Abstract
Brucellosis is endemic in livestock and humans in Uganda and its transmission involves a multitude of risk factors like consumption of milk from infected cattle. To shed new light on the epidemiology of brucellosis in Uganda the present study used phenotypic and molecular approaches to delineate the Brucella species, biovars, and genotypes shed in cattle milk. Brucella abortus without a biovar designation was isolated from eleven out of 207 milk samples from cattle in Uganda. These isolates had a genomic monomorphism at 16 variable number tandem repeat (VNTR) loci and showed in turn high levels of genetic variation when compared with other African strains or other B. abortus biovars from other parts of the world. This study further highlights the usefulness of MLVA as an epidemiological tool for investigation of Brucella infections.
1. Introduction
The genus Brucella has ten recognized species with more than 90% DNA homology [1, 2]. These species cause brucellosis that is of economic and public health importance in terrestrial and aquatic animals and humans [1, 3]. Species of B. melitensis, B. abortus, some B. suis biovars, B. canis, B. ceti, and B. inopinata are zoonotic and in humans the infection causes a debilitating disease with relapsing fever and flu-like symptoms with multiple organ involvement [4–6]. In cattle Brucella causes abortions, placentitis, orchitis, mastitis, and prenatal death [5, 7].
Brucellosis in cattle is almost exclusively caused by B. abortus [8], but B. melitensis and B. suis have been implicated in some herds [9, 10] making the vaccination of cattle using vaccines targeting only B. abortus less effective in preventing brucellosis in cattle and transmission to humans [11, 12]. Brucella biovars and genotypes are known to be regionally restricted in their distribution [13] but the evolution of international travel and trade and changing ecosystems have led to introduction of new biovars and genotypes into regions and hosts where they were not previously found [3]. A study done in Uganda in 1958 isolated B. abortus biovar 3 from a human patient [14] and in the neighboring Kenya B. melitensis biovar 1 and B. abortus biovar 3 have been isolated from cattle [9]. Phylogenetic analysis of B. melitensis isolates in Kenya showed a high degree of homology with isolates in Israel and the B. abortus isolates closely resembled that isolated in Uganda. In a related study in Egypt, B. abortus, B. suis, and B. melitensis were isolated from cattle and all had a high level of phylogenetic variability within each species although the isolates used in these studies were few [15]. The above findings indicate a complex epidemiology of brucellosis in cattle in the region and call for refined diagnostic methods beyond phenotypic typing.
High resolution phenotypic and molecular approaches have been developed for Brucella speciation, biotyping, and epidemiological trace-back [16, 17]. To date, advanced molecular technologies have not been widely used in low income countries where brucellosis is endemic in livestock and humans [7, 18]. Thus, information on the prevailing Brucella species, biovars, and genotypes/strains in such areas of endemicity may shed new light on the epidemiology of Brucella infection and the species and biovars circulating. Besides this generic scientific rationale for undertaking such investigations, increased understanding of the Brucella epidemiology is critical for refining control of brucellosis in resource weak countries where the same measures as in high income countries cannot be applied.
In northern and eastern Uganda where this study was performed, there was a considerable mixing of livestock species during years of insurgency in the 1990s, presenting ideal conditions for inter- and intraherd transmission of diseases such as brucellosis. Indeed, high herd and individual animal seroprevalences of up to 27% and 7.5%, respectively, were recorded in a recent survey in the region [19]. This high prevalence may pose a severe threat to public health as previous studies around the capital of Uganda suggest milk or milk products from cows as a major source of Brucella infection in humans [20, 21]. The present study aimed at isolating and molecular-typing Brucella from cattle milk in northern and eastern Uganda for better understanding of its epidemiology.
2. Materials and Methods
2.1. Study Design and Collection of Samples
Milk samples were collected from 207 lactating cows in urban and periurban areas of Gulu and Soroti towns of northern and eastern Uganda from May 2011 to March 2012 for isolation of Brucella. A total of 110 individual cow milk samples were collected from 72 herds in Gulu and a total of 97 individual cow milk samples were collected from 33 herds in Soroti. These herds were part of the 166 herds whose animals had been screened for brucellosis and were within a radius of 15 Km in both Gulu and Soroti towns, with the two towns being 200 Km apart. The cattle from which milk samples were taken had been screened for Brucella antibodies and in total 17 of the 207 cows were seropositive. In both towns, the seropositive herds from which the milk samples were taken were near each other. The numbers and selection of households and animals included are described in detail previously [19]. Midstream milk samples were collected from all quarters with 10–20 mL collected from each teat into sterile 100 mL falcon tubes. The samples were transported chilled to Makerere University College of Health Sciences microbiology laboratory, Kampala, Uganda, kept at 4°C, and cultured within three days.
2.2. Sample Preparation, Brucella Culturing, and Biotyping
The milk was centrifuged at 3000 ×g at 4°C for 15 minutes and the pellet and supernatant were plated on both Farrel and Centro de Investigación y Tecnología Agroalimentaria (CITA) selective media supplemented with calf serum [22]. Briefly, Farrel's medium was prepared from Brucella medium base (Oxoid, UK), sterilized at 121°C for 15 minutes, and supplemented with Brucella selective supplement (Oxoid, UK) according to the manufacturer's instructions. The CITA medium was prepared according to De Miguel et al. [22]. Inoculated plates were incubated at 37°C for 8 days in a 5–10% carbon-dioxide incubator and read every 24 hours from day three of incubation for colony growth. Resultant colonies were subcultured and biotyped based on their colony morphology, serum and carbon-dioxide requirement for growth, hydrogen sulphide production, urease activity, oxidase test, and growth in presence of dye basic fuchsin and agglutination of anti-Brucella IgG monospecific sera A (Animal Health and Veterinary Laboratories Agencies, Weybridge, UK), according to the OIE Terrestrial Manual [8]. Representative colonies are stored at −80°C in 20% glycerol for long-term storage.
2.3. Genomic DNA Extraction and Real-Time PCR Detection
The colonies that conformed to all the above phenotypic characteristics of Brucella were subjected to genomic DNA extraction using the Norgen bacterial genomic DNA isolation kit (Norgen Biotek Corp., Ontario, Canada). The extracted Brucella genomic DNA was used to run a real-time multiplex PCR assay with oligonucleotide primers, probes, reaction mixture, and PCR conditions according to Probert et al. [23]. Amplification and real-time fluorescence detection were done on the Rotor-Gene 3000 real-time PCR machine (Corbett Research-Corbett Life Sciences, Mort Lake Australia). Three positive and two negative controls were included in each run. When the cycle threshold (CT) value of the samples was ≤40, samples were evaluated as positive. This real-time multiplex PCR was designed to detect Brucella at both the genus and species levels for B. abortus and B. melitensis since it has the genus specific probe and the species specific probes for the two Brucella species commonly infecting cattle.
2.4. Brucella Species Confirmation
Positive samples on real-time PCR were analysed further for Brucella speciation using the Bruce-ladder multiplex PCR assay kit (Ingenasa, Spain). The oligonucleotide primers, reaction mixture, and PCR conditions were performed according to the manufacture's conditions in conformity with López-Goñi et al. [16]. However amplification was done in a MyCycler thermal cycler (BioRad).
2.5. Characterization of Isolates by MLVA Genotyping
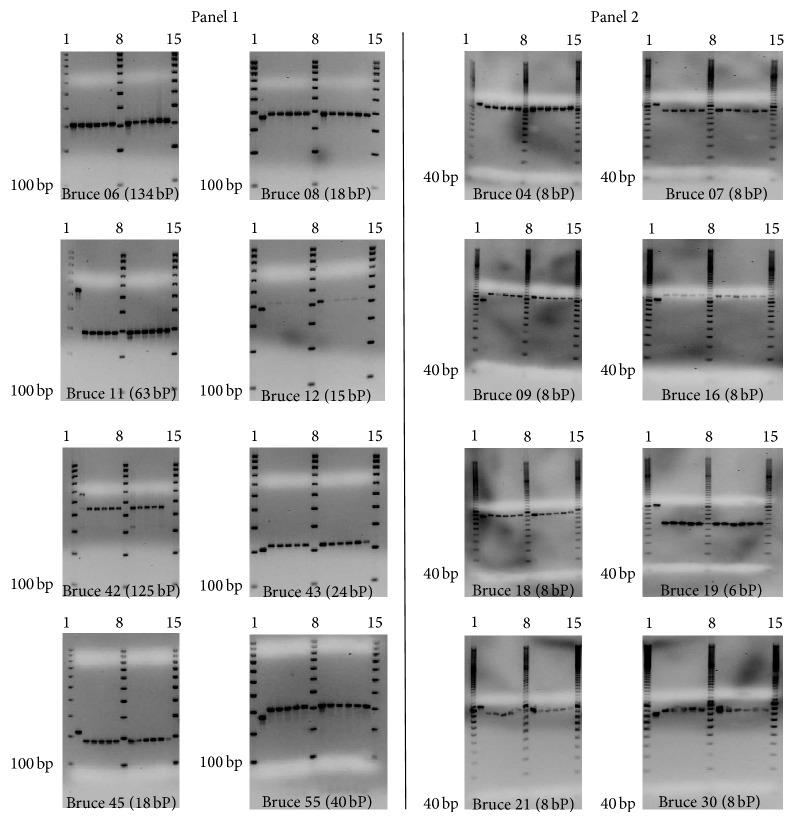
Genotyping was performed using the Multiple Locus Variable Number Tandem Repeat Analysis (MLVA), using the 16-primer-pair assay [17, 24–26]. The oligonucleotide primer pairs incorporated in the Brucella MLVA 16 assay target both the conserved and highly discriminatory regions of the Brucella genome. Each sample was run on three prescribed MLVA panels. Panel 1 consisted of moderately polymorphic minisatellite primer pairs targeting the highly conserved genomic regions of different Brucella species (bruce 06, bruce 08, bruce 11, bruce 12, bruce 42, bruce 43, bruce 45, and bruce 55). Panel 2A (bruce 18, bruce 19, and bruce 21) and panel 2B (bruce 04, bruce 07, bruce 09, bruce 16, and bruce 30) consisted of microsatellite primer pairs targeting the discriminatory genomic regions. The PCR amplification and genotyping were done according to le Flèche et al. [17] with only a modification in the total reaction volume to 30 μL. At each run, B. suis reference strain REF. 1330 (from Bruce-ladder kit) was included as shown in Figure 1.
Figure 1.
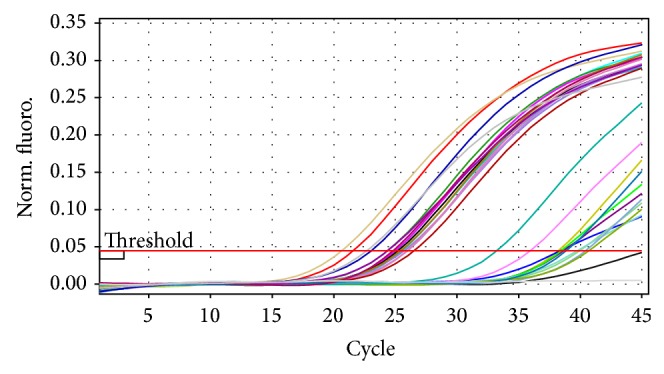
Triplex real-time PCR amplification pattern using the Brucella genus probe. Fluorescence ratio is plotted against the number of PCR cycles to monitor amplification in real-time mode. Isolates with weak Ct values (29.18 and above) had Brucella-like phenotypic characteristics and were included in this assay.
2.6. Gel-Electrophoresis Analysis of Panel 1 and Panel 2 Loci Amplification Products
Five microliters of the panel 1 and panel 2 loci amplification products were loaded into 3% and 2% agarose gel containing ethidium bromide (0.5 μg/mL), respectively, to visualize the banding pattern in the samples and positive controls, under UV illumination. The agarose gel was run on 8 V/cm current, and a 100 base pair and 20 base pair ladders (BioRad) were included per run for panel 1 and panel 2, respectively.
2.7. Sequencing the VNTR Locus Amplicons
In order to identify repeat copy number variation among the isolates in question the resulting PCR products were sequenced for each VNTR locus at Macrogen, Netherlands. Sequences were viewed using BioEDIT version 7.0.9.0. Sequencing was performed in both directions using the M13-primers according to Applied Biosystems (ABI). Since each of the VNTR locus primers was tagged with M13 primer ([M13-Forward] 5′-GTAAAACGACGGCCAGT-3′ and [M13-Rev] 5′-GCGGATAACAATTTCACACAGG-3′), this increases each VNTR locus PCR product size by 39 base pairs.
2.8. Analysis of MLVA Sequence Data
The MLVA PCR products were sequenced per loci and the forward and reverse sequences were assembled into a contig in the Bionumerics software version 5.0 (Applied Maths, Belgium). The M13 primer tags were trimmed from the contig and the allele designation was determined by comparing the fragment size with the published allele numbering system (version 3.6 http://mlva.u-psud.fr Brucella support website for MLVA typing). The number of tandem repeats per loci was queried in the Brucella MLVA 2012 public database (http://mlva.u-psud.fr/mlvav4/genotyping/) accessed on February 21, 2014, for genotyping of our isolates. The closest related known strains were determined based on the genetic distance (the minimum number of changes in the number of repeats of any locus that converts one genotype to another).
3. Results
3.1. Biotyping
Based on the biotyping (Table 1), B. abortus biovar 1, 3, or 7 was isolated in 11 (5.3%) out of 207 milk samples. These 11 positive samples were all from seropositive cows (i.e., 11 of 17). The colonies being smooth eliminated B. canis and B. ovis which have rough colonies. Production of hydrogen sulfide eliminated B. melitensis, B. ceti, B. microti and B. abortus biovars 5 and 6, and B. suis except B. suis biovar 1. Ability to grow in absence of serum eliminated B. abortus biovar 2 that generally requires serum for growth. Brucella abortus biovar 2, B. neotomae, and B. suis biovar 1 were eliminated by their inability to grow in basic fuchsin. Agglutination with anti-Brucella monospecific sera A eliminated B. abortus biovars 4 and 9.
Table 1.
Phenotypic characteristics of Brucella spp. isolated from cattle milk from northern and eastern Uganda in 2011-2012.
| Sample ID | Serological result | Colony morphology | Serum requirement | CO2 requirement | Oxidase test | Urease activity | Agglutination with monospecific sera A | H2S production | Growth in basic fuchsin | Biovar |
|---|---|---|---|---|---|---|---|---|---|---|
| S406/01 | + | S | − | − | + | + | + | + | + | 1 or 3 |
| S403/07 | + | S | − | − | + | + | + | + | + | 1 or 3 |
| S03/03 | + | S | − | − | + | + | + | + | + | 1 or 3 |
| S37/02 | + | S | − | − | + | + | + | + | + | 1 or 3 |
| S406/02 | + | S | − | − | + | + | + | + | + | 1 or 3 |
| G41/01 | + | S | − | − | + | + | + | + | + | 1 or 3 |
| G86/14 | + | S | − | − | + | + | + | + | + | 1 or 3 |
| S403/12 | + | S | − | − | + | + | + | + | + | 1 or 3 |
| S403/09 | + | S | − | − | + | + | + | + | + | 1 or 3 |
| S01/08 | + | S | − | − | + | + | + | + | + | 1 or 3 |
| S02/10 | + | S | − | − | + | + | + | + | + | 1 or 3 |
S: smooth colonies.
3.2. Molecular Characterization
3.2.1. Brucella DNA Detection by Real-Time PCR
DNA from all the 11 isolates that was judged as B. abortus by the biotyping was detected as Brucella DNA by the Brucella genus probe in the triplex real-time PCR (Figure 1). However the triplex real-time PCR was unable to detect the Brucella species involved using its B. melitensis probe (Figure 2) and B. abortus probe (Figure 3).
Figure 2.
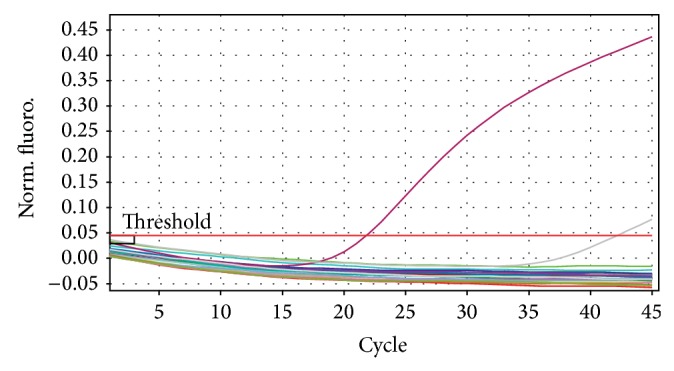
Triplex real-time PCR amplification pattern using the B. melitensis probe. Fluorescence ratio is plotted against the number of PCR cycles to monitor amplification in real-time mode. Only B. melitensis (positive control) was picked by this probe.
Figure 3.
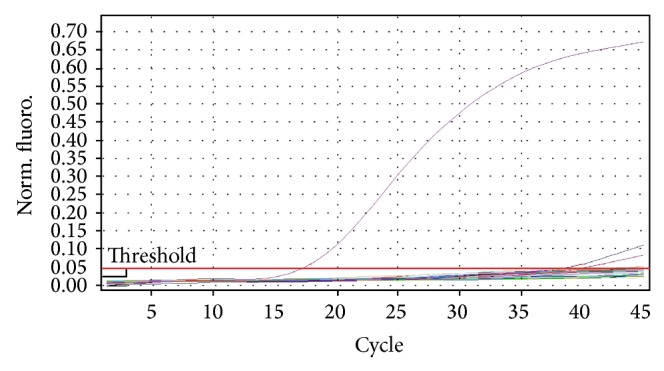
Triplex real-time PCR amplification pattern using the B. abortus probe. Fluorescence ratio is plotted against the number of PCR cycles to monitor amplification in real-time mode. Only B. abortus (positive control) was picked with a strong Ct value by this probe.
3.2.2. Brucella Species Confirmation by Bruce-Ladder
DNA from all the 11 isolates that were confirmed as belonging to the genus Brucella in the triplex real-time PCR was detected as B. abortus DNA by the Bruce-ladder multiplex PCR (Figure 4). B. abortus gives two bands of 1682 bp and 587 bp on Bruce-ladder PCR.
Figure 4.
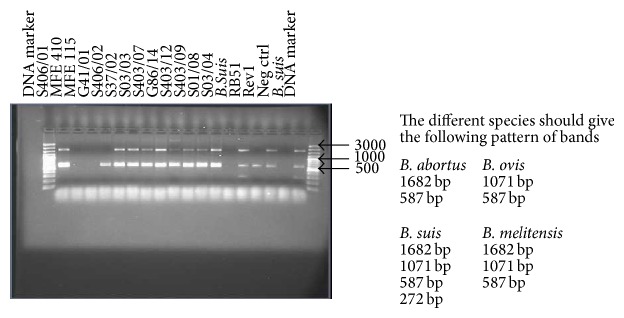
Bruce-ladder multiplex PCR agarose gel picture used to confirm the Brucella species isolated. Extreme left and right lanes are for 100 bp molecular weight marker; from left to right: lanes 2, 5–13 are for DNA from isolates detected as Brucella with strong Ct values; lanes 3, 4, and 14 are for DNA from Brucella-like isolates with weak Ct values; lanes 15 and 19 are for B. suis positive control DNA; lane 16 is for RB 51 positive control DNA; lane 17 is for Rev 1 positive control DNA; and lane 18 is for PCR grade water (negative control).
3.2.3. MLVA Genotyping
The MLVA-16 assay revealed that none of the 11 isolates did match any of the Brucella isolates in the Brucella MLVA 2012 database but closely resembled the former B. abortus biovar 7 strain 07-994-2411 from Kenya (Table 2). All isolates obtained were monomorphic at all loci as shown in Table 2 and Figure 5. We designated these isolates as UG Ba-m because they were isolated from cattle in Uganda and had a B. abortus profile on most biotyping assays but resembled both B. melitensis and B. abortus at genotyping. Both of the UG Ba-m isolates and the B. abortus strain 07-994-2411 showed close resemblance to a human B. melitensis biovar 1 (strain BCCN87-92) strain isolated from USA. When compared on MLVA-8 panel 1 loci the genetic distance was only zero between UG Ba-m isolates and B. abortus strain 07-994-2411 and one was between UG Ba-m isolates and B. melitensis biovar 1 strain BCCN87-92.
Table 2.
Brucella spp. isolates recovered from cattle milk samples in northern and eastern Uganda (2011-2012) and their MLVA 16 profile compared to that of some known Brucella strains from Brucella MLVA database and strains conforming to B. abortus former biovar 7.
| Sample ID/strains | Year | Region | Host | Specimen | Number of repeats for the polymorphic panel 1 and panel 2 Bruce loci | Species | Biovar | Genotype | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Panel 1 | Panel 2A | Panel 2B | |||||||||||||||||||||
| 6 | 8 | 11 | 12 | 42 | 43 | 45 | 55 | 18 | 19 | 21 | 4 | 7 | 9 | 16 | 30 | ||||||||
| G41/01 | 2011 | Gulu | Cattle | Milk | 2 | 4 | 2 | 12 | 3 | 2 | 3 | 3 | 5 | 9 | 6 | 4 | 2 | 9 | 8 | 4 | B. abortus | ND | UG Ba-m |
| S406/02 | 2012 | Soroti | Cattle | Milk | 2 | 4 | 2 | 12 | 3 | 2 | 3 | 3 | 5 | 9 | 6 | 4 | 2 | 9 | 8 | 4 | B. abortus | ND | UG Ba-m |
| S37/02 | 2012 | Soroti | Cattle | Milk | 2 | 4 | 2 | 12 | 3 | 2 | 3 | 3 | 5 | 9 | 6 | 4 | 2 | 9 | 8 | 4 | B. abortus | ND | UG Ba-m |
| S03/03 | 2012 | Soroti | Cattle | Milk | 2 | 4 | 2 | 12 | 3 | 2 | 3 | 3 | 5 | 9 | 6 | 4 | 2 | 9 | 8 | 4 | B. abortus | ND | UG Ba-m |
| S403/07 | 2012 | Soroti | Cattle | Milk | 2 | 4 | 2 | 12 | 3 | 2 | 3 | 3 | 5 | 9 | 6 | 4 | 2 | 9 | 8 | 4 | B. abortus | ND | UG Ba-m |
| S406/01 | 2012 | Soroti | Cattle | Milk | 2 | 4 | 2 | 12 | 3 | 2 | 3 | 3 | 5 | 9 | 6 | 4 | 2 | 9 | 8 | 4 | B. abortus | ND | UG Ba-m |
| G86/14 | 2011 | Gulu | Cattle | Milk | 2 | 4 | 2 | 12 | 3 | 2 | 3 | 3 | 5 | 9 | 6 | 4 | 2 | 9 | 8 | 4 | B. abortus | ND | UG Ba-m |
| S403/12 | 2012 | Soroti | Cattle | Milk | 2 | 4 | 2 | 12 | 3 | 2 | 3 | 3 | 5 | 9 | 6 | 4 | 2 | 9 | 8 | 4 | B. abortus | ND | UG Ba-m |
| S403/09 | 2012 | Soroti | Cattle | Milk | 2 | 4 | 2 | 12 | 3 | 2 | 3 | 3 | 5 | 9 | 6 | 4 | 2 | 9 | 8 | 4 | B. abortus | ND | UG Ba-m |
| S09/08 | 2012 | Soroti | Cattle | Milk | 2 | 4 | 2 | 12 | 3 | 2 | 3 | 3 | 5 | 9 | 6 | 4 | 2 | 9 | 8 | 4 | B. abortus | ND | UG Ba-m |
| S02/10 | 2012 | Soroti | Cattle | Milk | 2 | 4 | 2 | 12 | 3 | 2 | 3 | 3 | 5 | 9 | 6 | 4 | 2 | 9 | 8 | 4 | B. abortus | ND | UG Ba-m |
| *REF 1330 | — | USA | Swine | — | 2 | 3 | 6 | 10 | 4 | 1 | 5 | 2 | 4 | 38 | 9 | 6 | 6 | 5 | 5 | 3 | B. suis | Biovar 1 | 33 (MLVA-11) |
| aBCCN#87-92 | 1997 | USA | Human | — | 2 | 4 | 2 | 12 | 4 | 2 | 3 | 3 | 9 | 36 | 7 | 2 | 4 | 3 | 3 | 5 | B. melitensis | Biovar 1 | 126 (MLVA-11) |
| aREF 544 | 1942 | England | Bovine | — | 4 | 5 | 4 | 12 | 2 | 2 | 3 | 3 | 5 | 42 | 8 | 3 | 5 | 3 | 4 | 5 | B. abortus | Biovar 1 | 83 (MLVA-11) |
| aREF 86/8/59 | 1959 | England | Bovine | — | 4 | 5 | 4 | 12 | 2 | 1 | 3 | 3 | 6 | 42 | 8 | 3 | 4 | 3 | 3 | 5 | B. abortus | Biovar 2 | 80 (MLVA-11) |
| aREF Tulya | 1958 | Uganda | Human | — | 3 | 5 | 4 | 11 | 2 | 2 | 3 | 3 | 8 | 40 | 8 | 6 | 5 | 3 | 11 | 5 | B. abortus | Biovar 3 | 64 (MLVA-11) |
| aREF 292 | 1961 | England | Bovine | — | 4 | 5 | 4 | 12 | 2 | 2 | 3 | 2 | 6 | 42 | 8 | 3 | 4 | 3 | 3 | 5 | B. abortus | Biovar 4 | 78 (MLVA-11) |
| aREF B3196 | 1959 | England | Bovine | — | 3 | 5 | 3 | 12 | 2 | 2 | 2 | 3 | 7 | 42 | 8 | 6 | 7 | 3 | 3 | 3 | B. abortus | Biovar 5 | 67 (MLVA-11) |
| aREF 870 | 1959 | Africa | Bovine | — | 3 | 5 | 3 | 12 | 2 | 2 | 3 | 3 | 7 | 42 | 8 | 3 | 6 | 3 | 3 | 3 | B. abortus | Biovar 6 | 66 (MLVA-11) |
| aREF C68 | 1958 | England | Bovine | — | 3 | 5 | 3 | 12 | 2 | 2 | 2 | 3 | 7 | 42 | 8 | 6 | 6 | 3 | 3 | 3 | B. abortus | Biovar 9 | 67 (MLVA-11) |
| aBCCN#V1 (S19) | 1943 | USA | Bovine | — | 4 | 5 | 4 | 12 | 2 | 2 | 3 | 3 | 6 | 42 | 8 | 3 | 5 | 3 | 3 | 5 | B. abortus | Biovar 1 | 82 (MLVA-11) |
| aBCCN#V5 (RB51) | — | USA | Bovine | — | 4 | 5 | 4 | 12 | 2 | 3 | 3 | 3 | 6 | 42 | 8 | 3 | 7 | 3 | 3 | 5 | B. abortus | Biovar 1 | 79 (MLVA-11) |
| a13 | 2006-2007 | Egypt | Cattle | Spleen | 4 | 5 | 4 | 12 | 2 | 3 | 3 | 3 | 6 | — | 8 | 3 | 7 | 3 | 3 | 5 | B. abortus | — | — |
| a4 | 2002–2007 | Egypt | Cattle | — | 4 | 5 | 4 | 12 | 2 | 3 | 3 | 3 | 6 | — | 8 | 3 | 9 | 3 | 3 | 5 | B. abortus | — | — |
| a6-KEBa 1 | 2009 | Kenya | Cattle | Abortion material | 3 | 5 | 4 | 11 | 2 | 2 | 3 | 3 | 7 | 40 | 8 | 6 | 6 | 3 | 11 | 6 | B. abortus | Biovar 3 | 34 (MLVA-8) |
| a11-KEBa 2 | 2009 | Kenya | Cattle | Vaginal discharge | 3 | 5 | 4 | 11 | 2 | 2 | 3 | 3 | 7 | 40 | 8 | 6 | 5 | 3 | 12 | 5 | B. abortus | Biovar 3 | 34 (MLVA-8) |
| aBCCN#93-26 | 1993 | Sudan | Dromedary | — | 3 | 5 | 4 | 11 | 2 | 2 | 3 | 3 | 6 | 40 | 8 | 6 | 8 | 3 | 7 | 7 | B. abortus | Biovar 3 | 63 (MLVA-11) |
| b07-994-2411 | 1963 | Kenya | Bovine | — | 2 | 4 | 2 | 12 | 3 | 2 | 3 | 3 | 5 | 22 | 6 | 5 | 2 | 6 | 7 | 5 | B. abortus | ND | ND |
| b03-4923-239 | 2003 | Turkey | Bovine | — | 4 | 5 | 3 | 12 | 2 | 2 | 3 | 1 | 6 | 21 | 8 | 6 | 7 | 6 | 3 | 3 | B. abortus | ND | ND |
| b99-9971-135 | 1988 | Mongolia | Bovine | — | 4 | 5 | 5 | 12 | 2 | 2 | 2 | 2 | 6 | 21 | 8 | 5 | 6 | 4 | 3 | 3 | B. abortus | ND | ND |
| b99-9971-159 | 1993 | Mongolia | Bovine | — | 4 | 5 | 5 | 12 | 2 | 2 | 2 | 2 | 6 | 21 | 8 | 5 | 6 | 4 | 3 | 3 | B. abortus | ND | ND |
aStrains downloaded from Brucella MLVA database February 2014; http://mlva.u-psud.fr; *the reference strain used (from Bruce-ladder kit); ND: not designated; bstrains conforming to B. abortus former biovar 7.
Figure 5.
MLVA amplification pattern of isolates in this study and a B. suis as control. Lanes 1, 8, and 15 show DNA marker, lane 2 in each gel shows pattern for B. suis, and lanes 3–7 and 9–14 show the amplification pattern of isolates 1–11 (G41/01, S406/02, S37/02, S03/03, S403/07, S406/01, G86/14, S403/12, S403/9, S09/08, and S02/10).
4. Discussion
Here we present for the first time phenotypic and molecular characterization of Brucella isolates from cattle milk in Uganda. These results contribute to better understanding of geographical transmission patterns of Brucella in cattle in Uganda and are important if specific control measures are to be implemented in the future. In Uganda, most of the milk is marketed unprocessed through the informal milk marketing linkages, thus acting as a potential source of human brucellosis infections.
All UG Ba-m isolates were from Brucella seropositive cattle conforming to the well-known fact that Brucella infected lactating female animals shed the bacteria in their milk since the organism relocates to the udder from the pregnant uterus upon delivery [27]. This has public health implications in the region since most of the milk is consumed unpasteurized. One third of the seropositive cattle were not shedding the Brucella in the milk suggesting that these animals either had cleared the infection or were chronically sick, thus not shedding the bacteria as shown in a study by Capparelli et al. [28]. All UG Ba-m isolates being from seropositive cattle suggest an active infection. Notably, Brucella was not isolated from milk from any of the seronegative cows. This suggests that seroconversion precedes shedding of the Brucella in milk, and thus serological tests can be sufficient in predicting possible shedders.
The phenotypic characteristics of all UG Ba-m isolates matched those of B. abortus biovars 1, 3 or the former biovar 7. All isolates being B. abortus conform to the well-known fact that B. abortus is the predominant species in cattle [14]. Furthermore, all 11 UG Ba-m isolates having the same phenotypic profile suggest that they belong to the same biovar attesting to the suggestion of regional predominance of certain Brucella biovars in Africa, for instance, predominance of B. abortus biovar 6 in nomadic cattle in Western Sudan [29], B. abortus biovar 3 and B. melitensis biovar 1 in cattle in Kenya [9], and B. melitensis biovar 3 in ruminants in Egypt [15]. However the numbers of isolates in these studies were too few to make a solid basis for generalization.
Detection of all the 11 UG Ba-m isolates as Brucella by the Brucella genus specific probe in the triplex real-time PCR with strong signals confirmed them as Brucella. The inability of the B. melitensis probe in the triplex real-time PCR to detect the isolates as B. melitensis suggested that they are not B. melitensis. The inability of the B. abortus species specific probe in the triplex real-time PCR to detect the isolates as B. abortus suggested that they are not B. abortus biovars 1, 2, 3, 4, 5, 6, and 9 but could be the former B. abortus biovar 7 since the primers used were not targeting B. abortus biovar 7 [23, 30].
All the 11 UG Ba-m isolates were confirmed as B. abortus by the Bruce-ladder multiplex PCR. The inability of the triplex real-time PCR to detect these isolates at its B. abortus species specific probe contrary to the Bruce-ladder multiplex PCR suggests that these isolates belong to the former Brucella abortus biovar 7. This suggestion is based on the fact that the triplex real-time PCR was not designed to detect the former Brucella abortus biovar 7 with its B. abortus species specific probe (detecting only B. abortus biovars 1, 2, 3, 4, 5, 6, and 9), and the Bruce-ladder multiplex PCR was designed to detect all the B. abortus biovars including the former B. abortus biovar 7 [16, 23, 30]. This suggests that a diphasic PCR protocol involving the triplex real-time PCR by Probert et al. [23] and the Bruce-ladder multiplex PCR by López-Goñi et al. [16] could be used to replace the risky procedure of identifying the former B. abortus biovar 7 using the conventional biotyping methods by detecting particularly its agglutination with monospecific anti-sera A and M and its growth in both basic fuchsin and thionin dyes. This protocol needs, however, to be tested on all the former B. abortus biovar 7 isolates.
The evidence adduced using a combination of phenotypic and molecular approaches designated all UG Ba-m isolates as atypical B. abortus without a biovar designation. All the isolates were monomorphic at molecular analysis, which could be due to the isolates being from a small geographical region of approximately 15 Km radius per region (data not shown here) and having been collected in a short time frame making it possible for all the isolates to be from a common source as animals mix in the grazing grounds. The genetic monomorphism observed is partly congruent with that observed in five B. melitensis biovar 1 isolates obtained from bovine milk in central Kenya by Muendo et al. [9], although their finding was in a different Brucella species. Our results are further supported by findings by Garin-Bastuji et al. [31] who found similar monomorphism in isolates in Mongolia isolated 5 years apart in the same region. The genetic monomorphism exhibited at the minisatellite and microsatellite loci that are otherwise polymorphic even in highly genetic homogenous species like Brucella suggests that there is one or very few circulating strains of Brucella in this region of Uganda attesting to the regional predominance of Brucella biovars and strains.
The closest known strain for the 11 UG Ba-m isolates was a B. abortus strain 07-994-2411 isolated from cattle in the neighboring Kenya in 1963. This strain has no biovar designation but was formerly known as B. abortus biovar 7, before biovar 7 was suspended from the Brucella nomenclature (International Committee on Systematic Bacteriology, 1988). B. abortus biovar 7 was suspended from Brucella nomenclature because the reference strain (63/75) was thought to be a mixture of B. abortus biovars 3 and 5. A recent study by Garin-Bastuji et al. [31] proposed the reintroduction of B. abortus biovar 7 in the approved list of bacterial names having identified B. abortus strains from Turkey, Mongolia, and Kenya that perfectly matched the former B. abortus biovar 7 characteristics. B. abortus biovar 7 can be differentiated from other B. abortus biovars by its ability to agglutinate with anti-A and anti-B monospecific sera. B. abortus biovar 7 has smooth colonies, does not require carbon dioxide for growth, produces hydrogen sulphide, is oxidase and urease positive, does not agglutinate with monospecific anti-sera R, grows in the presence of dyes thionin and basic fuchsin at a concentration of 20 μg/mL, and is lysed by phages Tbilisi (Tb), Weybridge (Wb), Izatnagar 1 (LZ1), and R/C.
A genetic difference in the UG Ba-m isolates at 5 loci out of 16 polymorphic loci examined compared to the known closest related strain (07-994-2411) from Kenya in a period of half a century could be a result of mutations and proves the ability of MLVA to differentiate strains from different localities, a finding congruent with that of Verger et al. [2]. The observed genetic similarity between the Kenyan strain and the 11 UG Ba-m isolates compared to other isolates from distant places could be due to the cross-border transmission of Brucella in cattle that could have been facilitated by cattle rustling across the pastoral Karamoja subregion of Uganda and Kenya over the past years.
5. Conclusions
In conclusion, our findings suggest B. abortus without biovar designation (atypical B. abortus) as a cause of brucellosis in cattle in northern and eastern Uganda. The Ugandan isolates exhibited a single MLVA-16 pattern and show in turn high levels of genetic variation when compared with other African strains, highlighting the usefulness of MLVA as an epidemiological tool for investigation of Brucella infections. Furthermore, the ability of a diphasic PCR protocol involving the triplex real-time PCR by Probert et al. [23] and the Bruce-ladder multiplex PCR by López-Goñi et al. [16] to detect B. abortus could be used to replace the procedure of identifying the former B. abortus biovar 7 using the conventional biotyping methods.
Acknowledgments
This work was conducted in part using funds from the Swedish International Development Cooperation Agency (Sida), Carnegie Corporation of New York, Makerere University, and the SLU Global Food Security Research and Education Program 2010–2013, A Swedish Government Initiative, Ministry of Foreign Affairs, Sweden. The authors are grateful for the support and cooperation offered by the field veterinary staff and farmers in Gulu and Soroti during sample collection. They thank Professors Ignacio Moriyon and Amia Zuniga Ripa of Instituto de Salud Tropical, Universidad de Navarra, Spain, for their guidance and for providing part of the reagents used in this study.
Ethical Approval
This study entailed collection of milk samples from farmers' cattle. Ethical clearance was obtained from the Ethical Review Committee of the College of Veterinary Medicine, Animal Resources and Biosecurity, Makerere University. The farmers were informed of the study and their verbal consent was sought prior to commencement of data collection.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Godfroid J., Scholz H. C., Barbier T., et al. Brucellosis at the animal/ecosystem/human interface at the beginning of the 21st century. Preventive Veterinary Medicine. 2011;102(2):118–131. doi: 10.1016/j.prevetmed.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Verger J.-M., Grimont F., Grimont P. A. D., Grayon M. Brucella, a monospecific genus as shown by deoxyribonucleic acid hybridization. International Journal of Systematic Bacteriology. 1985;35(3):292–295. doi: 10.1099/00207713-35-3-292. [DOI] [Google Scholar]
- 3.Pappas G. The changing Brucella ecology: novel reservoirs, new threats. International Journal of Antimicrobial Agents. 2010;36(1):S8–S11. doi: 10.1016/j.ijantimicag.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Bruce D. Note on the discovery of a micro-organism in Malta fever. Practitioner. 1887;39:161–170. [Google Scholar]
- 5.Franco M. P., Mulder M., Gilman R. H., Smits H. L. Human brucellosis. The Lancet Infectious Diseases. 2007;7(12):775–786. doi: 10.1016/S1473-3099(07)70286-4. [DOI] [PubMed] [Google Scholar]
- 6.Scholz H. C., Nöckler K., Llner C. G., et al. Brucella inopinata sp. nov., isolated from a breast implant infection. International Journal of Systematic and Evolutionary Microbiology. 2010;60(4):801–808. doi: 10.1099/ijs.0.011148-0. [DOI] [PubMed] [Google Scholar]
- 7.McDermott J. J., Arimi S. M. Brucellosis in sub-Saharan Africa: epidemiology, control and impact. Veterinary Microbiology. 2002;90(1–4):111–134. doi: 10.1016/S0378-1135(02)00249-3. [DOI] [PubMed] [Google Scholar]
- 8.World Organisation for Animal Health. OIE Terrestrial Manual. chapter 2.4.3 2009. Bovine brucellosis. [Google Scholar]
- 9.Muendo E. N., Mbatha P. M., Macharia J., et al. Infection of cattle in Kenya with Brucella abortus biovar 3 and Brucella melitensis biovar 1 genotypes. Tropical Animal Health and Production. 2012;44(1):17–20. doi: 10.1007/s11250-011-9899-9. [DOI] [PubMed] [Google Scholar]
- 10.Ewalt D. R., Payeur J. B., Rhyan J. C., Geer P. L. Brucella suis biovar 1 in naturally infected cattle: a bacteriological, serological, and histological study. Journal of Veterinary Diagnostic Investigation. 1997;9(4):417–420. doi: 10.1177/104063879700900414. [DOI] [PubMed] [Google Scholar]
- 11.Schurig G. G., Sriranganathan N., Corbel M. J. Brucellosis vaccines: past, present and future. Veterinary Microbiology. 2002;90(1–4):479–496. doi: 10.1016/S0378-1135(02)00255-9. [DOI] [PubMed] [Google Scholar]
- 12.Moriyón I., Grilló M. J., Monreal D., et al. Rough vaccines in animal brucellosis: structural and genetic basis and present status. Veterinary Research. 2004;35(1):1–38. doi: 10.1051/vetres:2003037. [DOI] [PubMed] [Google Scholar]
- 13.Valdezate S., Navarro A., Villalón P., Carrasco G., Saéz-Nieto J. A. Epidemiological and phylogenetic analysis of Spanish human Brucella melitensis strains by multiple-locus variable-number tandem-repeat typing, hypervariable octameric oligonucleotide fingerprinting, and rpoB typing. Journal of Clinical Microbiology. 2010;48(8):2734–2740. doi: 10.1128/JCM.00533-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer M. E., Morgan W. J. B. Designation of neotype strains and of biotype reference strains for species of the genus Brucella Meyer and Shaw. International Journal of Systematic Bacteriology. 1973;23(2):135–141. doi: 10.1099/00207713-23-2-135. [DOI] [Google Scholar]
- 15.Menshawy A. M. S., Perez-Sancho M., Garcia-Seco T., et al. Assessment of genetic diversity of zoonotic Brucella spp. Recovered from livestock in Egypt using multiple locus VNTR analysis. BioMed Research International. 2014;2014:7. doi: 10.1155/2014/353876.353876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.López-Goñi I., García-Yoldi D., Marín C. M., et al. Evaluation of a multiplex PCR assay (Bruce-ladder) for molecular typing of all Brucella species, including the vaccine strains. Journal of Clinical Microbiology. 2008;46(10):3484–3487. doi: 10.1128/JCM.00837-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.le Flèche P., Jacques I., Grayon M., et al. Evaluation and selection of tandem repeat loci for a Brucella MLVA typing assay. BMC Microbiology. 2006;6, article 9 doi: 10.1186/1471-2180-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pappas G., Papadimitriou P., Akritidis N., Christou L., Tsianos E. V. The new global map of human brucellosis. The Lancet Infectious Diseases. 2006;6(2):91–99. doi: 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- 19.Mugizi R. D., Boqvist S., Nasinyama G. W. Prevalence of and factors associated with Brucella sero-positivity in cattle in urban and peri-urban farming systems in eastern and northern Uganda. Journal of Veterinary and Medical Science. In press. [DOI] [PMC free article] [PubMed]
- 20.Makita K., Fèvre E. M., Waiswa C., et al. Human Brucellosis in urban and peri-urban areas of Kampala, Uganda. Annals of the New York Academy of Sciences. 2008;1149:309–311. doi: 10.1196/annals.1428.015. [DOI] [PubMed] [Google Scholar]
- 21.Makita K., Fèvre E. M., Waiswa C., Eisler M. C., Welburn S. C. How human brucellosis incidence in urban Kampala can be reduced most efficiently? A stochastic risk assessment of informally-marketed milk. PLoS ONE. 2010;5(12) doi: 10.1371/journal.pone.0014188.e14188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Miguel M. J., Marín C. M., Muñoz P. M., Dieste L., Grilló M. J., Blasco J. M. Development of a selective culture medium for primary isolation of the main Brucella Species. Journal of Clinical Microbiology. 2011;49(4):1458–1463. doi: 10.1128/JCM.02301-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Probert W. S., Schrader K. N., Khuong N. Y., Bystrom S. L., Graves M. H. Real-time multiplex PCR assay for detection of Brucella spp., B. abortus, and B. melitensis. Journal of Clinical Microbiology. 2004;42(3):1290–1293. doi: 10.1128/JCM.42.3.1290-1293.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borriello G., Peletto S., Lucibelli M. G., Acutis P. L., Ercolini D., Galiero G. Link between geographical origin and occurrence of Brucella abortus biovars in cow and water buffalo herds. Applied and Environmental Microbiology. 2013;79(3):1039–1043. doi: 10.1128/AEM.02887-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al Dahouk S., Flèche P. L., Nöckler K., et al. Evaluation of Brucella MLVA typing for human brucellosis. Journal of Microbiological Methods. 2007;69(1):137–145. doi: 10.1016/j.mimet.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Whatmore A. M., Shankster S. J., Perrett L. L., et al. Identification and characterization of variable-number tandem-repeat markers for typing of Brucella spp. Journal of Clinical Microbiology. 2006;44(6):1982–1993. doi: 10.1128/JCM.02039-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tittarelli M., di Ventura M., de Massis F., et al. The persistence of Brucella melitensis in experimentally infected ewes through three reproductive cycles. Journal of Veterinary Medicine Series B: Infectious Diseases and Veterinary Public Health. 2005;52(9):403–409. doi: 10.1111/j.1439-0450.2005.00885.x. [DOI] [PubMed] [Google Scholar]
- 28.Capparelli R., Parlato M., Iannaccone M., et al. Heterogeneous shedding of Brucella abortus in milk and its effect on the control of animal brucellosis. Journal of Applied Microbiology. 2009;106(6):2041–2047. doi: 10.1111/j.1365-2672.2009.04177.x. [DOI] [PubMed] [Google Scholar]
- 29.Musa M. T., Jahans K. L., Fadalla M. E. Brucella biovars isolated from nomadic cattle in the southern Darfur Province of Western Sudan. Journal of Comparative Pathology. 1990;102(1):49–54. doi: 10.1016/S0021-9975(08)80006-0. [DOI] [PubMed] [Google Scholar]
- 30.Redkar R., Rose S., Bricker B., Delvecchio V. Real-time detection of Brucella abortus, Brucella melitensis and Brucella suis . Molecular and Cellular Probes. 2001;15(1):43–52. doi: 10.1006/mcpr.2000.0338. [DOI] [PubMed] [Google Scholar]
- 31.Garin-Bastuji B., Mick V., Le Carrou G., et al. Examination of taxonomic uncertainties surrounding Brucella abortus bv. 7 by phenotypic and molecular approaches. Applied and Environmental Microbiology. 2014;80(5):1570–1579. doi: 10.1128/AEM.03755-13. [DOI] [PMC free article] [PubMed] [Google Scholar]