Abstract
Dietary polyphenols present in a broad range of plant foods have been related to beneficial health effects. This review aims to update the current information about the modulation of the gut microbiota by dietary phenolic compounds, from a perspective based on the experimental approaches used. After referring to general aspects of gut microbiota and dietary polyphenols, studies related to this topic are presented according to their experimental design: batch culture fermentations, gastrointestinal simulators, animal model studies, and human intervention studies. In general, studies evidence that dietary polyphenols may contribute to the maintenance of intestinal health by preserving the gut microbial balance through the stimulation of the growth of beneficial bacteria (i.e., lactobacilli and bifidobacteria) and the inhibition of pathogenic bacteria, exerting prebiotic-like effects. Combination of in vitro and in vivo models could help to understand the underlying mechanisms in the polyphenols-microbiota-host triangle and elucidate the implications of polyphenols on human health. From a technological point of view, supplementation with rich-polyphenolic stuffs (phenolic extracts, phenolic-enriched fractions, etc.) could be an effective option to improve health benefits of functional foods such as the case of dairy fermented foods.
1. Introduction
More and more studies confirm the importance of the gut microbiota in host health, including mental health. Gut bacteria not only help us to maximize the absorption of nutrients and energy, but also are essential in the body health status [1]. In particular, microbial infections and imbalances in the composition of the gut microbiota are associated with intestinal disorders such as chronic inflammatory bowel diseases and with other immune related disorders [2, 3]. Although genetic and environmental factors are main determinants of gut microbiota composition, it is well established that diet influences microbial fermentation and total bacteria in the intestine. In fact, interindividual variation in gut microbiota may, in part, reflect differences in dietary intake, although the response of the gut microbiota to dietary change can also differ among individuals [4].
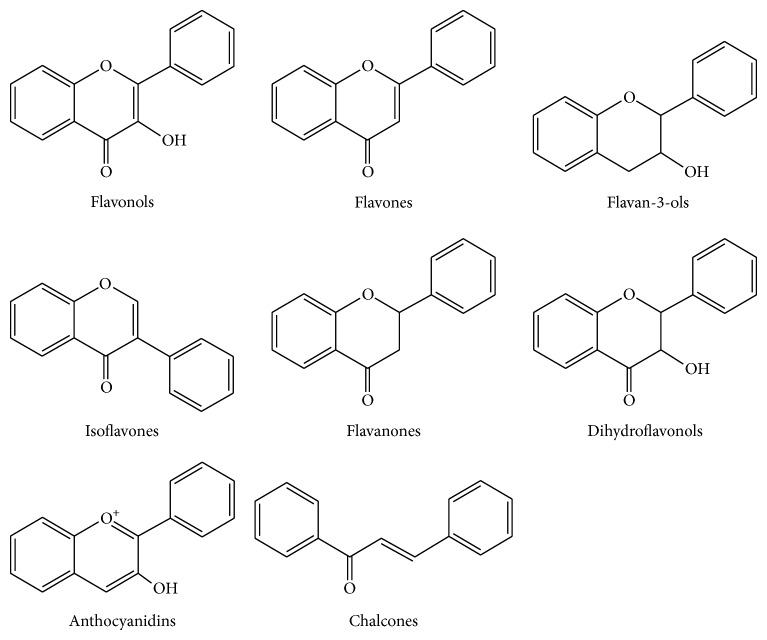
Phenolic compounds or polyphenols are secondary metabolites with a widespread occurrence in the plant kingdom. In nature, polyphenols can be classified into two major groups: flavonoids and nonflavonoids. Among flavonoids, various groups can be distinguished according to the C-heterocycle structure: flavonols, flavones, flavan-3-ols, isoflavones, flavanones, dihydroflavonols, anthocyanidins, and chalcones (Figure 1). Nonflavonoid phenolics include phenolic acids, hydrolysable tannins, and stilbenes, among others. Polyphenols also form part of the human diet, being present in a broad range of commonly consumed fruits, vegetables, and plant-derived products such as cocoa, tea, or wine. A number of epidemiological studies have shown that the intake of diets rich in fruits and vegetables is inversely associated with the risk of various chronic diseases, such as coronary heart disease, specific cancers, and neurodegenerative disorders [5–7]. Indeed, a range of pharmacological effects have been demonstrated for different phenolic compounds—especially flavonoids—through in vitro, ex vivo and animal assays [8, 9]. However, health effects of these compounds depend on their bioavailability and, therefore, it is important to understand how they are absorbed, metabolized, and eliminated from the body, in order to ascertain their in vivo actions.
Figure 1.
Common phenolic compounds in food.
Modulation of gut microbiota by polyphenols has been a topic of increasing attention by the scientific community in the last years. Several studies have been carried out by different authors ranging from the simplest experimental approaches on the effect of polyphenols on the growth of isolated intestinal bacteria to complex approximations implying the whole fecal microbiota, either in fermentation experiments (batch cultures and continuous simulators) or through compositional analysis of animal and human fecal samples. The existing knowledge about relationships between polyphenols and gut microbiota has been object of many reviews from different perspectives. Thus, some authors have put their attention on the impact of food constituents (polyphenols included) in the gut microbiome [10, 11], while others have focused on the effects of dietary polyphenols on microbial modulation and their potential implications in human health [12–15]. Selma et al. [16] wrote probably the first review trying to put together the concepts of microbial degradation of polyphenols and modulation of gut microbiota by polyphenols and phenolic metabolites. This two-way interaction between phenolics and intestinal bacteria has been also reviewed focusing on wine [17] and tea polyphenols [18]. The development of improved biology and microbial techniques has allowed notable advances in the knowledge of the gut microbiota and their modulation by dietary components and hence polyphenols. The potential of the novel metabolomic approaches in the study of the impact of polyphenols on gut microbiome has been recently reviewed [19].
Being aware of all this previous reviewing work, we have aimed to update the available information about modulation of gut microbiota by dietary polyphenols with a perspective based on the experimental approaches used. After two general sections covering relevant aspects about gut microbiota (Section 2) and dietary polyphenols (Section 3), studies are presented according to their experimental design: batch culture fermentations (Section 4), gastrointestinal simulators (Section 5), animal model studies (Section 6), and human intervention studies (Section 7). Main findings and general conclusions generated from the different types of studies are finally discussed (Section 8).
2. Gut Microbiota Composition and Analysis
The human gut is the natural habitat of a large, diverse population and dynamics of microorganisms, mainly anaerobic bacteria, which have adapted to life on mucosal surfaces in the gut lumen. The acquisition of gut microbiota begins at birth and is strongly influenced by a range of factors that include host genetics, immunological factors, antibiotic usage, and also dietary habits [20]. The microbial content of the gastrointestinal tract changes along its length, ranging from a narrow diversity and low numbers of microbes in the stomach to a wide diversity and high numbers in the large intestine, which can reach 1012 CFU/mL [21]. Most of intestinal bacteria belong to phylum Firmicutes (including Clostridium, Enterococcus, Lactobacillus, and Ruminococcus genera) and Bacteroidetes (including Prevotella and Bacteroides genera) which constitute over 90% of known phylogenetic categories and dominate the distal gut microbiota [22]. Recently, a novel classification of microbiota into three predominant “enterotypes,” dominated by three different genera, Bacteroides, Prevotella, and Ruminococcus, has been suggested [23]. In this line, Wu et al. [24] demonstrated that long-term diet high in animal proteins and fats versus simple carbohydrates clustered the human subjects into the previously described enterotypes Bacteroides and Prevotella. However, there is a current debate if the enterotypes should be seen discontinuous or as a gradient [25]. But in any case, a common observation is that homeostasis and resilience are coupled to a highly diverse gut microbiota in healthy people, whereas inflammatory and metabolic disorders are linked to perturbations in the composition and/or functions of the gut microbiota [26].
Culture-based techniques employed to bacteria identification are fairly cheap, laborious, and time-consuming and gives a limited view of the diversity and dynamics of the gastrointestinal microbiota, with less than 30% of gut microbiota members having been cultured to date [27]. Since 1990s, the introduction of novel molecular biological procedures has made it possible to overcome some of these limitations with the use of culture-independent methods [28]. These procedures are based on sequence divergences of the small subunit ribosomal RNA (16S rRNA) and include techniques such as denaturing gradient gel electrophoresis (DGGE), terminal restriction fragment length polymorphism (T-RFLP), fluorescence in situ hybridization (FISH), quantitative polymerase chain reaction (qPCR), DNA microarrays, and next-generation sequencing (NGS) of the 16S rRNA gene or its amplicons [29]. NGS techniques have promoted the emergence of new, high-throughput technologies, such as genomics, metagenomics, transcriptomics, and metatranscriptomics. Metagenomics gives a more in-depth, unbiased microbial analysis beyond the group level and involves multiple species, besides showing shorter sequencing speed, extended read length, and lower costs [30]. However, the enormous amount of data generated becomes cumbersome to analyze and requires lots of dedicated time as well as expertise to manage [29].
In the context of polyphenol-microbiota interactions, the emerging high-throughput meta-genomic, transcriptomic, and proteomic approaches can be adopted to identify genes and micro-organisms involved in polyphenol (in)activation and conversion, to reconstruct metabolic pathways, and to monitor how microbial communities adjust their metabolic activities upon polyphenol exposure [30]. Application of these technologies to human fecal samples requires further investigation to determine how these samples reflect metabolism inside the gut and, ultimately, to improve the understanding of the impact of polyphenols on host health [12, 31].
3. Dietary Polyphenols
It has been estimated that 90–95% of dietary polyphenols are not absorbed in the small intestine and therefore reach the colon [32], although absorption and metabolism are largely influenced by their chemical structure. Most flavonoids are poorly absorbed from the small intestine and are highly metabolized in the large intestine. Isoflavones seem to be the best absorbed dietary flavonoids; catechins, flavanones, and flavonol glycosides are intermediate, whereas proanthocyanidins, flavan-3-ol gallates, and anthocyanins would be the worst absorbed [33].
The first step in the metabolism of flavonoids, with the exception of flavan-3-ols (i.e., catechins and proanthocyanidins), is likely to be deglycosylation before absorption in the small intestine. Hydrolysis of some flavonoid glycoside might already occur in the oral cavity, as both saliva and oral microbiota show β-glucosidase activity. But the mechanism most usually assumed for flavonoid deglycosylation is hydrolysis by lactase phlorizin hydrolase (LPH) in the brush-border of the small intestine epithelial cells [34, 35], so that the resulting aglycones would enter the enterocyte by passive diffusion. The resulting aglycone is rapidly biotransformed by phase II enzymes within the enterocyte and further in the liver, so that conjugated metabolites (i.e., glucuronides, O-mehtylethers, and/or sulphates) through the respective action of UDP-glucuronosyltransferase (UGT), catechol-O-methyltransferase (COMT), and sulphotransferases would be the circulating forms in the human body [36, 37].
Generally, a relevant fraction of dietary flavonoids is not absorbed in the small intestine and, together with the conjugated metabolites that returned to the intestinal lumen via enterohepatic circulation, reaches the large intestine where compounds are subjected to the action of the colonic microbiota. Intestinal bacteria show diverse deglycosylating activities, thus releasing aglycones that might be absorbed in a lesser extent and, more probably, degraded to simpler phenolic derivatives [38, 39]. Degradation of flavonoid aglycones by colonic microbiota involves ring-C cleavage and reactions affecting functional groups, such as dehydroxylation, demethylation, or decarboxylation [39]. Various hydroxylated aromatic compounds derived from the A-ring (e.g., phloroglucinol, 3,4-dihydroxybenzaldehyde, or 3,4-dihydroxytoluene) and phenolic acids derived from the B-ring have been reported as relevant products of the colonic transformation of flavonoids [40]. It has become evident that the beneficial effects attributed to dietary polyphenols appear to be due more to phenolic metabolites formed in the gastrointestinal tract, mainly derived from the action of gut bacteria, rather than to the original forms found in food [41].
In subsequent sections, main findings related to the modulation of gut microbiota by polyphenols are presented as obtained from different methodological approaches and microbial analysis techniques.
4. Studies Using Batch Culture Fermentations
Although in vivo human or animal intervention trials are physiologically most relevant to study both polyphenol metabolism and microbial modulation, in vitro tools have been designed to simulate intestinal conditions. In combination with in vivo trials, in vitro experiments may help to elucidate the extent bioconversion processes mediated by the host itself [42, 43]. The complexity of in vitro gut models is diverse, ranging from simple static models (batch culture fermentation) to advanced continuous models (gastrointestinal simulators).
Simple, static gut models, also known as batch-type cultures, are generally closed systems using sealed bottles or reactors containing suspensions of fecal material that are maintained under anaerobic conditions. They are relatively easy to operate and cost-effective, have a fair throughput, and allow for parallel screening. This model approach is primarily used to assess the stability of polyphenols in the presence of human-derived gut microbiota and to evaluate which environmental conditions favor or limit polyphenol bioconversion. However, these static gut models are only adequate for simulating short-term conditions in the gut; for assessment of long-term adaptations of the gut microbial community, more complex dynamic models are needed [12].
Table 1 reports different studies of modulation of gut microbiota by dietary polyphenols using batch-type cultures. Details about fermentation conditions (fecal concentration, polyphenol origin and dose, and incubation time) and microbial techniques used, and main effects on bacteria groups (growth enhancement, growth inhibition, or no effect) have been included. As general characteristics, fecal fermentations employed feces concentration ≤10% (w/v) and lasted 48 h maximum. Both pure phenolic compounds and phenolic-rich extracts were added to the fecal medium at a final concentration <10% (w/v), and changes in specific bacterial groups were mainly assessed by FISH analysis. A first relevant experiment using batch culture fermentation was carried out by Tzounis et al. [44] who found that the flavan-3-ol monomers [(−)-epicatechin and (+)-catechin] promoted the growth of Clostridium coccoides-Eubacterium rectale group, which is known to produce large amounts of butyrate, a short-chain fatty acid (SCFA) with anti-inflammatory, and antineoplasic properties; (+)-catechin also increased the growth of Lactobacillus-Enterococcus spp., Bifidobacterium spp., and Escherichia coli but decreased the growth of Clostridium histolyticum. Also using standard compounds, Hidalgo et al. [45] found that anthocyanins (i.e., malvidin-3-glucoside and a mixture of anthocyanins) significantly enhanced the growth of Lactobacillus-Enterococcus spp. and Bifidobacterium spp. In addition, malvidin-3-glucoside showed a tendency to promote the growth of the C. coccoides-E. rectale group.
Table 1.
Studies using batch culture fermentation.
| Reference | Fecal concentration | Phenolic compound/food | Dose | Time of incubation | Microbial technique | Growth enhancement | Growth inhibition | No effect |
|---|---|---|---|---|---|---|---|---|
| Tzounis et al. (2008) [44] | 10%, w/v | (+)-catechin | 150 mg/L, 1000 mg/L | <48 h | FISH |
Lactobacillus-Enterococcus spp. Bifidobacterium spp. C. coccoides-E. rectale group E. coli |
C. histolyticum group | |
|
| ||||||||
| Molan et al. (2009) [46] | 0.1%, v/v | Blueberry extracts | 5, 10 and 25% | 48 h | FISH | Lactobacilli Bifidobacteria |
||
|
| ||||||||
| Bialonska et al. (2010) [47] | 10%, w/v | Pomegranate extract and punicalagin | 10% | 48 h | FISH | Total bacteria Bifidobacterium spp. Lactobacillus-Enterococcus spp. |
C. coccoides-E. rectale group C. histolyticum group |
|
|
| ||||||||
| Mandalari et al. (2010) [48] | 10%, w/v | Almond skins | 1%, w/v predigested almond skins | <24 h | FISH | Bifidobacteria C. coccoides-E. rectale group |
C. histolyticum group | |
|
| ||||||||
| Fogliano et al. (2011) [49] | 5%, w/v | Water-insoluble cocoa fraction | 1%, w/v | 36 h | FISH | Bifidobacteria Lactobacilli |
||
|
| ||||||||
| Cueva et al. (2013) [51] | 10%, w/v | Grape seed extract fractions | 300–450 mg/L | <48 h | FISH | Lactobacillus-Enterococcus spp. | C. histolyticum group | |
|
| ||||||||
| Hidalgo et al. (2012) [45] | 10%, w/v | Malvidin-3-O-glucoside Anthocyanidins mixture |
20 mg/L and 200 mg/L 4850 mg/L and 48500 mg/L |
<24 h | FISH |
Lactobacillus-Enterococcus spp. Bifidobacterium spp. C. coccoides-E. rectale group |
||
|
| ||||||||
| Sánchez-Patán et al. (2012) [52] | 1% w/v | Red wine extract | 600 mg/L | 48 h | FISH | C. histolyticum group | Lactobacillus-Enterococcus spp. | |
|
| ||||||||
| Barroso et al. (2013) [53] | Red wine extract | 500 mg/L | 48 h | qPCR |
Lactobacillus spp. Bifidobacterium spp. Bacteroides spp. Ruminococcus spp. |
|||
Similar results have been observed in batch culture fermentations with phenolic-rich extracts from different sources. Molan et al. [46] found that the addition of blueberry extracts to a mixture of fecal bacterial populations significantly increased the number of lactobacilli and bifidobacteria (Table 1). In the same line, Bialonska et al. [47] reported enhancement of the growth of total bacteria, Bifidobacterium spp., and Lactobacillus-Enterococcus spp. in response to a commercial extract of pomegranate, without influencing the C. coccoides-E. rectale and C. histolyticum groups (Table 1). Mandalari et al. [48] suggested a potential prebiotic effect for natural and blanched almond skins as these foodstuffs, in fermentations with fecal microbiota, significantly increased the populations of bifidobacteria and C. coccoides-E. rectale group and decreased the number of C. hystolyticum group. These authors related the possible prebiotic effect by almond skins not only to a high amount of dietary fibre, but also to some phenolic compounds such as ferulic acid, flavan-3-ols, and flavonols present in the almond skins [48]. Fogliano et al. [49] carried out an in vitro fermentation with a water-insoluble cocoa fraction in a three-stage continuous culture colonic model system. It was observed that this cocoa fraction presented prebiotic activity producing a significant increase in lactobacilli and bifidobacteria, as well as an increase in butyrate production. They concluded that the coexistence of fermentable polysaccharides and free flavanol monomers in cocoa, such as catechins, might be very effective in the modification of gut microbiota. Similar conclusions were drawn by Pozuelo et al. [50], who found a significant increase of the growth of Lactobacillus reuteri and Lactobacillus acidophilus in the presence of a grape antioxidant dietary fiber naturally obtained from red grapes. Our research group carried out several batch culture fermentations of two flavan-3-ol fractions with different degree of polymerisation and wine polyphenols, with fecal microbiota from different healthy volunteers [51, 52]. Both flavan-3-ol fractions promoted the growth of Lactobacillus/Enterococcus spp. and inhibited the C. histolyticum group during fermentation, although the effects were only statistically significant with the less polymerized fraction. Wine polyphenols only showed a slight inhibition in the C. histolyticum group, probably due to their lower content in flavan-3-ols.
Additionally, this type of fermentations has also been used to assess the contribution of certain probiotic strains to the colonic metabolism of polyphenols. In this sense, Barroso et al. [53] carried out fermentations of a red wine extract inoculated with human microbiota obtained from the colonic compartments of a dynamic simulator, in the presence and absence of the probiotic strain L. plantarum IFPL935. Microbial analysis by qPCR indicated that red wine polyphenols induced greater variations among in vitro batches harboring different colon-region (ascending colon, descending colon, and effluent) microbiota than those found when L. plantarum IFPL935 was added. Batches inoculated with microbiota from the ascending colon were shown to harbor the major proportion of saccharolytic bacteria (Bacteroides, Bifidobacterium, and Prevotella) whereas Clostridium groups were found in major numbers in the batches inoculated with microbiota simulating the distal regions [53] (Table 1).
5. Studies Using Human Gastrointestinal Simulators
In contrast to short-duration experiments with static gut models, longer-term experiments are required when the adaptation of the gut microbial community to dietary polyphenols needs to be assessed. To this end, dynamic in vitro gut models such as the “Reading” model [54], the Simulator of the Human Intestinal Microbial Ecosystem (SHIME), the TNO Intestinal Model 2 (TIM2) [55, 56], and the recent gastrointestinal simulator set up in our Institute (SIMGI) (unpublished work) have been developed where gut microbiota are cultured over a longer time frame (days to weeks) in one or multiple connected, pH controlled vessels representing different parts of the gastrointestinal tract.
As an example of the versatility and potential of human gastrointestinal simulators, Table 2 reports a series of studies about modulation of gut microbiota by polyphenols using the SHIME [57, 58]. This validated model comprises stomach and small intestinal sections for predigestion of food as well as vessels stimulating the ascending, transcending, and descending parts of the human colon, allowing assessment of changes in the different colonic areas that are very challenging to access in a human intervention. However, it should be underlined that this approach takes for granted that the extracts reach intact the colonic region, and no nutrient absorption is considered. The use of the SHIME to investigate the effects of a soy germ powder on the fermentative capacity of the simulated microbiota of the colon was the aim of a study carried out by De Boever et al. [57]. They observed that the addition of the soy germ powder in a 2-week treatment resulted into an overall increase of bacterial marker populations (Enterobacteriaceae, coliforms, Lactobacillus spp., Staphylococcus spp., and Clostridium spp.), with a significant increase of 2 log10 units in the Lactobacillus spp. population. More recently, Kemperman et al. [31], using the twin-SHIME model, studied the influence of a bolus dose and a 2-week continuous administration of complex dietary polyphenols from black tea or red wine grape extracts on the colonic microbiota. The Twin-SHIME system, involving two models that run in parallel, was inoculated with the same fecal sample for direct comparison of the effect of the two polyphenol types. A combination of analyses including cultivation, PCR-denaturing gradient gel electrophoresis (DGGE), quantitative PCR, and high throughput pyrosequencing of the 16S ribosomal RNA gene was applied to characterize microbial community changes. This study showed that complex polyphenols in the context of a model system can modulate select members of the human gut microbiota, revealing novel targets potentially involved in polyphenol metabolism and/or resistant microbes to be further investigated for polyphenol metabolism or resistance mechanisms [31].
Table 2.
Studies using the gastrointestinal simulators (i.e., SHIME).
| Reference | Simulator | Phenolic compound/food | Dose | Time | Microbial technique | Population increase | Population decrease |
|---|---|---|---|---|---|---|---|
| De Boever et al. (2000) [57] | SHIME | Soy germ powder | 2.5 g/day | 2 weeks | Plate count | Enterobacteriaceae Coliforms Lactobacillus spp. Staphylococcus spp. Clostridium spp. |
|
|
| |||||||
| Kemperman et al. (2013) [31] | Twin-SHIME | Black tea extract | 3 × daily dosing (1000 mg polyphenols as total daily dose) | 2 weeks | Plate count qPCR PCR-DGGE pyrosequencing |
Klebsiella spp. Enterococci Akkermansia spp. |
Bifidobacteria Blautia coccoides Anaeroglobus spp. Victivallis spp. |
|
| |||||||
| Kemperman et al. (2013) [31] | Twin-SHIME | Red wine-grape extract | 3 × daily dosing (1000 mg polyphenols as total daily dose) | 2 weeks | Plate count qPCR PCR-DGGE pyrosequencing |
Klebsiella spp. Alistipes spp. Cloacibacillus spp. Victivallis spp. Akkermansia spp. |
Bifidobacteria Blautia coccoides group Anaeroglobus spp. Subdoligranulum spp. Bacteroides |
6. Animal Models Studies
It is widely assumed that preliminary evidence should be warranted in animal models before human intervention trials. Animal models contribute to better understanding the mechanisms and biological effects that could be likely to happen in the human body. The metabolism of polyphenols has been object of numerous animal studies (mostly in rodents), especially for their impact on metabolic disorders [58], but only a few of these studies have followed the dynamics and composition of the intestinal microbiota in association with polyphenol metabolites retrieved from the host. Caution is required in extrapolating results to humans because culture-independent comparisons have revealed that most bacterial genera and species found in mice are not seen in humans, although the distal gut microbiota of mice and humans harbors the same bacterial phyla [59]. In this section, studies performed in animals in order to assess the effects of polyphenols on the modulation of intestinal microbiota are summarized (Table 3). Experiments were mainly performed in rats, although other larger animals such as chicks, calves, or pigs have also been used. Gut microbial communities were evaluated by diverse methodologies including culture-based methods (plate count), DGGE, FISH, T-RFLP, qPCR, and metagenomic sequencing.
Table 3.
Animal model studies.
| Reference | Animal | Phenolic compound/food | Dose | Treatment duration | Microbial technique | Population increase | Population decrease |
|---|---|---|---|---|---|---|---|
| Hara et al. (1995) [60] | Pigs | Tea polyphenols | 0.2% (free access) | 2 weeks | Plate count | Lactobacilli | Total bacteria Bacteroidaceae C. perfringens |
|
| |||||||
| Ishihara et al. (2001) [61] | Calves | Green tea extracts | 1.5 g/day | 4 weeks | Plate count |
Bifidobacterium spp. Lactobacillus spp. C. perfringens |
|
|
| |||||||
| Smith and Mackie (2004) [66] | Rats | Proantocyanidins extracted from Acacia angustissima | 0.7% (low tannin diet) and 2.0% (high tannin diet) | 3.5 weeks treatment + 3.5 weeks washout | PCR-DGGE Dot blot hybridization |
Bacteroides fragilis group Bacteroides-Prevotella-Porphyromonas group Enterobacteriaceae |
C. leptum group |
|
| |||||||
| Dolara et al. (2005) [62] | Rats | Red wine polyphenols powder | 50 mg/kg | 16 weeks | Plate count | Lactobacilli Bifidobacteria |
Propionibacteria Bacteroides Clostridia |
|
| |||||||
| Sembries et al. (2006) [63] | Rats | Apple juice | free access | 4 weeks | Plate count | Lactobacilli Bifidobacteria |
|
|
| |||||||
| Sembries et al. (2003) [64] | Rats | Apple pomace juice colloid | 5% suppl. diet (free access) | 6 weeks | Plate count FISH |
Bacteroidaceae | |
|
| |||||||
| Larrosa et al. (2009) [68] | Rats | Resveratrol | 1 mg/kg/day | 25 days | Plate count | Lactobacilli Bifidobacteria |
|
|
| |||||||
| Molan et al. (2010) [69] | Rats | Blackcurrant extracts (leaf or berry) | 3 times/week: (i) 30 mg/kg (leaf) (ii) 13.4 mg/kg (berry) |
4 weeks | FISH | Lactobacilli (berry extract) Bifidobacteria (leaf and berry extracts) |
|
|
| |||||||
| Viveros et al. (2011) [65] | Broiler chicks | Grape pomace concentrate (GPC) Grape seed extract (GSE) | 60 g/kg diet (GPC) 7.2 g/kg diet (GSE) (free access) |
21 days | Plate count T-RFLP |
E. coli
Enterococcus spp. Lactobacillus spp. |
|
|
| |||||||
| Lacombe et al. (2013) [70] | Rats | Lowbush wild blueberries | 20 g feed/day (eq. 24 ± 5.2 mg anthocyanin/day) |
6 weeks | Metagenomic sequencing | Thermonospora spp. Corynebacteria spp. Slackia spp. |
Lactobacillus spp. Enterococcus spp. |
Animal studies performed in pigs [60] and in calves [61] demonstrated that tea polyphenols administration contributed to the improvement in the composition of the intestinal microbiota. Thus, the administration of tea polyphenols in pigs significantly increased the levels of lactobacilli whilst it diminished the levels of total bacteria and Bacteroidaceae, and a tendency to decrease in lecithinase positive clostridia including C. perfringens was also observed [60]. However, the reduction rate of Bifidobacterium spp. and Lactobacillus spp. was slow, while that of C. perfringens decreased faster in calves supplemented with the green tea extract [61].
Dolara et al. [62] showed that treatment with wine polyphenols in carcinogen-treated F344 rats was associated with a strong variation in the colonic microbiota, compared to the control-fed rats. Although the total bacterial counts and anaerobe/aerobe ratio of microorganisms in the feces from polyphenol-treated rats were similar to that from control rats, propionibacteria, Bacteroides, and Clostridia decreased while lactobacilli and bifidobacteria increased. Based on additional experiments, these authors concluded that reduction of oxidative damage, modulation of colonic flora, and variation in gene expression may be all connected in the action of wine polyphenols on the intestinal function and carcinogenesis.
In other study, rats fed with apple juice instead of drinking water showed more lactobacilli and bifidobacteria in fresh feces that differed from the controls by one-log10 colony forming units [63]. The same research group studied the effect of colloids isolated from apple pomace extraction juices on the intestinal microbiota in Wistar rats. An increase of Bacteroidaceae in almost one-log10 higher counts was observed in feces of rats fed with apple juice colloid than control rats [64]. Another animal experiment conducted to study the effect on intestinal microbiota, of the inclusion of grape pomace extracts in the diet of broiler chicks [65], found that, for the cecum, birds fed grape extracts had higher populations of E. coli, Lactobacillus, and Enterococcus species than birds in any other treatment group. These authors concluded that grape polyphenol-rich products modified the gut morphology and intestinal microbiota and increased the biodiversity degree of intestinal bacteria in broiler chicks.
Inclusion of condensed tannins (proanthocyanidins) extracted from Acacia angustissima on rat diet resulted in a shift in the predominant bacteria towards tannin-resistant Gram-negative Enterobacteriaceaeand Bacteroides species and reduced the number of Gram-positive C. leptum group [66]. Compatible results were obtained in an experiment with rats fed a proanthocyanidin-rich cocoa preparation [67], where the authors found a significant decrease in the proportion of Bacteroides, Clostridium, and Staphylococcus genera in the feces of cocoa-fed animals. Interestingly, reductions in Clostridium species were found to correlate with weight loss and decrease in body mass index.
Larrosa et al. [68] observed an increase in lactobacilli and bifidobacteria when resveratrol (3,5,4′-trihydroxy-trans-stilbene), which naturally occurs in grapes and grape-derived foodstuffs such as red wine, was administered to rats. After induction of colitis by dextran sulphate sodium, proliferation of both E. coli and enterobacteria was lower in rats treated with resveratrol than in control rats. This could be the result of an indirect effect of resveratrol-supplemented diet, which increased bifidobacteria and lactobacilli counts preventing the colonization and invasion of tissues by enterobacteria including E. coli.
Prebiotic activity of wild blackcurrant extracts observed in in vitro experiments was further confirmed in rats by Molan et al. [69]. A significant increase in the population size of lactobacilli and bifidobacteria was observed after daily administration of those extracts to rats. Similarly, a grape antioxidant dietary fibre preparation was found to increase the population of Lactobacillus spp. when fed to rats, whereas populations of Bifidobacterium spp. decreased and changes in E. coli and Bacteroides vulgatus counts were not significant [50].
Recently, Lacombe et al. [70] studied the composition and functional potential of the colon microbiota from rats fed a diet enriched in lowbush wild blueberries. Application of novel metagenomic techniques (Illumina shotgun sequencing) revealed a significant reduction in the relative abundance of the genera Lactobacillus and Enterococcus associated with wild blueberries intake. In addition, hierarchical analysis showed a significant increase in the relative abundance of the phylum Actinobacteria, the order Actinomycetales, and several novel genera under the family Bifidobacteriaceae and Coriobacteriaceae in the blueberries group. The authors indicated that although the microbiome of rats differs from humans, the applied model was a powerful tool to study population dynamics and related metabolic functions. Metagenomic studies can determine microbial community profiles, gene presence/absence and abundance, and functional repertoire; however, they can only infer an observed phenotype since a gene presence does not imply its expression or functionality [71].
7. Human Intervention Studies
Investigations involving the use of humans potentially provide the best models for studying the interactions of food components (e.g., polyphenols) with microbiota, although in vivo intervention trials hold inevitable practical and ethical limitations [12]. The use of cross-over designs where volunteers serve as their own control permits multilevel analysis schemes that increase power but requires a relevant number of volunteers to allow for statistically significant multivariate models [72]. Up to now, only a few studies have examined the in vivo impact of dietary polyphenols on the human gut microbiota, and most of them were focused on single polyphenol molecules and selected bacterial populations. A summary of human intervention studies about effects of polyphenols in the modulation of the intestinal microbiota is collected in Table 4. In these studies, the polyphenol dose used was much dependent on the type of food preparation and its concentration, normally ranging from 0.1 to 4%; the treatment time was also variable, from 10 days to 2 months, and the applied microbial techniques were diverse (plate count, DGGE, FISH, T-RFLP, and qPCR).
Table 4.
Human intervention studies.
| Reference | Volunteer number | Phenolic compound/food | Dose | Treatment duration | Microbial technique | Population increase | Population decrease | No effect |
|---|---|---|---|---|---|---|---|---|
| Okubo et al. (1994) [73] | 8 | Green tea (Sunphenon) | 0.4 g/3 times per day | 4 weeks | Plate count |
C. perfringens
Clostridium spp. |
||
|
| ||||||||
| Yamakoshi et al. (2001) [76] | 9 | Proantocyanidin-rich extract from grape seeds | 0.5 g/day | 6 weeks | Plate count | Bifidobacterium spp. | Enterobacteriaceae | |
|
| ||||||||
| Mai et al. (2004) [74] | 15 | Black tea | 700 mg tea solids/5 times per day | 21 days | FISH DGGE | Total bacteria | No changes | |
|
| ||||||||
| Clavel et al. (2005) [83] | 39 | Isoflavones | 100 mg/day | 2 months | TTGE FISH |
C. coccoides-E. rectale group Bifidobacterium spp. Lactobacillus-Enterococcus spp. Faecalibacterium prausnitzii subgroup |
||
|
| ||||||||
| Costabile et al. (2008) [84] | 31 | Whole grain wheat cereals | 48 g/day | 3 weeks | FISH | Bifidobacteria Lactobacilli | Total bacteria Bacteroides spp. C. histolyticum/perfringens group Acetobacterium spp. |
|
|
| ||||||||
| Jaquet et al. (2009) [87] | 16 | Coffee | 3 cups/day | 3 weeks | FISH DGGE | Bifidobacterium spp. | ||
|
| ||||||||
| Carvalho-Wells et al. (2010) [85] | 32 | Whole grain maize cereals | 48 g/day | 3 weeks | FISH | Bifidobacteria | Total bacteria Bacteroides spp. C. histolyticum/perfringens group Acetobacterium spp. |
|
|
| ||||||||
| Gill et al. (2010) [80] | 10 | Raspberry puree | 20 g/day | 4 days | PCR-DGGE | No changes in the profile of colonic bacteria | ||
|
| ||||||||
| Shinohara et al. (2010) [79] | 8 | Apples | 2 apples/day | 2 weeks | Plate count |
Lactobacillus spp. Streptococcus spp. Enterococcus spp. |
Enterobacteriaceae lecithinase-positive clostridia including C. perfringens, Pseudomonas spp. | |
|
| ||||||||
| Tzounis et al. (2011) [77] | 22 | Cocoa flavanol | 494 mg/day 29 mg/day | 4 weeks | FISH |
Bifidobacterium spp. Lactobacillus spp. |
C. histolyticum/perfringens group | |
|
| ||||||||
| Vendrame et al. (2011) [81] | 15 | Wild blueberry drink | 25 g wild blueberries/day | 6 weeks | qPCR |
Bifidobacterium spp. L. acidophilus |
Bacteroides spp. Prevotella spp. Enterococcus spp. C. coccoides |
|
|
| ||||||||
| Queipo-Ortuño et al. (2012) [78] | 10 | Red wine | 272 mL/day | 20 days | qPCR |
Enterococcus spp. Prevotella spp. Bacteroides Bifidobacterium spp. Bacteroides uniformis Eggerthella lenta Blautia coccoides-E. rectale group |
Clostridium spp. C. histolyticum group |
Actinobacteria |
|
| ||||||||
| Jin et al. (2012) [75] | 10 | Green tea | 1000 mL/day | 10 days | T-RFLP qPCR |
Bifidobacterium spp. | ||
|
| ||||||||
| Guglielmetti et al. (2013) [82] | 15 | Wild blueberries drink | 25 g wild blueberries/day | 6 weeks | qPCR | B. longum subsp. infantis | ||
|
| ||||||||
| Cuervo et al. (2014) [86] | 38 | Dairy products Fruits Vegetables Cereals |
Food intake was recorded using an annual food frequency questionnaire | qPCR | Lactobacillus |
B. coccoides
C. leptum |
||
In a study with a reduced number of subjects (n = 8), Okubo et al. [73] reported a notably increase in the percentages of Bifidobacterium spp. in total fecal counts after an intervention with a product containing 70% of tea polyphenols. A significant decrease of C. perfringens and other Clostridium spp. was also observed during the intake period. However, in a crossover feeding study (number of volunteers not reported) that investigated the effects of black tea drinking on hypercholesterolemic volunteers, Mai et al. [74] found that although specific bacterial groups were not affected, the total amount of bacteria significantly decreased, highlighting large interindividual variations. More recently, an intervention study (n = 10) by Jin et al. [75] confirmed an overall tendency for the proportion of bifidobacteria to increase because of green tea consumption, even though there were interindividual differences in the Bifidobacterium species.
Yamakoshi et al. [76] showed that administration of a proanthocyanidin-rich extract from grape seeds to healthy volunteers (n = 9) significantly increased the fecal number of Bifidobacterium, whereas the number of putrefactive bacteria such as enterobacteria tended to decrease. The interaction between proanthocyanidins and intestinal bacteria was also confirmed in a randomized, double-blind, crossover, and controlled intervention study (n = 22) ingesting two cocoa drinks exhibiting low and high polyphenol content [77]. Compared with the consumption of the low-flavan-3-ol cocoa drink, the daily consumption of the high-flavan-3-ol cocoa drink significantly increased the bifidobacteria and lactobacilli populations but significantly decreased clostridia counts.
Queipo-Ortuño et al. [78] performed a randomized, crossover, and controlled trial (n = 10) consisting of the intake of red wine, dealcoholized red wine, and gin over three consecutive periods. After the red wine period, the bacterial concentrations of proteobacteria, fusobacteria, Firmicutes, and Bacteroidetes markedly increased compared with the washout period; significant increases in the number of Bifidobacterium spp. and Prevotella spp. were also observed. However, Lactobacillus spp., Clostridium spp., and C. histolyticum group concentrations remained unchanged throughout the study.
In a small-scale observational study (n = 8), Shinohara et al. [79] found that the number of bifidobacteria in feces significantly increased during apple intake and the numbers of Lactobacillus spp., Streptococcus spp., and Enterococcus spp. tended to increase. On the contrary, enterobacteria and lecithinase-positive clostridia, including C. perfringens and Pseudomonas species, tended to decrease. However, that study did not use culture-independent microbiology techniques and suffered from the lack of a control group. Also in relation to fruits, another small human intervention study (n = 10) with raspberry puree [80] did not observe statistically significant alterations in the profile of colonic bacteria, probably due to high interindividual variation in fecal bacteria, although the profiles of microbial metabolites of raspberry polyphenols varied greatly between individuals, indicating that the type of gut microbiota affects catabolite profiles released by bacteria in the colon. This lack of effect on the intestinal microbiota after the intake of raspberry puree might also be due to the short duration of the treatment, as well as the techniques employed to quantify the intestinal microbiota.
Vendrame et al. [81] studied the potential prebiotic activity of a drink elaborated from wild blueberries especially rich in anthocyanins, in a small intervention trial (n = 15). A significant increase in Bifidobacterium spp. and L. acidophilus group was detected, while no significant differences were observed for Bacteroides spp., Prevotella spp., Enterococcus spp., and C. coccoides. In a further paper of the same group [82], seven different intragenus bifidobacteria taxonomic clusters that were among the most common and abundant bifidobacteria species inhabiting the human gut were targeted in the same samples. It was found that B. adolescentis, B. breve, B. catenulatum/pseudocatenulatum, and B. longum subsp. longum were always present in the group of subjects enrolled, whereas B. bifidum and B. longum subsp. infantis were not. In spite of the large interindividual variability, a significant increase of B. longum subsp. infantis cell concentration was observed in the feces of volunteers after the wild blueberry drink treatment, which was attributed to the presence of prebiotic (bifidogenic) molecules from blueberries, possibly fibers and glycosylated anthocyanins.
In a study with postmenopausal women (n = 39), Clavel et al. [83] found that isoflavone supplementation stimulated dominant microorganisms of the C. coccoides-E. rectale cluster, Lactobacillus-Enterococcus group, Faecalibacterium prausnitzii subgroup, and Bifidobacterium genus. It was also suggested that the concentration of C. coccoides-E. rectale cluster was related to women capacity to excrete equol, an intestinal metabolite from daidzein. In two intervention studies with whole grain breakfast cereals from wheat (n = 31) and maize (n = 32) [84, 85], the ingestion of both products resulted in significant increases in fecal bifidobacteria and/or lactobacilli without changing the relative abundance of other dominant members of the gut microbiota. Little or no changes were observed in the numbers of total bacteria, Bacteroides spp., C. histolyticum/perfringens group, and Acetobacterium spp. present in the feces. However, as whole grains are good sources of dietary fiber, it is difficult to ascribe the observed effects only to the phenolic compounds present in these foods. In this respect, Cuervo et al. [86] have recently studied the correlations between the intake of fiber and polyphenols from diet and fecal microbiota composition in a cohort of apparently healthy subjects. Results showed that the intake of soluble pectins and flavanones from oranges presented a negative correlation with the levels of B. coccoides and C. leptum. By contrast, the intake of white bread, providing hemicellulose and resistant starch, was directly correlated with Lactobacillus.
Finally, another human trial (n = 16) carried out by Jaquet et al. [87] assessed the impact of a moderate consumption of instant coffee on the general composition of the human intestinal bacterial population. Coffee beverages contain significant amounts of soluble fibre (mainly galactomannans and arabinogalactan-proteins) and phenolic compounds (chlorogenic acids), which are well utilised by the human fecal microbiota. It was observed that although fecal profiles of the dominant microbiota were not significantly affected after the consumption of the coffee, the population of Bifidobacterium spp. increased, being the largest increase observed for those volunteers showing the lowest initial bifidobacteria levels.
8. Conclusions
This review has tried to summarize the current knowledge in relation to the phenolic metabolism by gut microbiota and the modulation of the gut microbiota by phenolic compounds and polyphenol-rich dietary sources. There are evidences that the beneficial effects attribute to dietary polyphenols depend on their biotransformation by the gut microbiota. Therefore, it is important to investigate the bacterial species implicated in the metabolism of dietary polyphenols, and further research is still needed in relation to the resultant microbial metabolites to ascertain their mechanisms of action. On the other hand, a great number of in vitro and in vivo (in animals and humans) studies showing the influence of dietary polyphenols on gut-inhabiting bacteria have been published in recent years. Although in vitro assays facilitate experimentation, caution must be taken in extrapolating results to in vivo situation, as many factors are acting upon this process. In general, in both in vitro and in vivo studies, polyphenols or polyphenol-rich sources have shown to influence the relative abundance of different bacterial groups within the gut microbiota, reducing numbers of potential pathogens, including C. perfringens and C. histolyticum, and certain Gram-negative Bacteroides spp. and enhancing mainly beneficial Clostridia, bifidobacteria and lactobacilli. A better understanding of the interaction between dietary polyphenols and gut microbiota through the emerging advances in high-throughput meta-genomic, transcriptomic, and proteomic approaches, would be essential in order to identify genes and micro-organisms involved in polyphenol (in)activation and conversion and thus, to elucidate the implications of diet on the modulation of microbiota for delivering health benefits.
Functional foods are considered to enhance the protective effects against diseases derived from some food components. In the last decades, dairy fermented foods have probably been one of the most-developed functional products and have deserved intensive research. In this expansion, dairy fermented foods have been supplemented with fruits, cereals, and other stuffs of plant origin, all of which represent a high percentage of the current market of the dairy industry. These products have a healthy appeal, which attracts consumers. Thus, fruit juices/concentrates, and prepared fruits (in the form of pieces, pulp, and even flour) have been successfully incorporated in dairy fermented foods as sources of prebiotic fibers and phytochemicals. Among these phytochemicals present in plant-derived foods, polyphenols have gained much interest due to their diverse potential beneficial effects in human health. The supplementation of dairy fermented products with rich-polyphenolic stuffs (phenolic extracts, phenolic-enriched fractions, etc.) seems to be an effective technological option to improve the benefits of these products in the balance of the intestinal microbiota, due not only to the action of the probiotics but also to the potential modulation effects exerted by polyphenols, as it has been described in this review. Further research in this area will aim to accomplish the benefits of both probiotic strains and polyphenols in relation to gut health.
Acknowledgments
The authors of this review were funded by the Spanish MINECO through different projects (AGL2012-40172-C02-01, AGL2010-17499, and BFU2012-35228) and the CONSOLIDER INGENIO 2010 programme (project FUN-C-FOOD, CSD2007-063), as well as Comunidad de Madrid (project ALIBIRD P2009/AGR-1469). Montserrat Dueñas would like to thank the Spanish “Ramón y Cajal” Programme for a contract.
Conflict of Interests
The authors declare no conflict of interests.
References
- 1.Power S. E., O'Toole P. W., Stanton C., Ross R. P., Fitzgerald G. F. Intestinal microbiota, diet and health. British Journal of Nutrition. 2014;111(3):387–402. doi: 10.1017/S0007114513002560. [DOI] [PubMed] [Google Scholar]
- 2.De Cruz P., Prideaux L., Wagner J., et al. Characterization of the gastrointestinal microbiota in health and inflammatory bowel disease. Inflammatory Bowel Diseases. 2012;18(2):372–390. doi: 10.1002/ibd.21751. [DOI] [PubMed] [Google Scholar]
- 3.Kramer A., Bekeschus S., Bröker B. M., Schleibinger H., Razavi B., Assadian O. Maintaining health by balancing microbial exposure and prevention of infection: the hygiene hypothesis versus the hypothesis of early immune challenge. Journal of Hospital Infection. 2013;83(1):S29–S34. doi: 10.1016/S0195-6701(13)60007-9. [DOI] [PubMed] [Google Scholar]
- 4.Rescigno M. Intestinal microbiota and its effects on the immune system. Cellular Microbiology. 2014;16(7):1004–1013. doi: 10.1111/cmi.12301. [DOI] [PubMed] [Google Scholar]
- 5.Hertog M. G. L., Feskens E. J. M., Hollman P. C. H., Katan M. B., Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. The Lancet. 1993;342(8878):1007–1011. doi: 10.1016/0140-6736(93)92876-U. [DOI] [PubMed] [Google Scholar]
- 6.Arts I. C. W., Hollman P. C. H. Polyphenols and disease risk in epidemiologic studies. The American Journal of Clinical Nutrition. 2009;81(1):317S–325S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 7.del Rio D., Rodriguez-Mateos A., Spencer J. P. E., Tognolini M., Borges G., Crozier A. Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxidants & Redox Signaling. 2013;18(14):1818–1892. doi: 10.1089/ars.2012.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez-Vizcaino F., Duarte J. Flavonols and cardiovascular disease. Molecular Aspects of Medicine. 2010;31(6):478–494. doi: 10.1016/j.mam.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Spencer J. P. E., Vafeiadou K., Williams R. J., Vauzour D. Neuroinflammation: modulation by flavonoids and mechanisms of action. Molecular Aspects of Medicine. 2012;33(1):83–97. doi: 10.1016/j.mam.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Laparra J. M., Sanz Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacological Research. 2010;61(3):219–225. doi: 10.1016/j.phrs.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 11.He X., Marco M. L., Slupsky C. M. Emerging aspects of food and nutrition on gut microbiota. Journal of Agricultural and Food Chemistry. 2013;61(40):9559–9574. doi: 10.1021/jf4029046. [DOI] [PubMed] [Google Scholar]
- 12.Hervert-Hernández D., Goñi I. Dietary polyphenols and human gut microbiota: a review. Food Reviews International. 2011;27(2):154–169. doi: 10.1080/87559129.2010.535233. [DOI] [Google Scholar]
- 13.Tuohy K. M., Conterno L., Gasperotti M., Viola R. Up-regulating the human intestinal microbiome using whole plant foods, polyphenols, and/or fiber. Journal of Agricultural and Food Chemistry. 2012;60(36):8776–8782. doi: 10.1021/jf2053959. [DOI] [PubMed] [Google Scholar]
- 14.Etxeberria U., Fernández-Quintela A., Milagro F. I., Aguirre L., Martínez J. A., Portillo M. P. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. Journal of Agricultural and Food Chemistry. 2013;61(40):9517–9533. doi: 10.1021/jf402506c. [DOI] [PubMed] [Google Scholar]
- 15.Cardona F., Andrés-Lacueva C., Tulipani S., Tinahones F. J., Queipo-Ortuño M. I. Benefits of polyphenols on gut microbiota and implications in human health. Journal of Nutritional Biochemistry. 2013;24(8):1415–1422. doi: 10.1016/j.jnutbio.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Selma M. V., Espín J. C., Tomás-Barberán F. A. Interaction between phenolics and gut microbiota: role in human health. Journal of Agricultural and Food Chemistry. 2009;57(15):6485–6501. doi: 10.1021/jf902107d. [DOI] [PubMed] [Google Scholar]
- 17.Requena T., Monagas M., Pozo-Bayón M. A., et al. Perspectives of the potential implications of wine polyphenols on human oral and gut microbiota. Trends in Food Science & Technology. 2010;21(7):332–344. doi: 10.1016/j.tifs.2010.04.004. [DOI] [Google Scholar]
- 18.Van Duynhoven J., Vaughan E. E., van Dorsten F., et al. Interactions of black tea polyphenols with human gut microbiota: implications for gut and cardiovascular health1-4. The American Journal of Clinical Nutrition. 2013;98(6):1631S–1641S. doi: 10.3945/ajcn.113.058263. [DOI] [PubMed] [Google Scholar]
- 19.Moco S., Martin F.-P. J., Rezzi S. Metabolomics view on gut microbiome modulation by polyphenol-rich foods. Journal of Proteome Research. 2012;11(10):4781–4790. doi: 10.1021/pr300581s. [DOI] [PubMed] [Google Scholar]
- 20.Buddington R. K., Sangild P. T. Companion animals symposium: development of the mammalian gastrointestinal tract, the resident microbiota, and the role of diet in early life. Journal of Animal Science. 2011;89(5):1506–1519. doi: 10.2527/jas.2010-3705. [DOI] [PubMed] [Google Scholar]
- 21.Tiihonen K., Ouwehand A. C., Rautonen N. Human intestinal microbiota and healthy ageing. Ageing Research Reviews. 2010;9(2):107–116. doi: 10.1016/j.arr.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Mariat D., Firmesse O., Levenez F., et al. The firmicutes/bacteroidetes ratio of the human microbiota changes with age. BMC Microbiology. 2009;9, article 123 doi: 10.1186/1471-2180-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arumugam M., Raes J., Pelletier E., et al. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu G. D., Chen J., Hoffmann C., et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeffery I. B., Claesson M. J., O'Toole P. W., Shanahan F. Categorization of the gut microbiota: enterotypes or gradients? Nature Reviews Microbiology. 2012;10(9):591–592. doi: 10.1038/nrmicro2861. [DOI] [PubMed] [Google Scholar]
- 26.de Vos W. M., Nieuwdorp M. Genomics: a gut prediction. Nature. 2013;498(7452):48–49. doi: 10.1038/nature12251. [DOI] [PubMed] [Google Scholar]
- 27.Eckburg P. B., Bik E. M., Bernstein C. N., et al. Microbiology: diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zoetendal E. G., Collier C. T., Koike S., Mackie R. I., Gaskins H. R. Molecular ecological analysis of the gastrointestinal microbiota: a review. Journal of Nutrition. 2004;134(2):465–472. doi: 10.1093/jn/134.2.465. [DOI] [PubMed] [Google Scholar]
- 29.Fraher M. H., O'Toole P. W., Quigley E. M. M. Techniques used to characterize the gut microbiota: a guide for the clinician. Nature Reviews Gastroenterology and Hepatology. 2012;9(6):312–322. doi: 10.1038/nrgastro.2012.44. [DOI] [PubMed] [Google Scholar]
- 30.Kemperman R. A., Bolca S., Roger L. C., Vaughan E. E. Novel approaches for analysing gut microbes and dietary polyphenols: challenges and opportunities. Microbiology. 2010;156(11):3224–3231. doi: 10.1099/mic.0.042127-0. [DOI] [PubMed] [Google Scholar]
- 31.Kemperman R. A., Gross G., Mondot S., et al. Impact of polyphenols from black tea and red wine/grape juice on a gut model microbiome. Food Research International. 2013;53(2):659–669. doi: 10.1016/j.foodres.2013.01.034. [DOI] [Google Scholar]
- 32.Clifford M. N. Diet-derived phenols in plasma and tissues and their implications for health. Planta Medica. 2004;70(12):1103–1114. doi: 10.1055/s-2004-835835. [DOI] [PubMed] [Google Scholar]
- 33.Crozier A., del Rio D., Clifford M. N. Bioavailability of dietary flavonoids and phenolic compounds. Molecular Aspects of Medicine. 2010;31(6):446–467. doi: 10.1016/j.mam.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Day A. J., Dupont M. S., Ridley S., et al. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver beta-glucosidase activity. FEBS Letters. 1998;436(1):71–75. doi: 10.1016/S0014-5793(98)01101-6. [DOI] [PubMed] [Google Scholar]
- 35.Day A. J., Cañada F. J., Díaz J. C., et al. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Letters. 2000;468(2-3):166–170. doi: 10.1016/S0014-5793(00)01211-4. [DOI] [PubMed] [Google Scholar]
- 36.Scalbert A., Williamson G. Dietary intake and bioavailability of polyphenols. Journal of Nutrition. 2000;130(8):2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 37.Rechner A. R., Kuhnle G., Hu H., et al. The metabolism of dietary polyphenols and the relevance to circulating levels of conjugated metabolites. Free Radical Research. 2002;36(11):1229–1241. doi: 10.1080/1071576021000016472. [DOI] [PubMed] [Google Scholar]
- 38.Aura A.-M., O'Leary K. A., Williamson G., et al. Quercetin derivatives are deconjugated and converted to hydroxyphenylacetic acids but not methylated by human fecal flora in vitro. Journal of Agricultural and Food Chemistry. 2002;50(6):1725–1730. doi: 10.1021/jf0108056. [DOI] [PubMed] [Google Scholar]
- 39.Aura A.-M. Microbial metabolism of dietary phenolic compounds in the colon. Phytochemistry Reviews. 2008;7(3):407–429. doi: 10.1007/s11101-008-9095-3. [DOI] [Google Scholar]
- 40.Monagas M., Urpi-Sarda M., Sánchez-Patán F., et al. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food and Function. 2010;1(3):233–253. doi: 10.1039/c0fo00132e. [DOI] [PubMed] [Google Scholar]
- 41.Williamson G., Clifford M. N. Colonic metabolites of berry polyphenols: the missing link to biological activity? British Journal of Nutrition. 2010;104(3):S48–S66. doi: 10.1017/S0007114510003946. [DOI] [PubMed] [Google Scholar]
- 42.Jacobs D. M., Gaudier E., van Duynhoven J., Vaughan E. E. Non-digestible food ingredients, colonic microbiota and the impact on gut health and immunity: a role for metabolomics. Current Drug Metabolism. 2009;10(1):41–54. doi: 10.2174/138920009787048383. [DOI] [PubMed] [Google Scholar]
- 43.Bolca S., Possemiers S., Maervoet V., et al. Microbial and dietary factors associated with the 8-prenylnaringenin producer phenotype: a dietary intervention trial with fifty healthy post-menopausal Caucasian women. British Journal of Nutrition. 2007;98(5):950–959. doi: 10.1017/S0007114507749243. [DOI] [PubMed] [Google Scholar]
- 44.Tzounis X., Vulevic J., Kuhnle G. G. C., et al. Flavanol monomer-induced changes to the human faecal microflora. British Journal of Nutrition. 2008;99(4):782–792. doi: 10.1017/S0007114507853384. [DOI] [PubMed] [Google Scholar]
- 45.Hidalgo M., Oruna-Concha M. J., Kolida S., et al. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. Journal of Agricultural and Food Chemistry. 2012;60(15):3882–3890. doi: 10.1021/jf3002153. [DOI] [PubMed] [Google Scholar]
- 46.Molan A. L., Lila M. A., Mawson J., De S. In vitro and in vivo evaluation of the prebiotic activity of water-soluble blueberry extracts. World Journal of Microbiology and Biotechnology. 2009;25(7):1243–1249. doi: 10.1007/s11274-009-0011-9. [DOI] [Google Scholar]
- 47.Bialonska D., Ramnani P., Kasimsetty S. G., Muntha K. R., Gibson G. R., Ferreira D. The influence of pomegranate by-product and punicalagins on selected groups of human intestinal microbiota. International Journal of Food Microbiology. 2010;140(2-3):175–182. doi: 10.1016/j.ijfoodmicro.2010.03.038. [DOI] [PubMed] [Google Scholar]
- 48.Mandalari G., Faulks R. M., Bisignano C., Waldron K. W., Narbad A., Wickham M. S. J. In vitro evaluation of the prebiotic properties of almond skins (Amygdalus communis L.) FEMS Microbiology Letters. 2010;304(2):116–122. doi: 10.1111/j.1574-6968.2010.01898.x. [DOI] [PubMed] [Google Scholar]
- 49.Fogliano V., Corollaro M. L., Vitaglione P., et al. In vitro bioaccessibility and gut biotransformation of polyphenols present in the water-insoluble cocoa fraction. Molecular Nutrition & Food Research. 2011;55(supplement 1):S44–S55. doi: 10.1002/mnfr.201000360. [DOI] [PubMed] [Google Scholar]
- 50.Pozuelo M. J., Agis-Torres A., Hervert-Hernández D., et al. Grape antioxidant dietary fiber stimulates Lactobacillus growth in rat cecum. Journal of Food Science. 2012;77(2):H59–H62. doi: 10.1111/j.1750-3841.2011.02520.x. [DOI] [PubMed] [Google Scholar]
- 51.Cueva C., Sánchez-Patán F., Monagas M., et al. In vitro fermentation of grape seed flavan-3-ol fractions by human faecal microbiota: changes in microbial groups and phenolic metabolites. FEMS Microbiology Ecology. 2013;83(3):792–805. doi: 10.1111/1574-6941.12037. [DOI] [PubMed] [Google Scholar]
- 52.Sánchez-Patán F., Cueva C., Monagas M., et al. In vitro fermentation of a red wine extract by human gut microbiota: changes in microbial groups and formation of phenolic metabolites. Journal of Agricultural and Food Chemistry. 2012;60(9):2136–2147. doi: 10.1021/jf2040115. [DOI] [PubMed] [Google Scholar]
- 53.Barroso E., Sánchez-Patán F., Martín-Alvarez P. J., et al. Lactobacillus plantarum IFPL935 favors the initial metabolism of red wine polyphenols when added to a colonic microbiota. Journal of Agricultural and Food Chemistry. 2013;61(42):10163–10172. doi: 10.1021/jf402816r. [DOI] [PubMed] [Google Scholar]
- 54.Gibson G. R., Cummings J. H., Macfarlane G. T. Use of a three-stage continuous culture system to study the effect of mucin on dissimilatory sulfate reduction and methanogenesis by mixed populations of human gut bacteria. Applied and Environmental Microbiology. 1988;54(11):2750–2755. doi: 10.1128/aem.54.11.2750-2755.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Molly K., Vande Woestyne M., Verstraete W. Development of a 5-step multi-chamber reactor as a simulation of the human intestinal microbial ecosystem. Applied Microbiology and Biotechnology. 1993;39(2):254–258. doi: 10.1007/BF00228615. [DOI] [PubMed] [Google Scholar]
- 56.Minekus M., Smeets-Peeters M., Bernalier A., et al. A computer-controlled system to simulate conditions of the large intestine with peristaltic mixing, water absorption and absorption of fermentation products. Applied Microbiology and Biotechnology. 1999;53(1):108–114. doi: 10.1007/s002530051622. [DOI] [PubMed] [Google Scholar]
- 57.De Boever P., Deplancke B., Verstraete W. Fermentation by gut microbiota cultured in a simulator of the human intestinal microbial ecosystem is improved by supplementing a soygerm powder. Journal of Nutrition. 2000;130(10):2599–2606. doi: 10.1093/jn/130.10.2599. [DOI] [PubMed] [Google Scholar]
- 58.Yang C. S., Wang X., Lu G., Picinich S. C. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nature Reviews Cancer. 2009;9(6):429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ley R. E., Bäckhed F., Turnbaugh P., Lozupone C. A., Knight R. D., Gordon J. I. Obesity alters gut microbial ecology. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(31):11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hara H., Orita N., Hatano S., et al. Effect of tea polyphenols on fecal flora and fecal metabolic products of pigs. The Journal of Veterinary Medical Science. 1995;57(1):45–49. doi: 10.1292/jvms.57.45. [DOI] [PubMed] [Google Scholar]
- 61.Ishihara N., Chu D.-C., Akachi S., Juneja L. R. Improvement of intestinal microflora balance and prevention of digestive and respiratory organ diseases in calves by green tea extracts. Livestock Production Science. 2001;68(2-3):217–229. doi: 10.1016/S0301-6226(00)00233-5. [DOI] [Google Scholar]
- 62.Dolara P., Luceri C., De Filippo C., et al. Red wine polyphenols influence carcinogenesis, intestinal microflora, oxidative damage and gene expression profiles of colonic mucosa in F344 rats. Mutation Research-Fundamental and Molecular Mechanisms of Mutagenesis. 2005;591(1-2):237–246. doi: 10.1016/j.mrfmmm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 63.Sembries S., Dongowski G., Mehrländer K., Will F., Dietrich H. Physiological effects of extraction juices from apple, grape, and red beet pomaces in rats. Journal of Agricultural and Food Chemistry. 2006;54(26):10269–10280. doi: 10.1021/jf0618168. [DOI] [PubMed] [Google Scholar]
- 64.Sembries S., Dongowski G., Jacobasch G., Mehrländer K., Will F., Dietrich H. Effects of dietary fibre-rich juice colloids from apple pomace extraction juices on intestinal fermentation products and microbiota in rats. British Journal of Nutrition. 2003;90(3):607–615. doi: 10.1079/BJN2003925. [DOI] [PubMed] [Google Scholar]
- 65.Viveros A., Chamorro S., Pizarro M., Arija I., Centeno C., Brenes A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poultry Science. 2011;90(3):566–578. doi: 10.3382/ps.2010-00889. [DOI] [PubMed] [Google Scholar]
- 66.Smith A. H., Mackie R. I. Effect of condensed tannins on bacterial diversity and metabolic activity in the rat gastrointestinal tract. Applied and Environmental Microbiology. 2004;70(2):1104–1115. doi: 10.1128/AEM.70.2.1104-1115.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Massot-Cladera M., Pérez-Berezo T., Franch A., Castell M., Pérez-Cano F. J. Cocoa modulatory effect on rat faecal microbiota and colonic crosstalk. Archives of Biochemistry and Biophysics. 2012;527(2):105–112. doi: 10.1016/j.abb.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 68.Larrosa M., Yañéz-Gascón M. J., Selma M. V., et al. Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. Journal of Agricultural and Food Chemistry. 2009;57(6):2211–2220. doi: 10.1021/jf803638d. [DOI] [PubMed] [Google Scholar]
- 69.Molan A.-L., Liu Z., Kruger M. The ability of blackcurrant extracts to positively modulate key markers of gastrointestinal function in rats. World Journal of Microbiology & Biotechnology. 2010;26(10):1735–1743. doi: 10.1007/s11274-010-0352-4. [DOI] [Google Scholar]
- 70.Lacombe A., Li R. W., Klimis-Zacas D., et al. Lowbush wild blueberries have the potential to modify gut microbiota and xenobiotic metabolism in the rat colon. PLoS ONE. 2013;8(6) doi: 10.1371/journal.pone.0067497.e67497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saad R., Rizkallah M. R., Aziz R. K. Gut Pharmacomicrobiomics: the tip of an iceberg of complex interactions between drugs and gut-associated microbes. Gut Pathogens. 2012;4(1, article 16) doi: 10.1186/1757-4749-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van Velzen E. J. J., Westerhuis J. A., van Duynhoven J. P. M., et al. Multilevel data analysis of a crossover designed human nutritional intervention study. Journal of Proteome Research. 2008;7(10):4483–4491. doi: 10.1021/pr800145j. [DOI] [PubMed] [Google Scholar]
- 73.Okubo T., Ishihara N., Takahashi H., et al. Effects of partially hydrolyzed guar gum intake on human intestinal microflora and its metabolism. Bioscience, Biotechnology and Biochemistry. 1994;58(8):1364–1369. doi: 10.1271/bbb.58.1364. [DOI] [Google Scholar]
- 74.Mai V., Katki H. A., Harmsen H., et al. Effects of a controlled diet and black tea drinking on the fecalmicroflora composition and the fecal bile acid profile of human volunteers in a double-blinded ramdomized feeding study. The Journal of Nutrition. 2004;134(2):473–478. doi: 10.1093/jn/134.2.473. [DOI] [PubMed] [Google Scholar]
- 75.Jin J.-S., Touyama M., Hisada T., Benno Y. Effects of green tea consumption on human fecal microbiota with special reference to Bifidobacterium species. Microbiology and Immunology. 2012;56(11):729–739. doi: 10.1111/j.1348-0421.2012.00502.x. [DOI] [PubMed] [Google Scholar]
- 76.Yamakoshi J., Tokutake S., Kikuchi M., Kubota Y., Konishi H., Mitsuoka T. Effect of proantocyanidin -rich extract from grape seed on human fecal flora and fecalodor. FASEB Journal. 2001;15(4):p. A633. [Google Scholar]
- 77.Tzounis X., Rodriguez-Mateos A., Vulevic J., Gibson G. R., Kwik-Uribe C., Spencer J. P. E. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. The American Journal of Clinical Nutrition. 2011;93(1):62–72. doi: 10.3945/ajcn.110.000075. [DOI] [PubMed] [Google Scholar]
- 78.Queipo-Ortuño M. I., Boto-Ordóñez M., Murri M., et al. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. American Journal of Clinical Nutrition. 2012;95(6):1323–1334. doi: 10.3945/ajcn.111.027847. [DOI] [PubMed] [Google Scholar]
- 79.Shinohara K., Ohashi Y., Kawasumi K., Terada A., Fujisawa T. Effect of apple intake on fecal microbiota and metabolites in humans. Anaerobe. 2010;16(5):510–515. doi: 10.1016/j.anaerobe.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 80.Gill C. I. R., Mcdougall G. J., Glidewell S., et al. Profiling of phenols in human fecal water after raspberry supplementation. Journal of Agricultural and Food Chemistry. 2010;58(19):10389–10395. doi: 10.1021/jf1017143. [DOI] [PubMed] [Google Scholar]
- 81.Vendrame S., Guglielmetti S., Riso P., Arioli S., Klimis-Zacas D., Porrini M. Six-week consumption of a wild blueberry powder drink increases Bifidobacteria in the human gut. Journal of Agricultural and Food Chemistry. 2011;59(24):12815–12820. doi: 10.1021/jf2028686. [DOI] [PubMed] [Google Scholar]
- 82.Guglielmetti S., Fracassetti D., Taverniti V., et al. Differential modulation of human intestinal Bifidobacterium populations after consumption of a wild blueberry (Vaccinium angustifolium) drink. Journal of Agricultural and Food Chemistry. 2013;61(34):8134–8140. doi: 10.1021/jf402495k. [DOI] [PubMed] [Google Scholar]
- 83.Clavel T., Fallani M., Lepage P., et al. Isoflavones and functional foods alter the dominant intestinal microbiota in postmenopausal women. Journal of Nutrition. 2005;135(12):2786–2792. doi: 10.1093/jn/135.12.2786. [DOI] [PubMed] [Google Scholar]
- 84.Costabile A., Klinder A., Fava F., et al. Whole-grain wheat breakfast cereal has a prebiotic effect on the human gut microbiota: a double-blind, placebo-controlled, crossover study. British Journal of Nutrition. 2008;99(1):110–120. doi: 10.1017/S0007114507793923. [DOI] [PubMed] [Google Scholar]
- 85.Carvalho-Wells A. L., Helmolz K., Nodet C., et al. Determination of the in vivo prebiotic potential of a maize-based whole grain breakfast cereal: a human feeding study. British Journal of Nutrition. 2010;104(9):1353–1356. doi: 10.1017/S0007114510002084. [DOI] [PubMed] [Google Scholar]
- 86.Cuervo A., Valdés L., Salazar N., et al. Pilot study of diet and microbiota: interactive associations of fibers and polyphenols with human intestinal bacteria. Journal of Agricultural and Food Chemistry. 2014;62(23):5330–5336. doi: 10.1021/jf501546a. [DOI] [PubMed] [Google Scholar]
- 87.Jaquet M., Rochat I., Moulin J., Cavin C., Bibiloni R. Impact of coffee consumption on the gut microbiota: a human volunteer study. International Journal of Food Microbiology. 2009;130(2):117–121. doi: 10.1016/j.ijfoodmicro.2009.01.011. [DOI] [PubMed] [Google Scholar]