Abstract
This investigation provides the first systematic determination of the cellular and molecular progression of vocal fold (VF) epithelium development in a murine model. We define five principal developmental events that constitute the progression from VF initiation in the embryonic anterior foregut tube to fully differentiated and functional adult tissue. These developmental events include (1) the initiation of the larynx and vocal folds with apposition of the lateral walls of the primitive laryngopharynx (embryonic (E) day 10.5); (2) the establishment of the epithelial lamina with fusion of the lateral walls of the primitive laryngopharynx (E11.5); (3) the epithelial lamina recanalization and separation of VFs (E13.5–18.5); (4) the stratification of the vocal folds (E13.5–18.5); and (5) the maturation of vocal fold epithelium (postnatal stages). The illustration of these morphogenetic events is substantiated by dynamic changes in cell proliferation and apoptosis, as well as the expression pattern of key transcription factors, FOXA2, SOX2 and NKX2-1 that specify and pattern the foregut endoderm. Furthermore, we documented the gradual conversion of VF epithelial cells from simple precursors expressing cytokeratins 8 and 18 in the embryo into mature stratified epithelial cells also expressing cytokeratins 5 and 14 in the adult. Interestingly, in the adult, cytokeratins 5 and 14 appear to be expressed in all cell layers in the VF, in contrast to their preferential localization to the basal cell layer in surrounding epithelium. To begin investigating the role of signaling molecules in vocal fold development, we characterized the expression pattern of SHH pathway genes, and how loss of Shh affects vocal fold development in the mutant. This study defines the cellular and molecular context and serves as the necessary foundation for future functional investigations of VF formation.
Keywords: Vocal fold, Epithelium, Differentiation, Stratification, Foregut
Introduction
Guided derivation of stem cells into various cell lineages represents a promising and valuable approach to generate functional units for tissue repair (Jiang et al., 2007; Wong et al., 2012; D'Amour et al., 2005). However, to successfully replicate the process of cell derivation in vitro, it is necessary to precisely define the dynamic cellular and molecular mechanisms underlying normal development in vivo in the mammalian embryo. Recently published derivation of vocal fold (VF) epithelial cells from induced pluripotent stem cells (Leydon et al., 2013) has reminded us that little is known about the development and differentiation of the VF epithelial cells as compared to those of other foregut epithelial surfaces including the esophagus (Yu et al., 2005; Jacobs et al., 2012), stomach (Kim et al., 2005, 2011), trachea and lung (Domyan and Sun, 2011; Morrisey and Hogan, 2010). Although we have shown that induced pluripotent stem cell-derived epithelial cells do stratify, they produce markers that are universal and are not tissue specific, thereby limiting their therapeutic applications for VF wound healing and regeneration (Leydon et al., 2013).
Existing studies describing the development of the larynx and VF in humans or other mammals such as mice consist of either morphological investigations based on conventional H&E staining (Hopp, 1955; Walander, 1955; Zaw-Tun and Burdi, 1985; Sanudo and Domenech-Mateu, 1990), or clinical investigations comparing normal versus pathological morphologies (Benjamin and Inglis, 1989; Kakodkar et al., 2012). These studies documented the basic steps of laryngeal cavity and VF development (Henick, 1993). The laryngeal cavity is tubular in shape and lined with mucosa. The superior aspect of the cavity (laryngeal vestibule) opens into the pharynx. The inferior aspect of the cavity is continuous with the lumen of the trachea. VFs are located in the inferior larynx at the top of the trachea. They open during inhalation and come together to close the glottis during swallowing and phonation. The luminal surface of the VF is covered by a protective layer of stratified squamous epithelium. On the surfaces of the epithelial cells are microridges and microvilli that help to spread and maintain a mucous coat (Hirano et al., 1983). Beneath the VF epithelium are lamina propria and vocalis muscle (thyroarytenoid muscle) that forms the body of VFs (Hirano and Bless, 1993). Stratified squamous epithelium of the VF transitions to a ciliated pseudostratified columnar epithelium at the supraglottis (rostral to VF), and infraglottis (caudal to VF). Recent studies comparing mature mouse and human VFs have documented similar laryngeal framework, epithelial and muscular organization, VF aging characteristics, as well as similar distribution of elastic and collagen fibers in the lamina propria (Watts et al., 2007, 2011). Proper patterning of these fibers is essential for effective vibration of VF during voice production in humans or during ultrasonic acoustic signal production in rodents (Johnson et al., 2010).
The laryngeal cavity emerges as primitive laryngopharynx and was a part of the foregut endoderm, which expresses Foxa2. This transcription factor plays an important role in initiating liver specification (Li et al., 2009) and in the differentiation of pancreatic α-cells (Lee et al., 2005). It also participates in the development of the normal bronchus and lung epithelium (Basseres et al., 2012). It is known that the epithelial lining of the trachea and lung arises from the ventral portion of the foregut endoderm that expresses Nkx2-1 (Que et al., 2007, 2009; Harris-Johnson et al., 2009; Domyan and Sun, 2011; Ferri et al., 2013). In contrast, the epithelial lining of the esophagus arises from the dorsal portion of the foregut endoderm that expresses Sox2 (Miller et al., 2012; Woo et al., 2011; Jacobs et al., 2012). In addition to being markers, Nkx2-1 and Sox2 are each essential for establishing the initial dorsal– ventral patterning of the foregut tube and proper development of the trachea versus esophagus (Que et al., 2007). At later stages in more distal tissue Nkx2-1 is required for early lung branching morphogenesis, while Sox2 is required for differentiation of airway and esophagus cell types (Kelly et al., 1996; Que et al., 2007, 2009). SOX2 interacts with another master transcription factor p63, which is best known for its requirement in the maintenance of the basal cell progenitor state (Daniely et al., 2004). Additional genes such as Wnt2, Wnt2b and Barx1 are expressed in the mesenchyme underlying the foregut epithelium and also play a role in trachea/esophagus development. While little is known in terms of nascent VF gene expression, based on its proximity to the trachea and esophagus, we hypothesize that some of the previously mentioned transcription factor genes may also be expressed in the VFs and may impact VF formation and VF epithelium differentiation.
In this study, we built upon existing knowledge and carried out a systematic determination of the cellular and molecular progression of VF epithelium development in mice. We examined the patterns of transcription factors, SOX2, NKX2-1 and FOXA2, and the patterns of cell proliferation and apoptosis during key morphogenetic events including apposition of the lateral walls of the primitive laryngopharynx, the formation of the epithelial lamina, and its recanalization. To trace the differentiation of VF epithelial cells, we investigated the temporo-spatial localization of simple epithelial markers, keratins (K) 8 and K18, versus stratified epithelial markers, K5 and K14, during the differentiation of VF epithelial cells. Based on our findings, we define, for the first time, five landmark events of VF development. We hope that this work provides the foundation for future elucidation of the mechanisms that drive the formation of a functional VF.
Materials and methods
Mouse matings and tissue collection
Wild-type Swiss Webster males and females were mated, and noon of the day when vaginal plugs were found was designated as Embryonic day (E) 0.5. Pregnant females were sacrificed at E10.5, E11.5, E13.5, E15.5, E16.5 and postnatal (P) stages, P0 and adult (6–8 weeks), following regulations of protocols approved by the University of Wisconsin Animal Care and Use Committee. Mouse larynges were dissected and immediately fixed in 4% paraformaldehyde in phospho-buffered saline at 4 °C/overnight, dehydrated in a gradient series of ethanol, treated with xylene and embedded in paraffin. Paraffin blocks were cut into serial sections (5 μm), dewaxed and rehydrated, heated to boiling in 10 mM citrate buffer pH=6 for antigen retrieval and then stained using standard IHC or IF protocols. Shh homozygous null mutants were generated by mating Shhcre/+ heterozygotes (Harfe et al., 2004).
Immunohistochemistry staining
Primary antibodies used were mouse anti-NKX2-1 antibody diluted at 1:100 (LS Bio; Seattle, WA, USA); rabbit anti-SOX2 antibody diluted at 1:500 (Novus Biologicals; Littleton, CO, USA); rabbit anti-K5 antibody diluted at 1:250 (Abcam; Cambridge, UK); rabbit anti-K8 antibody diluted at 1:250 (LS Bio; Seattle, WA, USA); rabbit anti-K18 antibody diluted at 1:250 (LS Bio; Seattle, WA, USA); rabbit anti-K14 antibody diluted at 1:250 (ProteinTech; Chicago, IL, USA); rabbit anti-FOXA2 antibody diluted at 1:2000 (Abcam; Cambridge, UK). Sections were incubated with the primary antibodies at 4 °C overnight. Secondary antibodies used were anti-mouse and anti-rabbit Ig ImmPress reagent kit peroxidase (Vector Laboratories; Peterborough, UK). Antigen detection was performed using a DAB kit (Vector Laboratories; Peterborough, UK). Slides were counterstained using hematoxylin.
Immunofluorescent staining
Primary antibodies used were mouse anti-NKX2-1 antibody diluted at 1:100 (LS Bio; Seattle, WA, USA), rabbit anti-SOX2 antibody diluted at 1:500 (Novus Biologicals; Littleton, CO, USA), rabbit anti-K8 antibody (LS Bio; Seattle, WA, USA) diluted at 1:250; mouse anti-p63 antibody (Santa Cruz Biotechnology; Dallas, Texas, USA) diluted at 1:200. Sections were incubated at 4 °C overnight. Secondary antibodies used were Cy3-conjugated anti-mouse antibodies (Jackson ImmunoResearch; West Grove, PA, USA) diluted at 1:200; FITC-conjugated anti-rabbit antibodies (Jackson ImmunoR-esearch; West Grove, PA, USA), diluted at 1:100 and applied 1 h at room temperature (RT). Slides were mounted using Vectashield (Vector Laboratories; Peterborough, UK). In case of whole mount IF staining, dissected respiratory structures were treated with rabbit anti-K8 antibody (LS Bio; Seattle, WA, USA) diluted at 1:250 overnight at RT and by secondary antibody Cy3-conjugated anti-rabbit antibody (Jackson ImmunoResearch; West Grove, PA, USA), diluted at 1:200, applied 4 °C/overnight. Slides were mounted using Vectashield (Vector Laboratories; Peterborough, UK).
TUNEL assay
TdT-mediated dUTP-biotin nick end labeling (TUNEL, Chemicon-Millipore; Billerica, USA) was employed to detect apoptosis. After rehydration, sectioned samples were treated with proteinase K at 20 μg/ml at room temperature for 15 min (Chemicon-Milli-pore; Billerica, MA, USA). After equilibration buffer, the reaction mixture was prepared as per manufacturer instructions and applied at 37 °C for 50 min. After anti-digoxigenin-peroxidase reaction 30 min/RT, positive cells were visualized by chromogen substrate diaminobenzidine (DAB kit, Vector Laboratories, Peter-borough, UK) and slides were counterstained with hematoxylin.
Cell proliferation assay
Pregnant females received an intraperitoneal injection of 100 μg EdU (Sigma Aldrich; St. Louis, MO, USA) per gram bodyweight 1 h before sacrifice. Embryos were fixed, processed to form paraffin-embedded sections. EdU detection was performed according to the manufacturer's protocol (Invitrogen; Carlsbad, CA, USA).
In situ hybridization
Whole mount in situ hybridization with digoxigenin-labeled probes was performed on transversal 200 μm thick vibratome sections at the level of developing larynx and vocal folds at embryonic stage E11.5 as described by Neubuser et al. (1997).
Results
To systematically characterize VF epithelium development, we selected stages based on known morphogenetic events, and isolated tissues at embryonic (E) 10.5, 11.5, 13.5, 15.5, and 16.5, postnatal (P) stages P0 and adult. H&E stained histological sections were shown together with schematics that illustrate sample orientation and morphology. Both supraglottic (rostral to VF) and infraglottic (caudal to VF) regions were included to provide tissue context. Our findings led us to define five landmark events of VF development: (1) apposition of the lateral walls of the primitive laryngopharynx, (2) fusion of the lateral walls and formation of the epithelial lamina, (3) epithelial lamina recanalization, (4) stratification of VFs, and (5) maturation of VF epithelium. As two of these events, recanalization and stratification occur concurrently, we organized the presentation of wild-type data into four sections below. We end by an investigation of the role of the signaling gene Shh in VF development, using knowledge gained from our study of the normal process.
Apposition of the laryngopharynx lateral walls (~E10.5)
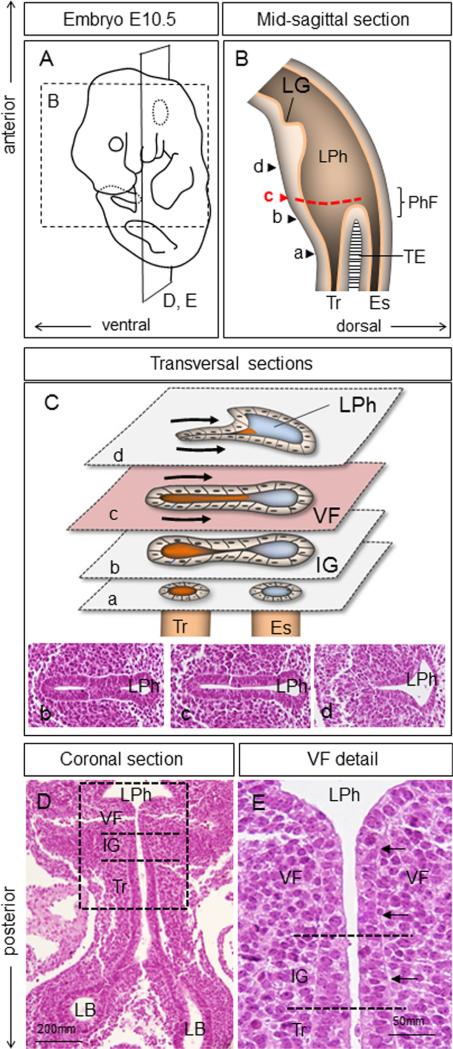
In the mouse at E10.5, prospective VF cells reside at the ventral side of the very anterior region of the foregut tube, known as the primitive laryngopharynx (LPh) (Fig. 1A and B). The primitive laryngopharynx is the segment of the foregut with the laryngeal groove (LG) at the level of the fourth pharyngeal pouch as its cranial border, and the primitive pharyngeal floor (PhF) as its caudal border (Fig. 1B). On the ventral side, the primitive laryngopharynx is continuous with the respiratory diverticulum, which grows caudally into the nascent trachea that splits into two lung buds. These buds give rise to the left and right primary bronchi that lead into lung lobes (Que et al., 2006; Morrisey and Hogan, 2010). On the dorsal side, the primitive laryngopharynx is continuous with the esophagus, which grows caudally and links onto the stomach and the remainder of the digestive system. The first indication of laryngeal formation occurs at E9.5, when the lateral walls of the primitive laryngopharynx start to move into the center of the lumen (Henick, 1993). By E10.5, in transverse sections at the level of the prospective VFs, ventral laryngopharynx epithelial cells are closely juxtaposed (Fig. 1C and Supplemental Fig. 1). By longitudinal section, VFs connect onto the trachea via the infraglottic region (Fig. 1D and E). Consistent with previous observations (Loebel et al., 2011), epithelial cells that cover the opposing walls of the prospective VF are pseudostratified and columnar, unlike at later stages (Fig. 1E). Similar morphology is observed in the epithelium rostral to VF. However, cells in the more caudal infraglottic and tracheal epithelium appear taller and their nuclei more closely packed (Fig. 1E).
Fig. 1.
Morphology of the primitive laryngopharynx at E10.5. (A) Schematic illustration of an embryo (E10.5) indicating the orientation of mid-sagittal (B) and coronal (D, E) sections. (B) Schematic illustration of the mid-sagittal section through the primitive laryngopharynx. Arrow heads (a–d) represent transversal sections illustrated in diagram (C). A red dashed line demonstrates the region where VFs develop. (C) Transversal sections through the primitive laryngopharynx at the levels indicated in diagram (B) including hematoxylin–eosin sections (b–d). Black arrows indicate bilateral compression leading to apposing of the lateral walls. (D) Hematoxylin & eosin coronal section demonstrating morphology of the airway structures at E10.5 including position of primitive laryngopharynx, vocal folds, trachea and lung lobes. (E) Magnified view of the boxed region in (D) to illustrate morphology of epithelial cells in the VF, infraglottic and trachea regions. Black arrows denote pseudostratified epithelium lining the prospective vocal folds. Abbreviations: LG—laryngeal groove; LPh—laryngopharynx; PhF—pharyngeal floor; IG—infraglottic region; TE—tracheoesophagal septum; Tr—trachea; Es—esophagus; VF—vocal folds; LB—lung buds.
At E10.5, epithelial cells of the VF uniformly express endoderm marker FOXA2 (Fig. 2A). Epithelial cells of future VF also express SOX2 (Fig. 2B). In contrast, the trachea and airway cells express NKX2-1 (Fig. 2C). Only a few epithelial and mesenchymal cells undergo programmed cell death (Fig. 2D). Less frequent proliferation was observed in the epithelial cells compared to the underlying mesenchyme (Fig. 2E and F). Among the keratins, K8 and K18 were expressed in the prospective VF epithelium, primitive laryngopharynx and trachea, consistent with the pseudostratified simple epithelium morphology (Fig. 2G and H). p63 is not expressed in the prospective VF epithelium at this stage (data not shown).
Fig. 2.
VF epithelial cell marker expression at E10.5. (A) Pattern of FOXA2 positive cells in brown (black arrows) in the VF and infraglottic regions. (B) Pattern of SOX2 positive cells in brown, black arrows denote positive cells in the vocal fold and infraglottic regions, red arrow denotes negative cells in the trachea. (C) Pattern of NKX2-1 positive cells in lung buds and the infraglottic region (black arrows) versus low expression of NKX2-1 in the region of prospective VF (red arrow). (D) TUNEL assay, black arrows demonstrate positive apoptotic cells in brown. (E, F) EdU proliferation assay (green) and K8 staining of VF epithelial cells in red, white arrows indicate examples of positively stained proliferating cells in the epithelium and mesenchyme. White dashed lines indicate the position of the EL. (G, H) Pattern of cytokeratins K8 and K18, black arrows denote positive cells in brown in the VF and IG regions. Abbreviations: LPh—primitive laryngopharynx; VF—vocal folds; IG—infraglottic region; LB—lung lobes.
Fusion of the lateral epithelium to form the epithelial lamina (~E11.5)
At E11.5 the lateral walls of the primitive laryngopharynx are squeezed closer at midline (Figs. 3 and 4). The two layers of epithelial cells fuse together, obliterate the ventral lumen and form the structure called the epithelial lamina (EL) (Figs. 3B and C and 4). In transversal sections, ventral to the EL is the proximal part of the trachea, dorsal is the laryngopharynx that is continuous with the esophagus (Fig. 3B and C). In coronal sections, EL is continuous with the infraglottic region and trachea below (Fig. 3D). During obliteration epithelial cells of the EL change their morphology from columnar to cuboidal and formally opposing cell layers become organized into two rows of closely packed cells (Fig. 3E). In contrast, epithelial cells located at the infraglottic region and trachea maintain their columnar phenotype (Fig. 3E).
Fig. 3.
Morphology of the laryngopharynx at E11.5. (A) Schematic illustration of an embryo (E11.5), including orientation of the mid-sagittal (B) and coronal (D, E) sections. (B) Schematic illustration of the mid-sagittal section through the laryngopharynx. A red dashed line denotes the position of the VF, arrow heads (a–c) demonstrate transversal sections illustrated in the diagram (C). (C) Transversal sections through the laryngopharynx as suggested at diagram (B); (b–d) transversal hematoxylin–eosin sections through the primitive laryngopharynx at the level of prospective VFs and infraglottis. Black arrows show bilateral compression, red lines demonstrate position of epithelial lamina. (D) Hematoxylin & eosin coronal section demonstrating morphology of the airway structures at E11.5. (E) Magnified view of the boxed region in (D) to illustrate morphology of vocal fold (VF) epithelial cells at the site of epithelial lamina (EL) and in the infraglottic region (black arrows). Abbreviations: EL—epithelial lamina; LPh—laryngopharynx; Tr—trachea; TE—tracheoesophagal septum; Es—esophagus; VF—vocal folds; IG—infraglottic region; RB—right bronchus and LB—left bronchus.
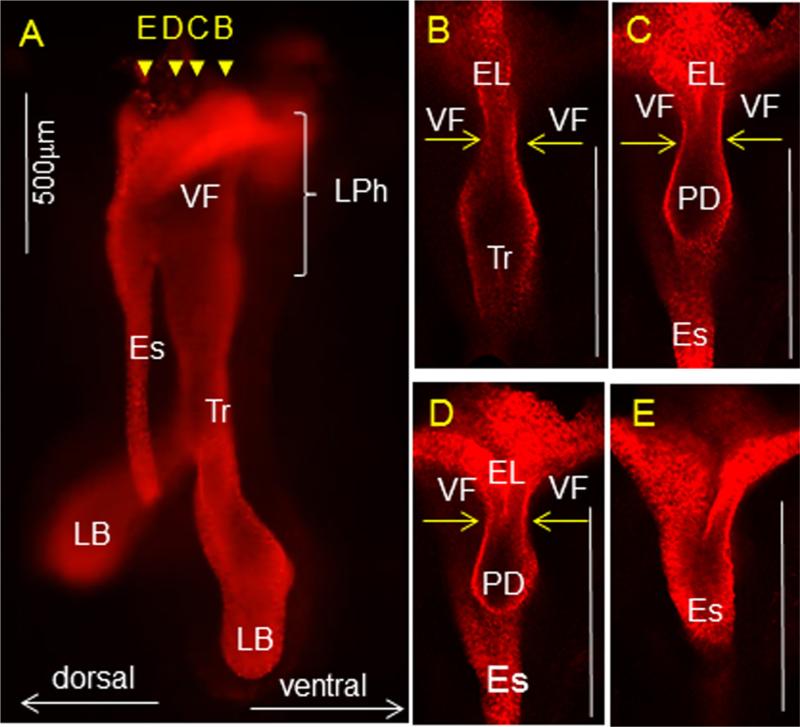
Fig. 4.
Whole mount IF staining for cytokeratin K8 at E11.5. (A) Whole mount IF staining of the isolated esophagus and respiratory structures including larynx, trachea and lung buds, yellow arrow heads represent coronal sections shown in B–E. (B–E) Yellow arrows indicate the bilateral compression of the laryngopharynx due to abutting of the lateral walls at midline. PD denotes the position of the pharyngoglottic duct that connects trachea and esophagus. Abbreviations: Es—esophagus; Tr—trachea; LB—lung buds; VF—vocal folds; EL—epithelial lamina; PD—pharyngoglottic duct.
To further illustrate tissue context beyond what can be gleaned from 2D sections, we performed whole mount IF staining for K8 and carried out 3D reconstruction of the anterior foregut (Fig. 4A). This analysis showed that lateral squeezing of laryngeal walls blocks the entrance into the trachea, except for at the pharyngoglottic duct (PD), which may remain open and is continuous with the trachea posteriorly and the esophagus dorsally (Fig. 4B–E).
Epithelial cells continue to express endoderm marker FOXA2 (Fig. 5A). SOX2 extends from the developing VFs to the infraglottic region and the trachea (Fig. 5B), while NKX2-1 remains present at high levels in the infraglottic region and the trachea and at low levels in the VFs (Fig. 5C). Similar to earlier stages, fewer cells proliferate in the epithelium compared to in the mesenchyme (Fig. 5D and E). A few apoptotic cells were detected in the infraglottic region (Fig. 5F). Similar to E10.5, K8 and K18 are expressed uniformly in the epithelial cells of prospective VF (Fig. 5G and H). Staining for p63 shows that p63 is highly expressed in epithelial cells of the future VF, but it is down-regulated in those that line the infraglottic region and the trachea (Fig. 5I).
Fig. 5.
VF epithelial cells marker expression at E11.5. (A) Distribution of FOXA2 positive cells in brown in vocal fold and infraglottic regions (black arrows). (B, C) Distribution of SOX2 and NKX2-1 positive cells in brown (black arrows), a red arrow denotes low expression of NKX2-1 in the developing VF. (D, E) EdU proliferation assay (green) and K8 staining of VF epithelial cells in red, white arrows denote proliferating epithelial cells of the epithelial lamina and adjacent mesenchyme. (F) TUNEL assay, black arrows denote positive apoptotic cells in brown. (G) IHC staining for K18, black arrows denote positive cells located in the vocal fold and infraglottic regions. (H) IF positive staining of K8 (white arrows). (I) IF staining of p63, white arrows denote positive p63 cells at the site of prospective VF and negative p63 cells in the infraglottic region. Abbreviations: LPh—laryngopharynx; VF—vocal folds; IG—infraglottis; Tr—trachea; EL—epithelial lamina.
Recanalization of EL and stratification of VFs (E13.5–18.5)
From E13.5 to E18.5 the EL is recanalized. At E13.5, in transversal sections, ventral to the EL is the laryngeal cecum (LC), which dilates from the laryngeal groove, dorsal is the pharyngoglottic duct (PD) that leads to the trachea (Fig. 6B–D). During EL recanalization the laryngeal cecum unites with pharyngoglottic duct. The process of recanalization is concurrent with the beginning of laryngeal cartilage chondrification and intrinsic laryngeal muscle formation. The laryngeal cavity further elongates along the A–P axis and dorsal–ventral (D–V) axis (Supplemental Fig. 1) to accommodate the growing VFs.
Fig. 6.
Morphology of the laryngopharynx at E13.5. (A) Schematic illustration of an embryo (E13.5) including orientation of the mid-sagittal (B) and transversal (D, E) sections. (B) Schematic illustration of the mid-sagittal section through the laryngopharynx. A red dashed line denotes the position of the VF, arrow heads (a–e) demonstrate transversal sections illustrated in diagram (C). (C) Transversal sections through the LPh as suggested in diagram (B) including position of the laryngeal cecum and pharyngoglottic duct. Black arrows denote initiation of EL separation. (D) Hematoxylin & eosin transverse section demonstrating morphology of the laryngeal cavity and VF at E13.5. (E) Detailed morphology of vocal fold (VF) epithelial cells (black arrows) at the site of the epithelial lamina. Abbreviations: LPh—laryngopharynx; LC—laryngeal cecum; EL—epithelial lamina; PD—pharyngoglottic duct; Tr—trachea; Es—esophagus; SG—supraglottic region, VF—vocal folds; IG—infraglottic region.
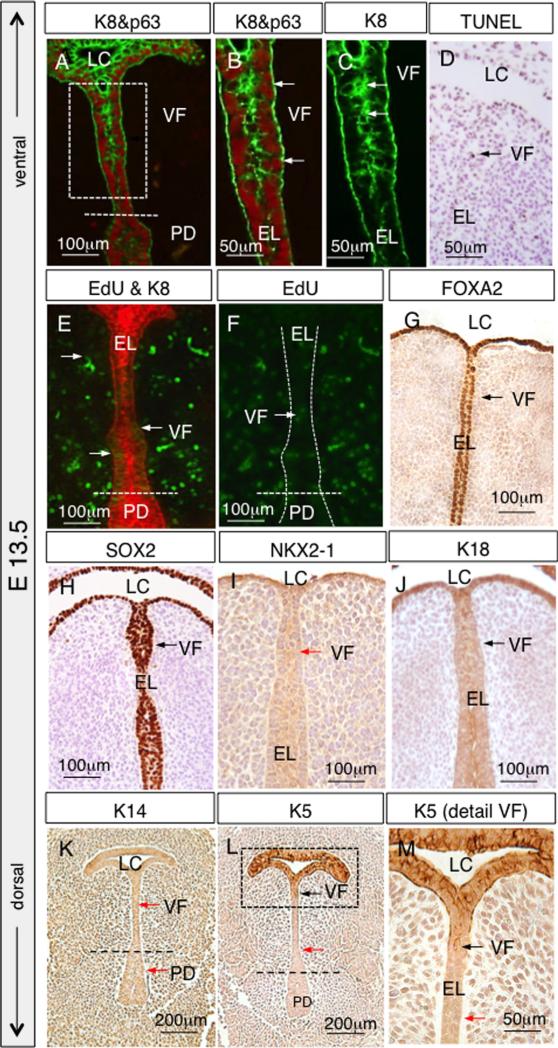
Concurrent with EL separation, the VF epithelium gains strati-fied morphology and initiates the expression of markers typical for stratified cells. To achieve stratification, some of the cuboidal cells elongate to form a basal cell layer and retain their p63 expression, the remainder of cells accumulate at the apical layer and down-regulate their p63 expression (Fig. 7A and B). Moreover, p63 was detected in the basal cells of the EL as well as the PD (Fig. 7A). Earlier uniform expression of K8 now exhibits more intensive staining at the apical layer of EL, especially at the level of prospective VFs (Fig. 7C). Only a few apoptotic cells were detected in the epithelium at the site of EL separation, suggesting that the separation is not driven by an increase in cell death (Fig. 7D). Lower cell proliferation in the EL and higher cell proliferation in the mesenchyme continues (Fig. 7E and F). FOXA2 and SOX2 expressions remain strong in the epithelial cells lining the laryngeal cecum and EL (Fig. 7G and H), in contrast to the production of NKX2-1, which was detected minimally (Fig. 7I). K18 expression appears to be uniform in VF epithelial cells (Fig. 7J). While little K14 expression was detected at this stage (Fig. 7K) compared to postnatal stages, K5 expression was detected in LC and a few cells in the adjacent VF epithelium, suggesting the initiation of epithelium stratification (Fig. 7L and M).
Fig. 7.
VF epithelial cells marker expression at E13.5. (A) Distribution of p63 positive basal cells and K8 positive suprabasal cells. (B) Magnified view of the boxed region in (A) to illustrate p63 and K8 expression in the VF epithelial cells. White arrows denote p63 positive basal cells. (C) Magnified view of the boxed region in (A) to illustrate K8 expression in the VF epithelial cells, which is mainly detected in cells localized at the center of EL (white arrows). (D) TUNEL assay, a black arrow denotes a positive apoptotic cell in brown. (E, F) EdU proliferation assay (green) and K8 staining of the epithelium in red, white arrows denote proliferating epithelial cells of the epithelial lamina and adjacent lamina propria. White dashed lines indicate the position of the EL. (G) Distribution of FOXA2 positive cells in brown (black arrows) in the prospective VF. (H, I) Distribution of SOX2 positive cells in brown (black arrow) and negative NKX2-1 cells (red arrow) in developing VF. (J) IHC staining for K18, black arrows denote positive cells in brown in prospective VF. (K) Negative IHC detection of K14 (red arrows) and (L, M) gradual upregulation of K5 in cells of EL (black arrows). Abbreviations: LC—laryngeal cecum; PD—pharyngoglottic duct; VF—vocal folds; EL—epithelial lamina.
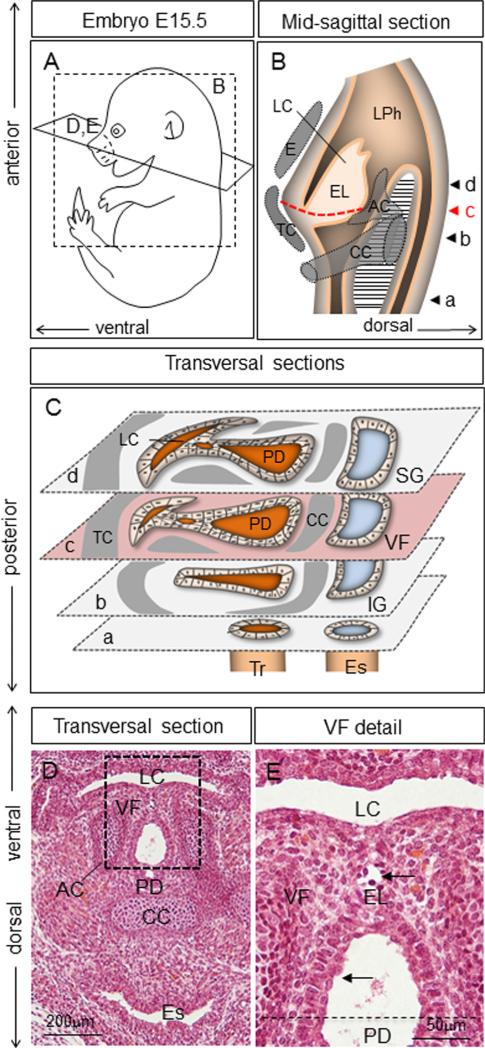
At E15.5, the dissociation of EL proceeds (Fig. 8A–E). Supporting cartilages are clearly visible (Fig. 8B–D). Epithelial cells covering the separated parts stratify (Fig. 8E). As observed in the previous stage, while a few apoptotic figures were observed, there is no massive cell death associated with this recanalization process (Fig. 9A). VF epithelial cells maintain their FOXA2 and SOX2 expressions (Fig. 9B and C), NKX2-1 expression is very low (Fig. 9D) compared to E16.5 expression. K18 expression appears to be uniform (Fig. 9E), while K8 expression continues to be slightly lower in the basal layer compared to the apical layer (Fig. 9F–J). Basal cells are positive for p63 in VF region (Fig. 9G) as well as in the PD (Fig. 9I). Concurrently with simple epithelial markers, expression of K5 is increased (Fig. 9K–M) and K14 expression initiates (Fig. 9N–P).
Fig. 8.
Morphology of the larynx at E15.5. (A) Schematic illustration of the embryo (E15.5) including orientation of the mid-sagittal (B) and transversal (D, E) sections. (B) Schematic illustration of the mid-sagittal section through the laryngopharynx. A red dashed line denotes the position of the VF, arrow heads (a–d) demonstrate transversal sections illustrated in the diagram (C). (C) Transversal sections through the LPh as suggested in the diagram (B) including the position of the laryngeal cecum, pharyngoglottic duct and laryngeal cartilages. (D) Hematoxylin & eosin transversal section describing morphology of the laryngeal cavity and VF at E15.5. (E) Detailed morphology of VF epithelial cells (black arrows) at the site of the epithelial lamina separation. Abbreviations: LC—laryngeal cecum; LPh—laryngopharynx; EL—epithelial lamina; CC—cricoid cartilage; TC—thyroid cartilage; E—epiglottis; AC—arytenoid cartilage; PD—pharyngoglottic duct; SG—supraglottic region; VF—vocal folds; IG—infraglottic region; Tr—trachea; Es—esophagus.
Fig. 9.
VF epithelial cells marker expression at E15.5. (A) TUNEL assay, a black arrow denotes a positive apoptotic cell in brown. (B–E) IHC staining for FOXA2, SOX2, NKX2-1 and K18 respectively, black arrows denote positive cells in brown located in the VF region. (F–I) Double IF K8 and p63 staining at the site of developing VF including details of VF (G, H) and pharyngoglottic duct (I, J). White arrows denote positive K8 superficial cells at the site of developing VF and positive p63 and K8 basal cells at the pharyngoglottic duct. (K, L, M) K5 detection in the VF including details of VF (L) and pharyngoglottic duct (M) respectively, positive cells in brown (black arrows). (N, O, P) Detail of K14 staining and its gradual upregulation in the developing VF (O) and pharyngoglottic duct (P) respectively. Black arrows denote positive cells in brown. Abbreviations: LC—laryngeal cecum; PD—pharyngoglottic duct; VF—vocal folds.
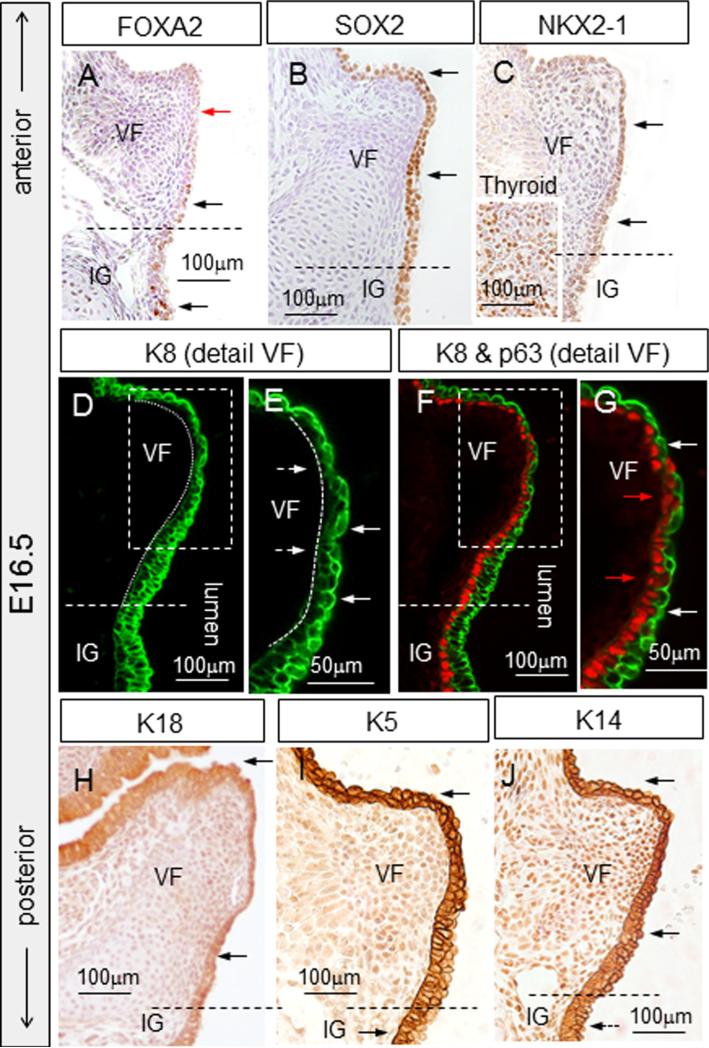
Between E16.5 and 18.5, VF separation proceeds to completion (Fig. 10A–E). Interestingly after EL separation, at E16.5, expression of FOXA2 in the VF epithelial cells is downregulated (Fig. 11A), SOX2 expression is maintained (Fig. 11B) and NKX2-1 expression has increased compared to previous stages but is lower than that of the thyroid gland (Fig. 11C). Among cytokeratins, K8 (Fig. 11D–G) and K18 (Fig. 11H) expression patterns are similar as at E15.5, with K8 expression very clearly biased in the apical layer. P63 is expressed in basal cells in the VF epithelium (Fig. 11G), as well as in the infraglottic region and trachea (Fig. 11F). K5 is detected in the VF in both epithelial cell layers (basal and apical), while in the infraglottic region K5 is preferentially detected in the basal layer (Fig. 11I). VFs also express K14 (Fig. 11J).
Fig. 10.
Morphology of the larynx at E16.5. (A) Schematic illustration of an embryo (E16.5) including orientation of the mid-sagittal (B) and coronal (D, E) sections. (B) Schematic illustration of the sagittal section through the laryngopharynx. A red dashed line denotes the position of the VF, arrow heads (a–d) demonstrate transversal sections illustrated in the diagram (C). (C) Transversal sections through the LPh as suggested in the diagram (B) including the position of laryngeal cartilages. Black arrows indicate the separation of VF. (D) Hematoxylin & eosin coronal section describing morphology of the laryngeal cavity and VF at E16.5. (E) Detail of the morphology of vocal fold (VF) epithelial cells. Abbreviations: LPh—laryngopharynx; TC—thyroid cartilage; CC—cricoid cartilage; AC—arytenoid cartilage; E—epiglottis; VF—vocal folds; SG—supraglottis; IG—infraglottis; Tr—trachea; Es—esophagus; TA—thyroarytenoid muscle (vocalis muscle); LP—lamina propria; EP—epithelial layer.
Fig. 11.
VF epithelial cells marker expression at E16.5. (A) Detection of FOXA2, black arrows denote FOXA2 positive cells in brown located in the infraglottic region, red arrows denote FOXA2 negative VF epithelial cells. (B) SOX2 staining, black arrows denote positive cells in brown. (C) NKX2-1 staining, black arrows denote positive NKX2-1 cells in brown. (D, E) IF K8 staining, dashed white arrows denote downregulation of K8 in the basal layer, solid white arrows denote K8 positive apical cells. (F, G) Double staining for p63 and K8, production of K8 is clearly shifted to the superficial layers (white arrows), red arrows denote p63 positive basal cells. (H) Weak detection of K18 (black arrows), (I) IHC detection of K5. Positive K5 cells were detected in both cell layers at the site of developing VF and they became restricted to basal cells at the site of infraglottis (black arrows). (J) IHC staining for K14—positive cells in brown. Abbreviations: VF—vocal folds; IG—infraglottic region.
Maturation of VF epithelial cells (postnatal stages)
During postnatal stages VFs are fully separated and firmly attached to the thyroid cartilage (ventrally) and arytenoid cartilages (dorsally) (Fig. 12A). Epithelial cells lining VF further stratify from the original two layers at P0 (Fig. 12B and C) to three or four layers in the adult (Fig. 13B–D). At P0, FOXA2 remains not expressed in the VF epithelial cells (Fig. 12D) in contrast to epithelial cells lining the trachea, which retain their FOXA2 expression (Fig. 12E). SOX2 expression remains strong (Fig. 12F), while NKX2-1 expression remains low as compared to the thyroid gland (Fig. 12G). K18 staining remains low and uniform in the epithelium (Fig. 12H), while K8 expression was no longer detected in the basal layer, but remained in the apical VF layer (Fig. 12I and J). p63 is expressed in the basal cell layer of VF epithelium (Fig. 12J). Interestingly, K5 was detected not only in the basal cells, but also in the apical cells in contrast to the supra- and infraglottic regions, where it was expressed predominantly in the basal cells (Fig. 12K and L). K14 was detected in the VF as well as in the supra- and infraglottic regions (Fig. 12M).
Fig. 12.
Morphology and VF epithelial cells marker expression for P0 stage of the VF development. (A) Schematic illustration of the larynx including orientation of the coronal sections (B–M) at P0. (B, C) Hematoxylin & eosin for assessment of the morphology of VF at P0, black arrows point to stratified epithelial cells. (D, E) FOXA2 staining in VF (D) and trachea (E), black arrows denote FOXA2 positive cells in brown, red arrows denote FOXA2 negative cells in VFs. (F) IHC staining for SOX2, black arrows denote positive cells in brown. (G) IHC staining for NKX2-1, black arrows denote positive cells in brown. (H) IHC staining for K18, black arrows denote positive cells in brown. (I) IF staining for K8, production of K8 is clearly shifted to the superficial layers (white arrows). (J) Magnified view of the boxed region in (I) IF double staining for K8 and p63, p63 stains the basal cells (red arrows) while K8 stains the superficial cell layer (white arrows). (K, L) IHC staining for K5 in the VF (K) and infraglottic regions (L). (M) K14 expression pattern in the VF region. K5 and K14 diffuse to the superficial layers of VF (black arrows). Abbreviations: EP—epithelium; LP—lamina propria; TA—thyroarytenoid muscle (vocalis muscle); VF—vocal folds; E—epiglottis; SG—supraglottic region; IG—infraglottic region; TC—thyroid cartilage; AC—arytenoid cartilage; CC—cricoid cartilage.
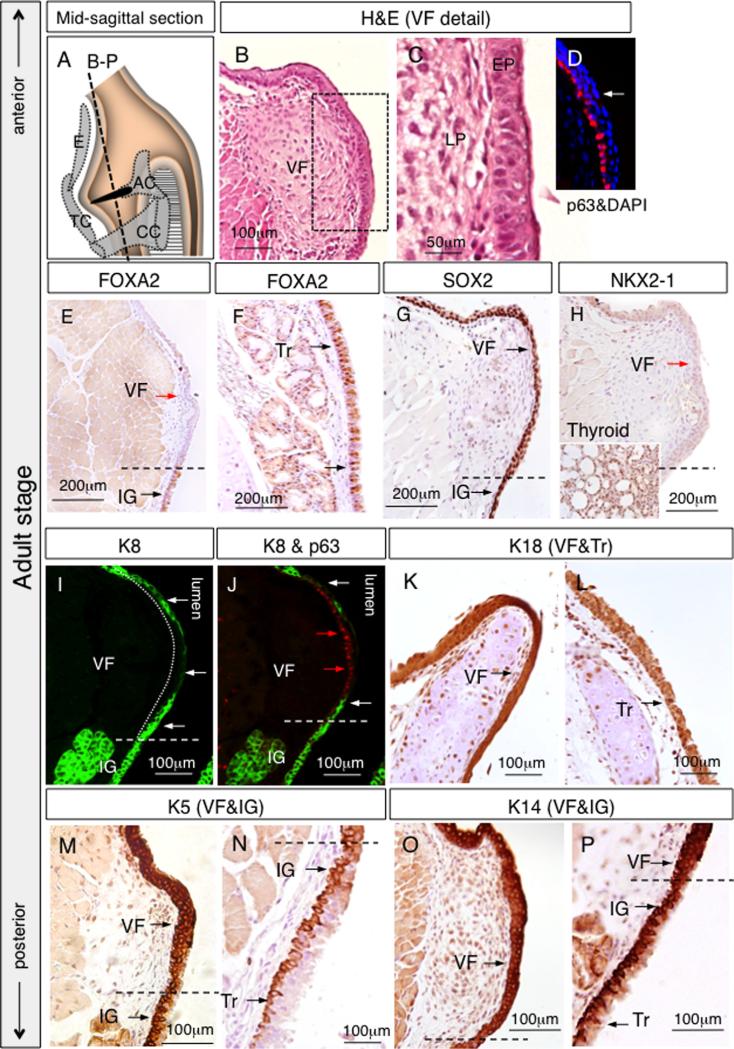
Fig. 13.
Morphology and VF epithelial cells marker expression in the adult stage of the VF development. (A) Schematic illustration of the larynx including orientation of the coronal sections (B–P) at the adult stage. (B) Hematoxilin & eosin for assessment of the morphology in adult VF. (C, D) Detail of the stratified epithelium of VF including IF data for p63 positive basal cells and p63 negative superficial layers (white arrow). (E, F) IHC FOXA2 staining in VF (E) and trachea (F), red arrows denote FOXA2 negative cells, black arrows denote FOXA2 positive cells in brown. (G) IHC SOX2 staining, black arrows denote SOX2 positive cells in brown. (H) IHC negative NKX2-1 staining in VF as compared to the positive NKX2-1 cells in the thyroid gland. (I) IF staining for K8, positive K8 cells were detected in the luminal layer of VF epithelium (white arrows), basal layer was p63 negative (a dashed white line). (J) IF double staining for K8 and p63, red arrows denote p63 positive basal cells while white arrows point to K8 superficial cells. (K, L) IHC staining for K18 in the VF (K) and trachea (L), black arrows denote positive cells in brown. (M, N) Expression of K5 was detected in all epithelial layers in the VF (M) in contrast to the trachea (N) (black arrows). (O, P) IHC staining for K14, K14 diffuses to the superficial layers of VF (O), while in the infraglottic region it is expressed predominantly by basal cells (P) (black arrows). Abbreviations: EP—epithelium; LP—lamina propria; Tr—trachea; IG—infraglottis; VF—vocal folds; E—epiglottis; TC—thyroid cartilage; AC—arytenoid cartilage; CC—cricoid cartilage.
In the adult, VFs are fully developed and functional (Fig. 13A). FOXA2 was not detected in epithelial cells lining the VFs (Fig. 13E), but was found in epithelial cells located in the infraglottic region and the trachea (Fig. 13E and F). SOX2 is expressed at a high level (Fig. 13G), while NKX2-1 expression is low, both compared to P0 stage as well to the thyroid gland (Fig. 13H). K8 expression is restricted to the luminal cell layer, and p63 stains the basal cells (Fig. 13I and J). In contrast, K18 staining was detected in all epithelial layers and showed slightly stronger intensity in the region of the VFs (Fig. 13K) as compared to the trachea (Fig. 13L). K5 is expressed in both basal and apical cells of the VF, similar to P0 (Fig. 13M). Interestingly, as the stratified epithelium transitions into pseudostratified epithelium in the infraglottic region and the trachea, K5 becomes restricted to the basal cell layer only (Fig. 13N). Similarly, uniform K14 expression was detected in the VF (Fig. 13O), while in the trachea, K14 expression was predominantly detected in the basal cell layer (Fig. 13P). This feature of K5 and K14 being in both the apical and basal layers seems to be unique to the VF.
Shh signaling is active in the prospective VF and essential for their development
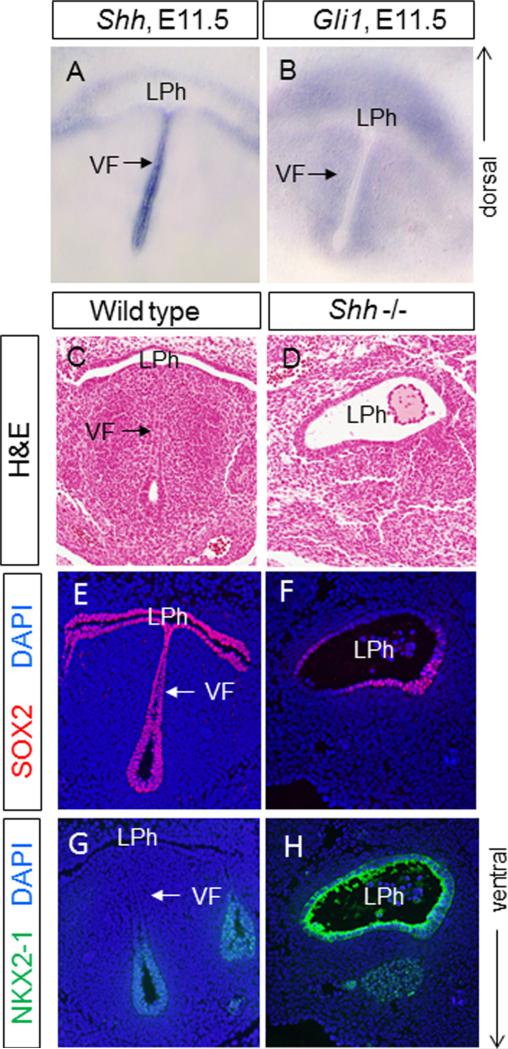
While many signaling pathway genes have been implicated in the development of other foregut tissues, little is known on their role in the development of the VF (Domyan and Sun, 2011). Here, we focused on SHH signaling pathway. At E11.5, Shh is highly expressed in epithelial cells lining the future VFs (Fig. 14A). Accordingly, SHH activity readout, Gli1 is expressed in the lamina propria and the adjacent mesenchyme (Fig. 14B).
Fig.14.
Shh expression in developing VFs. (A) Shh in situ hybridization in developing VF at E11.5 in a vibratome transversal section. (B) Gli1 expression in the developing VF in a vibratome transversal section. (C, D) Hematoxylin & eosin transversal sections demonstrating morphology of the laryngopharynx in wild type littermates (C) and Shh null mutants (D) at E11.5. (E, F) SOX2 expression (in red) in wild type littermates (E) and Shh null mutants (F). A white arrow indicates the position of the vocal folds in wild type littermates. (G, H) NKX2-1 expression (in green) in wild type littermates (G) and Shh null mutants (H). A white arrow indicates the position of the vocal folds in wild type littermates. Abbreviations: VF—vocal folds; LPh—laryngopharynx.
To address if SHH activity in the prospective VF region is essential for VF development, we studied the homozygous Shh null mutants (Harfe et al., 2004). By transverse sections, we found that at E11.5, while the primitive laryngopharynx can be identified in the Shh mutant embryos at a similar level as in the control, the lateral walls of the laryngopharynx do not approach each other, different from the control (Fig. 14C and D). As a result, the laryngopharynx in the mutant remains widely open.
We also examined how loss of Shh may affect early patterning of the prospective VF region, by examining the expression of NKX2-1 and SOX2. As shown above, in wild-type littermate, SOX2 is expressed strongly in the prospective VF as well as surrounding foregut endoderm (Fig. 14E), while NKX2-1 expression is negative at prospective VFs and it is only weakly expressed in the region ventral to the VFs, where the proximal part of the trachea is situated (Fig. 14H). In contrast, in the Shh null embryos, NKX2-1 is expressed more strongly in epithelial cells lining the laryngopharynx (Fig. 14I). On the other hand, SOX2 expression is decreased in the Shh mutant mice (Fig. 14G). These data suggest that Shh is essential for the development of the foregut structures that are essential for constructing the future VFs.
Discussion
In this comprehensive investigation, we focused on defining the developmental progression of VF epithelial cells and their gradual differentiation into a stratified squamous cell phenotype. Although the organization of stratified squamous tissue such as in the epidermis or esophagus has been extensively studied in recent years (Byrne et al., 1994; Jenson et al., 1999; Yu et al., 2005), the initiation and maturation of VF epithelium have not been resolved. For the first time, we have defined five principal developmental events that constitute the progression in vocal fold development. These developmental events include (1) the initiation of the larynx and vocal folds with apposition of the lateral walls of the primitive laryngopharynx (embryonic (E) day 10.5), (2) the establishment of the epithelial lamina with fusion of the lateral walls of the primitive laryngopharynx (E11.5), (3) epithelial lamina recanalization and separation of VFs (E13.5–18.5), (4) stratification of the vocal folds (E13.5–18.5), and (5) maturation of vocal fold epithelium (postnatal stages).
Our findings are consistent with the notion that the VF epithelium is derived from the foregut endoderm which develops from the definitive endoderm. VF epithelial cells express definitive endoderm marker FOXA2. Notably, when VFs separate from each other and the simple epithelial precursors differentiate into stratified epithelium, FOXA2 expression decreases on the contrary to the epithelial cells located in the trachea. The downregulation of FOXA2 seems to be essential for proper development VF epithelial cells. Moreover, at E10.5, there is precise juxtaposition of SOX2 and NKX2-1 expression domains in the foregut in both the D–V axis (SOX2 in esophagus dorsally, and NKX2-1 in trachea ventrally), and A–P axis (SOX2 in primitive LPh anteriorly and NKX2-1 in trachea and lung buds posteriorly). Interestingly, while future VF cells connect onto the trachea, which expresses NKX2-1, they express SOX2. As Sox2 has been shown to be essential in the differentiation of multiple foregut-derived epithelial surfaces, including that of the esophagus and airway (Que et al., 2009; Domyan et al., 2011), it is likely that this early expression of Sox2 in the VF precursors may also be essential for VF maturation. The strong SOX2 expression persists in the VF epithelium until adulthood, a contrast to NKX2-1, a marker of respiratory cells, which slightly upregulates its expression during EL recanalization and maturation of VF epithelium.
VF morphogenesis initiates in a mouse at E10.5, when the lateral walls of the primitive laryngopharynx appose. By this time, the trachea has separated from the esophagus and lung buds have branched into primary bronchi. Previously published data led to the proposal of two principle mechanisms responsible for the juxtaposition of lateral walls. It is either driven by intense proliferation of epithelial cells, thereby leading to epithelial bending which obliterates the lumen (Petrova, 1963; Lobcko et al., 1979; Sanudo and Domenech-Mateu, 1990), and/or by condensation of mesenchymal cells at the site of arytenoid swellings, thereby exerting the pressure on the lateral epithelial walls (Zaw-Tun and Burdi, 1985; Muller et al., 1985). Our data does not distinguish between these possibilities, as mitotic activity was detected in both epithelial cells and adjacent mesenchymal cells. However, there appears to be a higher percentage of EdU-positive cells in the mesenchyme compared to the epithelium at this stage, biasing towards the latter possibility.
At 11.5, as soon as the epithelial cells covering the prospective VF come into contact, obliterate the lumen, and create the EL, they undergo a very significant change in their morphology (Fig. 15). Original pseudostratified columnar epithelium of the foregut converts into a perfectly organized single layer of cuboidal cells. These simple epithelial precursors represent a transitional stage towards the stratified phenotype. This conversion from columnar into cuboidal epithelium is not observed in the adjacent esophagus or trachea (Yu et al., 2005; Domyan et al., 2011), and appears to be unique to VF. The mechanism of the VF epithelial morphology conversion requires further investigation. Our results indicate that despite intense proliferation, some epithelial cells of the developing VF are eliminated by apoptosis. The elimination of columnar cells and replacement by cuboidal cells may contribute to the overall cell shape change. It is also possible that some columnar cells directly transform into simple epithelial progenitors or transdifferentiate into mesenchymal cells of the lamina propria. Although the epithelial-to-mesenchymal transition has not been documented in normal foregut epithelium development, it does occur in dental epithelium during odontogenesis. A subset of epithelial cells of Hertwig's epithelial root sheath transforms into fibroblasts producing periodontal ligaments or cementoblasts of the root cementum, once they complete their function: to guide the growth of the tooth root and its supporting structures (Thomas, 1995; Zeichner-David et al., 2003; Luan et al., 2006).
Fig. 15.
A model for conversion and stratification of VF epithelial cells.
At E13.5 simple epithelial progenitors in EL change cell shape and further proliferate to transition from one layer to two layers of cells. Elongated cells constitute the basal layer, while rounded irregularly shaped cells lie on the top of them to constitute the apical layer (Fig. 15). This increase in cell number precedes and is likely required for proper EL recanalization and the final conversion from simple into multilayered or stratified epithelial architecture. Mechanism of EL recanalization is still unknown. It certainly requires precise coordination with the adjoining lamina propria. Failure of the EL to recanalize has been thought to give rise to a range of congenital disorders such as Congenital High Airway Obstruction Syndrome (CHAOS) including laryngeal webs and stenosis (Miller et al., 1984; Hartnick and Cotton, 2000; Wyatt et al., 2005; Ahmad and Soliman, 2007). Severe obstructions of the larynx and upper airway structures can lead to suffocation of a newborn. Our results show that only a few apoptotic cells were detected at the site of separation at E13.5 and E15.5, suggesting that apoptosis is unlikely to play a major role in EL recanalization. Rather, it is more likely that EL cells recanalize by changing their adhesive property as a response to signaling factors in their surroundings. Further functional experiments are needed to investigate the identity, localization and effect of these factors on EL separation.
Temporal and spatial expression patterns of the simple (K8 and K18) versus stratified (K5 and K14) epithelial markers correlated with changes in morphology. Initial uniform expression of K8 at E10.5 and E11.5 decreases in the basal layer as this layer emerges at E13.5, and then is absent in this layer at P0 and adult stages. In contrast, the most apical layer continues to express K8 through to adulthood. This expression pattern, together with the known role of this type of keratin, suggests that in addition to its structural and mechanical function, K8 may protect the VF epithelial cells from apoptosis and against stress and injury by serving as a phosphate “sponge” for stress-activated kinases (Ku and Omary, 2006). This non-mechanical or regulatory function of K8 protein is particularly advantageous for VF, as they make contact during phonation and undergo significant stresses and collisions (Bhattacharya and Siegmund, 2013).
Initiation of K5 and K14 expression (stratified squamous markers) in VF correlates with the switch from simple to stratified morphology. VF cells trigger their K5 and consequently K14 expression at E13.5 and E15.5, respectively, a similar time window when K8 expression is downregulated in VF basal cells. Our results also indicate that the beginning of VF stratification is synchronized with EL disintegration, suggesting that they may be controlled by the same factors. p63 has been shown to act as a master switch gene that controls the simple to stratified squamous cell transition (Daniely et al., 2004), and its expression pattern in VF is consistent with a role in this transition.
During postnatal stages (from P0 to adulthood) the VF epithelium further matures and stratifies to three or four cell layers (Fig. 15). Basal cells lose their columnar shape and become cuboidal. The more apical suprabasal layers terminally differentiate and become progressively more squamous towards the luminal surface of the VF. Expression of K5 and K14 is not restricted to the basal cell layer, as reported for the trachea or esophagus (Yu et al., 2005), but rather persists in all layers of the VF. The diffused pattern of K5 and K14 overlaps with the persistent K8 expression in the luminal layer, which seems to be unique for VF epithelial cells in a mouse. These cytokeratins may function to provide VF cells with increased mechanical stability and integrity, such that they can be subjected to higher mechanical pressure and abrasion as they vibrate.
Shh is one of the possible signals that may regulate the formation of the VFs and larynx. Previous observations showed that mice with targeted deletion of Shh develop defects such as tracheoesophagal atresia/fistula (Litingtung et al., 1998). We detected strong Shh and Gli1 expressions in prospective VF epithelial cells and surrounding mesenchyme, respectively. We found that in Shh knockout mutant embryos, the lateral walls of the LPh do not approach each other in the center of the LPh, such that the LPh remains open. The results presented here are consistent with the fact that lack of Shh signaling may down-regulate gene expression and proliferation of the adjacent mesenchymal target cells in the lamina propria. Accompanying the morphological phenotype is the alteration SOX2 and NKX2-1 expression. These data suggest that the requirement for Shh in foregut patterning may be a pre-requisite for proper apposition of the lateral walls, as unique requisite step for constructing the future VFs.
This first systematic study of the development of the VF epithelial cells has delineated the major morphogenetic events that occur during VF formation. Since both species, mice and humans, display similarities in the progress through developmental stages, knowledge obtained from our study could be successfully extrapolated to development of human VFs and their epithelial cells. Our findings offer as the cellular and molecular foundation for further functional experiments to uncover the genes that control normal VF development, and underlie VF-associated diseases.
Supplementary Material
Acknowledgments
This work was supported by grants of NIH NIDCD R01 DC004336 and R01 DC012773. We gratefully acknowledge Drew Ronnenberg for his expert assistance with the tissue samples preparation for this study. We thank Ciara Leydon for helpful suggestions.
Footnotes
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.ydbio.2014.12.037.
References
- Ahmad SM, Soliman AMS. Congenital anomalies of the larynx. Otolaryngol. Clin. N. Am. 2007;40:77–191. doi: 10.1016/j.otc.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Basseres DS, D'Alò F, Yeap BY, Löwenberg EC, Gonzalez DA, Yasuda H, Dayaram T, Kocher ON, Godleski JJ, Richards WG, Meyerson M, Kobayashi S, Tenen DG, Halmos B, Costa DB. Frequent downregulation of the transcription factor Foxa2 in lung cancer through epigenetic silencing. Lung Cancer. 2012;77(1):31–37. doi: 10.1016/j.lungcan.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin B, Inglis A. Minor congenital laryngeal clefts: diagnosis and classification. Ann. Otol. Rhinol. Laryngol. 1989;98:417–420. doi: 10.1177/000348948909800603. [DOI] [PubMed] [Google Scholar]
- Bhattacharya P, Siegmund T. A computational study of systemic hydration in vocal fold collision. Comput. Methods Biomech. Biomed. Eng. 2013;00:1–26. doi: 10.1080/10255842.2013.772591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne C, Tainsky M, Fuchs E. Programming gene expression in developing epidermis. Development. 1994;120:2369–2383. doi: 10.1242/dev.120.9.2369. [DOI] [PubMed] [Google Scholar]
- D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 2005;23(12):1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- Daniely Y, Liao G, Dixon D, Linnoila RI, Lori A, Randell SH, Oren M, Jetten AM. Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. Am. J. Physiol. Cell Physiol. 2004;287:171–18. doi: 10.1152/ajpcell.00226.2003. [DOI] [PubMed] [Google Scholar]
- Domyan ET, Sun X. Patterning and plasticity in development of the respiratory lineage. Dev. Dyn. 2011;240:477–485. doi: 10.1002/dvdy.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domyan ET, Ferretti E, Throckmorton K, Mishina Y, Nicolis SK, Sun X. Signaling through BMP receptors promotes respiratory identity in the foregut via repression of Sox2. Development. 2011;138:971–981. doi: 10.1242/dev.053694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri A, Favaro R, Beccari L, Bertolini J, Mercurio S, Nieto-Lopez F, Verzeroli C, La Regina F, Tonelli DP, Ottolenghi S, Bovolenta P, Nicolis SK. Sox2 is required for embryonic development of the ventral telencephalon through the activation of the ventral determinants Nkx2.1 and Shh. Development. 2013;140:1250–1261. doi: 10.1242/dev.073411. [DOI] [PubMed] [Google Scholar]
- Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Harris-Johnson KS, Domyan ET, Vezinab CM, Sun X. β-Catenin promotes respiratory progenitor identity in mouse foregut. Proc. Natl. Acad. Sci. USA. 2009;106(38):16287–16292. doi: 10.1073/pnas.0902274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartnick Ch.J., Cotton RT. Congenital laryngeal anomalies laryngeal atresia, stenosis, webs, and clefts. Otolaryngol. Clin. N. Am. 2000;33:1–10. doi: 10.1016/s0030-6665(05)70282-6. [DOI] [PubMed] [Google Scholar]
- Henick DH. Three dimensional analysis of murine laryngeal development. Ann. Otol. Rhinol. Larynol. Suppl. 1993;102:3–24. doi: 10.1177/00034894931020s301. [DOI] [PubMed] [Google Scholar]
- Hirano M, Kurita S, Nakashima T. Growth, development and aging of human vocal folds. In: Bless D, editor. Vocal Fold Physiology Contemporary Research and Clinical Issues. College-Hill Press; San Diego, Calif.: 1983. pp. 22–43. [Google Scholar]
- Hirano M, Bless DM. Videostroboscopic Examination of the Larynx. Singular Publishing; San Diego CA.: 1993. [Google Scholar]
- Hopp E. The development of the epithelium of the larynx. Laryngoscope. 1955;65(7):475–499. doi: 10.1288/00005537-195507000-00001. [DOI] [PubMed] [Google Scholar]
- Jacobs IJ, Ku WY, Que J. Genetic and cellular mechanisms regulating anterior foregut and esophageal development. Dev. Biol. 2012;369:54–64. doi: 10.1016/j.ydbio.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenson UB, Lowell S, Watt FM. The spatial relationship between stem cells and their progeny in the basal layer of human epidermis: a new view based on whole-mount labeling and lineage analysis. Development. 1999;126:209–218. doi: 10.1242/dev.126.11.2409. [DOI] [PubMed] [Google Scholar]
- Jiang J, Au M, Lu K, Eshpeter A, Korbutt G, Fisk G, Majumdar A. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells. 2007;25:1940–1953. doi: 10.1634/stemcells.2006-0761. [DOI] [PubMed] [Google Scholar]
- Johnson AM, Ciucci MR, Russell JA. Ultrasonic output from the excised rat larynx. J. Acoust. Soc. Am. 2010;128(2):75–79. doi: 10.1121/1.3462234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakodkar KA, Schroeder JW, Jr., Holinger LD. Laryngeal development and anatomy. Adv. Otorhinolaryngol. 2012;73:1–11. doi: 10.1159/000334108. [DOI] [PubMed] [Google Scholar]
- Kelly SE, Bachurski CJ, Burhans MS, Glasser SW. Transcription of the lung-specific surfactant protein C gene is mediated by thyroid transcription factor 1. J. Biol. Chem. 1996;271(12):6881–6888. doi: 10.1074/jbc.271.12.6881. [DOI] [PubMed] [Google Scholar]
- Kim BM, Buchner G, Miletich I, Sharpe PT, Shivdasani RA. The stomach mesenchymal transcription factor barx1 specifies gastric epithelial identity through inhibition of transient Wnt signaling. Dev. Cell. 2005;8:611–622. doi: 10.1016/j.devcel.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Kim BM, Woo J, Kanellopoulou Ch., Shivdasani RA. Regulation of mouse stomach development and Barx1 expression by specific microRNAs. Development. 2011;138:1081–1086. doi: 10.1242/dev.056317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku NO, Omary MB. Keratin 8 phosphorylation by p38 kinase regulates cellular keratin filament reorganization: modulation by a keratin 1-like disease causing mutation. J. Biol. Chem. 2006;277(13):10775–10782. doi: 10.1074/jbc.M107623200. [DOI] [PubMed] [Google Scholar]
- Lee CS, Sund NJ, Behr R, Herrera PL, Kaestner KH. Foxa2 is required for the differentiation of pancreatic α-cells. Dev. Biol. 2005;278(2):484–495. doi: 10.1016/j.ydbio.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Leydon C, Selekman JA, Palecek S, Thibeault SL. Human embryonic stem cell-derived epithelial cells in a novel in vitro model of vocal mucosa. Tissue Eng. A. 2013;19:2233–2241. doi: 10.1089/ten.tea.2012.0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, White P, Tuteja G, Rubins N, Sackett S, Kaestner KH. Foxa1 and Foxa2 regulate bile duct development in mice. J. Clin. Investig. 2009;119(6):1537–1545. doi: 10.1172/JCI38201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litingtung Y, Lei L, Westphal H, Chiang Ch. Sonic hedgehog is essential to foregut development. Nat. Genet. 1998;20:58–61. doi: 10.1038/1717. [DOI] [PubMed] [Google Scholar]
- Loebel DAF, Studdert JB, Power M, Radziewic T, Jones V, Coultas L, Jackson Y, Rao RS, Steiner K, Fossat N, Robb L, Tam PPL. Rhou maintains the epithelial architecture and facilitates differentiation of the foregut endoderm. Development. 2011;138:4511–4522. doi: 10.1242/dev.063867. [DOI] [PubMed] [Google Scholar]
- Lobcko PI, Petrova RM, Chaika EN. Functional anatomy of physiologic atresia in human and mammals embryogenesis. Anat. Anz. 1979;145:338–352. [PubMed] [Google Scholar]
- Luan X, Yoshihiro I, Diekwisch TGH. Evolution and development of Hertwig's epithelial root sheath. Dev. Dyn. 2006;235(5):1167–1180. doi: 10.1002/dvdy.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RH, Cagle PT, Pitcock JK, McGavran M. Laryngeal atresia: a detailed histologic study. Int. J. Pediatr. Otorhinolaryngol. 1984;7:273–280. doi: 10.1016/s0165-5876(84)80008-7. [DOI] [PubMed] [Google Scholar]
- Miller MF, Cohen ED, Baggs JE, Lu MM, Hogenesch JB, Morrisey EE. Wnt ligands signal in a cooperative manner to promote foregut organogenesis. Proc. Natl. Acad. Sci. USA. 2012;109(38):15348–15353. doi: 10.1073/pnas.1201583109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisey EE, Hogan BLM. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev. Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller F, O'rahilly R, Tucker JA. The human larynx at the end of the embryonic period proper. 2 The laryngeal cavity and the innervation of its lining. Ann. Otol. Rhinol. Laryngol. 1985;94:600–617. doi: 10.1177/000348948509400617. [DOI] [PubMed] [Google Scholar]
- Neubuser A, Peters H, Balling R, Martin GR. Antagonistic interactions between FGF and BMP signaling pathways: a mechanism for positioning the sites of tooth formation. Cell. 1997;90:247–255. doi: 10.1016/s0092-8674(00)80333-5. [DOI] [PubMed] [Google Scholar]
- Petrova RM. On development of laryngeal cavity in embryogenesis of man. Arch. Anat. Gistol. Embriol. 1963;44:100–104. [PubMed] [Google Scholar]
- Que J, Choi M, Ziel JW, Klingensmith J, Hogan BLM. Morphogenesis of the trachea and esophagus: current players and new roles for noggin and Bmps. Differentiation. 2006;74:422–437. doi: 10.1111/j.1432-0436.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- Que J, Okubo T, Goldenring JR, Nam KT, Kurotani R, Morrisey EE, Taranova O, Pevny LH, Hogan BLM. Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development. 2007;134:2521–2531. doi: 10.1242/dev.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que J, Luo X, Schwartz RJ, Hogan BL. Multiple roles for Sox2 in the developing and adult mouse trachea. Development. 2009;136:1899–1907. doi: 10.1242/dev.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanudo JR, Domenech-Mateu JM. The laryngeal primordium and epithelial lamina. A new interpretation. J. Anat. 1990;171:207–222. [PMC free article] [PubMed] [Google Scholar]
- Thomas HF. Root formation. Int. J. Dev. Biol. 1995;39:231–237. [PubMed] [Google Scholar]
- Walander A. Mechanisms of origin of congenital malformations of the larynx. Acta Otolaryngol. 1955;45(5):426–432. doi: 10.3109/00016485509124298. [DOI] [PubMed] [Google Scholar]
- Watt CR, Marler JA, Urban Z. The effects of supravalvular aortic stenosis elastin gene mutation on voice production. J. Med. Speech Lang. Pathol. 2007;15:679–698. [Google Scholar]
- Watts CR, Marler JA, Rousseau B. Qualitative characterization of elastic fiber distribution in mouse vocal fold: further development of an animal model. J. Voice. 2011;25(1):1–6. doi: 10.1016/j.jvoice.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Wong A, Bear Ch.E., Chin S, Pasceri P, Thompson TO, Huan LJ, Ratjen F, Ellis J, Rossant J. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat. Biotechnol. 2012;30(9):876–884. doi: 10.1038/nbt.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J, Miletich I, Kim BM, Sharpe PT, Shivdasani RA. Barx1-mediated inhibition of Wnt signaling in the mouse thoracic foregut controls tracheoesophageal septation and epithelial differentiation. Plos One. 2011;6(7):e22493. doi: 10.1371/journal.pone.0022493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt ME, Ernest B, Hartley J. Laryngotracheal reconstruction in congenital laryngeal webs and atresias. Otolaryngol. Head Neck Surg. 2005;132:232–238. doi: 10.1016/j.otohns.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Yu WY, Slack JMW, Tosh D. Conversion of columnar to stratified squamous epithelium in the developing mouse oesophagus. Dev. Biol. 2005;284:157–170. doi: 10.1016/j.ydbio.2005.04.042. [DOI] [PubMed] [Google Scholar]
- Zeichner-David M, Oishi K, Zhengyan S, Zakartchenko V, Chen L-S, Arzate H, Bringas P. Role of Hertwig's epithelial root sheath cells in tooth root development. Dev. Dyn. 2003;228:651–663. doi: 10.1002/dvdy.10404. [DOI] [PubMed] [Google Scholar]
- Zaw-Tun HA, Burdi AR. Reexamination of the origin and early development of the human larynx. Acta Anat. 1985;122:163–184. doi: 10.1159/000145998. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.