at the p<0.05 level for both overall mean mood and mean mood in each of the menstrual cycle phases (Table 2a, Figure 4a). The effect was most pronounced in the follicular phase.
Table 2.
GABAA receptor modulators and oral contraceptives interact to promote euthymia.
| 2A. GABAA receptor modulators not including lamotrigine | ||||||
|---|---|---|---|---|---|---|
| GABA− OCP− | GABA− OCP+ | GABA+ OCP− | GABA+ OCP+ | F(1,1) | p | |
| Mean mood overall | 49.4 | 46.6 | 43.9 | 50.4 | 7.854 | 0.006 |
| Mean follicular mood | 50.1 | 47.0 | 44.6 | 51.0 | 9.591 | 0.003 |
| Mean luteal mood | 48.6 | 46.9 | 43.1 | 49.8 | 4.640 | 0.034 |
| Mean flow mood | 48.4 | 45.9 | 42.9 | 48.8 | 4.475 | 0.037 |
| 2B. GABAA receptor modulators + lamotrigine | ||||||
|---|---|---|---|---|---|---|
| GABA− OCP− | GABA− OCP+ | GABA+ OCP− | GABA+ OCP+ | F(1,1) | p | |
| Mean mood overall | 51.2 | 41.2 | 45.6 | 50.4 | 9.344 | 0.003 |
| Mean follicular mood | 52.1 | 44.0 | 46.3 | 50.8 | 10.182 | 0.002 |
| Mean luteal mood | 51.8 | 43.0 | 44.4 | 50.0 | 8.546 | 0.004 |
| Mean flow mood | 50.8 | 41.3 | 44.4 | 49.1 | 7.946 | 0.006 |
Figure 4.
GABAergic medications interact with oral contraceptives to improve mood within the euthymic range.
Interestingly, when lamotrigine was included along with the other medications mentioned above, the significance level of this finding increased. Under this analysis, 76 women were using any medication acting at the GABAAR and 15 women were not using any medication acting at the GABAAR. The interaction between OCP and GABAAR modulators was significant by univariate ANOVA at the p<0.01 level for both overall mean mood and mean mood in each of the menstrual cycle phases (Table 2b, Figure 4b).
In a parallel analysis of the effect on sleep of medication combinations by univariate ANOVA, no synergistic effect on sleep of lamotrigine or other medications with OCP was observed (results not shown).
Discussion
The first finding of this study indicates that women with bipolar disorder, despite receiving appropriate psychiatric care, had generally lower mood ratings than women without bipolar disorder. This suggests that available treatments for bipolar disorder may not routinely bring sufferers into the range of euthymia enjoyed by healthy controls. Nightly sleep durations were also longer among women with treated bipolar disorder than among healthy controls, further underlining the importance of regular sleep for management of bipolar disorder (Plante et al. 2008, Bauer et al. 2006).
The second set of findings concerned the comparison between women with bipolar disorder receiving lamotrigine and those using other mood stabilizing medications. Our interest in this question was provoked by previous findings suggesting lamotrigine could be particularly useful for suppressing menstrually entrained mood cycling. Sepede and colleagues (2013) and Skokou and Gourzis (2013) both reported success with lamotrigine augmentation for intractable PMDD. Becker and colleagues (2004) also experienced success with lamotrigine, reporting a dose-response effect on mood variation in a woman with severe menstrually entrained, rapid-cycling BD. The only larger study available to date examined the incidental effects on mood lability of medications chosen primarily for antiepileptic activity. In this work, Herzog and colleagues (2011) examined mood stability in sixty women with comorbid epilepsy and PMDD, and found that higher lamotrigine serum levels corresponded with reduced severity of PMDD symptoms, a relationship that was not found for other antiepileptic medications in the study.
In the present study, women with bipolar disorder in the group not receiving lamotrigine did evince some variation in mood among menstrual cycle phases that was not present in controls, which supports our suggestion that menstrual entrainment of mood variability may be an unrecognized feature of many women with bipolar disorder. This result is in agreement with the findings of other researchers (Rasgon et al. 2003, Blehar et al. 1998, Cirillo et al. 2012) and indicates that monthly fluctuations in hormonal milieu could pose a unique and under-recognized difficulty for clinicians treating women with bipolar disorder.
Women with bipolar disorder in the group taking lamotrigine did not show significant differences in mood among menstrual cycle phases, which provides cautious support for our initial hypothesis that add-on lamotrigine could be specifically effective in the reduction of menstrually-entrained mood cyclicity. However this finding is tempered by the recognition that in this study sample, the degree of fluctuation in mood across the cycle was small even in the group that was not taking lamotrigine. Thus the effect may not be clinically significant, at least in groups not specifically complaining of menstrually entrained mood cyclicity. This study did not preselect for this complaint, which is a weakness in the study design. However the confirmation of a stabilizing effect of lamotrigine on mood variability among menstrual phases even in this unselected group is promising. Further research into this and other treatments that could potentially minimize menstrually entrained mood fluctuations is merited; however the effects might be more clearly demonstrated with study groups preselected for complaint of menstrually entrained mood fluctuation.
One unexpected aspect of our results was that mood state was lowest during the menstrual phase of the menstrual cycle for all women with BD. This stands in contrast to previous findings indicating that disrupted mood states tend to occur during the luteal phase (Rasgon et al. 2003, Shivakumar et al. 2008). However, a recent review (Teatero et al. 2013) which collated a large number of case reports and small studies found that while a slight majority (56%) of women who experienced cycle-linked mood disturbance were affected in the luteal phase, a significant minority experienced mood disturbances in other phases of the cycle.
Given the large degree of heterogeneity in the connection between cycle phase and mood disruption, it is possible that pooling subjects without regard to affected cycle phase may obscure significant relationships in these variables. This is another potential weakness of our study. Future work in this area might benefit from pre-selection of patients with documented menstrually-entrained mood disruption, such that subjects can be pooled according to affected cycle phase.
Another potential area of concern is the absence of an objective measure of ovulation: thus it is not known whether study subject cycles were ovulatory or anovulatory, and divisions between cycle phases are based only on the observation of menstrual flow. However the question of whether a given cycle was ovulatory or anovulatory is not of major importance to the study hypothesis, which concerns clinical manifestations of mood cyclicity, hence the question of underlying ovulation is mostly of academic interest. The issue of potential misclassification of cycle phases is more concerning, however this would bias more towards negative findings (since errors in cycle phase classification would tend to flatten out any true differences among cycle phases). Thus it would suggest that the true differences in mood among ovulation-demarcated phases might be rather greater than found here.
Additionally, because the study was open-label, we cannot exclude the possibility that women in the lamotrigine arm would experience a placebo effect from the addition of a new medication to their regimens. However the effects we found (small reduction in the degree of difference in mood state across menstrual cycle phases with lamotrigine use, reduction of hours spent awake in bed only during luteal and menstrual phases in the lamotrigine group, mood enhancement when OCP were combined with medications which act at the GABAA receptor) would all be unusually specific for a placebo effect, particularly given that the participants were not aware of the study hypotheses.
An additional novel finding was the articulation of a potential synergistic effect of OCP and GABAAR modulators on mood. While this synergistic effect was not so great as to push study participants into mania, it did move them to a higher point within the euthymic range, closer to the generally higher mood ratings of the healthy controls.
The molecular nature of such an interaction remains an open question. It is known that both the density and subunit composition of GABAARs in the central nervous system changes over the estrous cycle in response to fluctuations in neurosteroid levels in animal models (Maguire et al. 2005, Maguire et al. 2007, Maguire et al. 2009, Porcu et al. 2012). However it has been documented that the precise nature of these changes may differ between species (Porcu et al. 2014), and thus far, while there are several lines of evidence that point to changes in GABAergic activity that accompany alterations in steroid hormone milieu (Bäckström et al. 2003), it has not been possible to demonstrate directly the molecular nature of changes in GABAAR expression in response to hormonal fluctuation in humans.
We speculate that the improvement in mood produced by the combination of OCP and medications with activity at the GABAAR could be due to jointly effected alterations in the subunit composition of the GABAAR that might either potentiate the anxiolytic effects of the GABAergic medication or oppose the anxiogenic effects of endogenous neurosteroids, or possibly both.
It is interesting to note that lamotrigine also followed the pattern of the medications that act on the GABAAR. The most parsimonious explanation for this would postulate a common neuropharmacological function for these medications. A less parsimonious explanation could be that the same effect is achieved via distinct pathways.
Lamotrigine is hypothesized to work in part by potently inhibiting the presynaptic release of glutamate evoked by activation of voltage-dependent sodium channels (Leach et al. 1986), which does not at first offer a ready explanation of why it should interact with OCP in the same manner as modulators of the GABAAR. However, while lamotrigine does not appear to have acute GABAergic effects, chronic administration has been shown to induce alterations both in GABAA receptor subunit composition in primary cultures (Wang et al. 2001) and in levels of GABA in the central nervous system in vivo (Kuzniecky et al. 2002). This could suggest a potential mechanism by which lamotrigine could promote inhibitory neural activity, which could also contribute to the modulation of menstrually related mood lability.
Thus, while the most extensively documented mechanism of lamotrigine action involves changes in excitatory transmission (Leach et al. 1986), there have also been suggestions that chronic use of lamotrigine could alter the subunit composition of GABAARs (Wang et al. 2001) and affect GABAergic neurotransmission (Wang et al. 2001, Kuzniecky et al. 2002, Braga et al. 2002). Our work provides some indirect support for this latter hypothesis, and also suggests that clinicians treating women who use hormonal contraceptives would do well to consider how interactions between hormonal and psychotropic medications may potentially alter clinical observed effects.
In summary, we found that women treated for bipolar disorder manifested greater fluctuations in mood across menstrual cycle phases than healthy controls. The group of women with bipolar disorder whose regimens included lamotrigine demonstrated less intra-individual variability in mood across menstrual cycle phases, although the effect was small. These results suggest potential for the use of lamotrigine to reduce menstrual cycle-related fluctuations in mood in women with bipolar disorder.
Finally, direct modulation of cyclic alterations in reproductive hormones by use of hormonal contraception provides a complementary avenue by which to explore potential interactions between mood, psychotropic medication, and hormonal milieu. Previously we reported that women with bipolar disorder who were using oral contraceptives (OCP) did not have premenstrual worsening, in comparison to women with bipolar disorder who were not using OCP2.
Thus, an additional exploratory analysis was performed to examine potential interactions between lamotrigine use and oral contraceptives. In this portion of the study, average mood and sleep were compared across groups of women with bipolar disorder, based on use of lamotrigine and of OCP. A preliminary finding was that hormonal contraceptives may act synergistically with medications that modulate GABAAR activity to enhance mood; this finding merits further investigation with a targeted hypothesis and larger sample size. Meanwhile for the practitioner, we suggest that the pairing of oral contraceptives with lamotrigine may be a particularly useful combination in the management of women with bipolar disorder who exhibit menstrually entrained mood cyclicity.
Highlights.
Women treated for bipolar disorder manifested lower average mood, longer average nightly sleep duration, and greater fluctuations in mood and sleep across menstrual cycle phases than healthy controls.
Women with bipolar disorder who were taking lamotrigine had less fluctuation in mood both within and across menstrual cycle phases, and were more similar to the control group than to women with bipolar disorder who were not taking lamotrigine in this respect.
Lamotrigine and other medications with GABAA receptor modulating effects were found to result in improved mood ratings when combined with hormonal contraceptives in the treatment of women with bipolar disorder.
Supplementary Material
Table 1.
Demographic characteristics of the sample.
| Control (n=13) | BD Lam − (n=30) | BD Lam + (n=42) | |
|---|---|---|---|
| Age of Onset | N/A | 14.9±7.4 | 15.3±10.4 |
| Bipolar Subtype | |||
| Bipolar I | N/A | 11 | 12 |
| Bipolar II | N/A | 8 | 16 |
| Bipolar NOS | N/A | 11 | 14 |
| Education* | |||
| High School | 2 (15.4%) | 11 (36.7%) | 10 (23.8%) |
| College | 5 (38.5%) | 10 (33.3%) | 16 (38.1%) |
| Graduate School | 6 (46.2%) | 8 (26.7%) | 12 (28.6%) |
| Ethnicity* | |||
| Caucasian | 8 (61.5%) | 23 (76.7%) | 30 (71.4%) |
| Hispanic | 3 (23.1%) | 3 (10.0%) | 4 (9.5%) |
| Asian/Pacific Islander | 2 (15.4%) | 1 (3.3%) | 5 (11.9%) |
| African American | 0 | 0 | 0 |
| Other | 0 | 1 (3.3%) | 0 |
| Employment Status* | |||
| Full-time | 8 (61.5%) | 10 (33.3%) | 10 (23.8%) |
| Part-time | 2 (15.4%) | 5 (16.7%) | 5 (11.9%) |
| Student | 1 (7.7%) | 6 (20.0%) | 11 (26.2%) |
| Unemployed | 2 (15.4%) | 3 (10.0%) | 5 (11.9%) |
| Not in work force (other) | 0 | 5 (16.7%) | 7 (16.7%) |
| Marital Status* | |||
| Single, never married | 7 (53.8%) | 9 (30.0%) | 11 (26.2%) |
| Married or partnered | 5 (38.4%) | 19 (63.3%) | 19 (45.2%) |
| Divorced/Separated | 0 | 0 | 6 (14.3%) |
| Widowed | 0 | 0 | 1 (2.4%) |
Some columns may not sum to total due to missing data.
Acknowledgments
Role of the Funding Source
Financial support for this project was provided by the National Institutes of Health (R01 grant MH0066033), the National Center for Research Resources (M01 grant RR-00070), and by Glaxo Smith-Kline.
None of these funding sources had any role in study design beyond approving the study for funding; neither did they have any role in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
We thank Dr. Tasha Glenn of ChronoRecord Association, Inc., for invaluable assistance with data acquisition and management.
Footnotes
Author Contributions
Thalia Robakis contributed data entry, did the majority of the data analysis, contributed to data processing and background research, compiled figures and tables, and did the majority of the writing of the paper.
Jessie Holtzman contributed to data entry, data processing, data analysis, and background research, did some of the writing, compiled tables, and contributed proofreading and editing to the final paper.
Pascale Stemmle, Margaret May, and Heather Kenna did the original data collection, data entry, and some data processing, and contributed proofreading and editing to the final paper.
Natalie Rasgon conceived of the study, obtained the grant funding to support it, provided guidance and oversight throughout the project, and contributed proofreading and editing to the final paper.
Conflicts of Interest
We wish to draw the attention of the Editor to the following facts which may be considered as potential conflicts of interest and to significant financial contributions to this work.
Dr. Natalie Rasgon has received grant/research support and/or has been a consultant and/or received lecture honoraria from the following companies:
Current: Research support from Magceutics, Inc., the American Diabetes Association, and Corcept Pharmaceuticals. Consulting for Shire Pharmaceuticals, Sunovion Pharmaceuticals, and Takeda Pharmaceuticals.
Past: Bayer Pharmaceuticals (PI of a multi-site study), Abbot Laboratories, Inc. (past grant support), Bristol-Myers Squibb Company (past speaker), Forest Laboratories (past research support, speaker), GlaxoSmithKline (past research support), Pfizer, Inc. (past research support & past speaker), and Wyeth Pharmaceuticals (past research support, consultant).
We wish to confirm that the financial support available for this work (as detailed in the Role of the Funding Source) did not influence its outcome.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
We further confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bäckström T, Andersson A, Andree L, Birzniece V, Bixo M, Björn I, Lindblad C. Pathogenesis in menstrual Cycle-Linked CNS disorders. Ann N Y Acad Sci. 2003;1007(1):42–53. doi: 10.1196/annals.1286.005. [DOI] [PubMed] [Google Scholar]
- Baker FC, Driver HS. Self-reported sleep across the menstrual cycle in young, healthy women. J Psychosom Res. 2004;56(2):239–243. doi: 10.1016/S0022-3999(03)00067-9. [DOI] [PubMed] [Google Scholar]
- Batra NA, Seres-Mailo J, Hanstock C, Seres P, Khudabux J, Bellavance F, Hui E. Proton magnetic resonance spectroscopy measurement of brain glutamate levels in premenstrual dysphoric disorder. Biol Psychiatry. 2008;63(12):1178–1184. doi: 10.1016/j.biopsych.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Bauer MS, Crits-Christoph P, Ball WA, Dewees E, McAllister T, Alahi P, Whybrow PC. Independent assessment of manic and depressive symptoms by self-rating: Scale characteristics and implications for the study of mania. Arch Gen Psychiatry. 1991;48(9):807–812. doi: 10.1001/archpsyc.1991.01810330031005. [DOI] [PubMed] [Google Scholar]
- Bauer M, Grof P, Gyulai L, Rasgon N, Glenn T, Whybrow PC. Using technology to improve longitudinal studies: Self-reporting with ChronoRecord in bipolar disorder. Bipolar Disord. 2004;6(1):67–74. doi: 10.1046/j.1399-5618.2003.00085.x. [DOI] [PubMed] [Google Scholar]
- Bauer M, Grof P, Rasgon N, Bschor T, Glenn T, Whybrow PC. Temporal relation between sleep and mood in patients with bipolar disorder. Bipolar Disord. 2006;8(2):160–167. doi: 10.1111/j.1399-5618.2006.00294.x. [DOI] [PubMed] [Google Scholar]
- Bauer M, Wilson T, Neuhaus K, Sasse J, Pfennig A, Lewitzka U, Bschor T. Self-reporting software for bipolar disorder: Validation of ChronoRecord by patients with mania. Psychiatry Res. 2008;159(3):359–366. doi: 10.1016/j.psychres.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Becker O, Rasgon NL, Marsh WK, Glenn T, Ketter TA. Lamotrigine therapy in treatment-resistant menstrually-related rapid cycling bipolar disorder: A case report. Bipolar Disord. 2004;6(5):435–439. doi: 10.1111/j.1399-5618.2004.00146.x. [DOI] [PubMed] [Google Scholar]
- Blehar MC, DePaulo JR, Jr, Gershon ES, Reich T, Simpson SG, Nurnberger JI., Jr Women with bipolar disorder: Findings from the NIMH genetics initiative sample. Psychopharmacol Bull. 1998;34(3):239–43. [PubMed] [Google Scholar]
- Braga MF, Aroniadou–Anderjaska V, Post RM, Li H. Lamotrigine reduces spontaneous and evoked GABAA receptor-mediated synaptic transmission in the basolateral amygdala: Implications for its effects in seizure and affective disorders: Lamotrigine and inhibition in the amygdala. Neuropharmacology. 2002;42(4):522–529. doi: 10.1016/s0028-3908(01)00198-8. [DOI] [PubMed] [Google Scholar]
- Casas S, Giuliani F, Cremaschi F, Yunes R, Cabrera R. Neuromodulatory effect of progesterone on the dopaminergic, glutamatergic, and GABAergic activities in a male rat model of Parkinson’s disease. Neurol Res. 2013;35(7):719–25. doi: 10.1179/1743132812Y.0000000142. [DOI] [PubMed] [Google Scholar]
- Cirillo PC, Passos RBF, Bevilaqua Mario Cesar do Nascimento, López Jose Ramón, Arras Rodriguez, Nardi AE. Bipolar disorder and premenstrual syndrome or premenstrual dysphoric disorder comorbidity: A systematic review. Rev Bras Psiquiatr. 2012;34(4):467–479. doi: 10.1016/j.rbp.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS, Fairburn C. Grand challenges in global mental health. Nature. 2011;475(7354):27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldvary N, Perry M, Lee J, Dinner D, Morris HH. The effects of lamotrigine on sleep in patients with epilepsy. Epilepsia. 2001;42(12):1569–1573. doi: 10.1046/j.1528-1157.2001.46100.x. [DOI] [PubMed] [Google Scholar]
- Herrero AI, Del Olmo N, González-Escalada JR, Solís JM. Two new actions of topiramate: Inhibition of depolarizing GABAA-mediated responses and activation of a potassium conductance. Neuropharmacology. 2002;42(2):210–220. doi: 10.1016/s0028-3908(01)00171-x. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Smithson SD, Fowler KM, Krishnamurthy KB, Sundstrom D, Kalayjian LA, Garcia E. Premenstrual dysphoric disorder in women with epilepsy: Relationships to potential epileptic, antiepileptic drug, and reproductive endocrine factors. Epilepsy Behav. 2011;21(4):391–396. doi: 10.1016/j.yebeh.2011.05.024. [DOI] [PubMed] [Google Scholar]
- Jia F, Goldstein PA, Harrison NL. The modulation of synaptic GABA(A) receptors in the thalamus by eszopiclone and zolpidem. J Pharmacol Exp Ther. 2009;328(3):1000–1006. doi: 10.1124/jpet.108.146084. [DOI] [PubMed] [Google Scholar]
- Ketter TA, Manji HK, Post RM. Potential mechanisms of action of lamotrigine in the treatment of bipolar disorders. J Clin Psychopharmacol. 2003;23(5):484–495. doi: 10.1097/01.jcp.0000088915.02635.e8. [DOI] [PubMed] [Google Scholar]
- Kralic J, O’Buckley T, Khisti R, Hodge C, Homanics G, Morrow A. GABAA receptor alpha-1 subunit deletion alters receptor subtype assembly, pharmacological and behavioral responses to benzodiazepines and zolpidem. Neuropharmacology. 2002;43(4):685–694. doi: 10.1016/s0028-3908(02)00174-0. [DOI] [PubMed] [Google Scholar]
- Kuzniecky R, Ho S, Pan J, Martin R, Gilliam F, Faught E, Hetherington H. Modulation of cerebral GABA by topiramate, lamotrigine, and gabapentin in healthy adults. Neurology. 2002;58(3):368–372. doi: 10.1212/wnl.58.3.368. [DOI] [PubMed] [Google Scholar]
- Labyak S, Lava S, Turek F, Zee P. Effects of shiftwork on sleep and menstrual function in nurses. Health Care Women Int. 2002;23(6–7):703–714. doi: 10.1080/07399330290107449. [DOI] [PubMed] [Google Scholar]
- Leach MJ, Marden CM, Miller AA. Pharmacological studies on lamotrigine, a novel potential antiepileptic drug. Epilepsia. 1986;27(5):490–497. doi: 10.1111/j.1528-1157.1986.tb03573.x. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Ashman SB, Feldman-Naim S, Yonkers KA. Lack of relationship between menstrual cycle phase and mood in a sample of women with rapid cycling bipolar disorder. Biol Psychiatry. 1999;46(4):577–580. doi: 10.1016/s0006-3223(99)00023-2. [DOI] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle–linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8(6):797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Maguire J, Mody I. Steroid hormone fluctuations and GABAA-R plasticity. Psychoneuroendocrinology. 2009;34(S1):S84–S90. doi: 10.1016/j.psyneuen.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. Neurosteroid synthesis-mediated regulation of GABAA receptors: Relevance to the ovarian cycle and stress. J Neurosci. 2007;27(9):2155–2162. doi: 10.1523/JNEUROSCI.4945-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimaki T, Suzuki Y, Tagawa T, Karasawa T, Yabuuchi H. Interaction of zonisamide with benzodiazepine and GABA receptors in rat brain. Med J Osaka Univ. 1990;39(1–4):13–17. [PubMed] [Google Scholar]
- Placidi F, Marciani M, Diomedi M, Scalise A, Giacomini P, Gigli G. Effects of lamotrigine on nocturnal sleep, daytime somnolence and cognitive functions in focal epilepsy. Acta Neurol Scand. 2000;102(2):81–86. doi: 10.1034/j.1600-0404.2000.102002081.x. [DOI] [PubMed] [Google Scholar]
- Plante D, Winkelman J. Sleep disturbance in bipolar disorder: Therapeutic implications. Am J Psychiatry. 2008;165(7):830–843. doi: 10.1176/appi.ajp.2008.08010077. [DOI] [PubMed] [Google Scholar]
- Porcu P, Morrow AL. Divergent neuroactive steroid responses to stress and ethanol in rat and mouse strains: Relevance for human studies. Psychopharmacology. 2014;231(17):3257–3272. doi: 10.1007/s00213-014-3564-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, Mostallino MC, Sogliano C, Santoru F, Berretti R, Concas A. Long-term administration with levonorgestrel decreases allopregnanolone levels and alters GABAA receptor subunit expression and anxiety-like behavior. Pharmacol Biochem Behav. 2012;102(2):366–372. doi: 10.1016/j.pbb.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Rasgon N, Bauer M, Glenn T, Elman S, Whybrow PC. Menstrual cycle related mood changes in women with bipolar disorder. Bipolar Disorders. 2003;5(1):48–52. doi: 10.1034/j.1399-5618.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- Reynolds-May MF, Kenna HA, Marsh W, Stemmle PG, Wang P, Ketter TA, Rasgon NL. Evaluation of reproductive function in women treated for bipolar disorder compared to healthy controls. Bipolar Disord. 2014;16(1):37–47. doi: 10.1111/bdi.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepede G, Martinotti G, Gambi F, Salerno RM, Di Giannantonio M. Lamotrigine augmentation in premenstrual dysphoric disorder: A case report. Clin Neuropharmacol. 2013;36(1):31–33. doi: 10.1097/WNF.0b013e318279ee1f. [DOI] [PubMed] [Google Scholar]
- Shivakumar G, Bernstein IH, Suppes T. Are bipolar mood symptoms affected by the phase of the menstrual cycle? J Women’s Health. 2008;17(3):473–478. doi: 10.1089/jwh.2007.0466. [DOI] [PubMed] [Google Scholar]
- Sit D, Seltman H, Wisner KL. Menstrual effects on mood symptoms in treated women with bipolar disorder. Bipolar Disord. 2011;13(3):310–317. doi: 10.1111/j.1399-5618.2011.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skokou M, Gourzis P. Menstrually related cyclothymia: A case report. European Psychiatry. 2013;28(S1):1. [Google Scholar]
- Teatero ML, Mazmanian D, Sharma V. Effects of the menstrual cycle on bipolar disorder. Bipolar Disord. 2013;16(1):22–36. doi: 10.1111/bdi.12138. [DOI] [PubMed] [Google Scholar]
- Wang J, Sun X, Chen B, Young LT. Lamotrigine increases gene expression of GABAA receptor β3 subunit in primary cultured rat hippocampus cells. Neuropsychopharmacology. 2001;26:415–421. doi: 10.1016/S0893-133X(01)00385-2. [DOI] [PubMed] [Google Scholar]
- Zlotnik A, Gruenbaum BF, Mohar B, Kuts R, Gruenbaum SE, Ohayon S, Shaked G. The effects of estrogen and progesterone on blood glutamate levels: Evidence from changes of blood glutamate levels during the menstrual cycle in women. Biol Reprod. 2011;84(3):581–586. doi: 10.1095/biolreprod.110.088120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.