Abstract
Mammalian auditory hair cells do not spontaneously regenerate, unlike hair cells in lower vertebrates including fish and birds. In mammals, hearing loss due to the loss of hair cells is thus permanent and intractable. Recent studies in the mouse have demonstrated spontaneous hair cell regeneration during a short postnatal period, but this regenerative capacity is lost in the adult cochlea. Reduced regeneration coincides with a transition that results in a decreased pool of progenitor cells in the cochlear sensory epithelium. Here, we review the signaling cascades involved in hair cell formation and morphogenesis of the organ of Corti in developing mammals, the changing status of progenitor cells in the cochlea, and the regeneration of auditory hair cells in adult mammals.
Hair cells: Mechanotransducing cells of sensory end organs
Over the course of evolution, structures for sensing the flow or vibration of the external environment have developed in parallel with the neural networks to relay the resulting signals to the central nervous system (CNS). Hair cells, which are observed in a range of vertebrates, such as fish, amphibians, birds, and mammals, are highly specialized for this particular task. The cells have apically arranged hair bundles that vibrate in response to movements in the fluid filled labyrinth of the ear or the surrounding medium in aquatic species with motion-sensing lateral line hair cells; this vibration is coupled to mechanotransduction channels. The flow or vibration through the medium stimulates the bundles to generate action potentials via the opening of calcium-gated channels. Sources of this mechanical movement depend on the environment and include water flow, gravity and sound. The essential structures and mechanics of this process are evolutionarily conserved regardless of the source.
Sound is a vibration or traveling wave of medium (e.g., air, water) that is transduced into an electrophysiological signal by auditory organs. Action potentials serve as these signals, which are subsequently transmitted to the brain via bipolar auditory neurons. In mammals, there are two subtypes of hair cells in the cochlea to subserve different aspects of detecting sound: inner hair cells transmit the signal arising from mechanotransduction channels to the afferent neurons, and outer hair cells change length in response to sound, thus amplifying the mechanical vibration of the basilar membrane that forms the cochlear partition containing the organ of Corti. This organ comprises the sensory epithelium where the hair cells reside together with surrounding supporting cells.
Limited capacity for hair cell regeneration in mammals
Mammalian auditory hair cells do not spontaneously regenerate, unlike hair cells in lower vertebrates, and, as a result, hearing loss due to the loss of hair cells is permanent and intractable. Although recent mouse studies showed limited regenerative capacity of auditory sensory epithelium during a short postnatal period, hair cell regeneration does not occur in the adult cochlea (1–10).
The gradual loss of the regenerative capacity of cochlear hair cells in adult mammals may be an adaptation to the complexity of the organized structure of the cochlear amplifier, which is essential for inner ear function and could be disorganized by a regenerative response to insult.
Mechanisms that have been proposed to account for the decreased regenerative capacity of the adult mammalian cochlea are a reduced number of progenitor cells (11) or lower flexibility of the epithelium resulting from an accumulation of actin in cell-cell junctions (12) Understanding molecular mechanisms for the loss of regenerative capacity is critical both for designing molecular pathways for hair cell regeneration and for reconstituting the architecture of the epithelium such that function is restored.
This review surveys the literature on signaling cascades involved in development of hair cells and morphogenesis of the organ of Corti, the changing status of progenitor cells during the maturation of the cochlea, and the regeneration of auditory hair cells.
The generation and arrangement of hair cells in the developing cochlear sensory epithelium (Fig. 1)
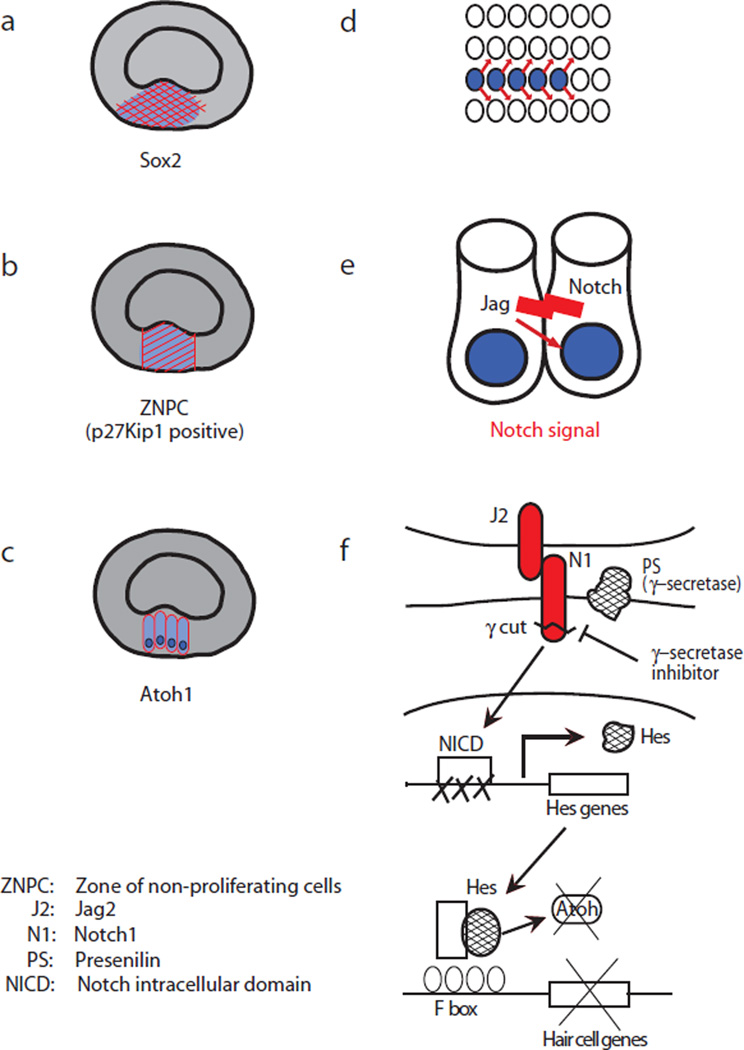
Fig. 1. Schematic of Notch signaling in the developing cochlea.
During differentiation of sensory epithelium in the mouse cochlea, a thickened area that expresses Sox2 is specified by embryonic day 12 (E12) (a). The cells destined to become the cochlear sensory epithelium exit the cell cycle in a region termed the zone of non-proliferating cells marked by the expression of cell cycle inhibitor. p27Kip1 (b). A master gene for hair cell differentiation, Atoh1, is observed within this area (c). Emerging Atoh1-positive cells (blue) start to express Notch ligands including jagged 2 and interact with neighboring cells through Notch signal-mediated “lateral inhibition” (d, e). In the surrounding cells, hair cell genes are shut down through the competition of Hes and Atoh1 (e). Therefore, the cells adjacent to hair cells differentiate into supporting cells, resulting in a checkerboard arrangement of hair cells and supporting cells (f). (ZNPC: Zone of non-proliferating cells; J2: jagged 2; N1: Notch 1; PS: Presenilin; NICD: Notch intracellular domain)
Regenerative medicine offers hope for the treatment of organs in which endogenous cell replacement does not occur. For any advances to be made, a thorough understanding of morphogenesis and cell-fate decisions, with a particular emphasis on underlying signaling cascades, is imperative. In regenerative biology as in developmental biology, gene-targeting techniques in transgenic mice have created the possibility of testing the in vivo significance of molecular pathways.
During development, a thickened area of proliferative cells first appears at the floor of the cochlear duct and becomes specified as the prosensory domain that will give rise to the cochlear sensory epithelium. In the mouse, these Sox2-positive cells (13) are specified by embryonic day 12 (E12) in the basal turn of the cochlea (Fig. 1a). Subsequently, the cells that are destined to become the cochlear sensory epithelium exit the cell cycle in a region termed the zone of non-proliferating cells (ZNPC) marked by the expression of cell cycle inhibitor, p27Kip1 (Cdkn1b) (14) (Fig. 1b). Expression of basic helix-loop-helix (bHLH) transcription factor, Atoh1, a master gene for hair cell differentiation, is observed within this area (1) (Fig. 1c). Notch signal-mediated “lateral inhibition” defines the checkered pattern of hair cells and supporting cells, the two developing sensory epithelial cell types in this area (Fig. 1d). Atoh1-positive cells have been reported to express Notch ligands including jagged2 and delta-like 1 at their cell surface (13) (Fig. 1e). Interaction between these ligands and Notch activates the pathway in the surrounding cells: Notch1 is cleaved beneath the cell membrane by γ-secretase in response to the ligand-receptor contact, and the intracellular domain of the protein is translocated into the nucleus. The downstream signals of the cascade include negative bHLH transcription factors and Hes genes, such as Hes5, which compete with proneuronal bHLH factor Atoh1 and shut off downstream hair cell genes (Fig. 1e). These findings were obtained in several mouse models in which Notch signaling was disrupted (1–10). The cells adjacent to hair cells differentiate into a supporting cell lineage, resulting in a checkerboard arrangement of hair cells and supporting cells.
Supporting cells have the potential to become progenitor cells but are dormant in adult mice
Neurosphere formation and culturing is used to selectively expand stem/progenitor cells that have self-renewal capacity and multipotency. Thus, the in vitro neurosphere protocol is widely used as a method of harvesting stem cell-enriched populations in the CNS (18). Oshima and colleagues revealed that the cells harvested from different parts of the cochlea gave rise to otospheres. In the organ of Corti, however, otoosphere-forming capacity rapidly decreased and was lost within the first several weeks after birth (19).
Evidence reported by several groups indicates that supporting cells adjacent to hair cells in the organ of Corti have a latent capacity to divide and regenerate until P14 (19, 20). White et al. showed that FACS-sorted, dissociated, p27Kip1-eGFP-positive supporting cells proliferated and gave rise to new hair cells, but the number of proliferating cells decreased dramatically from P2 to P14 (20). A subset of supporting cells that expressed Lgr5, a gene target of Wnt signaling (11, 21), efficiently proliferated and differentiated into hair cells upon Wnt pathway activation in the newborn cochlea (22).
As described above, stimulation of Notch signaling by Notch ligands on adjacent cells influences cell fate, and the inhibition of Notch signaling in immature supporting cells can convert them into hair cells. This phenomenon was reported (5, 6, 9, 23, 24) to occur up to the early postnatal period. Hair cell were produced by proliferation of supporting cells followed by their transdifferentiation during embryonic stages, whereas, in neonatal mice, hair cells were generated by transdifferentiation alone.
However, whereas inhibition of Notch in the perinatal period leads to supporting cell transdifferentiation to hair cells, the capacity for transdifferentiation and accumulation of supernumerary hair cells is not apparent in the adult in the absence of hair cell loss.
The insult-induced rejuvenation of supporting cells
Transdifferentiation of supporting cells giving rise to new hair cells has been studied in the perinatal organ of Corti from intact ears. In various adult organs, stem cells are quiescent under normal conditions and “re-activated” by tissue damage. In the central nervous system, where the lack of regeneration in adult mammals has long been studied, subventricular zone astrocytes or radial glia were reported to divide asymmetrically and produce neurons after damage, as observed in the middle cerebral artery occlusion model of brain ischemia (25). In the skin, hair follicle stem cells produce squamous epithelium when the surrounding skin is disrupted (26). Thus, tissue regeneration can be increased by cellular damage.
Bramhall et al. investigated the regenerative capacity of the cochlear sensory epithelium and revealed that supporting cells transformed into hair cells in the neonatal organ of Corti after hair cell death, even in the absence of treatment (27). Cox et al (28) found similar transdifferentiation as well as proliferation of supporting cells in the perinatal cochlea after death of hair cells from diphtheria toxin. The replacement of hair cells could be enhanced by Notch inhibition, and as demonstrated by elegant lineage tracing analyses, inner pillar cells and 3rd row Deiters cells gave rise to hair cells. A BrdU labeling analysis showed that the proliferation of supporting cells was limited and that the replacement of auditory hair cells was predominantly achieved through transdifferentiation (27). Pillar cells have been shown to act as a source of hair cells in other studies in the postnatal period (20, 29).
There is currently limited knowledge regarding in vivo stem cells in adult ears. Marker expression analyses showed that the stem cell markers Sox2, Lgr5, Abcg2, Musashi1, Notch, Prox1, islet1 and Nestin were expressed in supporting cells (11, 30–34). Recent studies showed that Nestin-driven reporters were induced in supporting cells after both gentamicin-induced ototoxicity and noise exposure (35, 36).
In vivo transdifferentiation of supporting cells to hair cells induces hearing recovery
The proper positioning of regenerating hair cells is crucial due to the structure-function relationship in the mammalian organ of Corti. With this in mind, transdifferentiation approaches have been tested for hair cell regeneration in the cochlea. Previously, the adenoviral gene transfer of Atoh1 after kanamycin-induced deafness was reported to induce hair cell regeneration and ameliorated hearing loss in guinea pigs (37). The above mentioned study by Watanabe et al showed the re-emergence of Nestin-LacZ expression in supporting cells up to 14 days after noise exposure (36). We identified a transient upregulation of Hes5 (a direct downstream target of Notch signaling), which suggested that the supporting cells were maintained as supporting cells through Notch signaling (38). Notch signaling increases were also found after damage due to gentamicin in the neonatal mouse (39). Without damage, overexpression of Atoh1 had a limited effect on supporting cells (10, 40).
Transdifferentiation approaches have now been tested with small molecules with the goal of future clinical use (38, 41). We successfully induced hair cells using LY411575, a potent γ-secretase inhibitor (Fig. 2). After the administration of LY411575, we observed an increased hair cell number. Using Sox2-CreER mice for lineage tracing (Fig. 3), we demonstrated that the added hair cells were derived from Sox2-positive supporting cells (Fig. 4b). Most of the hair cells were in the outer hair cell region and morphologically matured, while the few additional hair cells in the inner pillar cell region were immature (Fig. 2). The new cells derived from transdifferentation were in the organ of Corti (their normal location). Finally, tests of hearing by distortion product otoacoustic emission (DPOAE) and auditory brainstem response (ABR) showed a slight improvement in the LY411575-treated ear. The result demonstrated the successful transdifferentiation of supporting cells to hair cells that were functional (38). Similar findings were recently reported by Tona et al (41).
Fig. 2. Scanning electron microscopy (SEM) of the organ of Corti after LY411575 administration.
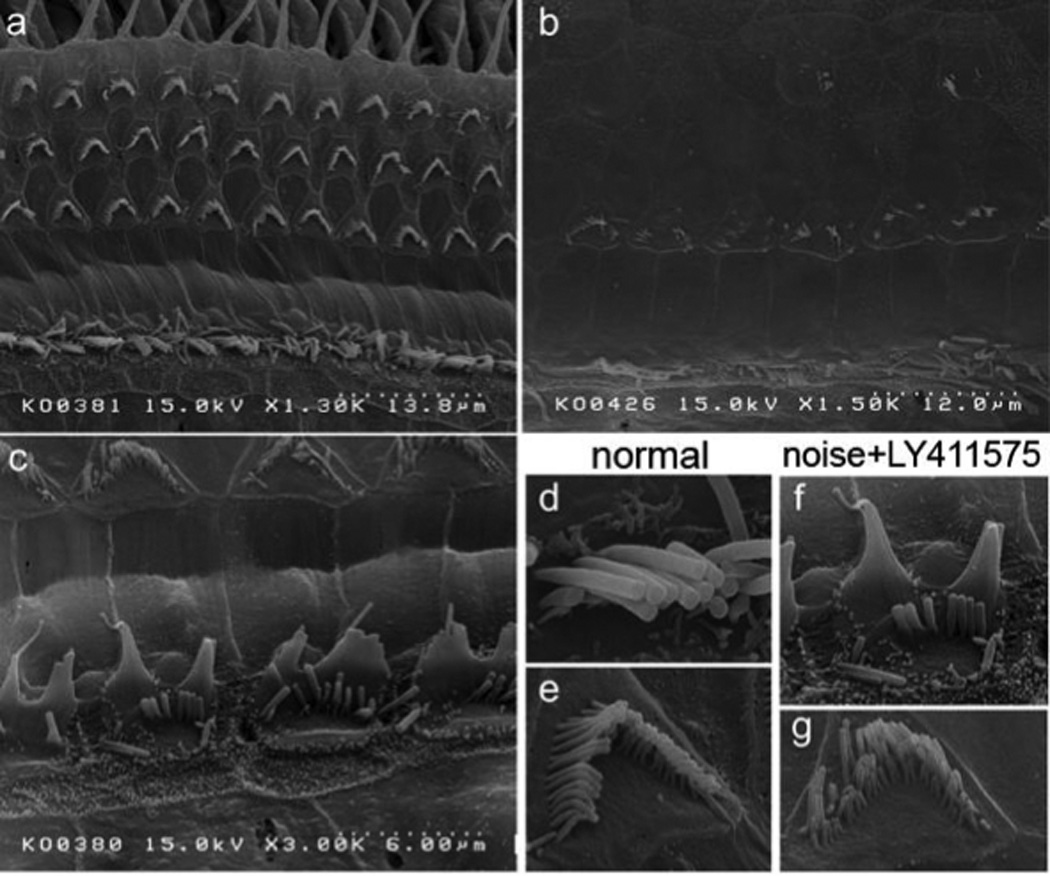
SEM images of the organ of Corti of young adult mice without treatment (a,d,e) and after acoustic trauma with (c,f,g) and without (b) administration of LY411575 to regenerate hair cells. Note that the cells in the inner pillar cell region (adjacent to inner hair cells) had disorganized “immature” stereociliary bundles (c, f), while bundles in the outer hair cells were apparently normal (g).
Fig. 3. Tracing the lineage of regenerated hair cells.
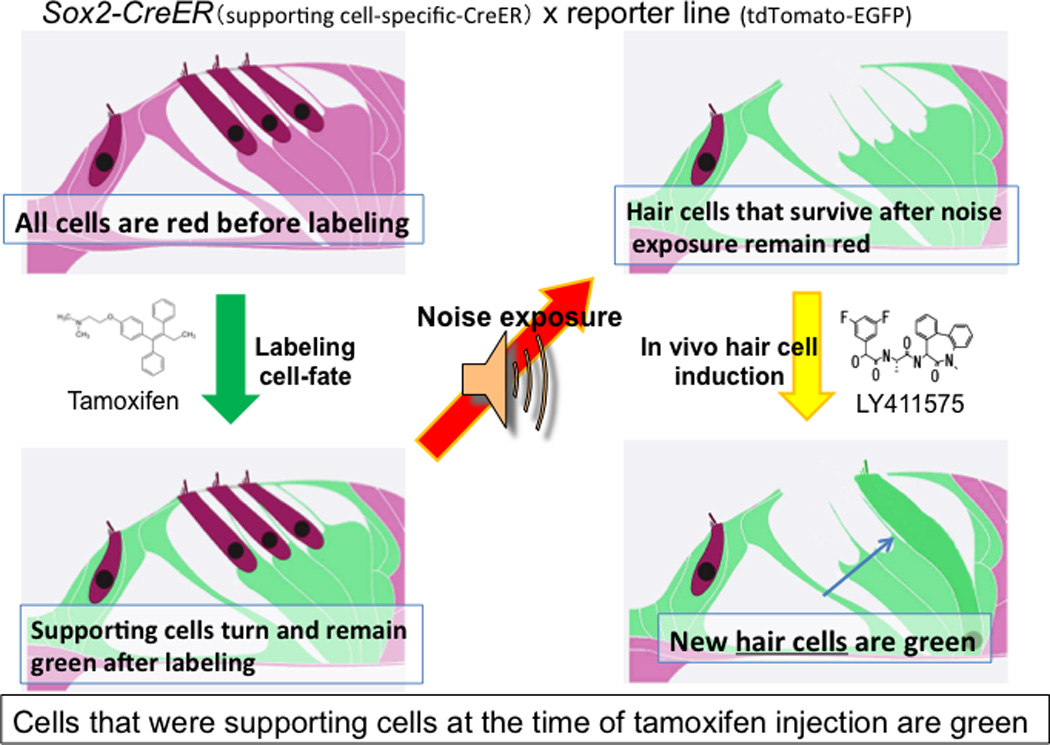
Prior to noise exposure, supporting cells were labeled in green by administration of tamoxifen to activate CreER (under a Sox2 promoter) in supporting cells Following LY411575 treatment after ototoxic insult, newly generated hair cells derived from supporting cells retained a green label, in contrast to the surviving hair cells which remained red.
Fig. 4. Regenerated hair cells.
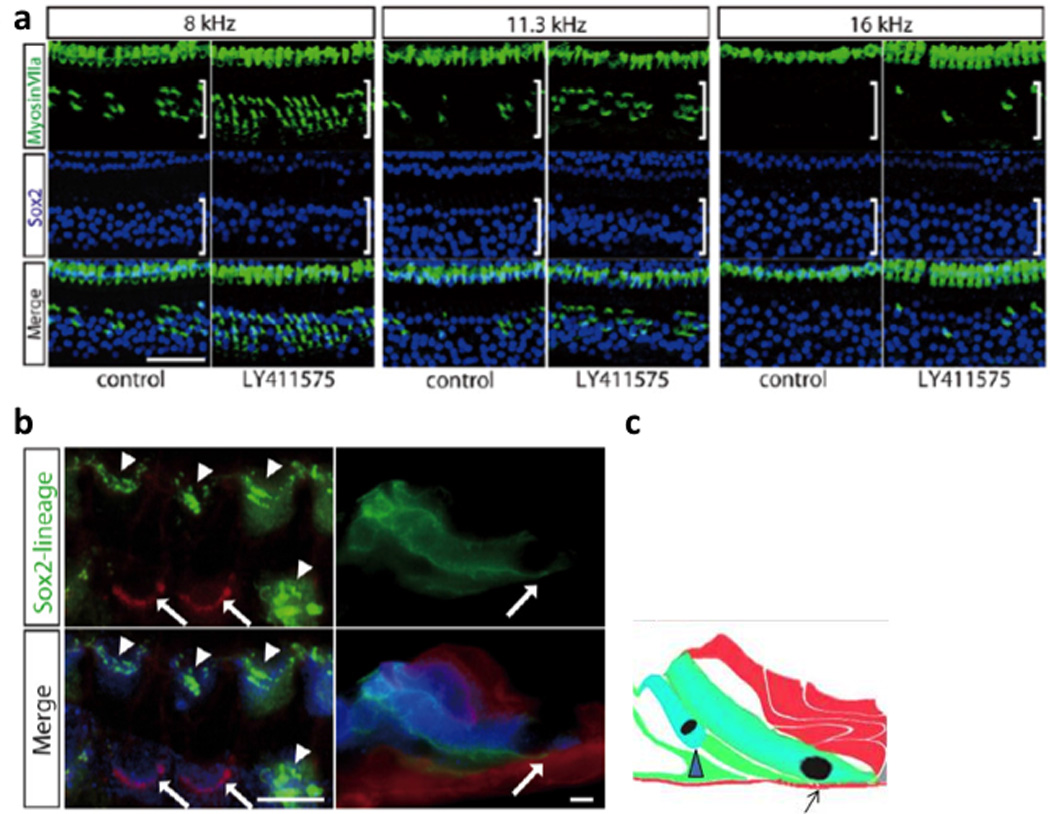
Hair cells and supporting cells were labeled by anti myosin VIIa (green) and anti Sox2 (blue) antibodies, respectively (a). hair cells number increased by approximately the same amount as the supporting cells number decreased. Supporting cell lineage was traced in green and other cells in red in Sox2-CreER; mT/mG double transgenic mice. (b). As described in Fig. 2, hair cells (marked by arrowheads) transdifferentiated from supporting cells were green, while surviving hair cells after noise exposure were red. Some of the green hair cells (right panel in the Z-stack) shown in the diagram (c) extended to the basilar membrane (arrow). The figure is reproduced with permission from Figure 4 and 5, Mizutari et al., Neuron Vol. 77 Issue 1.
These studies demonstrate that the in vivo differentiation of hair cells from surrounding cells is a feasible therapeutic approach for sensorineural hearing loss. Future efforts to uncover small molecules that will improve the efficiency of induction may bring this hair cell regeneration strategy closer to clinical use.
Conclusions and future directions
Spontaneous auditory hair cell regeneration was reported in birds in the late 1980s. This announcement was followed by similar reports in fish in the early 1990s. These findings suggested the potential for a new therapy to treat human hearing loss. Decades of subsequent research, accompanied by an expansion in the field of molecular biology, led to investigations into therapies that could replace adult mammalian auditory hair cells. Specifically, the understanding of and ability to manipulate signaling cascades has led to promising new therapeutic modalities.
Box 1 Lower vertebrates regenerate hair cells upon ototoxic damage.
The regenerative capacity observed in lower vertebrates has informed the search for pathways and mechanisms that might stimulate mammalian hair cell regeneration. The regeneration of hair cells after damage has been demonstrated in avian species (15, 16). Hair cell regeneration in birds is comprised of two modes, i.e. transdifferentiation from supporting cells and proliferation. Molecular mechanisms underlying each process with respect to relevant signaling pathways, such as Notch and its components, Atoh1, Wnt, and FGFs, have been elucidated in the last decade. Ku et al (17) reported a transcriptome analysis of utricle hair cell regeneration in the inner ear of the chick, showing that the enhanced expression of Atoh1, which was upregulated after 72 hr of aminoglycoside antibiotic treatment, led to enhanced levels of delta and jagged, thus activating Notch signaling and downstream Hes5 expression in adjoining cells (the cell-cell signaling described above). FGF signaling is also a regulator of inner ear development. Expression patterns of members of FGF signaling pathways were altered during regeneration (17).
Box 2 OUTSTANDING QUESTIONS.
Is the dual regeneration of functional inner and outer hair cells possible?
Can supporting cells be reprogrammed to generate new hair cells rather than glial scars?
Can a proliferative response be induced in the adult cochlea without leading to apoptosis?
Will this cell division replace supporting cells that have transdifferentiated to hair cells?
Are there options for treatment long after damage has occurred?
Can the findings in the mouse be extended to primates?
Are there treatment options for presbycusis?
These will be important questions for developing new therapies from the deepening molecular understanding of signaling pathways.
Highlights.
-
-
Regeneration of cochlear hair cells is a potential therapy for hearing loss
-
-
The lack of hair cell regeneration in adults is a major cause of hearing loss
-
-
Supporting cells can be recruited as facultative progenitors for hair cells
-
-
Small molecules and gene therapy are promising approaches for hair cell regeneration
Acknowledgements
We are grateful to Dr. T. Nagai for his excellent technical assistance in the electron microscopy. This work was supported by MEXT. KAKENHI (Grant-in-Aid for Scientific Research (C), 24592560), by MHLW (Comprehensive Research on Disability Health and Welfare), by Takeda Science Foundation to M.F, and by NIH grants RO1 DC007174, R21 DC010440, and P30 DC05209.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bermingham NA, et al. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284(5421):1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- 2.Lanford PJ, et al. Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat Genet. 1999;21(3):289–292. doi: 10.1038/6804. [DOI] [PubMed] [Google Scholar]
- 3.Zine A, et al. Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear. J Neurosci. 2001;21(13):4712–4720. doi: 10.1523/JNEUROSCI.21-13-04712.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woods C, Montcouquiol M, Kelley MW. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci. 2004;7(12):1310–1318. doi: 10.1038/nn1349. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi T, Cunningham D, Bermingham-McDonogh O. Loss of Fgfr3 leads to excess hair cell development in the mouse organ of Corti. Dev Dyn. 2007;236(2):525–533. doi: 10.1002/dvdy.21026. [DOI] [PubMed] [Google Scholar]
- 6.Doetzlhofer A, et al. Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti. Dev Cell. 2009;16(1):58–69. doi: 10.1016/j.devcel.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartman BH, et al. Hes5 expression in the postnatal and adult mouse inner ear and the drug-damaged cochlea. J Assoc Res Otolaryngol. 2009;10(3):321–340. doi: 10.1007/s10162-009-0162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan W, Jin Y, Stanger B, Kiernan AE. Notch signaling is required for the generation of hair cells and supporting cells in the mammalian inner ear. Proc Natl Acad Sci U S A. 2010;107(36):15798–15803. doi: 10.1073/pnas.1003089107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto N, Chang W, Kelley MW. Rbpj regulates development of prosensory cells in the mammalian inner ear. Dev Biol. 2011;353(2):367–379. doi: 10.1016/j.ydbio.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Kelly MC, Chang Q, Pan A, Lin X, Chen P. Atoh1 directs the formation of sensory mosaics and induces cell proliferation in the postnatal mammalian cochlea in vivo. J Neurosci. 2012;32(19):6699–6710. doi: 10.1523/JNEUROSCI.5420-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi F, Kempfle JS, Edge AS. Wnt-responsive lgr5-expressing stem cells are hair cell progenitors in the cochlea. J Neurosci. 2012;32(28):9639–9648. doi: 10.1523/JNEUROSCI.1064-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burns JC, et al. Reinforcement of cell junctions correlates with the absence of hair cell regeneration in mammals and its occurrence in birds. J Comp Neurol. 2008;511(3):396–414. doi: 10.1002/cne.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci. 2006;7(11):837–849. doi: 10.1038/nrn1987. [DOI] [PubMed] [Google Scholar]
- 14.Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126(8):1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- 15.Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240(4860):1772–1774. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- 16.Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240(4860):1774–1776. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- 17.Ku YC, et al. The transcriptome of utricle hair cell regeneration in the avian inner ear. J Neurosci. 2014;34(10):3523–3535. doi: 10.1523/JNEUROSCI.2606-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 19.Oshima K, et al. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J Assoc Res Otolaryngol. 2007;8(1):18–31. doi: 10.1007/s10162-006-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441(7096):984–987. doi: 10.1038/nature04849. [DOI] [PubMed] [Google Scholar]
- 21.Chai R, et al. Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc Natl Acad Sci U S A. 2012;109(21):8167–8172. doi: 10.1073/pnas.1202774109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi F, Hu L, Edge AS. Generation of hair cells in neonatal mice by beta-catenin overexpression in Lgr5-positive cochlear progenitors. Proc Natl Acad Sci U S A. 2013;110(34):13851–13856. doi: 10.1073/pnas.1219952110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto N, et al. Inhibition of Notch/RBP-J signaling induces hair cell formation in neonate mouse cochleas. J Mol Med (Berl) 2006;84(1):37–45. doi: 10.1007/s00109-005-0706-9. [DOI] [PubMed] [Google Scholar]
- 24.Jeon SJ, Fujioka M, Kim SC, Edge ASB. Notch signaling alters sensory or neuronal cell fate specification of inner ear stem cells. J. Neurosci. 2011;31(23):8351–8358. doi: 10.1523/JNEUROSCI.6366-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuwahara A, et al. Tcf3 Represses Wnt-beta-Catenin Signaling and Maintains Neural Stem Cell Population during Neocortical Development. PLoS One. 2014;9(5):e94408. doi: 10.1371/journal.pone.0094408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito M, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11(12):1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 27.Bramhall NF, Shi F, Arnold K, Hochedlinger K, Edge AS. Lgr5-positive supporting cells generate new hair cells in the postnatal cochlea. Stem Cell Reports. 2014;2(3):311–322. doi: 10.1016/j.stemcr.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox BC, et al. Spontaneous hair cell regeneration in the neonatal mouse cochlea in vivo. Development. 2014;141(4):816–829. doi: 10.1242/dev.103036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacques BE, Montcouquiol ME, Layman EM, Lewandoski M, Kelley MW. Fgf8 induces pillar cell fate and regulates cellular patterning in the mammalian cochlea. Development. 2007;134(16):3021–3029. doi: 10.1242/dev.02874. [DOI] [PubMed] [Google Scholar]
- 30.Oesterle EC, Campbell S, Taylor RR, Forge A, Hume CR. Sox2 and JAGGED1 expression in normal and drug-damaged adult mouse inner ear. J Assoc Res Otolaryngol. 2008;9(1):65–89. doi: 10.1007/s10162-007-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landt SG, et al. ChIP-seq guidelines and practices of the ENCODE and modENCODE consortia. Genome Res. 2012;22(9):1813–1831. doi: 10.1101/gr.136184.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bermingham-McDonogh O, et al. Expression of Prox1 during mouse cochlear development. J Comp Neurol. 2006;496(2):172–186. doi: 10.1002/cne.20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radde-Gallwitz K, et al. Expression of Islet1 marks the sensory and neuronal lineages in the mammalian inner ear. J Comp Neurol. 2004;477(4):412–421. doi: 10.1002/cne.20257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahlfeld J, et al. Sox2 requirement in sonic hedgehog-associated medulloblastoma. Cancer Res. 2013;73(12):3796–3807. doi: 10.1158/0008-5472.CAN-13-0238. [DOI] [PubMed] [Google Scholar]
- 35.Martone T, et al. Nestin expression and reactive phenomena in the mouse cochlea after kanamycin ototoxicity. Eur J Neurosci. 2014;39(11):1729–1741. doi: 10.1111/ejn.12576. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe R, et al. Nestin-expressing cells in the developing, mature and noise-exposed cochlear epithelium. Mol Cell Neurosci. 2012;49(2):104–109. doi: 10.1016/j.mcn.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Izumikawa M, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11(3):271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- 38.Mizutari K, et al. Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron. 2013;77(1):58–69. doi: 10.1016/j.neuron.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korrapati S, Roux I, Glowatzki E, Doetzlhofer A. Notch signaling limits supporting cell plasticity in the hair cell-damaged early postnatal murine cochlea. PLoS One. 2013;8(8):e73276. doi: 10.1371/journal.pone.0073276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Z, et al. Age-dependent in vivo conversion of mouse cochlear pillar and Deiters' cells to immature hair cells by Atoh1 ectopic expression. J Neurosci. 2012;32(19):6600–6610. doi: 10.1523/JNEUROSCI.0818-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tona Y, et al. Therapeutic potential of a gamma-secretase inhibitor for hearing restoration in a guinea pig model with noise-induced hearing loss. BMC Neurosci. 2014;15:66. doi: 10.1186/1471-2202-15-66. [DOI] [PMC free article] [PubMed] [Google Scholar]