Abstract
Exposure to hyposmotic solution causes release of ATP from lens cells via hemichannels. Because hemichannel opening feasibly could swamp the cells with calcium, we carried out studies to measure the magnitude of the increase in cytoplasmic calcium concentration caused by hemichannel opening. In studies on porcine lens epithelial cells in primary culture, propidium iodide (PI) uptake was measured as an index of hemichannel opening. PI uptake was increased significantly in cells exposed to hyposmotic solution. The PI increase under hyposmotic conditions was suppressed by GAP 27, a connexin inhibitor peptide. In studies on cells loaded with Fura-2, continuous exposure to hyposmotic solution caused a cytoplasmic calcium concentration increase that peaked within ~30 sec then remained elevated at or below the peak response for more than 60 min. The peak calcium concentration was 186 ± 2.3 nM compared to a baseline value of 98.0 ± 1.4 nM. The calcium concentration increased a lot further in cells exposed to A23187 (2.5 µM) or the sodium-calcium exchange inhibitor SN-6 (10 µM) added after the onset of the calcium rise in hyposmotic solution. The cytoplasmic calcium increase in hyposmotic solution was abolished by GAP 27. Calcium returned to baseline in cells exposed to hyposmotic solution then treated with GAP 27 starting 2 min after the onset of the calcium rise. The calcium increase in hyposmotic solution did not occur when calcium was eliminated from the bathing medium. The responses to hyposmotic and hyperosmotic stress were different. There was no detectable increase in calcium or PI entry in cells exposed to hyperosmotic solution (500 mOsm). In summary, GAP 27-sensitive accumulation of PI by cultured lens epithelium points to connexin hemichannel opening and associated calcium entry. Even though connexins form channels with a large carrying capacity, calcium entry does not increase the cytoplasmic calcium concentration beyond a tolerable physiological range.
Keywords: Calcium, connexin hemichannel, hyposmotic stress, lens epithelium, propidium iodide uptake
1. Introduction
Mature lens fiber cells, which make up the bulk of the lens, have little or no Na,K-ATPase activity. Ion and water homeostasis of the entire structure is made possible mostly by Na,K-ATPase activity in cells at or close to the anterior-equatorial lens surface: a single layer of epithelial cells and differentiating fibers that have not attained maturity (Gao et al., 2000). The level of Na,K-ATPase activity can change. A Src family tyrosine kinase-dependent signaling mechanism increases Na,K-ATPase activity in the epithelium when osmotic swelling causes release of cellular ATP via connexin and pannexin hemichannels, then subsequent purinergic receptor activation (Shahidullah et al., 2012a; Srivastava et al., 1997). Hemichannel-mediated ATP release is part of a Na,K-ATPase regulation mechanism.
Hemichannels are recognized as an unusually large conduit that, when open, allow passage of high molecular weight solutes as well as ions including calcium (Fiori et al., 2012; Saez et al., 2010; Schalper et al., 2010). Thus, when hyposmotic solution causes opening of hemichannels in order to release ATP from the epithelium, one of the consequences is likely to be a flow of extracellular calcium ions through the hemichannels into the cell. This raises the possibility that hemichannel opening might swamp the cells with calcium. While calcium plays an important role in signaling pathways, the duration and magnitude of calcium elevation is generally very tightly regulated. Cytoplasmic calcium overload can occur when calcium regulation is abnormal or suppressed, leading to cell death by necrosis or apoptosis (Li et al., 2010; Mattson and Chan, 2003). Calcium homeostasis is particularly critical for lens transparency and calcium elevation is associated with pathophysiology (Rhodes and Sanderson, 2009). Here we examine the magnitude of the calcium increase in cytoplasmic calcium. Because cytoplasmic calcium studies on the intact lens are not possible, the studies were carried out on cultured lens epithelial cells.
2. Materials and Methods
2.1. Materials
Propidium iodide, ethylene glycol tetraacetic acid (EGTA) and DMSO were purchased from Sigma (St Louis, MO, USA). Acetoxymethyl 2-[5-[bis[(acetoxymethoxy-oxo-methyl)methyl]amino]-4-[2-[2-[bis[(acetoxymethoxy-oxo- methyl)methyl]amino]-5-methyl-phenoxy]ethoxy]benzofuran-2-yl]oxazole-5-carboxylate (Fura2-AM) was purchased from Life Technologies (Grand Island, NY, USA). Depending on their solubility, test agents were dissolved either in water or in DMSO from freshly opened ampules. All other chemicals, including those for preparing the Krebs’ solution, were of analytical grade and purchased from Sigma. Krebs’ solution composition was (in mM); 119 NaCl, 4.7 KCl, 1.2 KH2PO4, 25 NaHCO3, 2.5 CaCl2, 1 MgCl2, and 5.5 glucose, equlibrated by bubbling with 5% CO2 for 40 min and adjusted to pH 7.4. The osmolarity of control Krebs solution was 300 mOsm. Hyposmotic Krebs’ solution (200 mOsm) contained 69 mM NaCl. Hyperosmotic Krebs solution (500 mOsm) contained an additional 200 mM mannitol. Osmolarity of the solutions was measured using a micro osmometer (μ Osmette, Precision System Inc., Florence AL, USA) and adjusted when necessary.
2.2. Cell culture
Primary culture of lens epithelium was obtained according to our published method (Shahidullah et al., 2012b). After removing extraocular muscles and fat, the eyes were washed with antibiotic mixed HBSS (200 U/ml penicillin and 200µg/ml streptomycin). Intact lenses were isolated as described earlier (Shahidullah et al., 2012a) under sterile condition in a laminar flow cabinet. The eyes were dissected open from the posterior pole, the vitreous was removed by gently pushing aside with a curved forceps, zonules were cut and intact lenses were isolated. Any remnants of ciliary epithelium or vitreous were removed by rolling the lens over sterile filter paper. The lens was then placed on a Petri dish with its posterior (more convex surface adjacent to the vitreous) face upward. The capsule-epithelium was isolated by making a small incision on the posterior capsule with a surgical blade and by holding and pulling apart the two cut margins using fine forceps. Care was taken to prevent curling or twisting of the capsule during isolation since the basement membrane of the lens epithelium is attached to the inner surface of the anterior capsule. Several capsule-epithelia (7–8) were placed on a large (100 mm diameter) Petri dish with the cell side facing upward. A small amount of complete medium (1.0 ml) was added along the edge of the Petri dish to prevent dehydration. The Petri dish was then placed in a 37°C incubator with a humified atmosphere of 95% air and 5% CO2 and incubated for 1–3 h to encourage firm attachment of the capsule to the plastic surface. 5.0 ml of complete medium was then added carefully to the Petri dish and incubated for 48 h. The complete medium consists of a mixture of epithelial culture medium (EpiCM, ScienCell, Cat #4101), 2.0% fetal bovine serum, epithelial cell growth factor (1.0 ml/100 ml medium, ScienCell Cat. # 4152) and 100 U/ml penicillin plus 100 µg/ml streptomycin. Medium was changed every alternate day after that. After 6–8 days, when cells had grown out of the explants but before reaching overconfluence, the cells were trypsinized for 3–5 min using 5 ml 1X trypsin EDTA (Gibco). Trypsin was immediately neutralized using 5 ml of a mixture of new born calf serum and FBS (1:1). The cell-capsule suspension was separated from the capsule fragments by spinning at low speed (350 rpm, ~20×g) for 5 min at 4°C and the cell suspension was collected from the top with a Pasteur pipette. The cell suspension was centrifuged at 1000 rpm (168g) for 10 min at 4°C, supernatant discarded and the cells were seeded in a 25 cm2 flask at a density of 2×104 cells/cm2 in 5 ml of complete medium. Medium was changed after 24 h and then every alternate day. Cell usually reached confluence in 3–4 days. Cells were then trypsinized and passaged for maintenance and propagation. Only cells from passage 2–3 were used.
2.3. Propidium iodide uptake
Cells were cultured to confluence in 24 well plates. Culture medium was aspirated and the cells were washed twice with the control Krebs’ solution comprising mM 119 NaCl, 4.7 KCl, 1.2 KH2PO4, 25 NaHCO3, 2.5 CaCl2, 1 MgCl2, and 5.5 glucose, equlibrated by bubbling with 5% CO2 for 40 min and adjusted to pH 7.4. Cells were then acclimatized in the Krebs solution for an hour. Following 20 min pre-incubation in control Krebs’ solution with the test reagent, the cells were further incubated for 30 min in hyposmotic Krebs’ solution containing 25 µM propidium iodide (PI, MW = 668.4) in the continued presence of the test agents. Control cells received only the vehicle for the respective test reagent. Hyposmotic solution was prepared by reducing Na in the above control Krebs, solution. Cells were then washed 3 times with the control Krebs’ solution, 300 µl of distilled water was added to each wells and homogenized for 1 min (4 strokes of 15 sec at 5 sec intervals) using Misonix S3000 sonicator at a 6W power setting (Misonix, New York, USA). 150 µl of the cell homogenate was transferred from each well to each well of a 96-well black plate (BD Falcon) and PI fluorescence intensity was measured at excitation and emission wavelengths of 535 nm and 617 nm respectively, using Varioskan flash multimode reader (Thermo Scientific, Barrington, IL, USA). Protein in the sample was measured by bicinchoninic acid assay (Smith et al., 1985). The results are expressed as relative fluorescence/mg protein.
2.4. Measurement of cytoplasmic Ca2+
Cytoplasmic calcium was measured in cells loaded with Fura-2 AM by measuring the fluorescence intensity at alternating excitation wavelengths of 340 nm and 380 nm, and the emission captured at 510 nm using a modified method described earlier (Mandal et al., 2010). Semi-confluent (70–80%) cells grown on 35 mm culture dish (Corning Cat. # 430165) was loaded for 40 min at 37°C with 5 µM Fura-2 -AM in a Krebs’ solution comprising (mM) 119 NaCl, 4.7 KCl, 1.2 KH2PO4, 25 NaHCO3, 2.5 CaCl2, 1 MgCl2, and 5.5 glucose. Prior to use the solution was equilibrated with 5% CO2 and adjusted to pH 7.4. After five washes, the dish with Fura-2 AM loaded cells was mounted on an open perfusion mini-incubator (Harvard Apparatus, Model PDMI-2) attached to the stage of an inverted microscope (Nikon Eclipse TS100). The cells were superfused continuously at a rate of 1.5 ml/min. Baseline values for cytoplasmic calcium was obtained by superfusing the cells for 5–10 min with the control Krebs’ solution. Cells were then exposed to drugs or hyposmotic solution by switching the feeding reservoir to one with a fixed concentration of the intended drug or with the hyposmotic solution. In some cases cells were pre-incubated to antagonist drugs for 15–20 min at the end of loading period. Pre-incubation duration with GAP 27 was 1 h.
The ratio of fluorescence intensity was determined at 340 nm vs 380 nm using an established method. The experimental fluorescence ratios were converted into free calcium concentration by the system-integrated software by plotting the respective data on the calibration curve. In each experiment data from 15–30 individual cells on the dish surface were averaged and considered as n=1. The calibration curve was obtained in the absence of cells using a calibration chamber and a Fura-2 Calcium Calibration Kit both supplied by Invitrogen (cat # C-3008MP) following the manufacturer’s recommended protocol. In brief, fluorescence ratios (340nm/380nm) were derived using a set of standards containing 0, 38, 100, 225, 351, 602 and 1350 nM concentrations of free calcium. A calibration curve was constructed by plotting the Fura-2 fluorescence ratios (F340nm/F380nm) against the free calcium concentrations.
2.5. Western blot
Confluent cells in 60 mm dishes were washed with the control Krebs solution twice. Krebs’ solution was removed and replaced with 200 µl ice-cold lysis buffer that contained (in mM) 50 HEPES, 150 NaCl, 1 EDTA, 10 sodium fluoride, 10 sodium pyrophosphate, 2 sodium orthovanadate, 10% glycerol, 1% Triton X-100, 1% sodium deoxycholate, protease inhibitor cocktail at manufacturer’s recommended concentration (Roche Applied Science, Mannheim, Germany) (pH of 7.5). The culture plates were then placed on ice for 5–10 min and the cells were scrapped and collected in pre-marked 1.7 ml Eppendorf tubes. Cells were then homogenized for 1 min (4 strokes of 15 sec at 5 sec interval) using a Misonix S3000 sonicator at 6W power setting (Misonix, New York, USA). The homogenate was placed in a centrifuge at 13,000g for 25 min at 4°C to remove nuclei, larger mitochondria and unbroken debris. Protein was measured by bicinchoninic acid assay (Smith et al., 1985). The supernatant was subjected to Western blot analysis. Proteins were separated by 7.5% SDS-PAGE electrophoresis and transferred to nitrocellulose membrane. The membrane was kept overnight at 4°C in blocking buffer (LICOR, Lincoln, NE) then incubated overnight at 4°C with rabbit anti-connexin 43 polyclonal antibody (2 mg/ml). After three 5-min washes with a mixture of Tris-buffered saline and Tween 20, the nitrocellulose membrane was incubated at room temperature for 60 to 90 min with goat anti-rabbit secondary antibody conjugated with IRDye 680 or goat anti-mouse secondary antibody conjugated with IR Dye 800 (LICOR, Lincoln, NE). Then the membrane was washed three times with Tris-buffered saline + Tween 20 and three times with PBS. Immunoreactive bands were detected using an Odyssey infrared scanner (LICOR, Lincoln, NE).
2.6. Statistical analysis
Results are expressed as the mean ± SE of data from a specified number of independent experiments. Statistical comparison was made by two sample Student “t” test and by one way analysis of variance followed by the Bonferroni post hoc multiple comparison test. A probability (P) value of <0.05 was considered significant.
3. Results
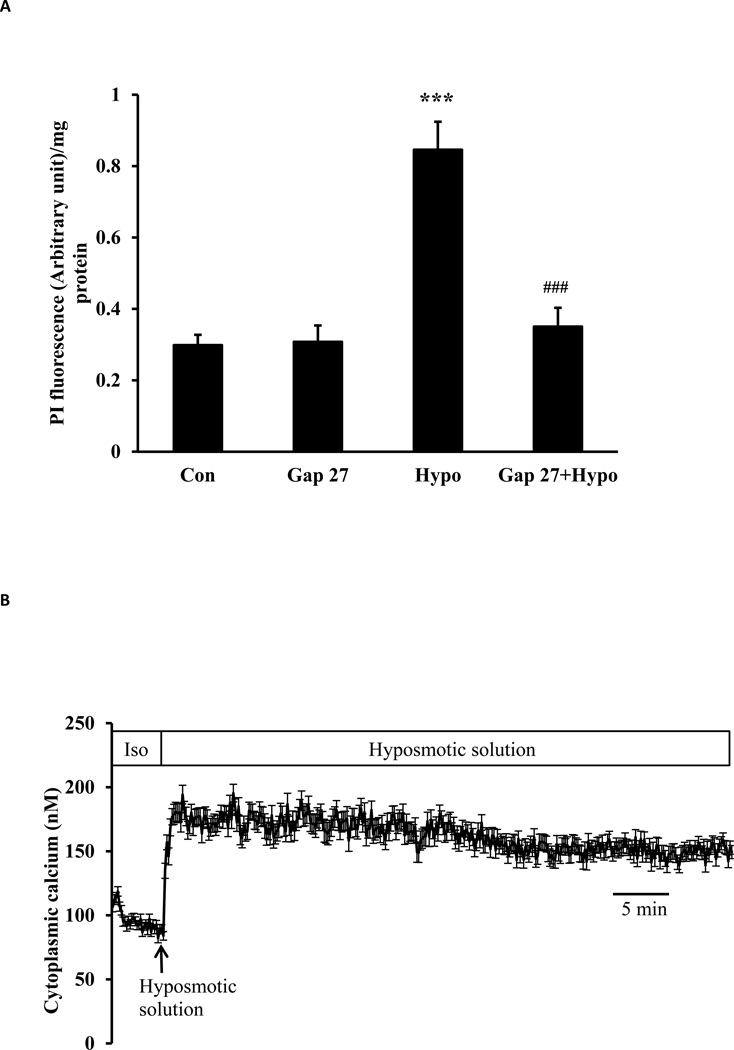
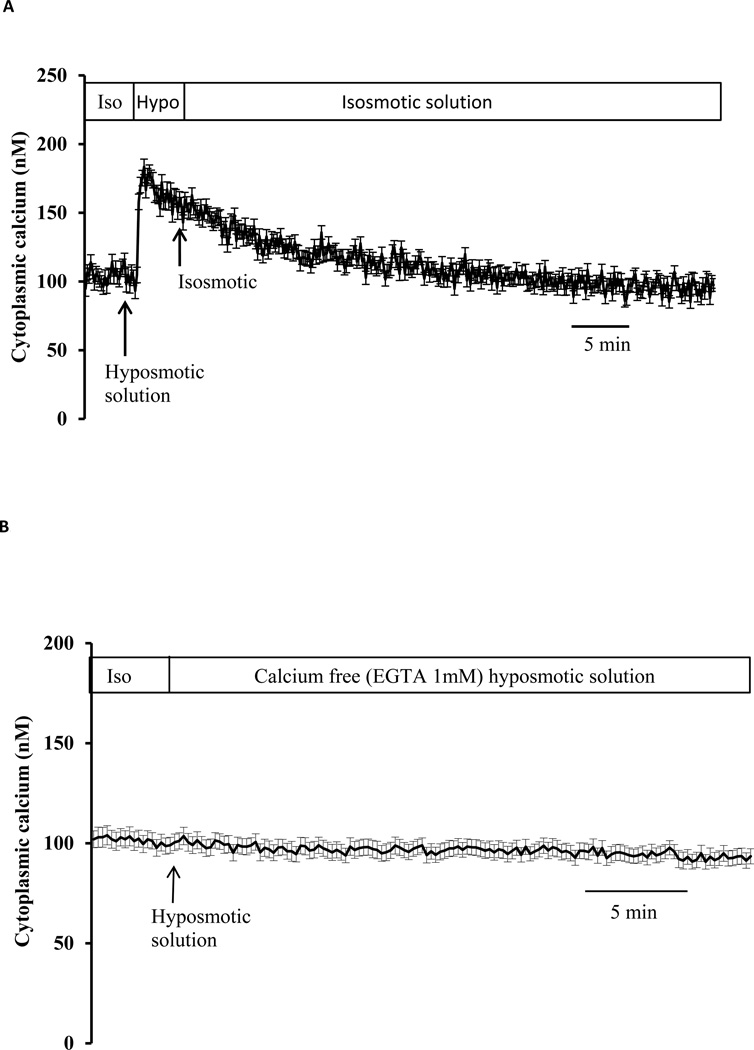
Studies were carried out using porcine lens epithelial cells grown in primary culture. Propidium iodide (PI) uptake, measured as an index of hemichannel opening, was increased in cells exposed to hyposmotic solution (200 mOsm) for 30 min (Fig. 1A). Under the same hyposmotic conditions, cytoplasmic calcium concentration rises sharply and remains elevated (Fig. 1B). The sustained plateau was slightly less than double the calcium concentration measured under control conditions, rising from a baseline value of 98.0 ± 1.4 to a peak value of 186 ± 2.3 nM (n=27). Calcium returned toward baseline in cells that were exposed to hyposmotic solution for 2 min then returned to control (isosmotic) solution (Fig. 2A). Hyposmotic solutions did not cause cytoplasmic calcium concentration to increase when calcium was eliminated from the bathing medium (Fig. 2B).
Fig. 1.
Studies on lens epithelial cells exposed to hyposmotic solution (200 mOsm). Panel A shows propidium iodide (PI) uptake. Cells were exposed to 25 µM PI for 30 min in hyposmotic (200 mOsm) solution (Hypo) or control isosmotic solution (Con). Some cells were pre-incubated for 60 min in isosmotic solution containing 200 µM GAP27, a connexin inhibitor, before being exposed to either PI/hyposmotic (GAP 27 + Hypo) or PI/isosmotic solution (GAP 27) in the continued presence of GAP 27. Following the PI uptake period, cells were washed, harvested, homogenized, and PI fluorescence intensity was measured. The results are expressed as relative fluorescence/mg protein. The values are mean ± SE of results from 6 independent experiments. *** indicates a significant difference (p< 0.001) compared to control and ### indicates a significant difference (p< 0.001) compared to hyposmotic treatment alone. Panel B shows the increase in cytoplasmic calcium concentration detected in cells exposed to hyposmotic solution. Using cells loaded with Fura-2, baseline calcium concentration was first measured for 5 min in control isosmotic solution (Iso). Hyposmotic solution (200 mOsm) was then introduced and calcium measurement continued for 1 hour. Data from 15–30 individual cells were averaged and considered as n=1. Results are means ± SE of 5 independent experiments.
Fig. 2.
To examine reversal, cytoplasmic calcium concentration was measured in cells exposed to hyposmotic solution (200 mOsm) for 2 min then returned to control (isosmotic) solution (Panel A). To examine dependence on extracellular calcium cytoplasmic calcium concentration was measured in cells exposed to calcium-free hyposmotic solution (200 mOsm) (Panel B). Baseline calcium concentration was first measured for 5 min in control isosmotic solution (Iso) then calcium-free hyposmotic solution (200 mOsm) was introduced and the measurement continued for a further 30 min. Data from 15–30 individual cells were averaged and considered as n=1. Results are means ± SE of 5 independent experiments.
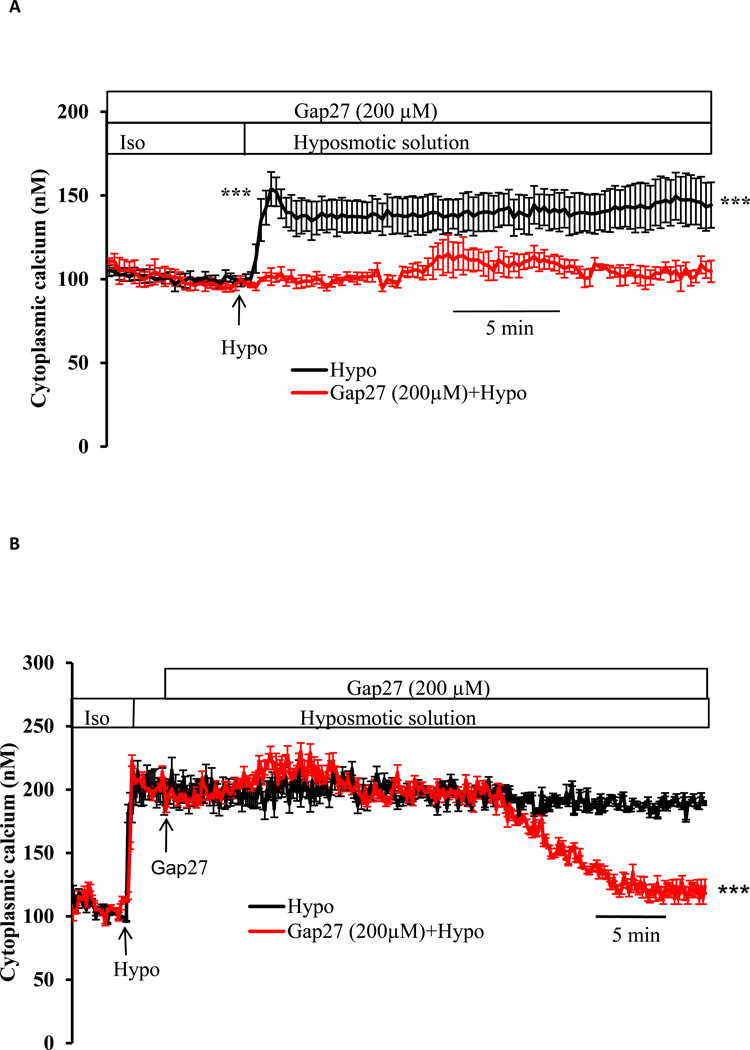
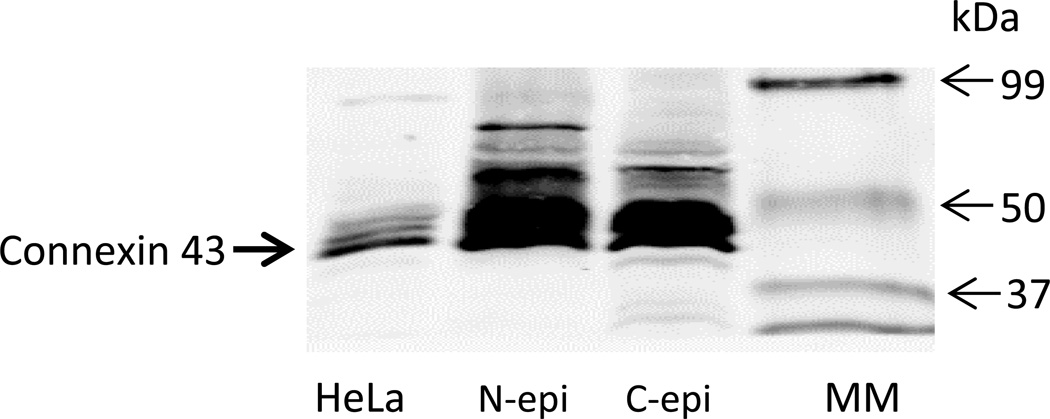
To examine the contribution of connexin hemichannels, cells were exposed to hyposmotic solution in the presence of GAP 27, a peptide mimetic connexin inhibitor, added 60 min beforehand. Under these conditions, GAP 27 suppressed the increase in PI uptake (Fig. 1A) and also prevented the increase in cytoplasmic calcium concentration caused by hyposmotic solution (Fig. 3A). In separate experiments, cytoplasmic calcium concentration was monitored in cells that were exposed to GAP 27 introduced 2 min after the onset of treatment with hyposmotic solution, when calcium was already elevated. Following a lag period of ~ 40 min, the calcium concentration in GAP 27-treated cells was observed to decrease back toward the baseline value (Fig. 3B). Western blot studies confirmed connexin 43 in both native and cultured lens epithelium (Fig 4).
Fig. 3.
The effect of GAP 27, a connexin inhibitor, on the cytoplasmic calcium response to hyposmotic solution. Panel A: cells were preincubated in isosmotic solution for 60 min with GAP 27 (200 µM) then baseline cytoplasmic calcium concentration was measured for 5 min before the cells were exposed to hyposmotic solution (200 mOsm) in the continued presence of GAP 27. Data from 15–30 individual cells were averaged and considered as n=1. The values are the mean ± SE of results from 3–5 independent experiments. *** indicates a significant difference (p< 0.001) between hyposmotic solution alone and hyposmotic solution plus GAP 27. Panel B shows reversal of the calcium response by GAP 27. Cells were exposed to hyposmotic solution (Hypo), causing an increase in cytoplasmic calcium concentration. GAP 27 (200 µM) was introduced 2 min later and calcium was monitored continuously in the hyposmotic GAP 27-containing solution. Data from 15–30 individual cells were averaged and considered as n=1. Results are means ±SE of 3–5 independent experiments. *** indicates a significant difference (p< 0.001) between hyposmotic solution alone and hyposmotic solution plus GAP 27.
Fig. 4.
Western blot detection of connexin 43 in native (N-epi) and cultured (C-epi) porcine lens epithelium. 50 µg of epithelial homogenate protein was loaded on each lane. HeLa cell homogenate (20 µg) was used as a positive control. MM=molecular size marker lane.
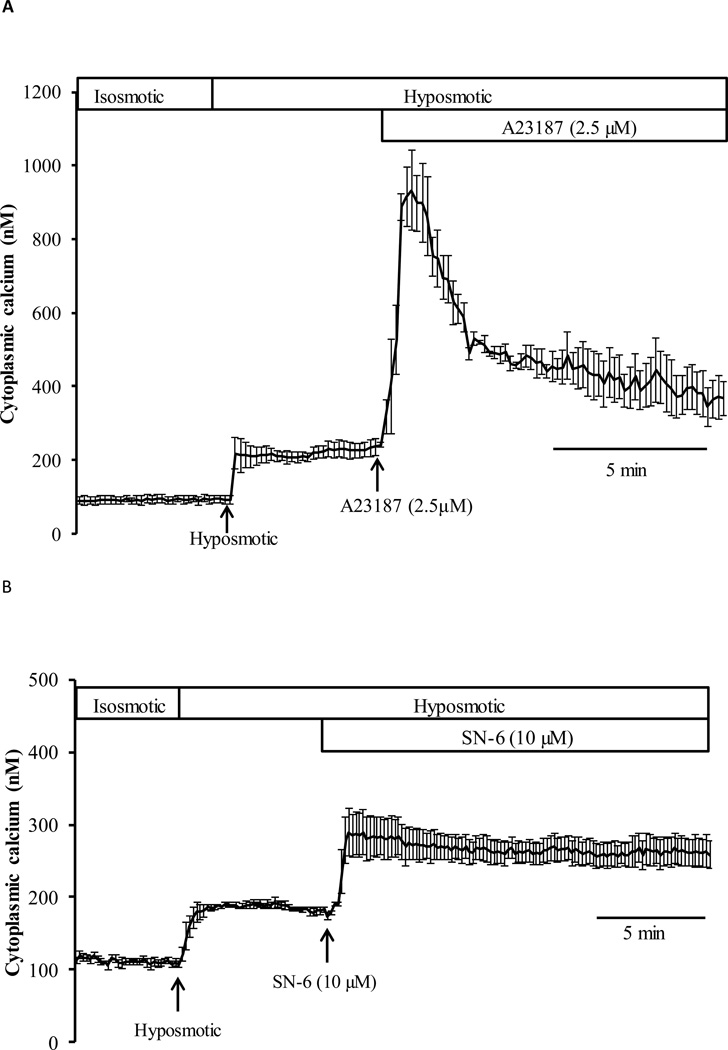
To confirm the ability of Fura-2 to detect higher calcium concentrations, cells were exposed to calcium ionophore A23187 (2.5 µM) added after the onset of the cytoplasmic calcium rise elicited by hyposmotic solution. A23187 caused a further calcium increase (Fig. 5A) to a transient peak concentration >800 nM followed by a decline to ~400 nM. These values greatly exceed the calcium concentration recorded in cells exposed to hyposmotic solution alone. The sodium-calcium exchange (NCX) inhibitor (SN-6) also elicited a further increase of calcium when added after the onset of the calcium rise in cells exposed to hyposmotic solution (Fig. 5B). In hyposmotic solution that contained SN-6 (10 µM) the sustained plateau concentration was ~265 nM compared to 186 nM in hyposmotic solution alone.
Fig. 5.
Calcium increases further in response to ionophore A23187 or NCX inhibitor SN-6. Panel A: cytoplasmic calcium response to A23187 (2.5 µM) added after the onset of the hyposmotic solution-induced increase in cytoplasmic calcium. Baseline calcium concentration was measured for 5 min in control isosmotic solution (Iso), the cells were then exposed to hyposmotic solution (200 mOsm) for another 5 min before A23187-containing hyposmotic solution was introduced for a further 10 min. Data from 25–30 individual cells were averaged and considered as n=1. Results are means ±SE of 4 independent experiments. Panel B shows cytoplasmic calcium measured in cells exposed to SN-6 (10 µM) added after the onset of the hyposmotic solution-induced increase in cytoplasmic calcium. After establishing a stable baseline, the cells were exposed to hyposmotic solution (200 mOsm) for 5 min before SN-6-containing hyposmotic solution was introduced for the remainder of the experiment. Data from 25–30 individual cells were averaged and considered as n=1. Results are means ±SE of 4 independent experiments.
To examine the contribution of pannexin channels to PI uptake, cells were exposed to hyposmotic solution in the presence of probenicid. PI fluorescence measured in the epithelium of lenses exposed to hyposmotic solution + 1mM probenecid for 30 min was not significantly different from that measured in lenses exposed to hyposmotic solution alone (0.82 ± 0.07 vs 0.85 ± 0.03; n=6) fluorescence units/mg protein respectively). Calcium studies in probenicid-treated cells were not feasible because probenicid interferes with the fluorescence signal of Fura-2.
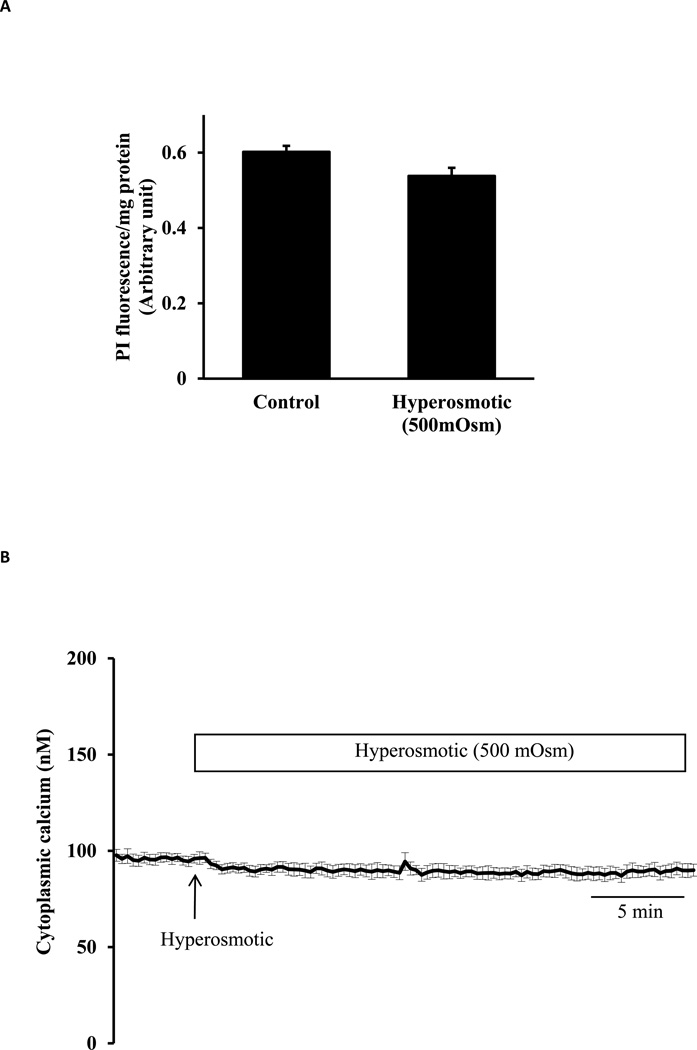
To compare responses to hyposmotic and hyperosmotic stress, cells were exposed to hyperosmotic solution with an osmolarity of 500 mOsm. Propidium iodide (PI) uptake was not increased in cells exposed to hyperosmotic solution for 30 min (Fig. 6A). When calcium was monitored continuously in cells loaded with Fura-2, the introduction of hyperosmotic solution failed to cause a detectable rise in cytoplasmic calcium concentration (Fig.6B).
Fig. 6.
Studies on lens epithelial cells exposed to hyperosmotic solution (500 mOsm). Panel A shows propidium iodide (PI) uptake measured in cells incubated for 30 min in hyperosmotic (500 mOsm) solution (Hyper) or control isosmotic solution (Control) that contained PI (25 µM). The results are expressed as relative fluorescence/mg protein. The values are mean ± SE of results from 6 independent experiments. Panel B shows cytoplasmic calcium concentration measured for 5 min in isosmotic solution (Iso) remained unchanged when hyperosmotic solution (500 mOsm) was introduced for a further 30 min. Data from 15–30 individual cells were averaged and considered as n=1. Results are means ± SE of 5 independent experiments.
4. Discussion
Cultured lens epithelial cells switched to hyposmotic solution displayed a significant increase in propidium iodide (PI) uptake. PI has an apparent molecular weight of 668 Da and its ability to enter cells subjected to hyposmotic shock, coupled with the ability of GAP 27 to prevent the response, indicates the opening of connexin hemichannels. GAP 27 is well known to as a functional inhibitor of connexin hemichannels. It is a connexin mimetic peptide derived from the extracellular loop of connexin 43. Cells swell under hyposmotic conditions and although hemichannel opening in hyposmotic solution is most likely the response to an abrupt change in cell size, there was no evidence of hemichannel opening in cells caused to shrink by exposure to hyperosmotic solution. PI uptake and cytoplasmic calcium concentration both were unchanged by 500 mOsm solution. The lens epithelium responses to osmotic swelling and shrinkage are quite different.
In addition to increased PI entry, cells exposed to hyposmotic solution displayed an increase in cytoplasmic calcium concentration that peaked within ~30 sec then remained elevated for 60 min or more. Baseline cytoplasmic calcium concentration was 98.0±1.4nM and the peak concentration was 186.0 ±2.3 nM. The findings suggest the sustained calcium elevation is due to calcium entry via connexin hemichannels. First, the immediate calcium rise and sustained plateau both are caused by entry of extracellular calcium as indicated by absence of the entire calcium response in cells that were exposed to calcium-free hyposmotic solution. Second, the cells express connexin 43 protein and the calcium response to hyposmotic solution was absent in cells pretreated with the connexin inhibitor GAP 27. Third, the plateau phase of the calcium response returned to baseline when GAP 27 was added after the onset of hyposmotic challenge. The length of time require for GAP 27 to reduce plateau calcium concentration is consistent with earlier reports of reduced gap junction-mediated dye transfer between cultured vascular cells exposed to GAP 27 for 30 min (Gao et al., 2004). In tracheal epithelial cells, reduction of gap junction calcium permeability by Gap 27 required 60 min (Dahm and Prescott, 2003).
Cell to cell flow of calcium through connexin gap junctions has been studied far more extensively than the inward movement of extracellular calcium via unpaired connexin hemichannels. Under most conditions the physiological concentration of extracellular calcium causes hemichannels to remain closed (Gomez-Hernandez et al., 2003). However, alkalinization, metabolic inhibition, certain inflammatory mediators and osmotic swelling are known to cause hemichannel opening (Schalper et al., 2010) (Saez et al., 2010; Sanchez et al., 2009). In the intact lens, hemichannel opening causes the release of ATP when lenses are exposed to hyposmotic solution (Shahidullah et al., 2012a). When open, hemichannels permit calcium flow (Fiori et al., 2012; Schalper et al., 2010) and it is interesting that HeLa cells transfected with connexin 43 display an approximate doubling of cytoplasmic calcium concentration in response to hemichannel opening maneuvers (Schalper et al., 2010). A similar doubling of baseline calcium was observed here in lens epithelium subjected to hyposmotic stress.
Although the evidence points to a sustained cytoplasmic calcium elevation due to opening of connexin hemichannels in cells subjected to hyposmotic stress, the calcium rise is not overwhelming. Hemichannel opening does not cause the cells to be swamped with calcium. The plateau calcium concentration in the presence of hyposmotic solution was <200 nM. This is far less than the 2 mM extracellular concentration, which explains why the ionophore A23187 was able to elicit a large additional increase in calcium concentration. The A23187 response also confirms Fura2 was not saturated under the experimental conditions. The sustained plateau concentration of less than 200 nM remains in the working range of the various transport mechanisms, SERCA, PMCA and NCX (Brini and Carafoli, 2011). The steady-state cytoplasmic calcium concentration under any given condition is determined by the balance between calcium entry and the combined operation of several export mechanisms on the plasma membrane. Sequestration in the ER and mitochondria also may play a role but in order to prevent a long-term gain, calcium entry and export must balance. The NCX inhibitor SN-6 increased the plateau calcium concentration from 186 to 265 nM suggesting that steady state cytoplasmic calcium concentration following exposure to hyposmotic solution in part reflects a balance between hemichannel-mediated entry and NCX-mediated export. NCX is a high capacity, low affinity calcium transporter (Blaustein and Lederer, 1999) and indeed NCX inhibitors have little detectable influence on baseline calcium concentration but impair calcium export at higher cytoplasmic calcium concentrations (Okafor et al., 2003). The contribution of PMCA is impossible to quantify because selective inhibitors are unavailable. The sustained calcium rise in hyposmotic solution was probably below the threshold for damage due to calcium overload since calcium was observed to return to baseline when the hyposmotic challenge was withdrawn after 2 min and also when GAP 27 was added during a 60 min period of exposure to hyposmotic solution. In various cell types, cytoplasmic calcium concentration rises to higher values during transient calcium spikes that occur due to agonist induced release of calcium stores (Verkhratsky, 2005) or store operated calcium entry (Yamamura et al., 2014).
Ion concentrations in the lens reflect a steady state balance between entry and export. While calcium that enters the lens via hemichannels should be taken into consideration, the relative significance of this route is unknown. We acknowledge the complexity of calcium homeostasis in the intact lens where calcium and other solutes circulate through the fiber mass (Gao et al., 2004). Calcium handling may be different in cultured lens epithelial cells and the epithelium in the intact lens. Differences between calcium regulation in lens epithelium and fiber cells are likely because the baseline cytoplasmic calcium in the epithelium (~100nM) is lower than that in the fibers where the concentration is ~300 nM in the outer fibers and ~700 nM in the inner fibers (Gao et al., 2004). NCX is thought to play a significant role in lens calcium regulation (Tomlinson et al., 1991) and binding also may be important (Dahm and Prescott, 2003). Failure of lens calcium homeostasis is associated with loss of transparency (Lee et al., 2008).
The GAP 27 studies point to PI uptake and calcium entry via functional connexin hemichannels. In contrast, the uptake of PI by cells subjected to hyposmotic stress was not significantly inhibited by probenicid, which suggests that pannexin channels do not contribute to PI entry under the conditions of these particular studies. Cytoplasmic calcium studies on probenicid-treated cells could not be carried out due to fluorescence interference with Fura-2. However, the ability of GAP 27 pretreatment to fully eliminate calcium rise in hyposmotic solution suggests pannexin channels contribute little to calcium entry. We cannot explain why pannexin channels contribute to the hyposmotic stress response in intact lens (Shahidullah et al., 2012a) but not cultured lens epithelium studied here. The nature of the hyposmotic challenge to cells in the intact lens is different because when the intact lens is placed in hyposmotic solution, epithelial cells are subjected not only to hyposmotic stress but also to stretching forces caused by swelling of the entire lens structure.
In summary, hyposmotic solution causes GAP 27-sensitive entry of propidium iodide in cultured lens epithelium, pointing to connexin hemichannel opening. Even though connexins form channels with a large carrying capacity, calcium entry does not increase the cytoplasmic calcium concentration beyond a tolerable physiological range. Physiological increases of cytoplasmic calcium typically activate various signaling pathways. It is possible that hemichannel-mediated calcium influx plays a signaling role associated with changes of epithelium function when the lens is subjected to hyposmotic stress.
Highlights.
Sustained Ca2+ rise in hyposmotic solution occurs via connexin hemichannel opening.
Hemichannel opening increases [Ca2+]i only to 186 nM from a baseline value of 97nM.
Although hemichannels open [Ca2+]i keeps within a tolerable physiological range.
Acknowledgement
The authors wish to thank the University of Arizona Meat Science Laboratory, West Valley Meat Processing, Buckeye, Phoenix and Hatfield Quality Meat, Pennsylvania for their continued willingness to supply porcine eyes. This research was supported by a grant from the National Institute of Health (Grant: NIH EY009532).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors do not have any conflict of interest to declare.
Authorship Contributions
Research concept and design: Mohammad Shahidullah and Nicholas A Delamere, Amritlal Mandal.
Conducted experiments: Amritlal Mandal and Mohammad Shahidullah.
Data analysis and interpretation: Mohammad Shahidullah and Amritlal Mandal.
Wrote manuscript: Mohammad Shahidullah and Nicholas A Delamere.
References
- Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiological Reviews. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- Brini M, Carafoli E. The plasma membrane Ca(2)+ ATPase and the plasma membrane sodium calcium exchanger cooperate in the regulation of cell calcium. Cold Spring Harbor perspectives in biology. 2011;3 doi: 10.1101/cshperspect.a004168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahm R, Prescott AR. Identification of a novel intercellular structure in late-stage differentiating lens cells. Ophthalmic Research. 2003;35:2–7. doi: 10.1159/000068196. [DOI] [PubMed] [Google Scholar]
- Fiori MC, Figueroa V, Zoghbi ME, Saez JC, Reuss L, Altenberg GA. Permeation of calcium through purified connexin 26 hemichannels. Journal of Biological Chemistry. 2012;287:40826–40834. doi: 10.1074/jbc.M112.383281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Sun X, Martinez-Wittinghan FJ, Gong X, White TW, Mathias RT. Connections between connexins, calcium, and cataracts in the lens. Journal of General Physiology. 2004;124:289–300. doi: 10.1085/jgp.200409121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Sun X, Yatsula V, Wymore RS, Mathias RT. Isoform-specific function and distribution of Na/K pumps in the frog lens epithelium. Journal of Membrane Biology. 2000;178:89–101. doi: 10.1007/s002320010017. [DOI] [PubMed] [Google Scholar]
- Gomez-Hernandez JM, de Miguel M, Larrosa B, Gonzalez D, Barrio LC. Molecular basis of calcium regulation in connexin-32 hemichannels. Proc Natl Acad Sci U S A. 2003;100:16030–16035. doi: 10.1073/pnas.2530348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HYY, Morton JD, Sanderson J, Bickerstaffe R, Robertson LJG. The involvement of calpains in opacification induced by Ca2+-overload in ovine lens culture. Veterinary Ophthalmology. 2008;11:347–355. doi: 10.1111/j.1463-5224.2008.00655.x. [DOI] [PubMed] [Google Scholar]
- Li G-Y, Fan B, Zheng Y-C. Calcium overload is a critical step in programmed necrosis of ARPE- 19 cells induced by high-concentration H2O2. Biomedical & Environmental Sciences. 2010;23:371–377. doi: 10.1016/S0895-3988(10)60078-5. [DOI] [PubMed] [Google Scholar]
- Mandal A, Shahidullah M, Delamere NA. Hydrostatic Pressure-Induced Release of Stored Calcium in Cultured Rat Optic Nerve Head Astrocytes. Invest. Ophthalmol. Vis. Sci. 2010;51:3129–3138. doi: 10.1167/iovs.09-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Chan SL. Calcium orchestrates apoptosis. Nat Cell Biol. 2003;5:1041–1043. doi: 10.1038/ncb1203-1041. [DOI] [PubMed] [Google Scholar]
- Okafor M, Tamiya S, Delamere NA. Sodium-calcium exchange influences the response to endothelin-1 in lens epithelium. Cell Calcium. 2003;34:231–240. doi: 10.1016/s0143-4160(03)00085-x. [DOI] [PubMed] [Google Scholar]
- Rhodes JD, Sanderson J. The mechanisms of calcium homeostasis and signalling in the lens. Experimental Eye Research. 2009;88:226–234. doi: 10.1016/j.exer.2008.10.025. [DOI] [PubMed] [Google Scholar]
- Saez JC, Schalper KA, Retamal MA, Orellana JA, Shoji KF, Bennett MVL. Cell membrane permeabilization via connexin hemichannels in living and dying cells. Experimental Cell Research. 2010;316:2377–2389. doi: 10.1016/j.yexcr.2010.05.026. [DOI] [PubMed] [Google Scholar]
- Sanchez HA, Orellana JA, Verselis VK, Saez JC. Metabolic inhibition increases activity of connexin-32 hemichannels permeable to Ca2+ in transfected HeLa cells. American Journal of Physiology - Cell Physiology. 2009;297:C665–C678. doi: 10.1152/ajpcell.00200.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalper KA, Sanchez HA, Lee SC, Altenberg GA, Nathanson MH, Saez JC. Connexin 43 hemichannels mediate the Ca2+ influx induced by extracellular alkalinization. American Journal of Physiology - Cell Physiology. 2010;299:C1504–C1515. doi: 10.1152/ajpcell.00015.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidullah M, Mandal A, Beimgraben C, Delamere NA. Hyposmotic stress causes ATP release and stimulates Na, K-ATPase activity in porcine lens. Journal of Cellular Physiology. 2012a;227:1428–1437. doi: 10.1002/jcp.22858. [DOI] [PubMed] [Google Scholar]
- Shahidullah M, Mandal A, Delamere NA. TRPV4 in porcine lens epithelium regulates hemichannel-mediated ATP release and Na-K-ATPase activity. American Journal of Physiology - Cell Physiology. 2012b;302:C1751–C1761. doi: 10.1152/ajpcell.00010.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Analytical Biochemistry. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Srivastava SK, Wang LF, Ansari NH, Bhatnagar A. Calcium homeostasis of isolated single cortical fibers of rat lens. Investigative Ophthalmology & Visual Science. 1997;38:2300–2312. [PubMed] [Google Scholar]
- Tomlinson J, Bannister SC, Croghan PC, Duncan G. Analysis of rat lens 45Ca2+ fluxes: evidence for Na(+)-Ca2+ exchange. Experimental Eye Research. 1991;52:619–627. doi: 10.1016/0014-4835(91)90065-m. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiological reviews. 2005;85:201–279. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- Yamamura H, Yamamura A, Ko EA, Pohl NM, Smith KA, Zeifman A, Powell FL, Thistlethwaite PA, Yuan JX. Activation of Notch signaling by short-term treatment with Jagged-1 enhances store-operated Ca2+ entry in human pulmonary arterial smooth muscle cells. American journal of physiology. Cell physiology. 2014;306:C871–C878. doi: 10.1152/ajpcell.00221.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]