Abstract
Background
Cervical cancer is one of the most important and widespread cancer which affects women. There are several causes of cervical cancer; among them HPV types 16 and 18 are the most prominent ones which are recurrent and persistent infections. These genotypes are currently about 70% of cervical cancer causes in developing countries. Due to the importance of these viruses in cervical cancer, we pioneered the production of Human Papilloma Virus type16 E6 oncoprotein as a recombinant protein in order to develop a vaccine. Two HPV oncoproteins, E6 and E7, are consistently expressed in HPV-associated cancer cells and are responsible for malignant transformation. These oncogenic proteins represent ideal target antigens for developing vaccine and immunotherapeutic strategies against HPV-associated neoplasm.
Methods
In the present study, the cloned E6-oncoprotein of HPV16 in pTZ57R/T-E6 vector was used to produce professional expression vector. The target gene was subcloned in a eukaryotic expression vector. The pcDNA3-E6 vector was propagated in E.coli strain DH5α and transfected into CHO cells 72 hours post-transfection.
Results
The transfected cells were harvested; mRNA detection and the interest protein production were confirmed by western blot analysis using specific anti E6 monoclonal antibody.
Conclusion
HPV16-E6 target protein recognized by specific antibody could be an appropriate form of protein, which can be used for further studies. Due to potential effect of this protein, its DNA construction can be used for DNA vaccine in future studies.
Keywords: E6 protein, Human Papilloma Virus type 16, Uterine Cervical Neoplasms, Gene expression
Introduction
There are approximately more than 100 different types of Human Papilloma Virus (HPV) [1,2], which can be further classified as low risk types, found primarily in benign lesions, and high risk types, found in malignant or potentially malignant tumors [3,4]. HPVs are intimately associated with development of cervical cancer [4], which is the second leading cause of cancer-related death among women worldwide [5, 6]. It was shown that in some region of Iran such as Gorgan, it is considered very important cancer as the genital cancer is the third malignancy [7]. More than 99% of cervical cancers contain DNA of HPV [8], particularly the high-risk HPV type 16 [9]. About 80% of 500,000 new cases of cervical cancer occur each year in developing countries [5, 10].
The oncogenic activity of high-risk HPV E6 gene has been shown in tissue culture and transgenic mouse model systems extensively [1, 8]. The E6 oncoprotein plays an important role in HPV-associated cervical carcinoma and also in the Human Papilloma Virus life cycle [11]. The E6 is expressed through all stages of cervical neoplasia [12]. A novel and powerful strategy for anti-tumor vaccine is direct inoculation of plasmid DNA encoding tumor-associated antigens. This technique called DNA immunization, which is known to induce both antigen-specific cellular and humoral immune response [13, 14]. DNA vaccine has become an appealing approach to generate antigen-specific immunotherapy as a result of simplicity, stability, safety, and capacity for repeated administration [9]. The major concern on HPV DNA vaccine is the possibility of DNA integration into the host genome [15].Several studies have demonstrated in different animal models that DNA immunization with plasmid encoding HPV oncogenic proteins can prevent or delay development of invasive carcinoma [16].
The HPV E6 protein is a small protein containing of approximately 150 amino acids [1, 11], which degrades p53 tumor suppressor through an ubiquitin-dependent pathway, interfering with both cell cycle control and apoptotic pathway [17, 18]. In this study we showed construction, expression, and characterization of E6 protein isolated from HPV 16 which can be used for DNA vaccine.
Materials and Methods
Construction of expression vector
The E6 gene of HPV16 was isolated and cloned in pTZ57R/T-E6 to use for initial cloning of HPV16-E6 gene and sequencing [19]. Preparative digestion was done by Hind III (all enzymes were obtained from Roch, Germany) and BamH I sites of cloning vector and then subcloned into eukaryotic expression vector pcDNA3 (Invitrogen, USA). Competent Ecoli cells were transformed using constructed pcDNA3-E6. The accuracy of constructed plasmid was confirmed by colony-PCR and restriction enzyme analysis. Using E6 forward
5´- TAATCGAAGCTTAAAACTAAGGGCCT -3´and reverse
5´-GCAAAGGATCCATATATTCATGCAATGTAG -3´ primers. The colonies were used directly as template in colony-PCR, using PCR mix (50 pmol each primer, 1.5 mM MgCl2, 0.2 mM each dNTP, 1U Taq DNA polymerase (Cinagen, Iran) in a total reaction volume of 15 μl). Amplification was carried out for 35 cycles (94˚C for 30s, 61˚C for 30s, 72˚C for 45s) after an initial denaturation at 94˚C for 5 min, on a Techne Thermal Cycler. The cycles were followed by a 5 min extension at 72˚C and PCR product was visualized by UV on a 1.5% agarose gel electrophoresis. Colonies containing expected plasmids were selected and purified after large scale preparation. Purified pcDNA3-E6 was confirmed by restriction enzyme analysis using Hind III and BamH I.
Cell culture
CHO cells were cultured in RPMI 1640 supplemented with 10% Fetal Calf Serum (FCS), 2mM L-glutamine, 100 U/ml of penicillin, 100 μg/ml of streptomycin, and 1 mM sodium pyrovate. The cells incubated at 37˚C in a humidified 5% CO2 atmosphere.
Transfection by calcium phosphate precipitation
Expressing E6 protein, from the respective plasmid construct was tested in transient transfection assay. In a transient transfection experiment, two well of microplate untransfected cells were used as negative controls. To monitor expression of E6 protein, CHO cells were seeded into 6-well microtiter plates and incubated overnight in complete medium without any antibiotic. The confluent CHO cells were transfected with pcDNA3-E6 or pcDNA3 (as negative control) using calcium-phosphate protocol [20, 21]. Fourty eight hours after transfection, the cells were washed with PBS and then were analyzed by RT-PCR.
RNA extraction and Reverse Transcription (RT)-PCR
Total RNA were isolated from the cells using RNX-plus solution (Cinagen, Iran) according to manufacturer's instructions. Contaminated DNA was removed by DNase I (Fermentase, Germany) and extracted RNA was used in RT-PCR.
The first strand of cDNA was synthesized at 37˚C for 60 min in presence of 1μg RNA and 200 U/MMLV reverse transcriptase (Fermentase, Germany), 20U RNase inhibitor, dNTP mix (final concentration of 1 mM) with oligo (dT) priming in a 20μl reaction. The PCR amplification of cDNA was carried out using E6 primers. The condition of PCR mix and programs were the same as previously described.
Western Blot analysis
For characterization of expressed HPV16-E6 protein, total cell lysates were prepared by sonication (60HZ, 0.5 amplitude) after 5 times freezing and thawing. The samples were lysed by addition of 2X sample buffer at 100˚C for 5 min on 12% gel and stained with coomassie blue. The proteins were also transferred into nitrocellulose paper by electrophoresis for 1 hour at 90 V, blocked with 3% BSA in PBS for more than 1 h. The proper antigenicity of E6 protein was assessed using mouse monoclonal anti-E6 antibody (abcam, UK) and specific bands were visualized with Horse-Radish- Peroxidase (HRP)-conjugated anti-mouse immunoglobulin G antibody (TEBSUN Company, Iran) and development was carried out using Diamino Benzidine (DAB) substrate (Biogene, Iran).
Results
Construction and expression of HPV 16-E6
The expression vector containing HPV16-E6 gene was constructed as described in Material and Methods. The accuracy of resulted plasmid was confirmed by colony-PCR using designed specific E6 primers (Figure1).
Figure 1.
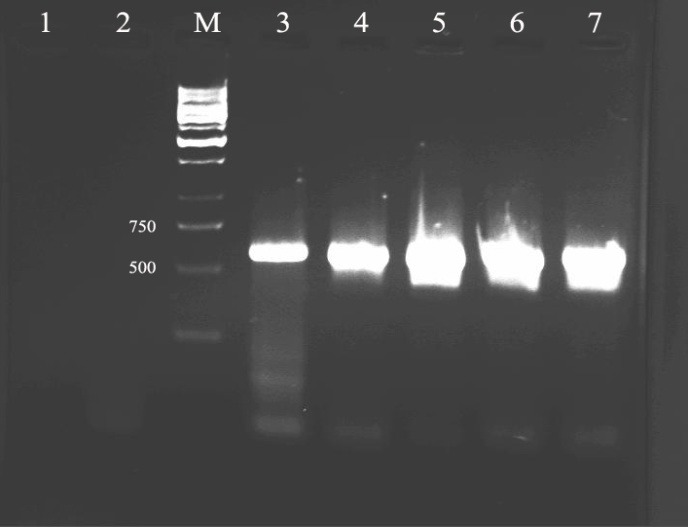
Shows agarose gel electrophoresis of colony PCR amplification products which stained with Ethidium Bromide and visualized under UV illumination.Lane 1: PCR negative control, lane 2: negative control plasmid (pcDNA3), lane M: DNA marker, lane 3: PCR positive control and lanes 4, 5, 6, 7: the colonies containing desired product of 600 bp belong to HPV16-E6 were confirmed.
The colonies containing desired plasmid were shown in lane 4, 5, 6 and 7 behind positive control in lane 3 on agarose gel and existence 600 bp bands belong to HPV16-E6.
The colonies which were positive in colony-PCR were subjected to restriction enzyme analysis using BamH I and Hind III (Figure 2).
Figure 2.
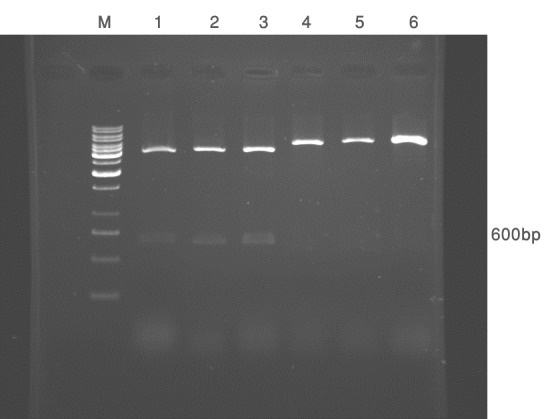
Shows digestion pattern of pcDNA3-E6 vector using BamH I and Hind III (lanes 1, 2 and 3) or HindIII (lanes 4, 5 and 6).
In vitro evaluation
Plasmid pcDNA3-E6 was transfected in CHO cells using calcium phosphate. The expression of HPV16-E6 was analyzed by RT-PCR 48 hr post-tarnsfection.
As shown in Figure 3, using E6 specific primers the 600 bp fragments was amplified on resulted cDNA of target produced mRNA on tranfected cells.
Figure 3.
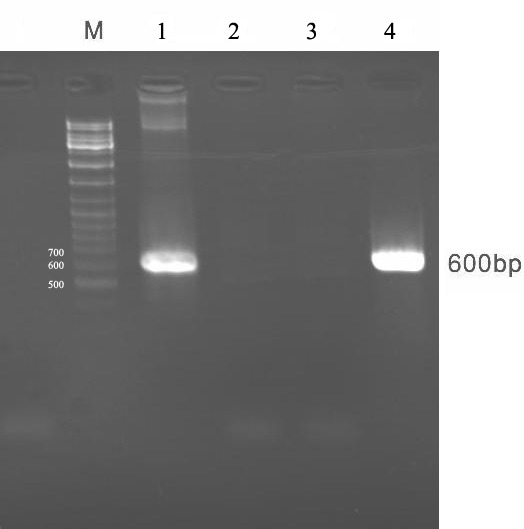
Shows confirmation of E6 mRNA production in transfected CHO cells.Lane M: DNA size marker, lane 1: PCR positive control, lane 2: PCR negative control, lane 3: untransfected cell, lane 4: transfected cell containing E6 mRNA which can be analyzed by RT-PCR.
Characterization of HPV16-E6 protein by western blotting
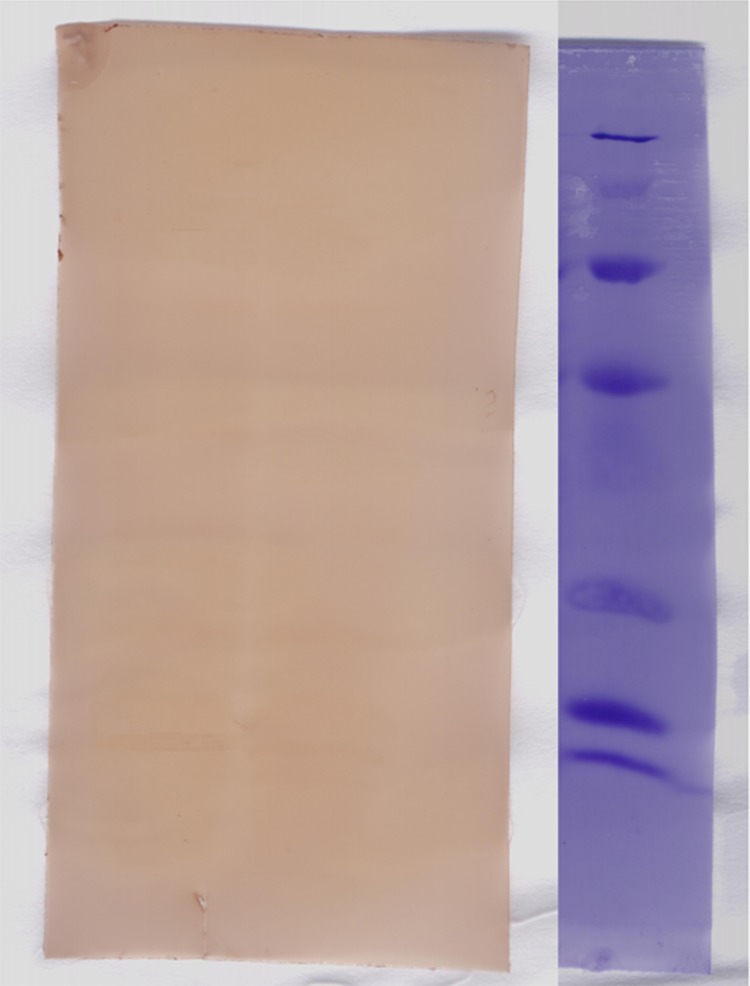
The transfected cells by pcDNA3-E6 plasmid were subjected to SDS-PAGE electrophoresis and transferred into a Polyvinylidene Fluoride (PVDF) membrane and confirmed by monoclonal antibody and visualized with 3, 3́-diaminibanzidine tetrahydrochloride. The calculated molecular mass of HPV16-E6 gene was approximately 17 KDa which were appeared in transfected cell extract. The band is absent in untransfected or mock transfected cells (Figure 4).
Figure 4.
Shows western blot analysis of HPV-E6 gene using mouse monoclonal antibody.Lane 1: protein size marker, lane 2: transfected cell, lane 3: untransfected cells.
Discussion
Considering the important role of Human Papilloma Virus in cervical cancer and high frequency genital infection of HPV, detecting high-risk genotype of HPV infection in high risk population is of paramount importance to prevent malignancy. As the incidence of cervical cancer continues to increase, the need for a safe and effective vaccine is more felt. Since the E6 and E7 proteins represent attractive target to develop anti-cancer vaccine, availability of HPV16-E6 constructions can greatly help for developing potent vaccines such as subunit or DNA vaccine [22].
In the present study, isolation of HPV 16 was subjected to isolation of E6 gene by PCR. The gene of interest was subcloned in pcDNA3 expression vector. After confirmation, the CHO cells were transfected with pcDNA3-E6 and protein production was evaluated by RT-PCR, SDS-PAGAE and western blotting. It was shown that there is always a minor expression of protein in transfected cells.
In optimal condition only 2.5% of total proteins in eukaryotic cells belong to recombinant protein. As shown by Rapp L, the E6 protein was presented at very low level in cells. In vitro analysis of E6 was very complicated [11]. In transfected CHO cell suspension E6 protein was detected as a faint band migrating approximately 17 KDa, according to its calculated molecular mass. The expression of E6 protein was done by using efficient expression vector and optimization of conditions. Previous attempts to express E6 protein have demonstrated that the level of expression was very low and immunoprecipitation was needed to detect the desired protein. These primary findings warrant further studies for DNA vaccine against HPV16.
Acknowledgments
This study was financially supported by Deputy of Research, Faculty of Medical Sciences, Tarbiat Modares University. The authors also acknowledge the valuable collaboration of Department of Allergy and Clinical Immunology, Children Medical Center, and Immunology, Asthma and Allergy Research Institute, Tehran, Iran for financial support.
Footnotes
Conflicts of Interest
The authors state that there is no conflict of interest.
Authors' Contribution
MH and MZ carried out the tests and helped in writing the paper. SH designed the study, supervised the project, interpreted the results, draft and revised the manuscript.
PZ and HZM contributed to the literature review. All authors read and approved the final manuscript.
REFERENCES
- 1.Munger K, Baldwin A, Edwards KM, Hayakawa H, Nguyen CL, Owens M, et al. Mechanisms of Human Papillomavirus-induced oncogenesis. J Virol. 2004;78(21):11451–60. doi: 10.1128/JVI.78.21.11451-11460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burd EM. Human Papilloma Virus and cervical cancer. Clin Microbial Rev. 2003;16(1):1–17. doi: 10.1128/CMR.16.1.1-17.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daniels PR, Sanders CM, Maitland NJ. Characterization of the interactions of Human Paillomavirus type 16 E6 with p53 and E6-associated protein in insect and human cells. J Gen Virol. 1998;79(Pt 3):489–99. doi: 10.1099/0022-1317-79-3-489. [DOI] [PubMed] [Google Scholar]
- 4.Guccione E, Massimi P, Bernat A, Banks L. Comparative analysis of the intracellular location of the high- and low- risk human Human papillomavirus Papilloma Virus oncoproteines. Virology. 2002;293(1):20–5. doi: 10.1006/viro.2001.1290. [DOI] [PubMed] [Google Scholar]
- 5.Pagliusi SR, Teresa Aguado M. Efficacy and other milestones for human Human papaillomavirus Papailloma Virus vaccine introduction. Vaccine. 2004;23(5):569–78. doi: 10.1016/j.vaccine.2004.07.046. [DOI] [PubMed] [Google Scholar]
- 6.Nakagawa M, Kim KH, Moscicki AB. Different methods of identifying new antigenic epitopes of human Human papillomavirus Papilloma Virus type 16 E6 and E7 proteins. Clin Diagn Lab Immunol. 2004;11(5):889–96. doi: 10.1128/CDLI.11.5.889-896.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moradi A, Bakhshandeh Nosrat S, Besharat S. Molecular epidemiology of High-Risk Types of Human Papillomaviruses (16, 18) in Pap-Smear, the North East of Iran. Iran J Cancer Prev. 2011;4(3):136–41. [PMC free article] [PubMed] [Google Scholar]
- 8.Jeckel S, Huber E, Stubenrauch F, Iftner TA. Transactivator function of cottontail rabbit papillomavirus E2 is essential for tumor induction in rabbits. J Virol. 2002;76(22):11209–15. doi: 10.1128/JVI.76.22.11209-11215.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng S, Ji H, Trimble C, He L, Tsai YC, Yeatermeyer J, et al. DNA vaccine targeting human Human papillomavirus Papillomavirus type 16 oncoprotein E6. J Virol. 2004;78(16):8468–76. doi: 10.1128/JVI.78.16.8468-8476.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frazer IH. Prevention of cervical cancer through papillomavirus vaccination. Nat Rev Immunol. 2004;4(1):46–54. doi: 10.1038/nri1260. [DOI] [PubMed] [Google Scholar]
- 11.Rapp L, Chen JJ. The papaillomavirus E6 proteins. Biochim Biophys Acta. 1998;1378(1):F1–19. doi: 10.1016/s0304-419x(98)00009-2. [DOI] [PubMed] [Google Scholar]
- 12.Stern PL, Brown M, Stcey SN, Kitchener HC, Hampson I, Abdel-Hady A, et al. Natural HPV immunity and vaccination strategies. J Clin Virol. 2000;19(1-2):57–66. doi: 10.1016/s1386-6532(00)00128-1. [DOI] [PubMed] [Google Scholar]
- 13.Weiner DB, Kennedy RC. Genetic vaccines. N Engl J Med. 1999;341:227–78. [Google Scholar]
- 14.Donnelly JJ, Ulmer JB, Shiver JW, Liu MA. DNA vaccines. Annu Rev Immunol. 1997;15:617–48. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 15.Cheung YK, Cheng SC, Sin FW. Plasmid encoding papillomavirus Type 16(HPV16) DNA constructed with codon optimization improved the immunogenicity against HPV infection. Vaccine. 2004;23(5):629–38. doi: 10.1016/j.vaccine.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Haupt K, Roggendorf M, Mann K. The potential of DNA vaccination against tumor-associated antigens for antitumor therapy. Exp Biol Med. 2002;227(4):227–37. doi: 10.1177/153537020222700403. [DOI] [PubMed] [Google Scholar]
- 17.Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human Human papillomavirus Papilloma Virus types 16 and 18 promotes the degradation of p53. Cell. 1990;63(6):1129–36. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 18.Scheffiner M, Huibregtse JM, Vierstrs RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as an ubiquitinprotein ligase in the ubiqitination of p53. Cell. 1993;75(3):495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- 19.Mirshahabi H, Solimanjahi H, Meshkat Z, Bamdad T, Hassan ZM. Isolation of Iranian human papillomavirus Papilloma Virus type 16 gene and construction of its cloning vector. Pak J Biol Sci. 2006;9(14):2652–6. [Google Scholar]
- 20.Sambrook J, Russell DW. Molecular cloning. 3rd. New York: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 21.Kaufman RJ. Overview of vector design for mammalian gene expression. Molecule Biotechnol. 2000;16(2):151–60. doi: 10.1385/MB:16:2:151. [DOI] [PubMed] [Google Scholar]
- 22.Villa LL. Vaccines against papillomavirus infections and disease. Salud Publica Mex. 2003;45(Suppl 3):S443–8. doi: 10.1590/s0036-36342003000900019. [DOI] [PubMed] [Google Scholar]