Abstract
The cephalosporin antibiotic ceftriaxone was evaluated as a potential therapeutic agent for the treatment of amyotrophic lateral sclerosis (ALS). The pharmacokinetics (PK) of ceftriaxone in plasma and cerebrospinal fluid (CSF) were investigated in 66 participants in a previously reported clinical trial. Their mean age was 51 years, and 65 % were male. Participants were randomly assigned to one of three treatment groups receiving intravenous infusions (mean duration: 25 minutes) every 12 hours of either: placebo and placebo; 2 grams ceftriaxone and placebo; or 2 grams ceftriaxone twice. Mean steady-state plasma PK variables were: volume of distribution, 14 liters (0.17 liters/kg); elimination half-life, 8 - 9 hours; total clearance, 17-21 mL/min (0.22 - 0.25 mL/min/kg). Values were not different between dosage groups. CSF PK analysis, determined through sparse CSF sampling, indicated apparent entry and elimination half-life values of 1.0 and 34 hours, respectively. With both dosage regimens, CSF concentrations were maintained above the target threshold of 1.0 μM (0.55 μg/mL) as determined from in vitro models. The plasma and CSF PK profile of ceftriaxone were used as a basis for planning the Phase 3 clinical trial of ceftriaxone in ALS.
Keywords: Amyotrophic lateral sclerosis, Ceftriaxone, Plasma pharmacokinetics, Cerebrospinal fluid uptake, HPLC Micromethod
Introduction
Amyotrophic lateral sclerosis (ALS) is a neurological disease that causes muscle weakness, disability, and eventually death by progressive loss of spinal and cortical motor neurons. ALS prevalence is approximately 5.2 people per 100,000 in western countries. The median age of onset for sporadic ALS is 64 years, and the average survival is 3-5 years 1. The incidence of ALS is __ greater in men than in women (M:F = 1.5 : 1). The cause of ALS is not fully known, but glutamate excitotoxicity may be a factor in disease progression2, 3.
Screening studies have indicated that beta-lactam antibiotics are active in the majority of ALS-related assays particularly those related to glutamate toxicity4-6. Ceftriaxone, an FDA-approved beta-lactam antibiotic, is neuroprotective in many in vitro and in vivo models by reducing glutamate excitoxicity7-9. Preclinical studies highlight ceftriaxone as a potential anti-excitoxicity therapy for patients with ALS4, 6, 7, 9-11 Ceftriaxone has a relatively long half-life (6 -9 hours in blood12-16 and 17 hours in CSF17,18), excellent penetration into extracellular fluid17-28 and extensive serum protein binding which is reported to be saturable 29-31. The actual CNS/CSF concentration necessary to be neuroprotective in human brain or to induce human excitatory amino acid transporter 2 (EAAT2) in vivo is not clearly established, and it is not known how CSF concentrations reflect concentrations in brain tissue. In any case, in vitro models suggest that ceftriaxone concentrations of at least 1 micromolar are effective in increasing expression of glutamate transporter 1, and in preventing toxicity caused by threo-hydroxyaspartate6-8.
As there is a large body of research supporting a possible neuroprotective role for ceftriaxone, understanding of the pharmacokinetics of ceftriaxone in patients with ALS is of importance in designing clinical dosage regimens. A recently completed randomized controlled trial evaluated the clinical efficacy and pharmacokinetics of ceftriaxone in patients with ALS3. A summary of the PK data has been previously reported. In this paper, we report a more detailed analysis of the pharmacokinetic profile of ceftriaxone in these patients.
Methods
Clinical Study Design
At screening, eligible participants had a diagnosis of probable or definite ALS by El Escorial Criteria28, a vital capacity (VC) ≥60% of the predicted normal value for height, age and gender, symptom duration of less than 3 years, and were either not on riluzole or were on a stable dose of riluzole for ≥ 30 days. Exclusion criteria included use of mechanical ventilation, known sensitivity to beta-lactam antibiotics, pregnancy, exposure to investigational agents within 30 days of screening, active gastrointestinal or biliary disease within 30 days of screening, history of antibiotic-induced colitis, or clinically significant abnormal safety laboratory values. Other screening procedures are described previously3.
The study (IND #68892) was approved by the Massachusetts General Hospital (MGH) Coordination Center Institutional Review Board (IRB) and by all participating center IRBs, including Harvard Partners, State University of New York Upstate Medical University, Washington University in St. Louis, Wake Forest University, California Pacific Medical Center, Methodist Neurological Institute, Carolinas Medical Center, Indiana University, Emory University, and University of Chicago. The study was listed on clinicaltrials.gov (NCT00349622).
66 subjects at ten clinical sites were enrolled and randomized equally into three study groups receiving intravenous infusions (mean duration: 25 minutes) every 12 hours (Table 1). A single-lumen tunneled central venous (CV) catheter was placed by trained personnel prior to randomization, for purposes of medication administration. The three treatment groups were:
Placebo in the morning and evening (Twice daily);
Two grams of ceftriaxone in the morning, and placebo in the evening (Total dose of 2 grams daily);
Two grams of ceftriaxone in the morning and evening (Total dose of 4 grams daily).
Table 1. Inter- and Intra-day Accuracy and Precision of Ceftriaxone Assay.
| Plasma Ceftriaxone | CSF Ceftriaxone | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Nominal Conc. (ug/mL) | Mean Measured Conc. (ug/mL) | Accuracy (%) | Precision (%) | Nominal Conc. (ug/mL) | Mean Measured Conc. (ug/mL) | Accuracy (%) | Precision (%) |
| Inter-day (n=6) | Inter-day (n=6) | ||||||
| 4 | 3.9 | 97.3% | 2.9% | 0.5 | 0.5 | 99.2% | 0.2% |
| 40 | 38.7 | 96.8% | 1.2% | 2.5 | 2.52 | 100.9% | 0.7% |
| 160 | 160.8 | 100.1% | 2.0% | 15 | 14.9 | 99.3% | 1.2% |
| Intra-day (n=6) | Intra-day (n=6) | ||||||
| 4 | 3.6 | 89.0% | 8.1% | 0.5 | 0.48 | 95.4% | 0.4% |
| 40 | 38.5 | 95.5% | 9.3% | 2.5 | 2.47 | 98.8% | 1.2% |
| 160 | 152.6 | 93.3% | 4.1% | 15 | 14.9 | 99.8% | 2.7% |
|
| |||||||
| QC Sample (inter-day) | |||||||
| 5 | 4.7 | 94.1% | 7.6% | ||||
| 50 | 48.5 | 97.0% | 5.3% | ||||
Placebo was pediatric multivitamin matching the study drug in appearance, taste, and odor. The study medication (ceftriaxone) was supplied by Baxter Healthcare Corporation. To assure that the steady state condition had been reached, the study day was always scheduled after at least 7 consecutive days of daily ceftriaxone or placebo dosage, Blood samples were obtained from a peripheral sampling site before the dose and at the following times after the start of the infusion: 0.5, 1, 2, 3, 4, 6, 8, 10 and 12 hours. Blood samples were collected in heparinized glass tubes and centrifuged immediately. Plasma specimens were transferred to glass scintillation vials, which were stored at -20°C until assayed.
The study day also included sampling of CSF by lumbar puncture. All subjects had a sample drawn prior to ceftriaxone dosing. A second CSF sample was drawn for each subject at a single post-dosage time of either 2, 4, 6, 8 or 10 hours after morning dosing. The specific time was based on random assignment. CSF specimens were frozen at -20 °C for analysis.
Analytic Method for Ceftriaxone in Plasma and CSF
Reagents and Chemicals
Ceftriaxone sodium salt and cefazolin sodium salt (the internal standard) were supplied by Sigma (St. Louis, MO. USA). Ammonium acetate, phosphoric acid, chloroform and triethanolamine were HPLC grade, obtained from Fisher Scientific Co., Waltham, MA USA. Acetonitrile and methanol (HPLC grade) also came from Fisher. All solvents and reagents were of analytical grade unless indicated otherwise. The solutions were prepared with deionized water (Milli-Q-quality).
Chromatographic Conditions
The system consisted of an Agilent 1100 HPLC equipped with a quaternary pump, a preparative auto-sampler, a micro vacuum degasser, and a variable wavelength detector. The system control and data processing were performed on Chemstation 32 software (Agilent, USA). The chromatographic separation was achieved on a reverse phase Waters® μBondapak™ C18 (3.9 × 300mm, 10μm 125A) column protected by a Phenomenex® guard cartridge.
The mobile phase consisted of water, methanol and triethylamine in proportions of 750:250:4 (V:V:V), the pH of which was adjusted to 3 by phosphoric acid. The UV detector was set at 270 nm, and the flow rate was 1 mL/min.
The total elution time is 20 min, with retention times of ceftriaxone at 8.3 min and cefazolin at 14.6 min.
Sample preparation
100 uL of plasma or CSF in a 2.0 mL conical centrifuge tube and 100 uL of 0.1 M ammonium acetate buffer (pH 5) containing cefazolin were combimed with 500 uL of acetonitrile, mixed in a vortex mixer for 1 minute, and centrifuged for 6 min at 14×1000 min-1 (Eppendorf Centrifuge 5415C). The clear supernatant was transferred to another conical centrifuge tube with an additional of 500 uL of chloroform, mixed in a vortex mixer for 1 min, and centrifuged for 6 min. 15 uL of the upper aqueous phase was injected onto the HPLC system32.
Preparation of Standards
The stock solution of ceftriaxone (0.5 mg/ml) was prepared in 50 mM phosphate buffer with pH=7.4 and then serially diluted to obtain working solutions. The stock solution of cefazolin (100 ng/mL) as internal standard was in 20 mM ammonium acetate. The criteria for the calibration standards for both matrices were at least 6 to 8 calibration points within 10% relative error (within 20% at LLQ). The above stock solutions and working solution were all stored at 4 °C.
Linearity and Limit of Quantification (LOQ)
Linearity of the calibration curve for plasma was proven from 5 to 400 μg/mL with regression coefficients of 0.9996. The range of linearity of calibration for CSF was 0.25 to 20 μg/mL with regression coefficients of 0.9998. The limit of quantification (LOQ) of the HPLC system was 5 ug/mL for plasma and 0.25 μg/mL for CSF.
Intra- and Inter-day Variability
Intra- and Inter-day accuracy and precision of the method were 93.3-100.1% and 1.2%-9.3% for plasma, and 95.4% - 100.9% and 0.2% -2.7% for CSF respectively (Table 1).
Quality control samples at two concentrations (5 and 50 μg/mL) were analyzed each day. The criteria for accuracy of the quality control samples was relative error <±15% for at least 2 samples at each of the 2 concentrations.
Data analysis
The pharmacokinetic profile of ceftriaxone in plasma was found to be consistent with a two-compartment model, and the following core equation33:
This equation was appropriately modified based on the assumption that all patients were in the steady-state condition. A zero order ceftriaxone infusion of duration INFT was assumed. During the infusion (0 ≤ t ≤ INFT), the concentration-time relationship is described by the following equation:
After termination of the infusion (INFT ≤ t ≤ TAU), the applicable equation is:
INFT is the infusion of time as described above, TAU is the interval between doses, and α and β are hybrid exponents having units of reciprocal time. The parameters U1 through U4 are defined as following:
V1 is the central compartment volume, and K21is the rate constant for drug transfer from peripheral to central compartment.
The equations, during and after infusion, were simultaneously fitted to data points by nonlinear regression, using Statistical Analysis System (SAS) PROC NLIN. Residual errors were weighted by the reciprocal of the observed concentration. Iterated variables were V1, K21, α, and β.
Parameters from the fitted function were used to determine the central compartment volume (V1), the total volume of distribution by the area method (Vd) calculated as (α·V1)/K21, the apparent half-life values of distribution and elimination (t1/2α, t1/2β) and total clearance calculated as Vd·β.
Because only one CSF sample was available per patient, all CSF values were analyzed in aggregate. CSF ceftriaxone concentrations were analyzed by nonlinear regression, assuming first-order entry into and removal from CSF. All data points at each dosage level were assumed to be independent, and analyzed simultaneously. The following equation was fitted to the data points:
Iterated variables were: B, a coefficient having units of concentration; Kout, the apparent rate constant for ceftriaxone efflux from CSF; and Kin, the apparent rate constant for entry into CSF. TAU was the interval between doses as described above.
For each subject, the CSF/plasma ratio was calculated as the CSF concentration divided by the plasma concentration determined at the same sampling time.
Statistical Analysis
Statistics methods included Student's t-test and linear regression.
Results
The analytical method was validated based on FDA industry guidelines for bioanalytical method validation for selectivity, linearity within the expected concentration range, recovery, precision, sensitivity, and stability. Relative standard deviations for between-day and within-day assays were lower than 9.3% for plasma and 2.7% for CSF (Table 1). The precision of quality control samples, done along with each analytical run, were 7.6% and 5.3% at concentrations of 5 μg/mL and 50 μg/mL respectively. Studies of drug stability during sample storage, sample preparation, and chromatography showed no degradation of ceftriaxone and the internal standard cefazolin.
A total of 66 patients participated in the study. Their overall mean age was 51 years, and 65% were male. The mean time elapsed since symptom onset was 1.5 years, with an average of 10 months since diagnosis, and an average of 18 months between symptom onset to screening for this study. None of the demographic characteristics differed among in the three groups.
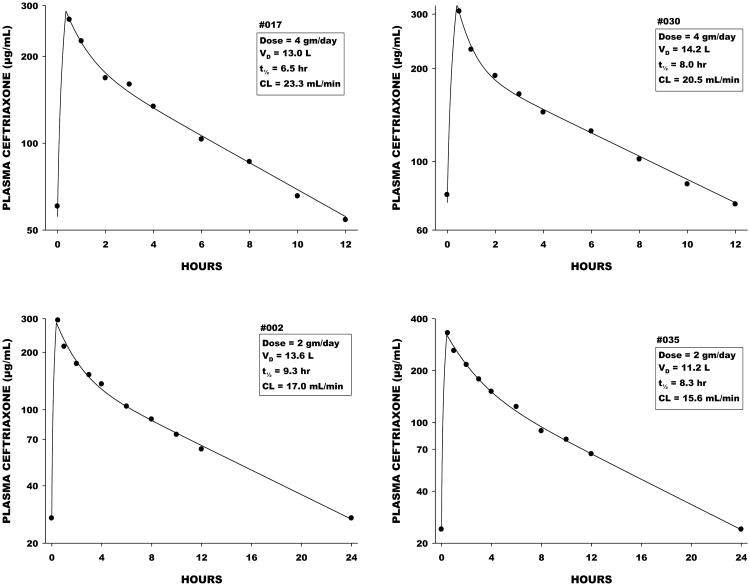
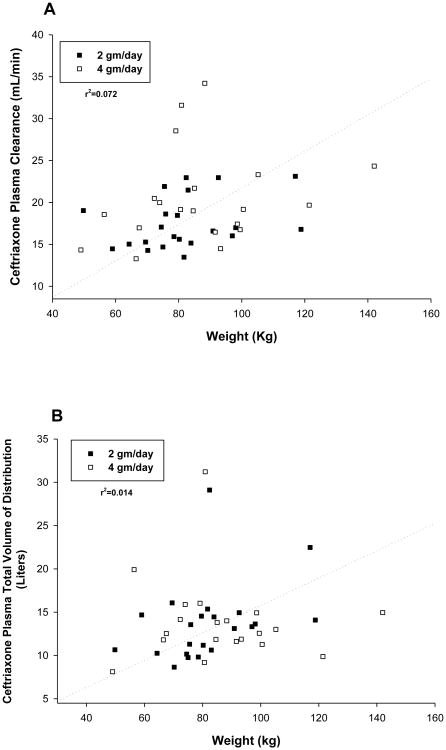
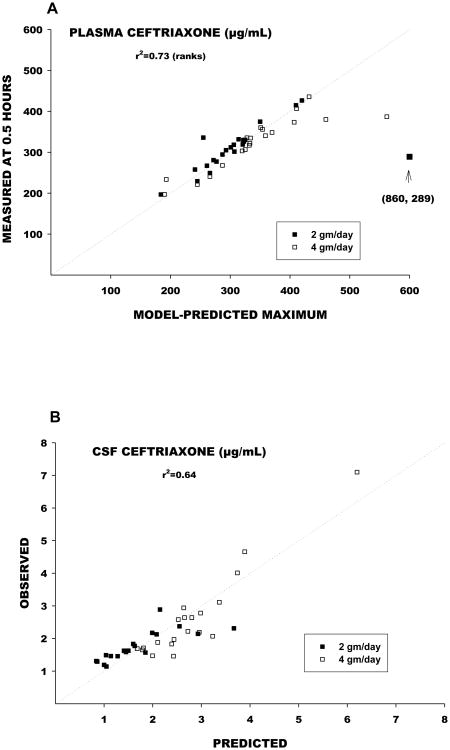
A total of 22 patients were randomized to receive the ceftriaxone dosage of 2 grams every 24 hours, and 20 received 2 grams every 12 hours. Figure 1 shows plasma ceftriaxone concentrations and pharmacokinetic functions determined by nonlinear regression analysis for representative patients in each group. Individual and mean pharmacokinetic variables are shown in Table 2. Vd averaged approximately 14 liters, elimination half-life 8 to 9 hours, and total clearance 17 to 21 mL/min. Body weight accounted for an insignificant fraction of the overall variance in Vd and clearance (Figure 2). Observed maximum plasma ceftriaxone concentrations were closely correlated with maximum concentrations predicted by the model (Figure 3).
Figure 1.
Representative plasma concentration data for patients receiving 2 grams every 12 hours (above) and 2 grams every 24 hours (below). The pictures show the actual plasma concentrations, along with the pharmacokinetic functions determined by nonlinear regression. Within the boxes are the calculated pharmacokinetic variables.
Table 2. Individual Pharmacokinetic Parameters for Ceftriaxone.
| Volumes of Distribution | Half-life | Clearance | r2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| Central | Total | Distribution | Elimination | ||||||
|
|
|
||||||||
| Patient | Dose (gm/day) | Baseline Weight (kg) | INFT (hr) | (Liters) | (Liters) | (hr) | (hr) | (mL/min) | |
| 002 | 2 | 98.1 | 0.367 | 7.1 | 13.6 | 0.96 | 9.3 | 17.0 | 1.00 |
| 005 | 2 | 49.8 | 0.417 | 6.2 | 10.7 | 1.46 | 6.5 | 19.0 | 0.99 |
| 007 | 2 | 83.9 | 0.333 | 6.9 | 14.5 | 0.96 | 11.0 | 15.2 | 0.99 |
| 012 | 2 | 117 | 0.417 | 11.7 | 22.5 | 1.75 | 11.2 | 23.1 | 0.99 |
| 015 | 2 | 75.5 | 0.417 | 8.0 | 11.3 | 0.98 | 6.0 | 21.9 | 0.99 |
| 018 | 2 | 74.5 | 0.367 | 6.1 | 10.2 | 1.10 | 6.9 | 17.1 | 0.99 |
| 019 | 2 | 59 | 0.433 | 5.9 | 14.7 | 1.25 | 11.7 | 14.5 | 0.99 |
| 025 | 2 | 78.5 | 0.367 | 0.6 | 9.8 | 0.05 | 7.1 | 15.9 | 1.00 |
| 029 | 2 | 69.5 | 0.533 | 8.0 | 16.1 | 3.05 | 12.2 | 15.3 | 1.00 |
| 032 | 2 | 90.9 | 0.450 | 6.2 | 13.1 | 1.57 | 9.1 | 16.6 | 1.00 |
| 035 | 2 | 80.2 | 0.417 | 6.3 | 11.2 | 1.31 | 8.3 | 15.6 | 1.00 |
| 037 | 2 | 75 | 0.500 | 6.1 | 9.8 | 0.79 | 7.7 | 14.7 | 1.00 |
| 040 | 2 | 97 | 0.500 | 7.4 | 13.3 | 0.71 | 9.6 | 16.0 | 0.99 |
| 045 | 2 | 79.6 | 0.367 | 8.0 | 14.5 | 1.98 | 9.1 | 18.5 | 1.00 |
| 048 | 2 | 81.7 | 0.500 | 7.1 | 15.4 | 1.63 | 13.2 | 13.5 | 0.99 |
| 049 | 2 | 92.6 | 0.350 | 7.1 | 14.9 | 1.22 | 7.5 | 23.0 | 0.99 |
| 052 | 2 | 75.9 | 0.400 | 8.6 | 13.6 | 1.39 | 8.4 | 18.6 | 0.96 |
| 055 | 2 | 83 | 0.417 | 6.6 | 10.6 | 1.38 | 5.7 | 21.5 | 0.98 |
| 060 | 2 | 70.2 | 0.417 | 4.7 | 8.7 | 0.81 | 7.0 | 14.3 | 1.00 |
| 063 | 2 | 118.8 | 0.400 | 6.6 | 14.1 | 0.83 | 9.7 | 16.8 | 0.98 |
| 065 | 2 | 82.4 | 0.417 | 8.0 | 29.1 | 1.55 | 14.6 | 23.0 | 0.91 |
| 069 | 2 | 64.3 | 0.583 | 4.4 | 10.3 | 1.14 | 7.9 | 15.0 | 0.97 |
| Mean | 81.7 | 0.426 | 6.7 | 13.7 | 1.27 | 9.1 | 17.5 | ||
| SD | 16.4 | 0.063 | 2.0 | 4.6 | 0.58 | 2.4 | 3.1 | ||
| SE | 3.5 | 0.013 | 0.4 | 1.0 | 0.12 | 0.5 | 0.7 | ||
| n | 22 | 22 | 22 | 22 | 22 | 22 | 22 | ||
|
| |||||||||
| 003 | 4 | 85 | 0.417 | 6.3 | 13.8 | 0.34 | 7.4 | 21.7 | 0.99 |
| 004 | 4 | 80.9 | 0.417 | 8.9 | 31.2 | 0.93 | 11.4 | 31.6 | 0.98 |
| 009 | 4 | 84.6 | 0.417 | 5.9 | 11.9 | 0.43 | 7.2 | 19.0 | 0.99 |
| 010 | 4 | 49 | 0.333 | 5.7 | 8.1 | 0.29 | 6.6 | 14.3 | 0.99 |
| 013 | 4 | 88.3 | 0.483 | 14.0 | 4.7 | 34.2 | 0.91 | ||
| 017 | 4 | 105.2 | 0.350 | 7.8 | 13.0 | 0.56 | 6.4 | 23.3 | 1.00 |
| 021 | 4 | 98.6 | 0.450 | 6.9 | 14.9 | 0.99 | 9.9 | 17.4 | 0.99 |
| 024 | 4 | 99.5 | 0.433 | 6.9 | 12.6 | 0.97 | 8.7 | 16.8 | 0.99 |
| 026 | 4 | 142.0 | 0.417 | 15.0 | 7.1 | 24.3 | 0.99 | ||
| 030 | 4 | 72.3 | 0.400 | 6.6 | 14.2 | 0.41 | 8.0 | 20.5 | 1.00 |
| 033 | 4 | 91.6 | 0.433 | 7.7 | 11.6 | 1.05 | 8.2 | 16.5 | 0.99 |
| 034 | 4 | 80.6 | 0.417 | 2.7 | 9.2 | 0.12 | 5.5 | 19.1 | 0.99 |
| 039 | 4 | 67.5 | 0.417 | 6.4 | 12.5 | 1.03 | 8.5 | 17.0 | 0.99 |
| 041 | 4 | 93.3 | 0.417 | 5.9 | 11.9 | 0.73 | 9.5 | 14.5 | 0.98 |
| 043 | 4 | 66.5 | 0.433 | 5.8 | 11.8 | 0.76 | 10.3 | 13.3 | 0.99 |
| 050 | 4 | 100.5 | 0.467 | 6.6 | 11.3 | 0.59 | 6.8 | 19.2 | 0.99 |
| 054 | 4 | 79.1 | 0.417 | 6.7 | 16.0 | 0.24 | 6.5 | 28.5 | 0.97 |
| 057 | 4 | 74 | 0.417 | 7.0 | 15.9 | 1.38 | 9.2 | 20.0 | 0.99 |
| 061 | 4 | 56.4 | 0.417 | 7.4 | 19.9 | 2.01 | 12.4 | 18.6 | 0.99 |
| 064 | 4 | 121.4 | 0.383 | 1.9 | 9.9 | 0.11 | 5.8 | 19.7 | 1.00 |
| Mean | 86.8 | 0.417 | 6.3 | 13.9 | 0.72 | 8.0 | 20.5 | ||
| SD | 21.5 | 0.034 | 1.7 | 4.9 | 0.48 | 2.0 | 5.6 | ||
| SE | 4.8 | 0.008 | 0.4 | 1.1 | 0.11 | 0.4 | 1.2 | ||
| n | 20 | 20 | 18 | 20 | 18 | 20 | 20 | ||
Figure 2.
Relation of ceftriaxone plasma volume of clearance (A) and distribution (B) to body weight.
Figure 3.
A: The relation between maximum plasma concentrations for each individual patient as predicted by the model (x-axis) versus the actual measured plasma concentration at 0.5 hours, which in most cases was shortly after termination of the infusion. Notice that in essentially all cases the model correctly predicts the maximum. In a few cases, the model over predicts the maximum. B: Predicted and observed CSF concentrations.
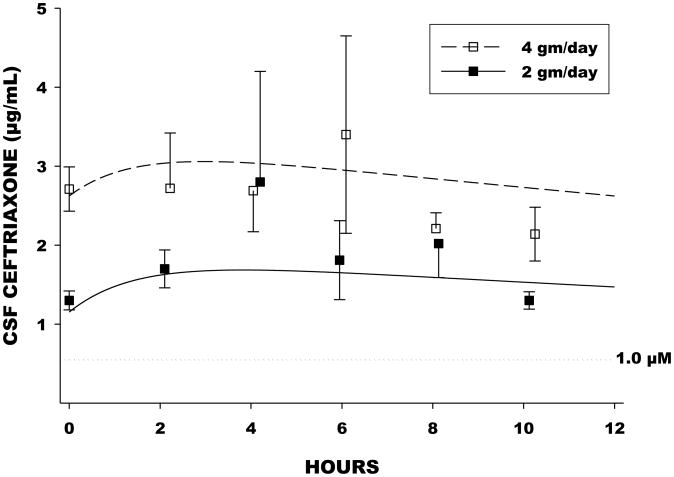
Ceftriaxone slowly entered and slowly effluxed from CSF (Figure 4). The entry rate constant (Kin) from nonlinear regression analysis was 0.693/hr, corresponding to an apparent entry half-life of 1.0 hours. The efflux rate constant (Kout) was 0.0203/hr, corresponding to a half-life of 34 hours. Observed and model-predicted CSF ceftriaxone concentrations were closely correlated (Figure 3). CSF entry of ceftriaxone was incomplete, with an overall CSF/plasma ratio of 0.043.
Figure 4.
Mean(±SE) CSF concentrations at corresponding times and the predicted “typical” concentration curves for the 2 gm/day and 4 gm/day dosage groups based on nonlinear regression. Also shown is the boundary of 1.0 micromolar, equivalent to 0.55μg/mL, repeated to be the effective target concentration based on in vitro studies.
Adverse events associated with the study have been described previously 3. There were no instances of toxicity related to the central nervous system with either dosage regimen, and no evidence of prolonged or excessive accumulation of ceftriaxone in CSF.
Discussion
The present study evaluated the steady-state pharmacokinetics of ceftriaxone in plasma and CSF in the context of an early clinical evaluation of ceftriaxone as a possible pharmacologic treatment of ALS. Candidate participants were randomly assigned to receive 2 grams of ceftriaxone intravenously every 12 hours (4 grams per day), 2 grams for the morning dose and placebo in the evening (2 grams per day), or placebo for both doses. Plasma sampling for the pharmacokinetic study was sufficiently frequent in every patient to allow a full pharmacokinetic profile for each individual. However CSF sampling was necessarily limited, and CSF pharmacokinetic analysis was accordingly based on the aggregated data.
The analysis of the pharmacokinetics of ceftriaxone in plasma assumed a two compartment model, with a further assumption that all patients were at steady-state at the time of the study. Pharmacokinetic parameters for ceftriaxone were consistent with previously published data12-17. Elimination half-life averaged in the range of 8 to 9 hours. Mean total volume of distribution was 14 liters (0.17 liters per kilogram), and total clearance averaged 17 to 21 mL/min (0.22 to 0.26 mL per minute per kilogram). Body weight explained only a small fraction of the variance in volume of distribution and clearance. Pharmacokinetic parameters did not differ significantly between the 2 grams/day and 4 grams/day dosage groups.
Consistent with previous reports17-28 the rate of entry of ceftriaxone into CSF was slow. The efflux rate also was slow, having an apparent half-life considerably longer than that in plasma. The profile yielded a “sustained” CSF concentration pattern over a 24 hour period for both dosing schedules. In both cases CSF concentrations on average remained in excess of the 1.0 micromolar (0.55 micrograms per mL) threshold reported to be the minimum effective concentration for favorable pharmacologic effects in the in vitro ALS models6-11. The CSF profile also allows, in principle, drug “holidays”, such that CSF concentrations remain in excess of 1.0 micromolar even if doses are missed3. It is also important to note that CSF entry of ceftriaxone was incomplete as well as slow. The overall CSF/plasma concentration ratio was approximately 0.04. Overall, 54 % of variability in CSF concentration was explained by plasma concentration. It is likely that the incomplete CSF uptake of ceftriaxone is explained by plasma protein binding, with free fraction previously reported to be in the range of 4 % of the total plasma concentration29-31. Since CSF ordinarily contains low concentration of protein, protein-binding of ceftriaxone in CSF is likely to be negligible.
The present study provided a pharmacokinetic basis for the planning of ceftriaxone dosage schedules for application to the ALS Phase 3 clinical trials. The findings might also be applied to planning of studies evaluating ceftriaxone for other neurological disorders.
Acknowledgments
Financial disclosure: This research was supported by grants from the National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (U01 NS049640-05) and from the Institute of Clinical and Translational Sciences (UL1 TR000448).
References
- 1.Wijesekera LC, Leigh PN. Amyotrophic lateral sclerosis. Orphanet J Rare Dis. 2009;4:3. doi: 10.1186/1750-1172-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothstein JD. Excitotoxic mechanisms in the pathogenesis of amyotrophic lateral sclerosis. Adv Neurol. 1995;68:7–20. [PubMed] [Google Scholar]
- 3.Berry JD, Shefner JM, Conwit R, Schoenfeld D, Keroack M, Felsenstein D, Krivickas L, David WS, Vriesendorp F, Pestronk A, Caress J, Katz J, Simpson E, Rosenfeld J, Pascuzzi R, Glass J, Rezania K, Rothstein J, Greenblatt DJ, Cudkowicz M. Design and initial results of a multi-phase randomized trial of cefriaxone in amyotropic lateral sclerosis. PLoS ONE. 2013;8:e61177. doi: 10.1371/journal.pone.0061177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vincent AM, Backus C, Taubman AA, Feldman EL. Identification of candidate drugs for the treatment of ALS. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6:29–36. doi: 10.1080/14660820510026171. [DOI] [PubMed] [Google Scholar]
- 5.Vincent AM, Sakowski SA, Schuyler A, Feldman EL. Strategic approaches to developing drug treatments for ALS. Drug Discov Today. 2008;13:67–72. doi: 10.1016/j.drudis.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 7.Lipski J, Wan CK, Bai JZ, Pi R, Li D, Donnelly D. Neuroprotective potential of ceftriaxone in in vitro models of stroke. Neuroscience. 2007;146:617–629. doi: 10.1016/j.neuroscience.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Rothstein JD, Kuncl RW. Neuroprotective strategies in a model of chronic glutamate-mediated motor neuron toxicity. J Neurochem. 1995;65:643–651. doi: 10.1046/j.1471-4159.1995.65020643.x. [DOI] [PubMed] [Google Scholar]
- 9.Thöne-Reineke C, Neumann C, Namsolleck P, Schmerbach K, Krikov M, Schefe JH, Lucht K, Hörtnagl H, Godes M, Müller S, Rumschüssel K, Funke-Kaiser H, Villringer A, Steckelings UM, Unger T. The beta-lactam antibiotic, ceftriaxone, dramatically improves survival, increases glutamate uptake and induces neurotrophins in stroke. J Hypertens. 2008;26:2426–2435. doi: 10.1097/HJH.0b013e328313e403. [DOI] [PubMed] [Google Scholar]
- 10.Nizzardo M, Nardini M, Ronchi D, Salani S, Donadoni C, Fortunato F, Colciago G, Falcone M, Simone C, Riboldi G, Govoni A, Bresolin N, Comi GP, Corti S. Beta-lactam antibiotic offers neuroprotection in a spinal muscular atrophy model by multiple mechanisms. Exp Neurol. 2011;229:214–225. doi: 10.1016/j.expneurol.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Traynor BJ, Bruijn L, Conwit R, Beal F, O'Neill G, Fagan SC, Cudkowicz ME. Neuroprotective agents for clinical trials in ALS: a systematic assessment. Neurology. 2006;67:20–27. doi: 10.1212/01.wnl.0000223353.34006.54. [DOI] [PubMed] [Google Scholar]
- 12.Perry TR, Schentag JJ. Clinical use of ceftriaxone: a pharmacokinetic-pharmacodynamic perspective on the impact of minimum inhibitory concentration and serum protein binding. Clin Pharmacokinet. 2001;40:685–694. doi: 10.2165/00003088-200140090-00004. [DOI] [PubMed] [Google Scholar]
- 13.Yuk JH, Nightingale CH, Quintiliani R. Clinical pharmacokinetics of ceftriaxone. Clin Pharmacokinet. 1989;17:223–235. doi: 10.2165/00003088-198917040-00002. [DOI] [PubMed] [Google Scholar]
- 14.Joynt GM, Lipman J, Gomersall CD, Young RJ, Wong EL, Gin T. The pharmacokinetics of once-daily dosing of ceftriaxone in critically ill patients. J Antimicrob Chemother. 2001;47:421–429. doi: 10.1093/jac/47.4.421. [DOI] [PubMed] [Google Scholar]
- 15.Reed MD, Rekate HL, Aronoff SC, Myers CM, Blumer JL. Single-dose plasma and cerebrospinal fluid pharmacokinetics of ceftriaxone in infants and children. Clin Pharm. 1983;2:558–563. [PubMed] [Google Scholar]
- 16.Garot D, Respaud R, Lanotte P, Simon N, Mercier E, Ehrmann S, Perrotin D, Dequin PF, Le Guellec C. Population pharmacokinetics of ceftriaxone in critically ill septic patients: a reappraisal. Br J Clin Pharmacol. 72:758–767. doi: 10.1111/j.1365-2125.2011.04005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nau R, Prange HW, Muth P, Mahr G, Menck S, Kolenda H, Sörgel F. Passage of cefotaxime and ceftriaxone into cerebrospinal fluid of patients with uninflamed meninges. Antimicrob Agents Chemother. 1993;37:1518–1524. doi: 10.1128/aac.37.7.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lutsar I, McCracken GH, Jr, Friedland IR. Antibiotic pharmacodynamics in cerebrospinal fluid. Clin Infect Dis. 1998;27:1117–1127. doi: 10.1086/515003. [DOI] [PubMed] [Google Scholar]
- 19.Chadwick EG, Yogev R, Shulman ST, Weinfeld RE, Patel IH. Single-dose ceftriaxone pharmacokinetics in pediatric patients with central nervous system infections. J Pediatr. 1983;102:134–137. doi: 10.1016/s0022-3476(83)80311-4. [DOI] [PubMed] [Google Scholar]
- 20.Cherubin CE, Eng RH, Norrby R, Modai J, Humbert G, Overturf G. Penetration of newer cephalosporins into cerebrospinal fluid. Rev Infect Dis. 1989;11:526–548. doi: 10.1093/clinids/11.4.526. [DOI] [PubMed] [Google Scholar]
- 21.Chandrasekar PH, Rolston KV, Smith BR, LeFrock JL. Diffusion of ceftriaxone into the cerebrospinal fluid of adults. J Antimicrob Chemother. 1984;14:427–430. doi: 10.1093/jac/14.4.427. [DOI] [PubMed] [Google Scholar]
- 22.Spector R. Ceftriaxone transport through the blood-brain barrier. J Infect Dis. 1987;156:209–211. doi: 10.1093/infdis/156.1.209. [DOI] [PubMed] [Google Scholar]
- 23.Latif R, Dajani AS. Ceftriaxone diffusion into cerebrospinal fluid of children with meningitis. Antimicrob Agents Chemother. 1983;23:46–48. doi: 10.1128/aac.23.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radouane A, Pehourcq F, Tramu G, Creppy EE, Bannwarth B. Influence of lipophilicity on the diffusion of cephalosporins into the cerebrospinal fluid. Fundam Clin Pharmacol. 1996;10:309–313. doi: 10.1111/j.1472-8206.1996.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 25.Lutsar I, Friedland IR. Pharmacokinetics and pharmacodynamics of cephalosporins in cerebrospinal fluid. Clin Pharmacokinet. 2000;39:335–343. doi: 10.2165/00003088-200039050-00003. [DOI] [PubMed] [Google Scholar]
- 26.Sullins AK, Abdel-Rahman SM. Pharmacokinetics of antibacterial agents in the CSF of children and adolescents. Paediatr Drugs. 15:93–117. doi: 10.1007/s40272-013-0017-5. [DOI] [PubMed] [Google Scholar]
- 27.Ogawa M, Suzuki H, Sawada Y, Hanano M, Sugiyama Y. Kinetics of active efflux via choroid plexus of beta-lactam antibiotics from the CSF into the circulation. Am J Physiol. 1994;266:R392–399. doi: 10.1152/ajpregu.1994.266.2.R392. [DOI] [PubMed] [Google Scholar]
- 28.Bradley JS, Farhat C, Stamboulian D, Branchini OG, Debbag R, Compogiannis LS. Ceftriaxone therapy of bacterial meningitis: cerebrospinal fluid concentrations and bactericidal activity after intramuscular injection in children treated with dexamethasone. Pediatr Infect Dis J. 1994;13:724–728. [PubMed] [Google Scholar]
- 29.Popick AC, Crouthamel WG, Bekersky I. Plasma protein binding of ceftriaxone. Xenobiotica. 1987;17:1139–1145. doi: 10.3109/00498258709167406. [DOI] [PubMed] [Google Scholar]
- 30.Stoeckel K, McNamara PJ, Brandt R, Plozza-Nottebrock H, Ziegler WH. Effects of concentration-dependent plasma protein binding on ceftriaxone kinetics. Clin Pharmacol Ther. 1981;29:650–657. doi: 10.1038/clpt.1981.90. [DOI] [PubMed] [Google Scholar]
- 31.Bourget P, Fernandez H, Quinquis V, Delouis C. Pharmacokinetics and protein binding of ceftriaxone during pregnancy. Antimicrob Agents Chemother. 1993;37:54–59. doi: 10.1128/aac.37.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demotes-Mainard FM, Vincon GA, Jarry CH, Albin HC. Micromethod for determination of ceftriaxone in plasma and urine by high-performance liquid chromatography. J Pharm Biomed Anal. 1988;6:407–413. doi: 10.1016/0731-7085(88)80006-2. [DOI] [PubMed] [Google Scholar]
- 33.Morgan DJ, Raymond K. The effect of duration of intravenous infusion on maximum and threshold blood concentrations for drugs exhibiting biexponential elimination kinetics. Journal of Pharmacokinetics and Biopharmaceutics. 1982;10:93–107. doi: 10.1007/BF01059185. [DOI] [PubMed] [Google Scholar]