Abstract
Objectives: Estrogen deficit following menopause results in cognitive behaviors impairment. This study aimed to evaluate the effects of pomegranate seed extract (PGSE) on avoidance memories after permanent bilateral common carotid arteries occlusion (2CCAO) in ovariectomized (OVX) rats.
Materials and Methods: Adult female Wistar rats were divided randomly into eight groups with 8 rats in each group: 1) Sham-operated for ovaries and 2CCAO (ShO); 2) OVX and sham operated for ischemia (OShI); 3-7) OVX with 2CCAO (OI) received PGSE (100, 200, 400 and 800 mg/2ml/kg or normal saline, orally) for 14 days (OI+E100, 200, 400, 800 or OI+Veh); 8) OShI received most effective dose of PGSE (200 and 400 mg/kg for passive and active avoidance memories respectively). Active and passive avoidance tasks were measured in Y-maze and two-way shuttle box respectively. Data were analyzed with one-way and RM-ANOVA followed by HSD post-hoc test.
Results: Sensorimotor impaired in OShI+Veh and OI+Veh (P<0.001 vs. ShO). PGSE improved it significantly in dose dependently manner (P<0.001 vs. OI+Veh). Both types of memories were significantly impaired in OVX rats before and after 2CCAO (P<0.001). PGSE treatment significantly improved memories in OI groups (P<0.05, P<0.01 and P<0.001) compared with OI+Veh. No toxicity was observed with PGSE consumption (800 mg/kg, 2 weeks, orally).
Conclusion: PGSE exhibits therapeutic potential for avoidance memories, which is most likely related at least in part to its phytoestrogenic and also antioxidative actions.
Key Words: Ovariectomy, Cerebralischemia, Pomegranate, Memory, Rat
Introduction
The effects of combined gonadal hormones deprivation and cerebral hypoperfusion/ischemia on memory is unknown. Therefore, we hypothesized that phytoestrogenic natural substances such as pomegranate seed extract (PGSE) serves as a neurotrophomodulatory substance for some brain areas neurons thought to be involved in learning and memory.
Gonadal hormones, such as estrogen, can alter cognitive performance. Estrogen can have positive mnemonic effects in the inhibitory avoidance task (Rhodes & Frye, 2004 ▶). Ovarian hormones differently affect female memory in an age-dependent manner (Aguiar et al., 2006 ▶). Surgically menopausal women and estrogen deficits in rats (ovariectomy) impair memory and cognition incur a 2- to 5-fold increased risk for dementia and mortality from neurological diseases, but the mechanisms underlying these increased risks remain unclear (Ben et al., 2010 ▶; Scott et al., 2013 ▶).
Estrogen deficit is associated with mental health disorders, emotional difficulties, memory impairment, and other cognitive failures (Sarkaki et al., 2008 ▶). Recently, considerable attention has been paid to medicinal plants and fruits with various physiological activities such as pomegranate and soy as phytoestrogens to alleviate symptoms related to estrogen deficit (Varadinova et al., 2009 ▶). Phytoestrogens are naturally occurring plant-derived compounds that are present in the human diet and are considered selective estrogen receptor (ER) modulators. The phytoestrogens are potent isoflavonoid, with binding affinities for both ER-alpha and ER-beta that are comparable to those of 17 b-estradiol (Canal Castro et al., 2012 ▶).
Stroke is a major cause of adult-onset disability and dependency (Sheorajpanday et al., 2011 ▶). Neuropsychological impairment after stroke when no motor sensory or language deficits are left remains understudied (Planton et al., 2012 ▶). The outcome for menopause women with hypoxic-ischemic brain injury (HIBI) is often poor. It is important to establish an accurate prognosis as soon as possible after the insult to guide management (Howard et al., 2012 ▶). Cerebral ischemia resulted from low oxygen and glucose supply evidently decreases the formation of ATP (Aviram et al., 2002 ▶; Aquilano et al., 2008 ▶). Damage to brain tissue resulting from cerebral ischemia is a major cause of adult disability. It can lead to cognition problems, seizures and death (Aviram et al., 2000 ▶; Aviram & Dornfeld, 2001 ▶). Transient global cerebral ischaemia (forebrain ischaemia), occurring during cardiorespiratory arrest in patients or in experimental animals, induces selective and delayed neuronal cell death (Ben Nasr et al., 1996 ▶; Block, 1999 ▶; Aviram et al., 2000 ▶; Banerjee et al., 2003 ▶). The hippocampus plays a central role in the brain network that is essential for memory function. Paradoxically, the hippocampus is also the brain structure that is most sensitive to hypoxic-ischemic episodes (Lavenex et al., 2011 ▶). Pyramidal neurons in the CA1 region of the hippocampus are particularly vulnerable and die after global ischemia. Hippocampal CA1 sector injury is observed a few days after untreated forebrain ischemia in the rat (Block, 1999 ▶; Aviram et al., 2000 ▶; Aviram & Dornfeld, 2001 ▶; Aviram et al., 2002 ▶; Banerjee et al., 2003 ▶), gerbil and human (Aviram et al., 2000 ▶; Cechetti et al., 2010 ▶). Transient global ischemia induces selective, delayed neuronal death of pyramidal neurons in the hippocampal CA1. Whereas long term treatment of middle-aged female rats with estradiol at physiological doses ameliorates neuronal death, the signaling pathways that mediate the neuroprotection are, as yet, unknown (De Butte-Smith et al., 2012 ▶).
Extracts from natural substances have the ability to protect neurons from ischemic damage (Meyer et al., 1987 ▶; McCarty, 2000 ▶). The extracts have several functions including antioxidant effects in neuroprotection from ischemic insults (Evans, 1993 ▶). Polyphenols have been found to possess antioxidant properties as well as having effects on gene expression (Ito et al., 1975 ▶). Recent studies indicate that among foods which contain polyphenols. Recent studies indicate that among foods that contain polyphenols, juice extracted from the pomegranate has the highest concentration of measurable polyphenols (Takeda et al., 2005 ▶; Jee et al., 2008 ▶). The pharmacologic actions of pomegranate juice include antiatherosclerotic, antibacterial, and antiproliferative properties (Kaplan et al., 2001 ▶; Kim et al., 2002 ▶). Studies of dietary supplementation with pomegranate juice have shown protective effects against atherogenesis and atherosclerosis as well as reductions in serum angiotensin-converting enzyme activity with subsequent reductions in systolic blood pressure (32–34). Also, maternal dietary supplementation with pomegranate juice results in protection against neonatal brain injury (Lau et al., 2005 ▶).
During recent years, phytoestrogens have been receiving an increasing amount of interest, as several lines of evidence suggest a possible role in preventing a range of diseases. The presence of these phytoestrogenic compounds in pomegranate has been shown to exert suppressive effects on disease (van Elswijk et al., 2004 ▶). Another study via biochemical analysis revealed that pomegranate with highest antioxidant capacity was found in leaves followed by peel, pulp, and seed (O'Keefe & Conway, 1978 ▶). In our previous work (Sarkaki & Rezaiei, 2013 ▶) the beneficial effects of PGSE on adult male and female rats suffering with cerebral hypoxia-ischemia (HI) and Parkinson’s disease were determined (Sarkaki et al., 2013b ▶).
With regard to the several beneficial effects mentioned for pomegranate, it seems that administration of its seed extract (PGSE) can be effective for the improvement of post-ischemic injuries. On the other hand, female sex hormones such as estrogen are neuroprotective and PGSE also contains phytoestrogenic substances with antioxidative properties, we decided therefore to test the effect of PGSE on cognition deficiency induced by HI in adult female rats.
The present study investigated the effects of different doses of PGSE on passive and active avoidance memories following cerebral hypoxia-ischemia induced via bilateral common carotid artery occlusion.
Materials and Methods
Animals
Eighty adult male albino rats of Wistar strain (250±20g, 3-4 months) obtained from Ahvaz Jundishapur University of Medical Sciences (AJUMS) laboratory animal center were used in this study. Animals were housed in standard cages under controlled room temperature (20±2 ◦C), humidity (55-60%) and light exposure conditions 12:12 h light–dark cycle (lighted on 07:00 am). All experiments carried out during the light phase of the cycle (8:00 am to 6:00 pm). Access to food and water were ad libitum except during the experiments. Animal handling and experimental procedures performed under observance of the University and Institutional legislation, controlled by the Local Ethics Committee for the Purpose of Control and Supervision of Experiments on Laboratory Animals. All efforts were made to minimize animal suffering, to reduce the number of animals used. Prior to the onset of behavioral testing, all rats were gentle handled for 3 days (daily 10 min). After one week, animals of seven groups were ovariectomized (O) under anesthesia induced by injection of ketamine hydrochloride (90 mg/kg, i.p., Rotex Medica, Trittau, Germany) and Xylazine (10 mg/kg, i.p., Miles Laboratories, Shawnee, Kansas, USA). While ovaries remained intact in sham operated groups (ShO). Bilateral common carotid arteries were occluded and dissected (2CCAO) in ovariectomized rats (OI groups). Animals were divided randomly into eight groups with 8 in each: 1) sham-operated for OVX (ShO); 2) ovariectomized and sham operated for ischemia with manipulation of both common carotids arteries without occlusion received vehicle (OShI+Veh); 3-7) ovariectomized with 2CCAO (OI) received PGSE (100, 200, 400 and 800 mg/2ml/kg or normal saline as vehicle, orally) for 14 days (OI+E100, 200, 400, 800 or OI+Veh); 8) OShI received most effective dose of PGSE which was different in two cognition tests (200 mg/kg, OShI+E200 in shuttle box test and 400 mg/kg, OShI+E400 in Y-maze test).
2CCAO procedure
Cechetti’s method (2012) with little modification was used. Briefly, rats were anesthetized with ketamine/xylazine (50/5mg/kg, i.p). A neck ventral midline incision was made and the common carotid arteries were then exposed and gently separated from the vagus nerve. Carotids were occluded with three days interval between interventions, the right common carotid being the first to be assessed and the left one being occluded three days later. Sham-operated rats were under same surgical procedures without carotid artery ligation and occlusion (Cechetti et al., 2012 ▶).
PGSE preparation
Pomegranate fruits (Punica granatum L.) as large fruit with red barriers were purchased from Shahreza granatum gardens- Iran. Seeds removed from the fruits, air dried in shade for one week and milled to fine powder (electric mill, Panasonic Co. Japan). The seeds powder was macerated in 70% ethanol for 72 hours at room temperature. The ethanol extract evaporated (Rotary Ovaporator, Heidolph Co. Germany) to remove ethanol and PGSE was obtained as a lyophilized powder (yield 17±2%).
Treatment
Different doses of extract were administrated to each animal in separate groups via forced oral administration (gavage) everyday 8:00–9:00 am for 2 weeks, starting on the 5 days post ischemic injury. Sham treated animals (OI+Veh) were received same volume of normal saline (2ml) for same period.
Sensorimotor evaluation
It consisted of two tests developed and described by Garcia (Garcia et al., 1995 ▶) with some modifications as discussed below. The scores assigned to each rat at the end of each examination is the sum of the two test scores. The minimum neurological score is three and the maximum is four. I/R rats with at least score 3 in each test were selected in this study.
Spontaneous activity
The animal is observed for 5 min in its normal cage. Scores indicate the following: (1) rat moves around, explores the environment, and approaches at least three walls of the cage; (2) rat moves around in the cage but does not approach all sides and hesitates to move, although it eventually reaches at least one upper rim of the cage (height=10 cm); (3) rat dose not rise up at all and barely moves in the cage; (4) rat does not move at all.
Symmetry in the movement of four limbs
The rat is held in the air by the tail to observe symmetry in the movement of the four limbs. Scores indicate the following: (1) all four limbs extended symmetrically; (2) limbs on one side extended less or more slowly than those on the other side; or slow extension of the four limbs; (3) limbs on one or both sides show minimal movements; (4) forelimbs on one or both sides do not move at all.
Passive avoidance task
The apparatus used for evaluation the passive avoidance task was two-way shuttle box (Borj Sanaat Co. Tehran, Iran), which consisted of two adjacent plexiglas compartments of identical dimensions (27×14.5×14 cm) with grid floors. The floor of two compartments has been covered with stainless steel bars (2 mm diameter) with 1 cm distance. Light compartment was illuminated by a 5 W lamp mounted on its wall just below a movable transparent plexiglas ceiling. The Tamburella’s procedure with little modification was used for passive avoidance memory test (Tamburella et al., 2012 ▶). Briefly, each rat was allowed a 10 minutes adaptation period with free access to either the light or dark compartment of the box to avoidance training and after being placed in a shuttle-box (in order to familiar with instruments). Following this adaptation period, on the second day (initial phase) rats were placed into the illuminated compartment and 10 seconds later the sliding door was raised. Initial latency was recorded as learning phase. Upon entering the dark compartment the door was closed and a 1.5 mA constant-current was applied to animal fore and hind paws for 3 seconds as electrical shock. After 60 seconds (in order to consolidation and return to normal psychological state) the rat was removed from the dark compartment and placed into the home cage. In order to test short-term memory, 24 hours after receiving foot shock, the rats were placed in illuminated chamber again and the sliding door was raised at 30 seconds later. The latency of entering the dark compartment was recorded again as memory test (step-through latency). The maximum time that considered in this procedure were 300 seconds (Mahut et al., 1982 ▶; Levy et al., 1985 ▶; Lipton & Rosenberg, 1994 ▶).
Active avoidance task
The apparatus used for evaluation the active avoidance task was 3-equal arms Y-maze (Made in Ahvaz, Iran). The Yu and Besnard procedures with little modifications were used for active avoidance memory test (Besnard et al., 2012 ▶; Yu et al., 2012 ▶). Sham operated, ischemic and all treated rats were trained in equal 3-arms Y-maze with using an A/D converter, special software on a PC as active avoidance learning. Training was done, 30 trails daily for 4 days as four sessions. Animals were conditioned, using a 12 watts light as conditioned stimulus (CS) and 20-25 volts, 3 mA electrical foot shock as unconditioned stimulus (UCS). Inter-trials interval (ITI) and inter-stimuli interval (ISI) were 60 and 5 seconds, respectively. Trained animals left the dark arms and enter in light arm during 5 seconds delay time (ISI). This effort was counted as conditioned response. Criterion condition response (CCR) was 90 percents correct responses fearing from unsafe locations in Y-maze (darken arms) to lighted (safe arm) in last session of training as optimum level of learning. Training session’s number was same for all rats in different groups.
Statistical analysis
Data were expressed as mean ±SEM of values for sensorimotor and avoidance memories tests. Statistical analysis was performed by one-way ANOVA (for sensorimotor and passive avoidance task) and repeated measures (RM) ANOVA (for active avoidance task) followed by HSD post hoc test. A P-value less than 0.05 were assumed to denote a significant difference and levels of significance are indicated by symbols: * vs. ShO group and # vs. OShI+Veh or OI+Veh groups.
Results
Motor evaluation
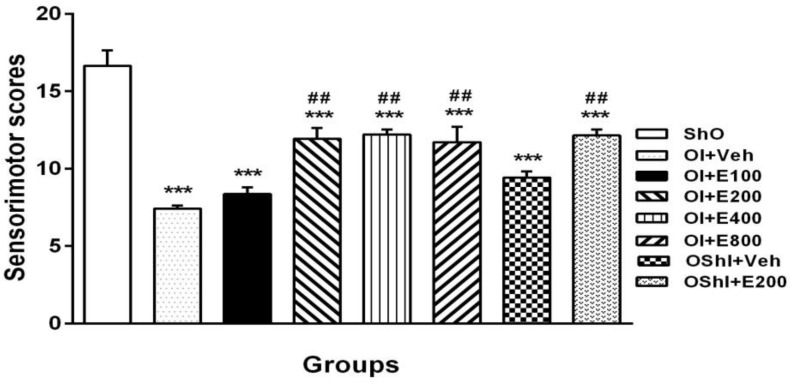
Figure 1 shows the sensorimotor scores 5 days after 2CCAO operation. There was significant sensorimotor impairment in OShI+Veh and OI+Veh groups when compared to ShO group (P<0.001).
Figure 1.
Mean±SEM of sensorimotor scores in different groups 5 days after 2CCAO induction. ***P<0.001 for significant difference with ShO group and ##P<0.01 for significant difference versus OI+Veh groups. One-way ANOVA analysis that followed by HSD post hoc test. ShO: sham operated for ovariectomy, OI: ovariectomized rats with 2CCAO, OI+E100-800: ovariectomized rats with 2CCAO received different doses of PGSE, OI+Veh: ovariectomized rats with 2CCAO received normal saline, OShI+E200 and OShI+Veh: ovariectomized rats with sham operated for 2CCAO received 200 mg/kg PGSE and normal saline respectively.
Treatment with different doses of PGSE for two weeks improved sensorimotor scores significantly in OI+E200, OI+E400 and OI+E 800 groups, but not with dose 100 mg/kg (P<0.001 vs. OI+Veh), while there was not any difference between them. There were significant differences between OShI+Veh vs. ShO (P<0.001) and OShI+E200 vs. OShI+Veh groups respectively.
Passive avoidance memory
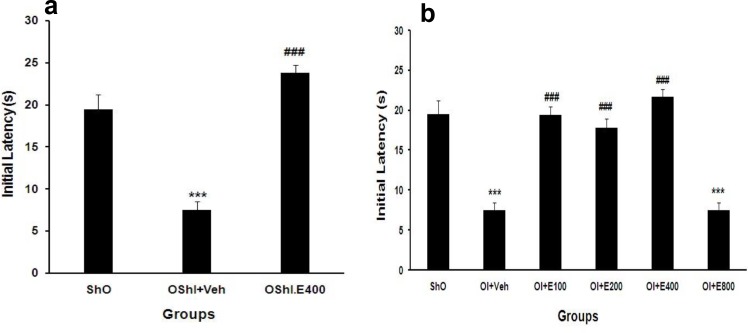
As shown in figure 2 the initial latency for leaving rats from lighted to darken compartment of shuttle box before exposing them to any serious stimulus (electrical shock) in ovariectomized rats without 2CCAO that received normal saline (OShI+Veh) was decreased significantly (P<0.001) when compared with sham operated (ShO) group. In this test, 200 mg/kg of PGSE was the best effective dose in passive avoidance memory and treatment OShI group with this dose (OShI+E200) reversed it toward to normal range (P<0.001, figure 2a).
Figure 2.
Mean±SEM of initial latency in different groups after two weeks treatment with PGSE in ovariectomized without and with 2CCAO groups. ***P<0.001 for significant difference with ShO group and ###P<0.001 for significant difference versus OI+Veh groups. One-way ANOVA analysis that followed by HSD post hoc test. ShO: sham operated for ovariectomy, OI: ovariectomized rats with 2CCAO, OI+E100-800: ovariectomized rats with 2CCAO received different doses of PGSE, OI+Veh: ovariectomized rats with 2CCAO received normal saline, OShI+E200 and OShI+Veh: ovariectomized rats with sham operated for 2CCAO received 200 mg/kg PGSE and normal saline respectively.
Treatment OVX animals without ischemia with this effective dose of PGSE (OShI+E200) compensated it and reversed its level in ShO group (fig.2a). Treatment of OI rats with oral administration of 100, 200 and 400 mg/kg of PGSE for 14 days improved initial latency significantly when compared with OI+Veh group (P<0.001), while initial latency of OI+E800 was reversed significantly when compared with ShO as well as other treated groups with PGSE (P<0.001, fig. 2b). Short-term passive avoidance memory (24 hours after exposing the electrical shock to paws of rats) in all groups was evaluated with same procedure while no shock delivered to animals.
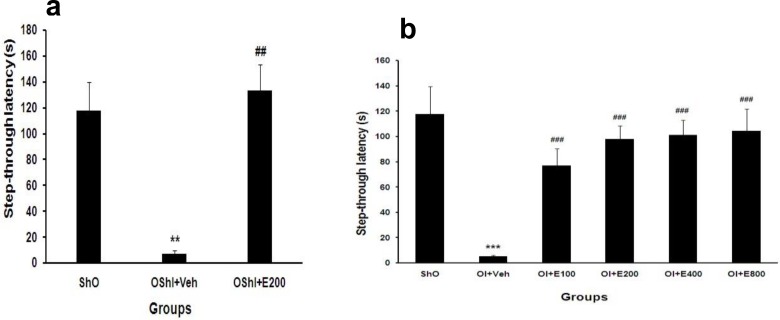
As shown in figure 3 step-through latency (memory) was impaired significantly in both OShI+Veh and OI+Veh groups (P<0.01 and P<0.001 vs. ShO respectively, fig.3a). Treatment of ovariectomized animals without ischemia with best effective dose of PGSE (200 mg/kg) and ovariectomized animals with ischemia with different doses of extract improved the memory significantly when compared with OI+Veh rats (P<0.001, fig. 3b).
Figure 3.
Mean±SEM of step-through latency in different groups after two weeks treatment with PGSE in ovariectomized without and with 2CCAO groups. ***P<0.001 for significant difference with ShO group and ###P<0.001 for significant difference versus OI+Veh groups. One-way ANOVA analysis that followed by HSD post hoc test. ShO: sham operated for ovariectomy, OI: ovariectomized rats with 2CCAO, OI+E100-800: ovariectomized rats with 2CCAO received different doses of PGSE, OI+Veh: ovariectomized rats with 2CCAO received normal saline, OShI+E200 and OShI+Veh: ovariectomized rats with sham operated for 2CCAO received 200 mg/kg PGSE and normal saline respectively.
Active avoidance memory
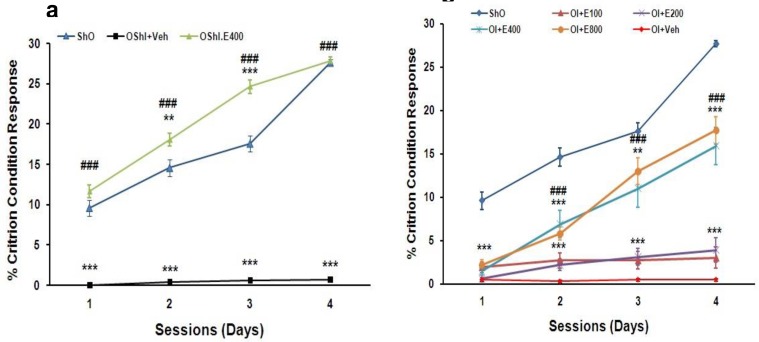
Figure 4 shows that the percents of criterion condition responses (%CCRs) was lower significantly after ovariectomy without 2CCAO group (OShI+Veh) during 4 days training in Y-maze (P<0.001). In this test, 400 mg/kg of PGSE was the best effective dose in active avoidance memory and treatment OShI group with this dose (OShI+E400) reversed it to normal range (P<0.001, fig. 4a).
Figure 4.
The percents of criterion condition responses (%CCRs) during four sessions training in Y-maze in different groups after two weeks treatment with PGSE in ovariectomized without and with 2CCAO groups. ***P<0.001 for significant difference with ShO group and ###P<0.001 for significant difference versus OShI+Veh and OI+Veh groups. Repeated measured (RM) ANOVA analysis that followed by HSD post hoc test. ShO: sham operated for ovariectomy, OI: ovariectomized rats with 2CCAO, OI+E100-800: ovariectomized rats with 2CCAO received different doses of PGSE, OI+Veh: ovariectomized rats with 2CCAO received normal saline, OShI+E200 and OShI+Veh: ovariectomized rats with sham operated for 2CCAO received 200 mg/kg PGSE and normal saline respectively.
Results of treatment the ovariectomized with 2CCAO groups with different doses of PGSE showed that only doses 400 and 800 mg/kg could improve %CCRs during 2-4 sessions significantly (P<0.001), with no significant difference. But it is steel lower than ShO group significantly during those training sessions (P<0.01 and P<0.001, fig. 4b).
Discussion
Our results show that passive and active avoidance tasks impair after ovarectomy in rats because of removing peripheral source of estrogen and treatment them with PGSE as a natural product with phytoestrogenic content agent could reverse avoidance tasks significantly. On the other hand, cerebral hypoperfusion induction in ovariectomized rats with 2CCAO impaired both passive and active avoidance tasks significantly while administration of doses 200 mg/kg (for passive avoidance task) and 400 mg/kg (for active avoidance task) had significant improving effects. These results show that 2CCAO induction causes oxidative stress progression and free radicals production in brain tissues and PGSE as an estrogenic compound and a potent antioxidant could activate estrogen beta receptors (Sarkaki et al., 2013a ▶; Sarkaki et al., 2013b ▶; Sarkaki & Rezaiei, 2013 ▶) as well as scavenge free radicals from brain regions involving the cognitive functions.
Ischemic stroke is a devastating condition, for which there is still no effective therapy. Acute ischemic stroke is associated with high concentrations of glutamate in the blood and interstitial brain fluid. The inability of the tissue to retain glutamate within the cells of the brain ultimately provokes neuronal death. Increased concentrations of interstitial glutamate exert further excitotoxic effects on healthy tissue surrounding the infarct zone (Godino Mdel et al., 2013 ▶).
Ovariectomy is known as 'surgical menopause' with decreased levels of oestrogen in female rodents and its reported risks and adverse effects include cognitive impairment. In the brain, oestrogen exerts effects through its receptors, oestrogen receptor alpha (ERalpha) and beta (ERbeta) (Qu et al., 2013 ▶). We hypothesize that menopause is an aging phenomenon that combines with oxidative stress and free radicals generation. Estrogen deficit following menopause results in cognitive behaviors impairment (Sarkaki et al., 2008 ▶). Phytoestrogens and other plant-derived compounds and extracts have been developed for the treatment of menopause-related complaints and disorders. Since estrogens have been discussed to enhance the risk for hormone-sensitive cancers, research activities try to find alternatives. Phytoestrogens like genistein, ellagic acid, and resveratrol presented in PGSE as well as other plant-derived compounds are capable of substituting for estrogens to some extent (Schilling et al., 2014 ▶).
Estrogens are not only critical for sexual differentiation; it is well-known for the role of 17beta-estradiol (E2) in the adult brain modulating memory, learning, mood and acts as a neuroprotector. As estrogen levels change with age, especially in females, it is important to know the effects of low E2 levels on ERalpha distribution; results from previous studies are controversial regarding this issue (Navarro et al., 2013 ▶).
Treatments for protection against neuronal cell death induced by hypoxia-ischemia (HI) and reperfusion have been developed in recent years, but none has been highly successful. A fundamental process believed to be responsible for HI damage to neurons is excitotoxicity, triggered mainly by elevated extracellular glutamate concentration (Choi & Rothman, 1990 ▶). This ischemia-induced release of glutamate likely occurs in man as well, and possibly underlies selective damage to the human hippocampus (Chun et al., 2008 ▶). Glutamate may cause ischemic neuronal death by acting at excitatory N-methyl-Daspartate (NMDA) receptors (Colbourne et al., 1999 ▶) which play an important physiological role in memory (Collingridge et al., 1983 ▶). Thus, the high concentration of NMDA excitatory receptors on the dendrite trees of hippocampal CA1 pyramidal cells (Esposito et al., 2002 ▶) likely explains the long-known selective vulnerability of the CA1 zone of the hippocampus to ischemic brain damage.
Estrogen deprivation after menopause is associated with increased oxidative stress. Oxidative stress and inflammation result in the development of ovariectomy-induced pathophysiological changes (Kaur et al., 2013 ▶). Reactive oxygen species (ROS) are generated within brain tissue during HI and play a role in the development of cerebral damage. They may be directly involved in glutamate release (Floyd, 1999 ▶) and more importantly, they may participate in the excitotoxic process itself. ROS are extremely reactive and attack lipids, proteins, and nucleic acids, which eventuates in tissue injury and cell death (Gil et al., 2000 ▶). Although there is strong evidence that total destruction of hippocampal CA1 neurons is sufficient to cause a memory deficit (Greenamyre et al., 1985 ▶), it is still presently unclear to what degree subtotal ischemic hippocampal damage may occur without an ensuing memory deficit (Hashimoto et al., 2003 ▶). The free radicals are neutralized by an elaborate antioxidant defense system consisting of enzymes and numerous non-enzymatic antioxidants, including vitamins A, E and C, glutathione, ubiquinone, and flavonoids (Dumlu & Gurkan, 2007 ▶; Pilsakova et al., 2010 ▶).
Hormone therapy to postmenopausal females increases the risk and severity of ischemic stroke. Previous work using an animal model of menopause (reproductive senescence) showed that middle cerebral artery occlusion (MCAO) causes a larger cortical-striatal infarct in this older acyclic group compared with younger females. Moreover, although estrogen treatment is neuroprotective in younger females, estrogen paradoxically increases infarct volume in acyclic females. These data support the hypothesis that stroke severity in older females is associated with decreased IGF-1 and further indicate that short-term post-ischemic IGF-1 therapy may be beneficial for stroke (Selvamani & Sohrabji, 2010 ▶).
The results of present study shows the PGSE is a natural product with power phytoestrogenic and antioxidative effects without any side effects could improve the cognitive deficit during menopause period and specially as followed by stress oxidative and possible cerebral hypoperfusion induced by vascular obstruction.
Our results show that passive and active avoidance tasks impair after ovarectomy in rats because of removing peripheral source of estrogen and treated them with PGSE as a natural product with phytoestrogenic and antioxidant contents could reverse both active and passive avoidance tasks significantly in OVX rats suffer with cerebral HI. Our previous results showed that 2CCAO induction causes oxidative stress progression and free radicals production in brain tissues (Mansouri et al., 2013 ▶) and PGSE as a potent phytoestrogenic and antioxidant may have compensating effects for peripheral estrogen deficit and scavenge produced free radicals after HI from brain regions involving the cognitive functions.
Acknowledgment
This work was supported financially by Ahvaz Jundishapur University of Medical Sciences, Research Affairs, Ahvaz Physiology Research Center (Grant No. APRC-86).
References
- Aguiar , RB , Dickel , OE , Cunha , RW , Monserrat , JM , Barros , DM , Martinez PE. Estradiol valerate and tibolone: effects on memory. Pharmacol Biochem Behav. 2006;85:689–696. doi: 10.1016/j.pbb.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Aquilano K, Baldelli S, Rotilio G, Ciriolo MR. Role of nitric oxide synthases in Parkinson's disease: a review on the antioxidant and anti-inflammatory activity of polyphenols. Neurochem Res. 2008;33:2416–2426. doi: 10.1007/s11064-008-9697-6. [DOI] [PubMed] [Google Scholar]
- Aviram M, Dornfeld L. Pomegranate juice consumption inhibits serum angiotensin converting enzyme activity and reduces systolic blood pressure. Atherosclerosis. 2001;158:195–198. doi: 10.1016/s0021-9150(01)00412-9. [DOI] [PubMed] [Google Scholar]
- Aviram M, Dornfeld L, Kaplan M, Coleman R, Gaitini D, Nitecki S, Hofman A, Rosenblat M, Volkova N, Presser D, Attias J, Hayek T, Fuhrman B. Pomegranate juice flavonoids inhibit low-density lipoprotein oxidation and cardiovascular diseases: studies in atherosclerotic mice and in humans. Drugs Exp Clin Res. 2002;28:49–62. [PubMed] [Google Scholar]
- Aviram M, Dornfeld L, Rosenblat M, Volkova N, Kaplan M, Coleman R, Hayek T, Presser D, Fuhrman B. Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: studies in humans and in atherosclerotic apolipoprotein E-deficient mice. Am J Clin Nutr. 2000;71:1062–1076. doi: 10.1093/ajcn/71.5.1062. [DOI] [PubMed] [Google Scholar]
- Banerjee AK, Mandal A, Chanda D, Chakraborti S. Oxidant, antioxidant and physical exercise. Mol Cell Biochem. 2003;253:307–312. doi: 10.1023/a:1026032404105. [DOI] [PubMed] [Google Scholar]
- Ben J, Soares FM, Scherer EB, Cechetti F, Netto CA, Wyse AT. Running exercise effects on spatial and avoidance tasks in ovariectomized rats. Neurobiol Learn Mem. 2010;94:312–317. doi: 10.1016/j.nlm.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Ben Nasr C, Ayed N, Metche M. Quantitative determination of the polyphenolic content of pomegranate peel. Z Lebensm Unters Forsch. 1996;203:374–378. doi: 10.1007/BF01231077. [DOI] [PubMed] [Google Scholar]
- Besnard S, Machado ML, Vignaux G, Boulouard M, Coquerel A, Bouet V, Freret T, Denise P, Lelong-Boulouard V. Influence of vestibular input on spatial and nonspatial memory and on hippocampal NMDA receptors. Hippocampus. 2012;22:814–826. doi: 10.1002/hipo.20942. [DOI] [PubMed] [Google Scholar]
- Block F. Global ischemia and behavioural deficits. Prog Neurobiol. 1999;58:279–295. doi: 10.1016/s0301-0082(98)00085-9. [DOI] [PubMed] [Google Scholar]
- Canal Castro C, Pagnussat AS, Orlandi L, Worm P, Moura N, Etgen AM, Alexandre Netto C. Coumestrol has neuroprotective effects before and after global cerebral ischemia in female rats. Brain Res. 2012;1474:82–90. doi: 10.1016/j.brainres.2012.07.025. [DOI] [PubMed] [Google Scholar]
- Cechetti F, Pagnussat AS, Worm PV, Elsner VR, Ben J, da Costa MS, Mestriner R, Weis SN, Netto CA. Chronic brain hypoperfusion causes early glial activation and neuronal death, and subsequent long-term memory impairment. Brain Res Bull. 2012;87:109–116. doi: 10.1016/j.brainresbull.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Cechetti F, Worm PV, Pereira LO, Siqueira IR, C AN. The modified 2VO ischemia protocol causes cognitive impairment similar to that induced by the standard method, but with a better survival rate. Braz J Med Biol Res. 2010;43:1178–1183. doi: 10.1590/s0100-879x2010007500124. [DOI] [PubMed] [Google Scholar]
- Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- Chun HS, Kim JM, Choi EH, Chang N. Neuroprotective effects of several korean medicinal plants traditionally used for stroke remedy. J Med Food. 2008;11:246–251. doi: 10.1089/jmf.2007.542. [DOI] [PubMed] [Google Scholar]
- Colbourne F, Li H, Buchan AM, Clemens , JA Continuing postischemic neuronal death in CA1: influence of ischemia duration and cytoprotective doses of NBQX and SNX-111 in rats. Stroke. 1999;30:662–668. doi: 10.1161/01.str.30.3.662. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Kehl SJ, McLennan H. The antagonism of amino acid-induced excitations of rat hippocampal CA1 neurones in vitro. J Physiol. 1983;334:19–31. doi: 10.1113/jphysiol.1983.sp014477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Butte-Smith M, Zukin RS, Etgen AM. Effects of global ischemia and estradiol pretreatment on phosphorylation of Akt, CREB and STAT3 in hippocampal CA1 of young and middle-aged female rats. Brain Res. 2012;1471:118–128. doi: 10.1016/j.brainres.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumlu MU, Gurkan E. Elemental and nutritional analysis of Punica granatum from Turkey. J Med Food. 2007;10:392–395. doi: 10.1089/jmf.2006.295. [DOI] [PubMed] [Google Scholar]
- Esposito E, Rotilio D, Di Matteo V, Di Giulio C, Cacchio M, Algeri S. A review of specific dietary antioxidants and the effects on biochemical mechanisms related to neurodegenerative processes. Neurobiol Aging. 2002;23:719–735. doi: 10.1016/s0197-4580(02)00078-7. [DOI] [PubMed] [Google Scholar]
- Evans PH. Free radicals in brain metabolism and pathology. Br Med Bull. 1993;49:577–587. doi: 10.1093/oxfordjournals.bmb.a072632. [DOI] [PubMed] [Google Scholar]
- Floyd , RA Antioxidants, oxidative stress, and degenerative neurological disorders. Proc Soc Exp Biol Med. 1999;222:236–245. doi: 10.1046/j.1525-1373.1999.d01-140.x. [DOI] [PubMed] [Google Scholar]
- Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- Gil MI, Tomas-Barberan FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;48:4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- Godino Mdel C, Romera VG, Sanchez-Tomero JA, Pacheco J, Canals S, Lerma J, Vivancos J, Moro MA, Torres M, Lizasoain I, Sanchez-Prieto J. Amelioration of ischemic brain damage by peritoneal dialysis. J Clin Invest. 2013;123:4359–4363. doi: 10.1172/JCI67284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenamyre JT, Olson JM, Penney JB Jr, Young AB. Autoradiographic characterization of N-methyl-D-aspartate-, quisqualate- and kainate-sensitive glutamate binding sites. J Pharmacol Exp Ther. 1985;233:254–263. [PubMed] [Google Scholar]
- Hashimoto T, Yonetani M, Nakamura H. Selective brain hypothermia protects against hypoxic-ischemic injury in newborn rats by reducing hydroxyl radical production. Kobe J Med Sci. 2003;49:83–91. [PubMed] [Google Scholar]
- Howard RS, Holmes PA, Siddiqui A, Treacher D, Tsiropoulos I, Koutroumanidis M. Hypoxic-ischaemic brain injury: imaging and neurophysiology abnormalities related to outcome. QJM. 2012;105:551–561. doi: 10.1093/qjmed/hcs016. [DOI] [PubMed] [Google Scholar]
- Ito U, Spatz M, Walker JT Jr, Klatzo I. Experimental cerebral ischemia in mongolian gerbils. I. Light microscopic observations. Acta Neuropathol. 1975;32:209–223. doi: 10.1007/BF00696570. [DOI] [PubMed] [Google Scholar]
- Jee YS, Ko IG, Sung YH, Lee JW, Kim YS, Kim SE, Kim BK, Seo JH, Shin MS, Lee HH, Cho HJ, Kim CJ. Effects of treadmill exercise on memory and c-Fos expression in the hippocampus of the rats with intracerebroventricular injection of streptozotocin. Neurosci Lett. 2008;443:188–192. doi: 10.1016/j.neulet.2008.07.078. [DOI] [PubMed] [Google Scholar]
- Kaplan M, Hayek T, Raz A, Coleman R, Dornfeld L, Vaya J, Aviram M. Pomegranate juice supplementation to atherosclerotic mice reduces macrophage lipid peroxidation, cellular cholesterol accumulation and development of atherosclerosis. J Nutr. 2001;131:2082–2089. doi: 10.1093/jn/131.8.2082. [DOI] [PubMed] [Google Scholar]
- Kaur A, Jindal S, Kaur IP, Chopra K. Effect of sesamol on the pathophysiological changes induced by surgical menopause in rodents. Climacteric. 2013;16:426–437. doi: 10.3109/13697137.2012.696292. [DOI] [PubMed] [Google Scholar]
- Kim ND, Mehta R, Yu W, Neeman I, Livney T, Amichay A, Poirier D, Nicholls P, Kirby A, Jiang W, Mansel R, Ramachandran C, Rabi T, Kaplan B, Lansky E. Chemopreventive and adjuvant therapeutic potential of pomegranate (Punica granatum) for human breast cancer. Breast Cancer Res Treat. 2002;71:203–217. doi: 10.1023/a:1014405730585. [DOI] [PubMed] [Google Scholar]
- Lau FC, Shukitt-Hale B, Joseph JA. The beneficial effects of fruit polyphenols on brain aging. Neurobiol Aging. 2005;26(Suppl 1):128–132. doi: 10.1016/j.neurobiolaging.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Sugden SG, Davis RR, Gregg JP, Lavenex PB. Developmental regulation of gene expression and astrocytic processes may explain selective hippocampal vulnerability. Hippocampus. 2011;21:142–149. doi: 10.1002/hipo.20730. [DOI] [PubMed] [Google Scholar]
- Levy DE, Caronna JJ, Singer BH, Lapinski RH, Frydman H, Plum F. Predicting outcome from hypoxic-ischemic coma. JAMA. 1985;253:1420–1426. [PubMed] [Google Scholar]
- Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- Mahut H, Zola-Morgan S, Moss M. Hippocampal resections impair associative learning and recognition memory in the monkey. J Neurosci. 1982;2:1214–1220. doi: 10.1523/JNEUROSCI.02-09-01214.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri MT, Farbood Y, Sameri MJ, Sarkaki A, Naghizadeh B, Rafeirad M. Neuroprotective effects of oral gallic acid against oxidative stress induced by 6-hydroxydopamine in rats. Food chemistry. 2013;138:1028–1033. doi: 10.1016/j.foodchem.2012.11.022. [DOI] [PubMed] [Google Scholar]
- McCarty MF. Up-regulation of endothelial nitric oxide activity as a central strategy for prevention of ischemic stroke - just say NO to stroke! Med Hypotheses. 2000;55:386–403. doi: 10.1054/mehy.2000.1075. [DOI] [PubMed] [Google Scholar]
- Meyer FB, Sundt TM Jr, Yanagihara T, Anderson RE. Focal cerebral ischemia: pathophysiologic mechanisms and rationale for future avenues of treatment. Mayo Clin Proc. 1987;62:35–55. doi: 10.1016/s0025-6196(12)61523-7. [DOI] [PubMed] [Google Scholar]
- Navarro A, Del Valle E, Ordonez C, Martinez E, Perez C, Alonso A, Gonzalez C, Tolivia J. Aging and substitutive hormonal therapy influence in regional and subcellular distribution of ERalpha in female rat brain. Age (Dordr) 2013;35:821–837. doi: 10.1007/s11357-012-9415-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J, Conway DH. Hippocampal place units in the freely moving rat: why they fire where they fire. Exp Brain Res. 1978;31:573–590. doi: 10.1007/BF00239813. [DOI] [PubMed] [Google Scholar]
- Pilsakova L, Riecansky I, Jagla F. The physiological actions of isoflavone phytoestrogens. Physiol Res. 2010;59:651–664. doi: 10.33549/physiolres.931902. [DOI] [PubMed] [Google Scholar]
- Planton M, Peiffer S, Albucher JF, Barbeau EJ, Tardy J, Pastor J, Januel AC, Bezy C, Lemesle B, Puel M, Demonet JF, Chollet F, Pariente J. Neuropsychological outcome after a first symptomatic ischaemic stroke with 'good recovery'. Eur J Neurol. 2012;19:212–219. doi: 10.1111/j.1468-1331.2011.03450.x. [DOI] [PubMed] [Google Scholar]
- Qu N, Wang L, Liu ZC, Tian Q, Zhang Q. Oestrogen receptor alpha agonist improved long-term ovariectomy-induced spatial cognition deficit in young rats. Int J Neuropsychopharmacol. 2013;16:1071–1082. doi: 10.1017/S1461145712000958. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. Estrogen has mnemonic-enhancing effects in the inhibitory avoidance task. Pharmacol Biochem Behav. 2004;78:551–558. doi: 10.1016/j.pbb.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Sarkaki A, Badavi M, Hoseiny N, Gharibnaseri M, Rahim F. Postmenopausal effects of intrastriatal estrogen on catalepsy and pallidal electroencephalogram in an animal model of Parkinson's disease. Neuroscience. 2008;154:940–945. doi: 10.1016/j.neuroscience.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Sarkaki A, Hajipour S, Mansouri MT, Pilevarian A, Rad MR. Pomegranate seed hydroalcoholic extract improves memory deficit due to permanent cerebral hypoperfusion/ischemia in male rats. HealthMed. 2013a;7:863–871. [Google Scholar]
- Sarkaki A, Norooz Zare F, Farbood Y, Pileverian AA. Impaired movements in 6-OHDA induced Parkinson's rat model improves by pomegranate seed hydroalcoholic extract. HealthMed. 2013b;7:348–358. [Google Scholar]
- Sarkaki A, Rezaiei M. Improving Active and Passive Avoidance Memories Deficits Due to Permanent Cerebral Ischemia by Pomegranate Seed Extract in Female Rats. The Malaysian journal of medical sciences: MJMS. 2013;20:25–35. [PMC free article] [PubMed] [Google Scholar]
- Schilling T, Ebert R, Raaijmakers N, Schutze N, Jakob F. Effects of phytoestrogens and other plant-derived compounds on mesenchymal stem cells, bone maintenance and regeneration. The Journal of steroid biochemistry and molecular biology. 2014;139:252–261. doi: 10.1016/j.jsbmb.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Scott EL, Zhang QG, Han D, Desai BN, Brann DW. Long-term estrogen deprivation leads to elevation of Dickkopf-1 and dysregulation of Wnt/beta-Catenin signaling in hippocampal CA1 neurons. Steroids. 2013;78:624–632. doi: 10.1016/j.steroids.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvamani A, Sohrabji F. The neurotoxic effects of estrogen on ischemic stroke in older female rats is associated with age-dependent loss of insulin-like growth factor-1. J Neurosci. 2010;30:6852–6861. doi: 10.1523/JNEUROSCI.0761-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheorajpanday RV, Nagels G, Weeren AJ, van Putten MJ, De Deyn PP. Quantitative EEG in ischemic stroke: correlation with functional status after 6 months. Clin Neurophysiol. 2011;122:874–883. doi: 10.1016/j.clinph.2010.07.028. [DOI] [PubMed] [Google Scholar]
- Takeda A, Tamano H, Tochigi M, Oku N. Zinc homeostasis in the hippocampus of zinc-deficient young adult rats. Neurochem Int. 2005;46:221–225. doi: 10.1016/j.neuint.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Tamburella A, Micale V, Mazzola C, Salomone S, Drago F. The selective norepinephrine reuptake inhibitor atomoxetine counteracts behavioral impairments in trimethyltin-intoxicated rats. Eur J Pharmacol. 2012;683:148–154. doi: 10.1016/j.ejphar.2012.02.045. [DOI] [PubMed] [Google Scholar]
- van Elswijk DA, Schobel UP, Lansky EP, Irth H, van der Greef J. Rapid dereplication of estrogenic compounds in pomegranate (Punica granatum) using on-line biochemical detection coupled to mass spectrometry. Phytochemistry. 2004;65:233–241. doi: 10.1016/j.phytochem.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Varadinova MG, Docheva-Drenska DI, Boyadjieva NI. Effects of anthocyanins on learning and memory of ovariectomized rats. Menopause. 2009;16:345–349. doi: 10.1097/gme.0b013e3181847619. [DOI] [PubMed] [Google Scholar]
- Yu X, Wang LN, Du QM, Ma L, Chen L, You R, Liu L, Ling JJ, Yang ZL, Ji H. Akebia Saponin D attenuates amyloid beta-induced cognitive deficits and inflammatory response in rats: Involvement of Akt/NF-kappaB pathway. Behav Brain Res. 2012;235:200–209. doi: 10.1016/j.bbr.2012.07.045. [DOI] [PubMed] [Google Scholar]